Abstract
The extent of neocortical gyrification is an important determinant of a species’ cognitive abilities, yet the mechanisms regulating cortical gyrification are poorly understood. We uncover long-range regulation of this process originating at the telencephalic dorsal midline, where levels of secreted Bmps are maintained by factors in both the neuroepithelium and the overlying mesenchyme. In the mouse, the combined loss of transcription factors Lmx1a and Lmx1b, selectively expressed in the midline neuroepithelium and the mesenchyme respectively, causes dorsal midline Bmp signaling to drop at early neural tube stages. This alters the spatial and temporal Wnt signaling profile of the dorsal midline cortical hem, which in turn causes gyrification of the distal neocortex. Our study uncovers early mesenchymal-neuroepithelial interactions that have long-range effects on neocortical gyrification and shows that lissencephaly in mice is actively maintained via redundant genetic regulation of dorsal midline development and signaling.
Subject terms: Developmental neurogenesis, Cell type diversity
The contribution of long-range signaling to cortical gyrification remains poorly understood. In this study, authors demonstrate that the combined genetic loss of transcription factors Lmx1a and Lmx1b, expressed in the telencephalic dorsal midline neuroepithelium and head mesenchyme, respectively, induces gyrification in the mouse neocortex
Introduction
Neocortical gyrification, defined as folding of the outer pial surface and underlying neuronal layers of the brain but a smooth ventricular surface, results in an uneven regional thickness of the neocortex and is associated with enhanced species’ cognitive abilities1–4. Neocortical gyrification is a variable trait across mammalian species ranging from complete absence of gyrification in lissencephalic mammals, such as the mouse, to highly elaborate gyrification in many primates, such as humans. It is believed that the most recent common mammalian ancestor had a gyrencephalic (folded) cortex and that several transitions between gyrencephaly and lissencephaly (smooth cortex) happened during mammalian evolution as adaptations to each species’ environment and lifestyle5–7.
Recent studies have revealed a coordinated sequence of neurogenic events in the developing neocortex of naturally gyrencephalic mammals, which begins with an early accumulation of apical cortical progenitors (APs) in the neocortical ventricular zone (VZ), followed by expansion of basal progenitors (BPs), including intermediate progenitors (IPs) and basal radial glia (bRG). BPs arise from APs and expand in the emerging gyri. It is believed that extensive generation of BPs, especially bRG, at the peak of neurogenesis and the subsequent production of neurons from these progenitors is a major force driving cortical gyrification in higher mammals3,8–12. A number of genes that promote gyrification acting locally in cortical progenitors have been identified4,13–21. During development, however, many cortical features are regulated by both locally acting mechanisms and long-range signaling. The contribution of signaling molecules, in particular long-range signaling molecules, to cortical gyrification remains poorly understood. Shh and Fgf signaling are known to promote cortical gyrification16,22–24. In humans, Shh is expressed and acts locally in cortical progenitors, while the sources of gyrification-related Fgf signaling remain unknown16,22.
Although there have been extensive efforts devoted to identify the mechanisms that promote cortical folding in gyrencephalic mammals, the endogenous mechanisms that maintain lissencephaly (suppress gyrification) in naturally lissencephalic mammals have received little attention and remain virtually unknown. One possibility is that lissencephalic species simply lack genes or regulatory elements necessary to drive cortical gyrification13–15,18,19. Alternatively, genes in the genome of lissencephalic species actively maintain lissencephaly, suppressing gyrification. To date, several mouse models with a gyrencephalic neocortex have been described. The vast majority of these, however, have been generated via local or transient manipulation of gene function and, therefore, alone are insufficient to dissect the endogenous mechanisms that maintain lissencephaly in the mouse brain. The only exception is the Flrt1−/−;Flrt3−/− mouse model25. Dual loss of the adhesion genes Flrt1 and Flrt3 affects migration of differentiating neurons and results in folding of the mouse neocortex. In this mouse model, however, cortical folding develops without the predominant expansion of BPs observed in higher mammals25.
