Abstract
Introduction
Peficitinib is a novel orally bioavailable, once-daily Janus kinase (JAK) inhibitor approved in Japan for the treatment of rheumatoid arthritis (RA). This 2-year extension study of two global phase IIb trials investigated the long-term safety and effectiveness of peficitinib.
Methods
All eligible patients with moderate-to-severe RA including patients in the placebo group who participated in one of two global phase IIb trials (‘with methotrexate’ or ‘without methotrexate’) were included in this 2-year open-label extension study and were converted to peficitinib 100 mg once daily. The primary objective was to evaluate an additional 2 years of safety by assessing treatment-emergent adverse events (AEs) and clinical laboratory evaluations for 105 weeks. Evaluation of an additional 2 years of effectiveness using American College of Rheumatology (ACR) 20/50/70 responses was the exploratory objective.
Results
Overall, 611 patients were enrolled in the extension study: 319 (52.2%) patients completed the study and 292 (48%) discontinued treatment, including for withdrawal of patient consent (n = 96), failure to achieve low disease activity (n = 62), and AE not including death (n = 41). AEs were reported in 463 (76%) patients. The most common AEs (per 100 patient-years) were upper respiratory tract infections (9.9) and urinary tract infections (7.2). Serious AEs were reported in 80 (13%) patients, with incidences per 100 patient-years of serious infections 2.7, herpes zoster 1.5 (including one herpes zoster ophthalmic), and malignancies 0.6 (most frequently basal cell carcinoma). At week 105, 269 (44%) patients demonstrated an ACR20 response relative to their respective phase IIb trial baselines.
Conclusion
Among 319 patients who completed this 2-year extension of two global phase IIb studies, peficitinib 100 mg once daily demonstrated a stable safety profile and sustained effectiveness in patients with moderate-to-severe RA.
Trial Registration
ClinicalTrials.gov identifier, NCT01711814. Registered 19 October 2012.
Funding
Astellas Pharma Global Development, Inc.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-019-00167-6) contains supplementary material, which is available to authorized users.
Keywords: Janus kinase (JAK) inhibitor, Long-term extension, Peficitinib, Rheumatoid arthritis
Introduction
The use of early, aggressive treatment strategies, as well as the development of biologic therapies, has substantially improved outcomes in patients with rheumatoid arthritis (RA) [1]. Although there is no cure for RA [1], potential therapies include the Janus kinase (JAK) inhibitors, which target various cytokine signalling pathways and suppress the activation and proliferation of inflammatory cells [2]. Two JAK inhibitors are currently approved in the USA, Europe and Asia for treatment of RA in adults: tofacitinib (a pan-JAK inhibitor) and baricitinib (a JAK1 and JAK2 selective inhibitor) [3–8]. A third JAK inhibitor, peficitinib (a pan-JAK inhibitor), is approved in Japan [9]. Peficitinib, an orally bioavailable, once-daily JAK inhibitor that inhibits JAK1, JAK3, and TYK2 with greater specificity than JAK2, may modulate crucial cytokine receptor pathways in RA [10, 11].
The clinical efficacy and safety of peficitinib have been demonstrated in phase II, randomized, double-blind, placebo-controlled trials of patients with moderate-to-severe RA [12–14]. In a Japanese phase II trial, peficitinib 50 mg, 100 mg, and 150 mg monotherapy for 12 weeks provided dose-dependent and significantly higher American College of Rheumatology 20% criteria (ACR20) response rates compared with placebo and had an acceptable safety profile [14]. Two global, phase IIb, randomized, double-blind, placebo-controlled trials assessed peficitinib for 12 weeks. In the first trial [‘without methotrexate (MTX)’], peficitinib 50 mg, 100 mg, or 150 mg once-daily plus limited csDMARDs provided significant, dose-dependent improvements in ACR20 compared with placebo [12]. In the second trial (‘with MTX’), significant improvements in ACR20 response with peficitinib plus MTX were observed with peficitinib 50 mg compared with placebo plus MTX [13]. In both phase IIb trials, peficitinib was well tolerated over the 12-week treatment periods.
Patients enrolled in this long-term extension study had previously completed treatment with either placebo or peficitinib in a global phase IIb, double-blind trial of adults with moderate-to-severe RA. In the ‘without MTX’ trial, 289 patients with long-term refractory disease and inadequate response or intolerance to csDMARDs received placebo or peficitinib 25–150 mg once daily in combination with limited csDMARDs [12]. In the ‘with MTX’ trial, 378 patients with an inadequate response to MTX received placebo or peficitinib 25–150 mg once daily added to existing MTX treatment [13]. After 12 weeks of double-blind treatment in either study, all eligible patients entered the extension study and received open-label peficitinib 100 mg once daily for 2 years. The primary objective was to assess the long-term tolerability and safety profile of peficitinib in adults with moderate-to-severe RA.
Methods
Design and Patients
This global open-label, single-arm, non-comparative, long-term extension study of peficitinib in adults with RA included patients who had completed one of two double-blind, placebo-controlled, 12-week phase IIb trials [12, 13]. The long-term extension study was conducted in 51 centres in the USA, Poland, Hungary, Colombia, Czech Republic, Mexico, Bulgaria, and Belgium (NCT01711814). Patients aged ≥ 18 years were eligible for the open-label extension study if they continued to fulfil the inclusion criteria for the double-blind trials [12, 13].
In the double-blind trials, patients received placebo or peficitinib 25–150 mg once daily in combination with limited csDMARDs (‘without MTX’; NCT01565655) or in combination with MTX (‘with MTX’; NCT01554696). This extension study included patients from both trials. All patients from both clinical trials were continued on 100 mg peficitinib daily for 105 weeks. There were 13 visits between the extension study baseline and week 105, with the end-of-study visit 30 days later.
An Institutional Review Board/Independent Ethics Committee-approved written informed consent form was obtained from each patient or from a legally authorized representative prior to the initiation of any study-specific procedures. This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, the EU Clinical Trials Directive, and applicable laws and regulations. The central IRB for the USA was Quorum IRB. Details of individual IRBs are included in Supplement 7.
Discontinuation Criteria
The patient discontinuation criteria, including a failure to achieve either low disease activity or remission, are shown in Supplement 1. Patients were free to withdraw from the study treatment and/or study for any reason and at any time without giving a reason. Patients were discontinued from the study if they withdrew consent, were non-compliant with the protocol in a way that might affect evaluation of treatment, or had an ongoing adverse event (AE) or an unresolved laboratory result. If a patient discontinued because of an AE, the event was followed until it resolved [15].
There were six protocol amendments during the study, including the criteria for withdrawals that were adopted to be consistent with EULAR and ACR treatment guidelines [16]. Amendment 4 (2 April 2013) changed the discontinuation criterion from no ACR20 response by week 13 to patients without a 20% improvement in tender and swollen joints. Protocol amendment 6 (24 March 2015) superseded amendment 4 and mandated patients to discontinue from the study for low disease activity or remission at their most recent visit, or if at their most recent visit they had recurrence of moderate or high disease activity for two consecutive visits after a previous response with low disease activity or remission.
Objectives
The primary objective was to evaluate 2-year safety by assessing adverse events (AEs) and clinical laboratory evaluations, respectively. The safety endpoints were AEs, serious AEs (SAEs), AEs of special interest, pregnancy outcomes, and laboratory assessments.
All AEs were coded using the Medical Dictionary for Regulatory Activities version 14.0; investigators designated the causal relationship between study treatment and AEs.
The exploratory objective was to evaluate 2-year effectiveness. The endpoints were ACR20/50/70 response, DAS28 (CRP) and DAS28 (ESR) scores, DAS28 (CRP) remission, Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI).
Statistical Analyses
All safety analyses were descriptive. Values were tabulated and provided as number and percentage reporting the event. Rates of AEs [per 100 patient-years (PYs)] were also calculated as the number of AEs for all patients in the treatment cohort divided by the total PYs in the treatment cohort, multiplied by 100.
The full analysis set included all enrolled patients who had completed a double-blind trial and who received at least one dose of open-label peficitinib. The double-blind trials have been previously published [12, 13].
ACR20/50/70 responses and the maintenance of ACR20 response in patients who achieved an ACR20 response at week 12 in the double-blind trials were analysed using observed data and non-responder imputation.
As the control group is not available in the long-term extension study, Kaplan-Meier analyses were performed. Survival plots were based on prior treatment arms and included time to withdrawal from the study due to AEs or lack of efficacy (LOE) (including failure to meet ACR20 and failure to achieve LDAS) for all patients, patients from the preceding phase IIb trial with MTX add-on, and patients from the preceding phase IIb trial without MTX add-on. For each patient who withdrew from the study because of AEs or LOE (i.e., event = 1), the time to withdrawal from the study due to AEs or LOE was defined as the number of days from the date of initial dose of study drug to the date of withdrawal from the study due to AEs or LOE and calculated as: date of withdrawal from the study due to AEs or LOE—date of initial dose of study drug + 1. For each patient who did not withdraw from the study because of AEs or LOE (i.e., event = 0), the event time (in days) was censored at the date of last dose of study drug.
Results
Patients
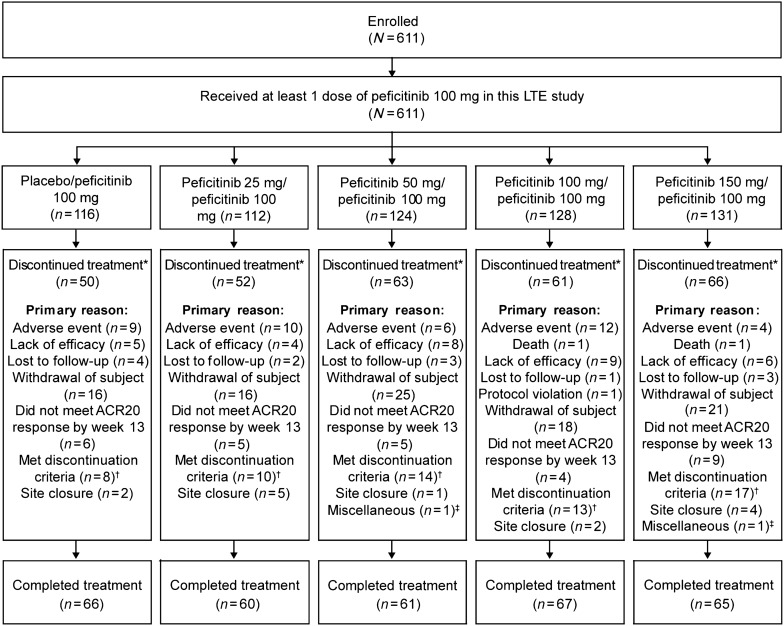
A total of 611 patients entered the long-term extension study and received ≥ 1 dose(s) of peficitinib 100 mg and were included in the FAS (Fig. 1). Baseline demographic and disease characteristics were similar across the study arms in the double-blind trial (Table 1). A total of 319 (52.2%) patients completed the study; among the 292 patients who discontinued, the main reasons were withdrawal of patient consent (n = 96; 32.9%), failure to achieve low disease activity (n = 62; 21.2%), AE not including death (n = 41; 14.0%), and lack of efficacy (n = 32; 11.0%).
Fig. 1.
Disposition of patients. *‘Discontinued treatment’ refers to any patient who discontinued at any time between receiving their first and last dose of peficitinib 100 mg. †Patients had not achieved either low disease activity or remission by their most recent visit, as determined by DAS28(CRP) < 3.2, DAS28(ESR) < 3.2 or SDAI ≤ 11 (if CRP was available) or CDAI ≤ 10 (if CRP was not available). Patients also met the discontinuation criteria if, after achieving low disease activity or remission, they experienced moderate or high disease activity for two consecutive visits as determined by DAS28(CRP) ≥ 3.2 (if CRP was available), DAS28(ESR) ≥ 3.2 (if ESR was available), and SDAI > 11 (if CRP was available) or CDAI > 10 (if CRP was not available). ‡Two patients discontinued treatment because of ‘miscellaneous’ reasons (one patient in the peficitinib 50 mg/100 mg group because of entering the study in error and one patient in the peficitinib 150 mg/100 mg group because of moving further from the study site). ACR20 American College of Rheumatology 20% improvement criteria, CDAI Clinical Disease Activity Index, CRP C-reactive protein, DAS28 Disease Activity Score in 28 joints, ESR erythrocyte sedimentation rate, LTE long-term extension, SDAI Simplified Disease Activity Index
Table 1.