While studying the role of transcription factors Lmx1a and Lmx1b in the cerebellum26, we observed that Lmx1a/b double, but not single, mutants had unexpected neocortical gyrification. We found that neither Lmx1a nor Lmx1b were expressed in the neocortex. In contrast to more posterior central nervous system26, in the telencephalon, these two genes were not even coexpressed: Lmx1a was expressed in the telencephalic dorsal midline neuroepithelium (DMe), while Lmx1b was expressed in head mesenchyme. Cortical gyrification in Lmx1a−/−;b−/− mutants was associated with expansion of neocortical BPs and resulted from the disruption of spatial and temporal dynamics of Bmp and Wnt signaling cascades originating at the distantly located dorsomedial telencephalon. Our study identifies an unexpected role of dorsal midline signaling in the long-range regulation of cortical gyrification and shows that mesenchymal–neuroepithelial interactions are necessary to maintain lissencephaly in mice.
Results
Cortical gyrification in Lmx1a−/−;b−/− mice
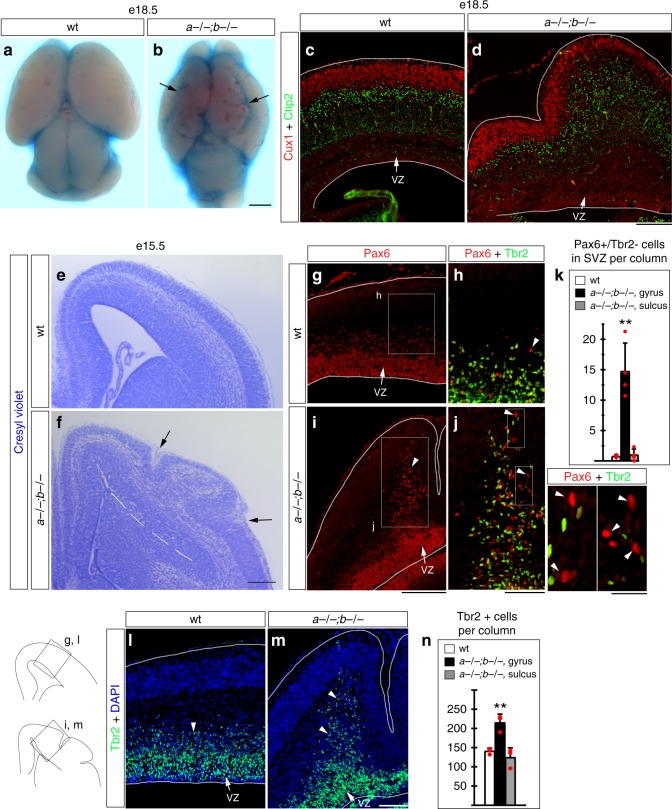
Transcription factors Lmx1a and Lmx1b redundantly regulate development of several cellular populations during embryonic development, such as the hindbrain roof plate and midbrain dopaminergic neurons26,27. While studying the role of Lmx1 genes in cerebellar development26, we found that Lmx1a−/−;b−/− mutants had unexpected and striking gyrification of the neocortex (Fig. 1a–f, Supplementary Fig. 1a–d). All 12 Lmx1a−/−;b−/− mutants that we dissected at late embryonic stages had macroscopic cortical folds apparent in both left and right hemispheres (Fig. 1b) yet the cortex remained lissencephalic in littermates with loss of either single gene (n = 20–25 mutants for each genotype) (Supplementary Fig. 2). Analysis of postnatal stages was precluded by neonatal death caused by a requirement for Lmx1b during mid/hindbrain development26. To compare location of cortical gyri in different e18.5 embryos, we divided the neocortical ventricular surface into 10 equally sized segments and measured cortical thickness in the middle of each of these segments (Supplementary Fig. 1e). Average cortical thickness was similar throughout wild-type cortex yet was uneven between different regions of Lmx1a−/−;b−/− neocortex, illustrating non-random locations of cortical gyri (Supplementary Fig. 1g, h). In both hemispheres, the most prominent was a dorsal gyrus located in cortical segments 2 (and also in segment 3 in right hemisphere), while the neuroepithelium in segment 4 (and also in segments 5 and 6 in right hemisphere) was thinner. More ventral gyri were less prominent (Supplementary Fig. 1b–d, h). Consistent with cortical gyrification, neocortical area (measured between the dorsal cortical bend and lateral ganglionic eminence, LGE) was increased in Lmx1a−/−;b−/− mutants compared to wild-type littermates (Supplementary Fig. 1f).