Demographics and baseline disease characteristics
| Patient characteristic | Dose received in preceding phase II trial | Total (N = 611) | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 116) | Peficitinib 25 mg (n = 112) | Peficitinib 50 mg (n = 124) | Peficitinib 100 mg (n = 128) | Peficitinib 150 mg (n = 131) | ||
| Prior trial participation, n (%) | ||||||
| ‘With MTX’ trial | 67 (57.8) | 61 (54.5) | 76 (61.3) | 77 (60.2) | 71 (54.2) | 352 (57.6) |
| ‘Without MTX’ trial | 49 (42.2) | 51 (45.5) | 48 (38.7) | 51 (39.8) | 60 (45.8) | 259 (42.4) |
| Female, n (%) | 99 (85.3) | 93 (83.0) | 102 (82.3) | 106 (82.8) | 104 (79.4) | 504 (82.5) |
| Age, mean (SD), years | 52.4 (12.1) | 52.2 (11.5) | 53.3 (11.5) | 54.5 (12.1) | 54.2 (12.4) | 53.4 (11.9) |
| BMI, mean (SD), kg/m2 | 28.6 (6.2) | 28.7 (6.1) | 29.0 (6.6) | 29.0 (7.0) | 28.2 (6.1) | 28.7 (6.4) |
| Hispanic or Latino, n (%) | 27 (23.3) | 34 (30.4) | 36 (29.0) | 36 (28.1) | 29 (22.1) | 162 (26.5) |
| RA duration, mean (SD), years | 8.4 (7.9) | 9.0 (7.2) | 8.4 (8.2) | 9.1 (8.4) | 8.8 (7.1) | 8.8 (7.8) |
| Prior bDMARD use, n (%) | 42 (36.2) | 39 (34.8) | 44 (35.5) | 44 (34.4) | 48 (36.6) | 217 (35.5) |
| Number of previous bDMARDs, n (%) | ||||||
| 1 | 15 (12.9) | 12 (10.7) | 19 (15.3) | 16 (12.5) | 17 (13.0) | 79 (12.9) |
| 2 | 11 (9.5) | 16 (14.3) | 14 (11.3) | 15 (11.7) | 16 (12.2) | 72 (11.8) |
| ≥ 3 | 16 (13.8) | 11 (9.8) | 11 (8.9) | 13 (10.2) | 15 (11.5) | 66 (10.8) |
| Prior anti-TNF use, n (%) | 39 (33.6) | 32 (28.6) | 41 (33.1) | 40 (31.3) | 38 (29.0) | 190 (31.1) |
| Concomitant MTX use, n (%) | 70 (60.3) | 62 (55.4) | 77 (62.1) | 80 (62.5) | 77 (58.8) | 366 (59.9) |
| Concomitant corticosteroids, n (%) | ||||||
| Prednisone | 27 (23.3) | 28 (25.0) | 40 (32.3) | 31 (24.2) | 34 (26.0) | 160 (26.2) |
| Methylprednisolone | 29 (25.0) | 27 (24.1) | 28 (22.6) | 37 (28.9) | 21 (16.0) | 142 (23.2) |
| Prednisolone | 3 (2.6) | 2 (1.8) | 3 (2.4) | 3 (2.3) | 2 (1.5) | 13 (2.1) |
| Dexamethasone | 2 (1.7) | 3 (2.7) | 2 (1.6) | 4 (3.1) | 1 (0.8) | 12 (2.0) |
| Beclometasone | 1 (0.9) | 1 (0.9) | 0 | 1 (0.8) | 0 | 3 (0.5) |
| Liothyronine | 1 (0.9) | 2 (1.8) | 0 | 0 | 0 | 3 (0.5) |
| Diprosan | 0 | 0 | 2 (1.6) | 0 | 0 | 2 (0.3) |
| Corticosteroidsa | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Concomitant SSZ | 17 (14.7) | 9 (8.0) | 2 (1.6) | 9 (7.0) | 11 (8.4) | 48 (7.9) |
| Concomitant anti-malarial | 7 (6.0) | 18 (16.1) | 13 (10.5) | 15 (11.7) | 10 (7.6) | 63 (10.3) |
| Geographic region, n (%) | ||||||
| North America | 48 (41.4) | 46 (41.1) | 54 (43.5) | 52 (40.6) | 56 (42.7) | 256 (41.9) |
| Europe | 51 (44.0) | 47 (42.0) | 49 (39.5) | 56 (43.8) | 55 (42.0) | 258 (42.2) |
| Latin America | 17 (14.7) | 19 (17.0) | 21 (16.9) | 20 (15.6) | 20 (15.3) | 97 (15.9) |
| Baseline disease activity, mean (SD) | ||||||
| SDAI | 24.7 (16.7) | 26.1 (16.7) | 20.3 (15.4) | 20.3 (16.6) | 19.3 (17.3) | 22.0 (16.7) |
| CDAI | 23.4 (16.2) | 25.0 (16.3) | 19.4 (14.9) | 19.3 (16.1) | 18.5 (16.9) | 21.0 (16.2) |
| TJC68 | 14.2 (13.1) | 14.9 (14.0) | 11.3 (12.6) | 12.3 (13.3) | 12.0 (15.2) | 12.9 (13.7) |
| SJC66 | 7.7 (9.0) | 8.5 (8.4) | 5.7 (6.9) | 6.4 (7.6) | 5.9 (7.8) | 6.8 (8.0) |
| CRP, mg/dl | 1.3 (1.6) | 1.1 (1.3) | 1.0 (1.3) | 1.0 (1.5) | 0.8 (1.3) | 1.0 (1.4) |
| ESR, mm/h | 31.9 (21.5) | 31.1 (19.1) | 32.0 (24.1) | 27.2 (19.0) | 23.5 (19.5) | 29.0 (20.9) |
| SGAP (100 mm VAS) | 48.7 (28.0) | 49.5 (27.1) | 41.5 (28.2) | 37.8 (27.0) | 37.2 (26.1) | 42.6 (27.7) |
| SGA (100 mm VAS) | 48.6 (26.7) | 50.7 (25.9) | 41.0 (26.8) | 38.6 (26.9) | 37.1 (25.5) | 42.8 (26.8) |
| PGA (100 mm VAS) | 39.2 (25.5) | 40.3 (26.3) | 30.7 (24.0) | 29.3 (24.4) | 28.0 (25.1) | 33.2 (25.5) |
| HAQ-DI | 1.2 (0.7) | 1.2 (0.7) | 1.1 (0.7) | 1.0 (0.7) | 1.0 (0.7) | 1.1 (0.7) |
| DAS28(ESR) | 5.0 (1.5) | 5.1 (1.6) | 4.6 (1.6) | 4.4 (1.7) | 4.2 (1.7) | 4.6 (1.7) |
| DAS28(CRP) | 4.4 (1.5) | 4.5 (1.6) | 4.0 (1.5) | 3.9 (1.6) | 3.8 (1.5) | 4.1 (1.6) |
Baseline characteristics were measured at baseline for this LTE study
bDMARD biological disease-modifying anti-rheumatic drug; BMI body mass index; CDAI Clinical Disease Activity Index; CRP C-reactive protein; DAS28 disease activity score in 28 joints; ESR erythrocyte sedimentation rate; HAQ-DI Health Assessment Questionnaire-Disability Index; MTX methotrexate; SD standard deviation; SDAI Simplified Disease Activity Index; SGA subject global assessment; SGAP subject global assessment of arthritis pain; PGA physician global assessment; RA rheumatoid arthritis; SJC swollen joint count; SSZ sulfasalazine; TJC tender joint count; TNF tumor necrosis factor; VAS visual analog scale
aOne patient that previously received placebo in their preceding phase II trial was recorded as having received concomitant corticosteroids during this study; it is unknown which corticosteroid was administered
During the study, 86.7% (n = 530) received MTX, 67.6% (n = 413) received folic acid, 27.2% (n = 166) received prednisone, and 23.9% (n = 146) received methylprednisolone. In the total population, the median (range) duration of peficitinib treatment was 720.0 days (1–771). Seven patients had a dose reduction during the study [they had previously received peficitinib 25 mg (n = 2), 50 mg (n = 3), 100 mg (n = 1), and 150 mg (n = 1)]: six patients discontinued because of AEs, and one patient fulfilled the pre-defined discontinuation criterion of absolute lymphocytopenia of < 500 cells/µl. Survival curves of patients who withdrew from the study are shown in Supplement 2.
Safety
Adverse Events
In total, there were 1813 AEs (182.4 AEs/100 PYs) (Table 2). Patients were grouped according to the dose received in the preceding phase II trial. The most frequently reported AEs per 100 PYs were upper respiratory tract infection (URTI) [n = 98 (9.9 events/100 PYs)], urinary tract infection (UTI) [n = 72 (7.2 events/100 PYs)], nasopharyngitis [n = 56 (5.6 events/100 PYs)], and bronchitis [n = 52 (5.2 events/100 PYs)]. There were no clinically meaningful differences in the frequency of any AEs between treatment groups and the number of AEs per 100 PYs was similar in patients from the ‘with MTX’ trial (373.2 events/100 PYs) and patients from the ‘without MTX’ trial (385.1 events/100 PYs). A total of 222 (36.3%) were considered by the investigator to have drug-related events, the most common being URTI (6.7%), hypercholesterolaemia (4.3%), and bacterial UTI (3.4%).