Fig. 1.
Gyrification with BPs expansion in the Lmx1a−/−;b−/− mouse cortex. Dorsal view of whole mount telencephalon with fast green dye applied on the telencephalic surface (a, b) and coronal cresyl violet or antibody-stained sections (c–j, l, m) at indicated stages. a, b Cortical surface of e18.5 Lmx1a−/−;b−/− mutants, but not wild-type littermates, showed groves (arrows) that extended along the anterior-posterior axis in each cortical hemisphere. c, d Cux1/Ctip2-immunostained sections showing that despite gyrification of the outer cortical surface (faces up), cortical layering was not grossly disrupted in Lmx1a−/−;b−/− mutants. Corresponding ventricular surface (faces down) was not folded. VZ - ventricular zone. e, f Arrows point to sulci that develop on the outer cortical surface of Lmx1a−/−;b−/− embryos at e15.5 (f). Dashed line demarcates cortical ventricular surface and lateral ganglionic eminence. g–k Numerous basally located Pax6+ cells were present in the emerging gyri (i, arrowhead) but not sulci in e15.5 Lmx1a−/−;b−/− mutants (i). The number of Pax6+/Tbr2− cells located basal to the dense band of Tbr2 + IPs (arrowheads in h, j, j insets) was dramatically increased in cortical gyri but not sulci relative to wild-type littermates (**p < 0.01, n = 4 embryos per genotype). l–n In e15.5 Lmx1a−/−;b−/− mutants, Tbr2 + IPs (arrowheads) were increased in the number in cortical gyri but not sulci relative to wild-type littermates (**p < 0.01, n = 3 embryos per genotype). All data are mean ± s.d., all p values are from two-tailed t-test after Bonferroni correction for multiple comparisons. Source data for panels k and n are provided as Source Data File. Scale bars: 1 mm (a, b); 200 μm (c–g, i); 100 μm (h, j, l, m); 25 μm (insets in panel j)
Similar to wild-type littermates28, in Lmx1a−/−;b−/− mutants, Cux1+ (upper layer) neurons occupied more superficial positions relative to Ctip2+ (lower layer) neurons, revealing largely preserved cortical lamination (Fig. 1c, d). Thus, Lmx1a and Lmx1b function redundantly to maintain lissencephaly in the mouse, and simultaneous loss of these genes is sufficient to induce gyrification of the mouse cortex.
Aberrant expansion of progenitors in Lmx1a−/−;b−/− cortex
To determine whether cortical gyrification in Lmx1a−/−;b−/− mutants was associated with expansion of cortical BPs, we analyzed embryos at e15.5, during mid-neurogenesis, when Lmx1a−/−;b−/− double mutant cortex first displays evidence of gyrification (cortical plate folding relative to ventricular surface) (Fig. 1e, f). In the emerging cortical gyri, but not sulci, of e15.5 Lmx1a−/−;b−/− mutants, more Pax6+/Tbr2− and Tbr2+ cells were observed in basal locations relative to wild-type controls (Fig. 1g–n), consistent with focal expansion of bRG and IPs16,29,30.