Table 2.
Summary of AEs and those occurring with a frequency of ≥ 25 adverse events/100 PYs
| n (%) | Dose received in preceding phase II trial | Total (N = 611) | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 116) | Peficitinib 25 mg (n = 112) | Peficitinib 50 mg (n = 124) | Peficitinib 100 mg (n = 128) | Peficitinib 150 mg (n = 131) | ||
| AE | 80 (69.0) | 85 (75.9) | 98 (79.0) | 101 (78.9) | 99 (75.6) | 463 (75.8) |
| Serious AE | 14 (12.1) | 11 (9.8) | 14 (11.3) | 25 (19.5) | 16 (12.2) | 80 (13.1) |
| Death | 0 | 0 | 0 | 1 (0.8) | 1 (0.8) | 2 (0.3) |
| Malignancy | 1 (0.9) | 0 | 1 (0.8) | 3 (2.4) | 1 (0.8) | 6 (1.0) |
| Herpes zoster | 1 (0.9) | 2 (1.8) | 4 (3.2) | 2 (1.6) | 5 (3.8) | 14 (2.3) |
| Herpes zoster ophthalmic | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Grade ≥ 3* | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) |
| AE leading to discontinuation | 9 (7.8) | 9 (8.0) | 8 (6.5) | 11 (8.6) | 6 (4.6) | 43 (7.0) |
| Serious AEs leading to discontinuation | 2 (1.7) | 1 (0.9) | 3 (2.4) | 5 (3.9) | 2 (1.5) | 13 (2.1) |
| Anaemia | 2 (1.7) | 0 | 0 | 1 (0.8) | 0 | 3 (0.5) |
| Diverticulitis | 1 (0.9) | 0 | 0 | 0 | 1 (0.8) | 2 (0.3) |
| Herpes zoster ophthalmic | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Myocardial infarction | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| Pericarditis | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| Chronic lymphocytic leukaemia | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| Chronic myeloid leukaemia | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| Headache | 0 | 0 | 1 (0.8) | 0 | 0 | 1 (0.2) |
| Lacunar infarction | 0 | 1 (0.9) | 0 | 0 | 0 | 1 (0.2) |
| Drug hypersensitivity | 0 | 0 | 1 (0.8) | 0 | 0 | 1 (0.2) |
| Road traffic accident | 0 | 0 | 0 | 0 | 1 (0.8) | 1 (0.2) |
| Renal failure acute | 0 | 0 | 1 (0.8) | 0 | 0 | 1 (0.2) |
| Interstitial lung disease | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Events (rate) | 100 PYs = 192.5 | 100 PYs = 182.7 | 100 PYs = 193.4 | 100 PYs = 212.0 | 100 PYs = 213.3 | 100 PYs = 993.9 |
|---|---|---|---|---|---|---|
| AE | 360 (187.0) | 313 (171.4) | 342 (176.8) | 465 (219.3) | 333 (156.1) | 1813 (182.4) |
| URTI | 24 (12.5) | 12 (6.6) | 21 (10.9) | 21 (9.9) | 20 (9.4) | 98 (9.9) |
| UTI | 19 (9.9) | 13 (7.1) | 8 (4.1) | 23 (10.8) | 9 (4.2) | 72 (7.2) |
| Nasopharyngitis | 7 (3.6) | 11 (6.0) | 16 (8.3) | 16 (7.5) | 6 (2.8) | 56 (5.6) |
| Bronchitis | 17 (8.8) | 12 (6.6) | 14 (7.2) | 3 (1.4) | 6 (2.8) | 52 (5.2) |
| Hypercholesterolaemia | 6 (3.1) | 9 (4.9) | 11 (5.7) | 6 (2.8) | 10 (4.7) | 42 (4.2) |
| Diarrhea | 10 (5.2) | 4 (2.2) | 7 (3.6) | 15 (7.1) | 4 (1.9) | 40 (4.0) |
| Blood CPK increase | 10 (5.2) | 9 (4.9) | 4 (2.1) | 5 (2.4) | 8 (3.8) | 36 (3.6) |
| Headache | 7 (3.6) | 5 (2.7) | 8 (4.1) | 13 (6.1) | 3 (1.4) | 36 (3.6) |
| RA | 7 (3.6) | 4 (2.2) | 12 (6.2) | 8 (3.8) | 3 (1.4) | 34 (3.4) |
| UTI bacterial | 5 (2.6) | 7 (3.8) | 4 (2.1) | 8 (3.8) | 9 (4.2) | 33 (3.3) |
| Influenza | 3 (1.6) | 4 (2.2) | 10 (5.2) | 10 (4.7) | 5 (2.3) | 32 (3.2) |
| Nausea | 10 (5.2) | 2 (1.1) | 5 (2.6) | 9 (4.2) | 2 (0.9) | 28 (2.8) |
| Hypertension | 3 (1.6) | 4 (2.2) | 7 (3.6) | 6 (2.8) | 7 (3.3) | 27 (2.7) |
*Grade 3 event of ophthalmic herpes zoster led to treatment discontinuation
CPK creatine phosphokinase, RA rheumatoid arthritis, AE treatment-emergent adverse event, URTI upper respiratory tract infection, UTI urinary tract infection
Serious Adverse Events
A total of 80 patients (13%) experienced an SAE; they had previously received placebo (12%), peficitinib 25 mg (10%), 50 mg (11%), 100 mg (20%), and 150 mg (12%) (Supplement 3). The most frequently reported SAEs were pregnancy [n = 8 (1%)], RA [n = 6 (1%)], ovarian cyst [n = 4 (1%)], UTI [n = 3 (< 1%)], anaemia [n = 3 (< 1%)], and acute renal failure [n = 3 (< 1%)]. Two patients who experienced SAEs died, one from a road traffic accident and the other a cardiac arrest. Incidences of pulmonary embolism and chest pain [n = 1 (< 1%)], pulmonary thrombosis and thrombosis [n = 1 (< 1%)], and deep vein thrombosis [n = 1 (< 1%)] were not considered by the investigator to be drug related. All events were resolved and the patients recovered.
A total of 78 patients (13%) had grade ≥ 3 AEs; they had previously received placebo (11%), peficitinib 25 mg (9%), 50 mg (12%), 100 mg (18%), and 150 mg (13%). The most frequently reported grade ≥ 3 AEs were RA [n = 5 (1%)], anaemia [n = 3 (< 1%)], appendicitis [n = 3 (< 1%)], headache [n = 3 (< 1%)], and UTI [n = 3 (< 1%)] (Table 3).
Table 3.
Grade ≥ 3 AEs
| Dose received in preceding phase II trial | Total (N = 611) | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 116) | Peficitinib 25 mg (n = 112) | Peficitinib 50 mg (n = 124) | Peficitinib 100 mg (n = 128) | Peficitinib 150 mg (n = 131) | ||
| RA | 1 (0.9) | 1 (0.9) | 2 (1.6) | 1 (0.8) | 0 | 5 (0.8) |
| Appendicitis | 1 (0.9) | 0 | 0 | 1 (0.8) | 1 (0.8) | 3 (0.5) |
| UTI | 0 | 0 | 0 | 2 (1.6) | 1 (0.8) | 3 (0.5) |
| Anaemia | 1 (0.9) | 0 | 0 | 2 (1.6) | 0 | 3 (0.5) |
| Headache | 0 | 1 (0.9) | 1 (0.8) | 1 (0.8) | 0 | 3 (0.5) |
| Pregnancya | 1 (0.9) | 0 | 1 (0.8) | 1 (0.8) | 0 | 3 (0.5) |
| Gastroenteritis | 0 | 0 | 1 (0.8) | 0 | 1 (0.8) | 2 (0.3) |
| Musculoskeletal pain | 0 | 0 | 0 | 1 (0.8) | 1 (0.8) | 2 (0.3) |
| Medical device complication | 0 | 0 | 1 (0.8) | 0 | 1 (0.8) | 2 (0.3) |
| Non-cardiac chest pain | 0 | 0 | 0 | 2 (1.6) | 0 | 2 (0.3) |
| Abdominal pain | 0 | 1 (0.9) | 0 | 1 (0.8) | 0 | 2 (0.3) |
| Nausea | 1 (0.9) | 0 | 0 | 1 (0.8) | 0 | 2 (0.3) |
| COPD | 1 (0.9) | 1 (0.9) | 0 | 0 | 0 | 2 (0.3) |
| Angina pectoris | 0 | 0 | 0 | 1 (0.8) | 1 (0.8) | 2 (0.3) |
| Cholelithiasis | 1 (0.9) | 0 | 1 (0.8) | 0 | 0 | 2 (0.3) |
| Blood CPK increase (> 1.5 × ULN) | 0 | 1 (0.9) | 0 | 0 | 1 (0.8) | 2 (0.3) |
All data are represented as n (%)
COPD chronic obstructive pulmonary disease, CPK creatine phosphokinase, RA rheumatoid arthritis, AE treatment-emergent adverse event, ULN upper limit of normal, UTI urinary tract infection
aOf the three patients who experienced a grade 3 AE of pregnancy during this study, one patient had an induced abortion and discontinued the study (previously received placebo in their respective preceding global phase II trial), and two patients had unknown pregnancy outcomes and discontinued the study (previously received peficitinib 50 mg and peficitinib 100 mg in their respective preceding global phase II trial)
A total of 43 patients (7%) discontinued study treatment because of an AE. Of these, 9 patients had received placebo, and 9, 8, 11, and 6 patients had received peficitinib 25 mg, 50 mg, 100 mg, and 150 mg in the prior phase IIb clinical trials. Overall, the most frequent AEs leading to treatment discontinuation were pregnancy [n = 5 (< 1%)], blood creatine phosphokinase (CPK) increase > 1.5 × upper limit of normal [ULN; n = 4 (< 1%)], anaemia [n = 3 (< 1%)], and headache [n = 3 (< 1%)].
Adverse Events of Special Interest
The incidence of serious infections was 2.7 events per 100 PYs (Table 3). UTI had the highest incidence (0.4 events/100 PYs), followed by appendicitis (0.3 events/100 PYs). Diverticulitis, gastroenteritis, and pneumonia each had two occurrences (0.2 events/100 PYs), and there was one event each of bronchitis, bronchitis bacterial, cellulitis staphylococcal, chikungunya virus infection, clostridial infection, herpes zoster, herpes zoster ophthalmic, influenza, nasopharyngitis, pneumonia bacterial, pneumonia primary atypical, pneumonia staphylococcal, URTI, and viral infection (0.1 events/100 PYs). There were no reports of tuberculosis or opportunistic infections. The incidence of herpes zoster (including herpes zoster and herpes zoster ophthalmic) was 1.6 events per 100 PYs, and the incidence of malignancies was 0.6 events per 100 PYs. There were no thrombotic events observed.
The overall incidence of AE events/100 PYs was 182.4, which included URTI (9.9), UTI (7.2), nasopharyngitis (5.6), and bronchitis (5.2). The most frequent AEs were infections and infestations (48.4%), including upper respiratory tract (12.6%), urinary tract (8.7%), and bronchitis (6.9%). The incidence of AEs was similar in patients with and without concomitant MTX treatment.
Clinical Laboratory Evaluations
The reported incidence of liver enzyme changes was 2.3 events per 100 PYs. Hepatic enzyme increase had the highest incidence (0.6 events/100 PYs) followed by gamma-glutamyltransferase increase (0.4 events/100 PYs). Alanine aminotransferase (ALT) increase, abnormal liver function test, and transaminases increase each had three occurrences (0.3 events/100 PYs), and there were two occurrences of aspartate aminotransferase (AST) increase (0.2 events/100 PYs) and one occurrence of hyperbilirubinaemia and liver injury (0.1 events/100 PYs).
The shift from baseline to week 105 for clinical laboratory values was similar to that observed in both the phase IIb trials (‘with MTX’ and ‘without MTX’) (Table 4). Compared with baseline measurements, there were reductions in absolute neutrophil and lymphocyte counts and increases in fasting cholesterol and high-density lipoprotein levels at week 105 (Table 5).
Table 4.