Since proliferation of BPs and the subsequent production of neurons from these progenitors are major mechanisms of cortical gyrification in higher mammals3,8–12, we studied BPs in the emerging cortical gyri of Lmx1a−/−;b−/− mutants in more detail. Co-labeling with a progenitor marker Ki67 confirmed that basal Pax6+/Tbr2− and Tbr2+ cells in Lmx1a−/−;b−/− mutants were indeed progenitors (Supplementary Fig. 3a, b). A short (90 min) BrdU pulse was used to label proliferating cells in the S-phase of the cell cycle. This analysis revealed numerous basally located BrdU+ cells in the emerging gyri of Lmx1a−/−;b−/− embryos (Supplementary Fig. 3c, g, arrowheads). The fraction of basally located BrdU+ cells was significantly higher in the emerging gyri of Lmx1a/b double mutants compared to wild-type controls (Supplementary Fig. 3c, g, k) arguing that similar to naturally gyrencephalic mammals3,9,11, in Lmx1a−/−;b−/− cortical gyri, proliferation was shifted to basal positions. Triple BrdU/Pax6/Tbr2 labeling revealed that in Lmx1a−/−;b−/− mutants, many BPs incorporated BrdU and, thus, were proliferating (Supplementary Fig. 3c–e, g–i). There was a significant increase in the numbers of both S-phase BrdU+/Pax6+/Tbr2− bRG and BrdU+/Tbr2+ IPs, but not apical BrdU+/Pax6+ cells in cortical gyri of Lmx1a−/−;b−/− mutants (Supplementary Fig. 3c–f, g–j, l–n). Since Pax6+/Tbr2− bRG cells were very rare in wild-type embryos, we were unable to compare the fraction of BrdU+ (S-phase) bRG cells between wild-type and Lmx1a−/−;b−/− embryos. However, we found that a similar fraction (~20%) of Tbr2+ IPs were BrdU+ (S-phase) in both wild-type and Lmx1a−/−;b−/− cortex (Supplementary Fig. 3o), suggesting a similar proliferation rate31 of IPs in wild-type and Lmx1a−/−;b−/− mutants.
A longer (20 h) BrdU pulse revealed an increased fraction of progenitors exiting the cell cycle in nascent gyri of Lmx1a−/−;b−/− embryos (Supplementary Fig. 3p–r), consistent with increased neurogenesis23. Analysis of Lmx1a−/−;b−/− mutants at e15.5, the time of gyri development, revealed a similar density of apoptotic (activated Caspase 3+) cells in cortical areas producing gyri and sulci (Supplementary Fig. 4), indicating that localized increase in apoptosis is not a major mechanism of cortical gyrification in Lmx1a−/−;/b−/− mutants. Thus, cortical gyrification in Lmx1a−/−;b−/− mutants was associated with expansion of cortical BPs and increased neurogenesis.
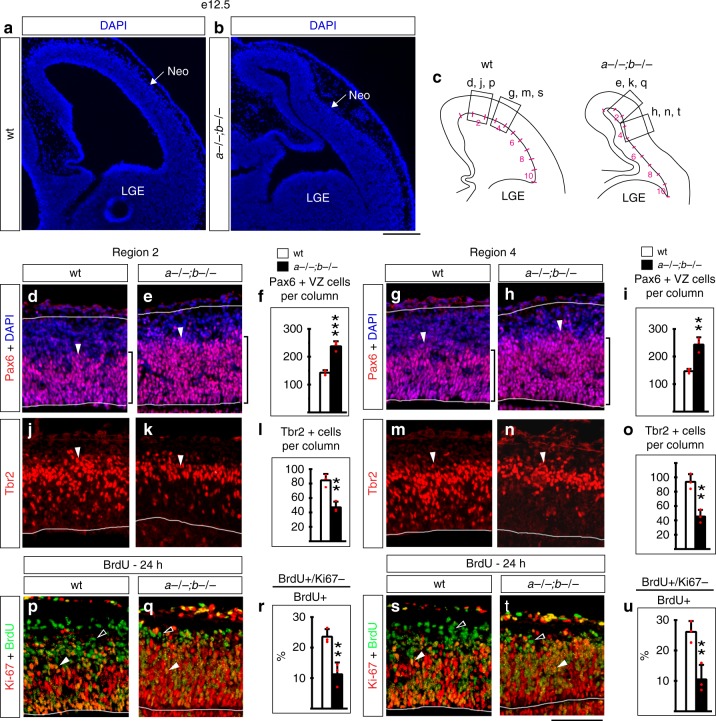
Next, we analyzed e12.5 embryos, at the beginning of cortical neurogenesis when Lmx1a−/−;b−/− mutants were not yet gyrified (Fig. 2a, b). We studied progenitor populations in two cortical regions—cortical segment 2 (the most consistent location of a future gyrus) and cortical segment 4 (a region with the lowest cortical thickness at e18.5) (Fig. 2c, Supplementary Fig. 1h). At this early stage, compared to controls, both cortical regions of the double mutants had increased numbers of Pax6+ APs, increased radial thickness of the VZ (Fig. 2d–i), reduced numbers of Tbr2+ IPs (Fig. 2j–o), and reduced cell-cycle exit (Fig. 2p–u). Thus, in Lmx1a/b double mutants, global overproduction of APs precedes local accumulation of BPs at later stages.