Summary of events of special interest and clinical laboratory values
| Events (rate) | Dose received in preceding phase II trial | Total (N = 611) 100 PYs = 993.9 |
|||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 116) 100 PYs = 192.5 |
Peficitinib 25 mg (n = 112) 100 PYs = 182.7 |
Peficitinib 50 mg (n = 124) 100 PYs = 193.4 |
Peficitinib 100 mg (n = 128) 100 PYs = 212.0 |
Peficitinib 150 mg (n = 131) 100 PYs = 213.3 |
|||
| Serious infections occurring in ≥ 2 patients | |||||||
| UTI | 0 | 0 | 0 | 3 (1.4) | 1 (0.5) | 4 (0.4) | |
| Appendicitis | 1 (0.5) | 0 | 0 | 1 (0.5) | 1 (0.5) | 3 (0.3) | |
| Diverticulitis | 1 (0.5) | 0 | 0 | 0 | 1 (0.5) | 2 (0.2) | |
| Gastroenteritis | 0 | 0 | 1 (0.5) | 0 | 1 (0.5) | 2 (0.2) | |
| Pneumonia | 0 | 1 (0.5) | 0 | 0 | 1 (0.5) | 2 (0.2) | |
| Herpes zoster | 1 (0.5) | 2 (1.1) | 5 (2.6) | 2 (0.9) | 5 (2.3) | 15 (1.5) | |
| Herpes zoster ophthalmic | 1 (0.5) | 0 | 0 | 0 | 0 | 1 (0.1) | |
| Malignancies | |||||||
| Basal cell carcinoma | 0 | 0 | 1 (0.5) | 1 (0.5) | 0 | 2 (0.2) | |
| Chronic lymphocytic leukaemia | 0 | 0 | 0 | 1 (0.5) | 0 | 1 (0.1) | |
| Chronic myeloid leukaemia | 0 | 0 | 0 | 1 (0.5) | 0 | 1 (0.1) | |
| Gastrointestinal carcinoma | 0 | 0 | 0 | 0 | 1 (0.5) | 1 (0.1) | |
| Thyroid cancer | 1 (0.5) | 0 | 0 | 0 | 0 | 1 (0.1) | |
| Liver enzyme changes | |||||||
| Hepatic enzyme increase | 0 | 2 (1.1) | 2 (1.0) | 1 (0.5) | 1 (0.5) | 6 (0.6) | |
| GGT increase | 2 (1.0) | 0 | 0 | 1 (0.5) | 1 (0.5) | 4 (0.4) | |
| ALT increase | 0 | 2 (1.1) | 0 | 0 | 1 (0.5) | 3 (0.3) | |
| Liver function test abnormal | 1 (0.5) | 0 | 0 | 1 (0.5) | 1 (0.5) | 3 (0.3) | |
| Transaminases increase | 0 | 3 (1.6) | 0 | 0 | 0 | 3 (0.3) | |
| AST increase | 0 | 0 | 0 | 1 (0.5) | 1 (0.5) | 2 (0.2) | |
| Hyperbilirubinaemia | 0 | 0 | 0 | 1 (0.5) | 0 | 1 (0.1) | |
| Liver injury | 1 (0.5) | 0 | 0 | 0 | 0 | 1 (0.1) | |
| Shift from baseline to week 105 for laboratory values, n (%) | |||||||
| ANC, cells/µl | |||||||
| < 500 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 500 to < 1000 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1000 to < 1500 | 1 (0.9) | 0 | 1 (0.8) | 1 (0.8) | 0 | 3 (0.5) | |
| ALC, cells/µl | |||||||
| < 200 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 200 to < 500 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hb, g/dl | |||||||
| < 8.0 | 0 | 1 (0.9) | 0 | 0 | 0 | 1 (0.2) | |
| 8.0 to < 10.0 | 0 | 2 (1.8) | 0 | 0 | 0 | 2 (0.3) | |
| Grade 2 or 3 | 2 (1.7) | 2 (1.8) | 0 | 1 (0.8) | 0 | 5 (0.8) | |
| Plt, cells/µl | |||||||
| ≤ 2 × 104 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 × 104 to ≤ 5 × 104 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CPK | |||||||
| > 3 × ULN to ≤ 5 × ULN | 0 | 0 | 0 | 0 | 0 | 0 | |
| > 5 × ULN to ≤ 10 × ULN | 0 | 0 | 0 | 0 | 1 (0.8) | 1 (0.2) | |
| > 10 × ULN | 0 | 0 | 0 | 0 | 0 | 0 | |
| ALT | |||||||
| > 2 × ULN to ≤ 3 × ULN | 1 (0.9) | 2 (1.8) | 0 | 1 (0.8) | 0 | 4 (0.7) | |
| > 3 × ULN to ≤ 5 × ULN | 1 (0.9) | 0 | 0 | 1 (0.8) | 0 | 2 (0.3) | |
| > 5 × ULN | 0 | 0 | 0 | 0 | 0 | 0 | |
| AST | |||||||
| > 2 × ULN to ≤ 3 × ULN | 0 | 1 (0.9) | 0 | 0 | 0 | 1 (0.2) | |
| > 3 × ULN to ≤ 5 × ULN | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.2) | |
| > 5 × ULN | 0 | 0 | 0 | 0 | 0 | 0 | |
| Creatinine | |||||||
| > 1.5 × baseline to ≤ 3.0 × baseline | 1 (0.9) | 0 | 0 | 0 | 0 | 1 (0.2) | |
| > 3.0 × baseline | 0 | 0 | 0 | 0 | 0 | 0 | |
| LDL, mg/dl | |||||||
| > 160 | 3 (2.6) | 10 (8.9) | 6 (4.8) | 7 (5.5) | 4 (3.1) | 30 (9.6) | |
| ≤ 160 | 60 (51.7) | 47 (42.0) | 55 (44.4) | 57 (44.5) | 62 (47.3) | 281 (46.0) | |
All shift data are reported as shift from this LTE study baseline to week 105
ALC absolute lymphocyte count, ALT alanine aminotransferase, ANC absolute neutrophil count, AST aspartate aminotransferase, CPK creatine phosphokinase, GGT gamma glutamyltransferase, Hb haemoglobin, LDL low-density lipoprotein, Plt platelets, PY patient-year, ULN upper limit of normal, UTI urinary tract infection
Table 5.
Summary of shifts from baseline to week 105 for haematology and fasting lipid profile values
| Analyte (unit) | Patients (n) | Mean change | Standard deviation |
|---|---|---|---|
| Haematology values | |||
| ANC (106/l) | 549 | − 353.1 | 2293.74 |
| Neutrophils (%) | 552 | − 1.01 | 9.559 |
| Basophils (106/l) | 549 | 5.7 | 27.03 |
| Basophil/leukocyte fraction | 552 | 0.0011 | 0.00352 |
| Eosinophils (106/l) | 549 | 4.8 | 213.30 |
| Eosinophil/leukocyte fraction | 552 | 0.0012 | 0.01733 |
| Haematocrit fraction | 543 | 0.0134 | 0.03674 |
| Haemoglobin (g/l) | 549 | 1.0 | 11.41 |
| Lymphocytes (106/l) | 549 | − 154.6 | 687.43 |
| Lymphocyte/leukocyte fraction | 552 | − 0.0046 | 0.08525 |
| Monocytes (106/l) | 549 | 57.8 | 202.63 |
| Monocyte/leukocyte fraction | 552 | 0.0121 | 0.02363 |
| Platelets (109/l) | 549 | 10.5 | 67.48 |
| Erythrocytes (1012/l) | 549 | 0.097 | 0.3098 |
| Leukocytes (109/l) | 549 | − 0.44 | 2.672 |
| Fasting lipid profile values (mmol/l) | |||
| Cholesterol | 315 | 0.266 | 0.9781 |
| HDL cholesterol | 315 | 0.213 | 0.3341 |
| LDL cholesterol | 311 | 0.060 | 0.7566 |
| Triglycerides | 314 | − 0.051 | 1.4337 |
All shift data are reported as shift from this long-term extension study baseline to week 105
ANC absolute neutrophil count, HDL high-densty lipoprotein, LDL low-density lipoprotein
Pregnancy Outcomes
Eight patients (1%) became pregnant during the study. Four reported abortions (one spontaneous, one induced, and two with no further information). Of the four pregnancies, one had a normal vaginal delivery, one had a caesarean delivery, and the outcome in two patients was lost to follow-up and was not reported. The study drug was discontinued in five patients with pregnancy.
Effectiveness
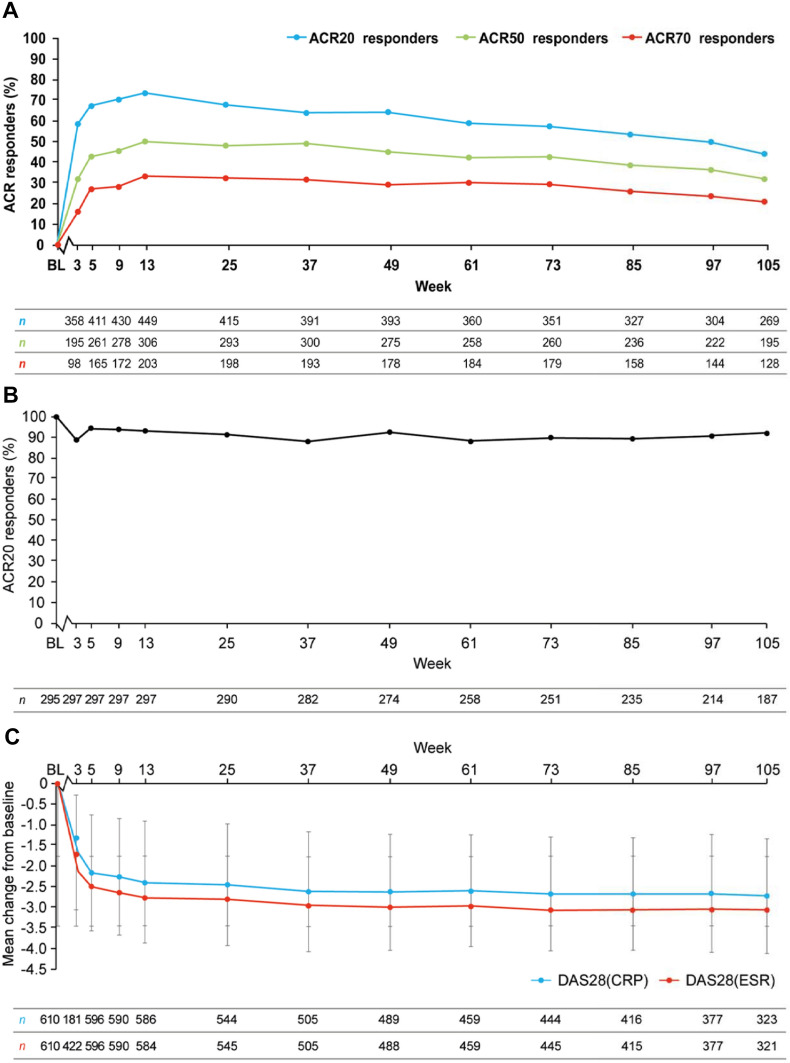
From baseline to week 105, the ACR20/50/70 response was 44% (n = 269), 32% (n = 195), and 21% (n = 128), respectively (Fig. 2a). In the extension study, comparable ACR20 response rates were observed at baseline and week 105 in all patients treated with peficitinib (Supplement 4).
Fig. 2.
a ACR20/50/70 responses, b maintenance of ACR20 responses, and c DAS28(CRP) and DAS28(ESR) over time. All data are relative to phase II trial baselines and were analysed using observed data. a ACR20/50/70 responses [percentages are calculated based on total number of patients in the FAS (N = 611)]. b Maintenance of ACR20 responses in patients who achieved an ACR20 response in their previous respective phase II trial. c Mean (± standard deviation) change from baseline DAS28(CRP) and DAS28(ESR). ACR20 American College of Rheumatology criteria for 20% improvement, ACR50 ACR 50% response, ACR70 ACR 70% response, BL baseline, DAS28(CRP) disease activity score in 28 joints using C-reactive protein DAS28(ESR) disease activity score in 28 joints using erythrocyte sedimentation rate
ACR20 response was observed in 302 (49.4%) patients at week 12 in the double-blind trials, and all of those patients continued in the extension study, of which 171 (57%) patients had an ACR20 response at week 105 when analysed using non-responder imputation (Fig. 2b).
DAS28(CRP) and DAS28(ESR) decreased during the first 9 weeks of the extension study relative to the start of the double-blind trials and then remained stable to week 105 (Fig. 2c). When analysed by previous treatment group, no differences were observed between treatment groups.
At baseline and week 105 in the extension study, 128 (20.9%) and 135 (22.0%) patients at baseline and week 105, respectively, achieved DAS28(CRP) < 2.6. The number of patients achieving DAS28(CRP) < 2.6 was similar when patients were stratified by previous treatment group.
CDAI and SDAI statuses at baseline and week 105 are shown in Supplements 5 and 6, respectively. At week 105, the proportions of patients achieving remission across the peficitinib groups were 95/323, 29.4% (SDAI ≤ 3.3) and 103/324, 31.8% (CDAI ≤ 2.8). Similarly for LDA, the proportions were 215/323, 66.6% (SDAI ≤ 11) and 215/324, 66.4% (CDAI ≤ 10).
Discussion
This phase IIb, open-label, single-arm global study showed that peficitinib 100 mg once daily had a safety profile that was consistent with JAK inhibitors licensed for the treatment of RA and sustained effectiveness over 2 years in adults with moderate-to-severe RA in individuals who remained in the study.
One unusual finding was the number of pregnancies that occurred in the extension study. There are no studies of JAK inhibitors in pregnant women, and licensed JAK inhibitors are contraindicated during pregnancy [1–5]. Women comprised 82.5% of this extension study population, and the protocol stated that women of childbearing potential had to be using highly effective contraception consisting of two forms of birth control. Eight (1%) patients became pregnant, and peficitinib treatment was stopped in five patients. Most of the pregnancies occurred towards the end of the study period, and it is possible that women became less diligent with contraception in the latter stages of the study. In this extension study, two pregnant women were lost to follow-up with ongoing pregnancy, but had stopped treatment before discontinuing; the known pregnancy outcomes were four spontaneous/elective abortions and two live births (both women had stopped study treatment).
Long-term RA outcomes based on various endpoints were evaluated in an exploratory analysis of the effectiveness of peficitinib from week 12 in the double-blind trials to week 105 of the extension study. Overall, 57% of patients who achieved an ACR20 response at week 12 continued with sustained and similar ACR20 responses at the end of the extension study. However, it should be noted that the main limitation of the study was the low completion rate (52%), which was influenced by the rate of patient withdrawals after the protocol amendment to be consistent with EULAR [15] and ACR treatment guidelines [16] was adopted. The latest amendment mandated that patients who did not achieve a low disease activity within 6 months of initiation of peficitinib therapy should be discontinued from the study. The amendment specified that dose intensification beyond 100 mg of peficitinib once daily in non-responders was not allowed. This amendment is one of the main reasons for the high level of discontinuation in this study. The study protocol was amended following a decision not to further investigate peficitinib outside of Japan and to ease the burden of study procedures on investigators and patients.
Additionally, patients who received placebo during the double-blind trials and switched to peficitinib during the extension trial showed greater improvements in ACR20 response to peficitinib compared with patients who received peficitinib during the double-blind trials. This difference was possibly due to higher disease activity in placebo-treated patients, in contrast to the peficitinib-treated patients, who had reported improvements during the double-blind trials or the open-label nature of the extension trial. Furthermore, although DAS28(CRP) and DAS28(ESR) decreased during the first 9 weeks of the extension study, as expected, responders were more likely to continue with treatment than were non-responders. Further limitations were the open-label design and the potential selection bias in the extension study for patients who responded to treatment and had acceptable safety during the double-blind trial.