Fig. 2.
Accumulation of APs precedes gyrification of the Lmx1a−/−;b−/− cortex. Coronal sections at e12.5. a, b No evidence of cortical gyrification (outgrowths on outer cortical surface without bending of the corresponding ventricular surface) was detected in Lmx1a−/−;b−/− mutants at e12.5. Neo-neocortical primordium. LGE lateral ganglionic eminence. c Ventricular surface in wild-type and Lmx1a−/−;b−/− neocortical primordium (between the dorsal cortical bend and LGE) was subdivided into 10 equally sized regions (segments 1–10) and cells were quantified in segment 2 (d–f, j–l, p–r) and segment 4 (g–i, m–o, s–u). d–i Pax6+ APs (d, e, g, h, arrowheads) were increased in the number (f, i) (***p < 0.001 and **p < 0.01) and Pax6+VZ zone (bracket) was thicker in Lmx1a−/−b−/− mutants in both segments of the neocortical primordium. j–o Tbr2+ IPs (j, k, m, n, arrowheads) were reduced in the number (l, o) (**p < 0.01) in Lmx1a−/−;b−/− mutants in both segments of the neocortical primordium. p–u Mice were injected with BrdU 24 h prior to collecting embryos. p, q, s, t Arrowheads point to cells that re-entered the cell cycle (BrdU+/Ki67+ cells), open arrowheads—to cell that exited the cell cycle (BrdU + /Ki67− cells). A lower fraction of cells exited the cell cycle (the number of BrdU+/Ki67− cells divided by the number of BrdU+ cells) (**p < 0.01) in Lmx1a−/−;b−/− mutants in both segments of the neocortical primordium. All data are mean ± s.d., all p values are from two-tailed t-test, n = 3 embryos per genotype. Source data for panels f, i, l, o, r, u are provided as Source Data File. Scale bars: 200 μm (a, b); 100 μm (all other panels)
Lmx1a/b are not coexpressed in the developing telencephalon
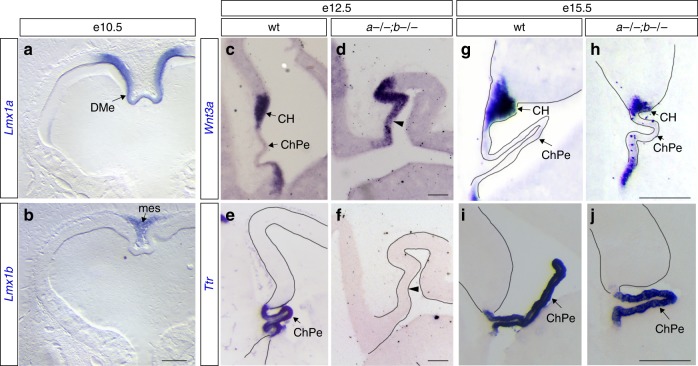
We expected the Lmx1a and Lmx1b to be coexpressed in the developing telencephalon, based on their co-expression in other cellular populations redundantly regulated by these genes26,27. Our extensive in situ hybridization, immunohistochemical and fate-mapping analyses revealed, however, that these genes are never coexpressed in the developing telencephalon. Further, neither is even expressed in neocortical neuroepithelium. Lmx1a expression was always restricted to the telencephalic DMe, beginning at the tips of the neural folds at e8.5 and through differentiation of the DMe into the medial choroid plexus epithelium (ChPe) and the cortical hem (CH). Lmx1b expression was restricted to the head mesenchyme, including the mesenchyme located adjacent to the DMe (Fig. 3a, b, Supplementary Figs. 5 and 6). Loss of one Lmx1 gene did not alter expression pattern of the remaining Lmx1 gene (Supplementary Fig. 7). When considered together with the Lmx1a−/−;b−/− cortical phenotype, the Lmx1a and Lmx1b expression pattern suggests that redundant Lmx1a/b-dependent mesenchymal and DMe signaling pathways regulate gyrification of distal neocortex.