Conclusion
This long-term study of open-label peficitinib in adults with moderate-to-severe RA showed that treatment had a favourable benefit-risk ratio, and although half of the population discontinued treatment during the 2-year study, among patients who completed, ACR20 responses at week 12 were sustained up to week 105.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
This study and the Rapid Service Fee were funded by Astellas Pharma Global Development, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing support was provided by Matthew Reynolds and Leigh Church of SuccinctChoice Medical Communications (London, UK), and Sharon Rayner for Cello Health MedErgy (Farnham, United Kingdom) funded by Astellas Pharma Global Development, Inc. The authors are grateful to Anil Kumar, MD, FRCS, FACS, an employee of Astellas Pharma, Inc., who assisted in the critical review and editing of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors made substantial contributions to the conception or design of this study, acquisition of the data, analysis and interpretation of the data and drafting of the publication, and are accountable for all aspects of the submitted work. We would like to acknowledge all other investigators for their participation in this study.
Disclosures
Employees of the study sponsor were involved in study conception, design and conduct, and in data collection and analysis. All authors had full access to study data and had final responsibility to submit for publication. Mark Genovese has received personal fees from AbbVie, Eli Lilly & Company, Galapagos NV, and Pfizer and has received grants from AbbVie and Pfizer. Maria Greenwald has received faculty/speaker fees from Pfizer and received grants from AbbVie, Astellas, Gilead, Lilly, and Pfizer. Mario Cardiel has received personal fees from AbbVie, Eli Lilly and Company, Pfizer, and Roche. Sergio Gutierrez-Ureña has received personal fees from Astellas Pharma Global Development, Inc., Celltrion, Human Genome Sciences, Inc., and Merck & Company. Anna Zubrzycka-Sienkiewicz has received grants from Auven Therapeutics, Celltrion, Galapagos NV, Janssen, Mabion, Merck & Company, Roche, and UCB SA. Christine Codding has received principal investigator fees from AbbVie, Bristol-Myers Squibb, Celgene, Coherus, Daiichi Sankyo, Eli Lilly & Company, Genentech, Inc., Gilead, Janssen, Merck & Company, Mesoblast, Novartis, Pfizer, Sandoz, Sanofi, and UCB. Alan Kivitz has received personal fees from AbbVie, Astellas Pharma Global Development, Inc., Eli Lilly & Company, Galapagos NV, Genentech, Inc., Johnson & Johnson, Pfizer, and Vertex Pharmaceuticals. Annie Wang is employed by Astellas Pharma Global Development, Inc. Rebecca Amos is employed by Astellas Pharma Global Development, Inc. Raul Vinueza is employed by Astellas Pharma Global Development, Inc. Weizhong He is employed by Astellas Pharma Global Development, Inc. Xuegong Wang is employed by Astellas Pharma Global Development, Inc. Jay Garg was employed by Astellas Pharma Global Development, Inc., during the conduct of this study and is now employed by Genentech. Jeffrey Poiley has nothing to disclose.
Compliance with Ethics Guidelines
An Institutional Review Board/Independent Ethics Committee-approved written informed consent form was obtained from each patient or from a legally authorized representative prior to the initiation of any study-specific procedures. This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines, the EU Clinical Trials Directive, and applicable laws and regulations. The central IRB for the United States was Quorum IRB. Details of individual IRBs are included in Supplement 7.
Data Availability
Access to anonymized individual participant level data collected during the study, in addition to supporting clinical documentation, is planned for studies conducted with approved product indications and formulations as well as compounds terminated during development. Studies conducted with product indications or formulations that remain active in development are assessed after study completion to determine if Individual Participant Data can be shared. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on http://www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan, and clinical study report. Access to participant-level data is offered to researchers after publication of the primary manuscript (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data sharing environment after receipt of a signed Data Sharing Agreement.
Footnotes
At the time of the study, J. Garg was an employee of Astellas Pharma Global Development, Inc. He is now an employee of Genentech, Inc., San Francisco, CA, USA.
Enhanced Digital Features
To view enhanced digital features for this article go to: 10.6084/m9.figshare.8976692.
References
- 1.Cheung TT, McInnes IB. Future therapeutic targets in rheumatoid arthritis? Semin Immunopathol. 2017;39:487–500. doi: 10.1007/s00281-017-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72 Suppl 2:ii111-5. 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed]
- 3.Eli Lilly and Company Limited. Summary of Product Characteristics: Olumiant 2 mg Film-Coated Tablets [Internet]. https://www.medicines.org.uk/emc/product/2434/smpc. Accessed 5 Aug 2019.
- 4.Pfizer Laboratories Div Pfizer Inc. Prescribing information: XELJANZ- tofacitinib tablet, film coated [Internet]. 2012; http://labeling.pfizer.com/showlabeling.aspx?id=959. Accessed 5 Aug 2019.
- 5.Pfizer Limited. Summary of Product Characteristics: XELJANZ 5 mg film-coated tablets [Internet]. 2018; https://www.medicines.org.uk/emc/product/2500/smpc. Accessed 5 Aug 2019.
- 6.Pfizer provides update on global regulatory approvals and launches of Xeljanz® (tofacitinib citrate) for the treatment of rheumatoid arthritis. 2013; https://www.pfizer.com/news/press-release/press-release-detail/pfizer_provides_update_on_global_regulatory_approvals_and_launches_of_xeljanz_tofacitinib_citrate_for_the_treatment_of_rheumatoid_arthritis. Accessed 6 Aug 2019
- 7.Korea approves Olumiant pills for treatment of rheumatoid arthritis. 2017; http://www.koreabiomed.com/news/articleView.html?idxno=2098. Accessed 6 Aug 2019.
- 8.Japan Ministry of Health Labour and Welfare. Xeljanz Tablets 5 mg report. 2013; https://www.pmda.go.jp/files/000153609.pdf. Accessed 6 Aug 2019.
- 9.Astellas Pharma, Inc. Oral JAK Inhibitor Smyraf® Tablets Approved in Japan for the Treatment of Rheumatoid Arthritis (including prevention of structural joint damage) in Patients Who Have an Inadequate Response to Conventional Therapies. 2019; https://www.astellas.com/en/news/14651. Accessed 5 Aug 2019.
- 10.Ito M, Yamazaki S, Yamagami K, Kuno M, Morita Y, Okuma K, et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133:25–33. doi: 10.1016/j.jphs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Hamaguchi H, Amano Y, Moritomo A, Shirakami S, Nakajima Y, Nakai K, et al. Discovery and structural characterization of peficitinib (ASP015 K) as a novel and potent JAK inhibitor. Bioorg Med Chem. 2018;26:4971–4983. doi: 10.1016/j.bmc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Genovese MC, Greenwald M, Codding C, Zubrzycka-Sienkiewicz A, Kivitz AJ, Wang A, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ) 2017;69:932–942. doi: 10.1002/art.40054. [DOI] [PubMed] [Google Scholar]
- 13.Kivitz AJ, Gutierrez-Urena SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol (Hoboken, NJ) 2017;69:709–719. doi: 10.1002/art.39955. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015 K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064. doi: 10.1136/annrheumdis-2015-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to anonymized individual participant level data collected during the study, in addition to supporting clinical documentation, is planned for studies conducted with approved product indications and formulations as well as compounds terminated during development. Studies conducted with product indications or formulations that remain active in development are assessed after study completion to determine if Individual Participant Data can be shared. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on http://www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan, and clinical study report. Access to participant-level data is offered to researchers after publication of the primary manuscript (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data sharing environment after receipt of a signed Data Sharing Agreement.