Fig. 3.
Dual loss of complementary expressed Lmx1a/b causes transient DMe mispatterning. a, b In situ stained wild-type coronal telencephalic sections at e10.5 showing specific expression of Lmx1a in the DMe and Lmx1b—in medial mesenchyme (mes.). c–f At e12.5, wild-type CH expressed Wnt3a (c), while ChPe—Ttr (e). In Lmx1a−/−;b−/− mutants, Wnt3a expression domain was enlarged, expanding into the prospective ChPe territory (d, arrowhead), while Ttr was not expressed (f, arrowhead). g–j DMe patterning was restored in e15.5 Lmx1a−/−;b−/− mutants, with Ttr expression in the ChPe and Wnt3a—in the CH. Wnt3a+ CH domain, however, was abnormally small (g, h). Scale bars: 200 μm (a, b, g, h); 150 μm (c–f); 250 μm (i, j)
Aberrant DMe and Wnt signaling in Lmx1a−/−;b−/− mutants
By e12.5, the wild-type telencephalic DMe differentiates into the medial Ttr+ ChPe and adjacent CH that expresses secreted Wnt3a32–34 (Fig. 3c, e). In Lmx1a−/−;b−/− mutants at this stage, however, Ttr expression was absent in a thickened DMe while Wnt3a expression expanded across the presumptive ChPe, resulting in an enlarged Wnt3a expression domain at the dorsal midline compared to controls (Fig. 3c-f). This phenotype was not observed in either single-gene mutant (Supplementary Fig. 8). In double mutants, the thickened medial neuroepithelium showed increased proliferation in the presumptive ChPe as early as at e10.5 (Supplementary Fig. 9a–g). By e15.5, there was partial amelioration of Lmx1a−/−;b−/− DMe deficits: Ttr ChPe expression was present and Wnt3a expression was restricted to the CH (Fig. 3g–j), although ChPe proliferation was still elevated compared to controls (Supplementary Fig. 9h, i). The double mutant CH also displayed increased apoptosis (Supplementary Fig. 9j–l), resulting in a small Wnt3a+ CH at e15.5 (Fig. 3g, h).
Early expansion of Wnt3a+ CH in e12.5 Lmx1a−/−;b−/− mutants was associated with elevated nuclear β-catenin, a definitive readout of canonical Wnt signaling35 (Supplementary Fig. 10) and elevated expression of downstream Wnt-β-catenin target genes Lef1 and Axin236 in the neocortical primordium (Supplementary Fig. 11). However, at e15.5, consistent with the dramatic reduction of size of the double-mutant Wnt3a+ CH, nuclear β-catenin was undetectable in the Lmx1a−/−;b−/− neocortex, and Lef1 and Axin2 levels were significantly reduced relative to comparably staged controls (Supplementary Fig. 12). These data define two distinct phases of canonical Wnt signaling and reception in the Lmx1a−/−;b−/− embryonic telencephalon: an increased early phase and a decreased late phase.
In addition to producing Wnt signaling molecules, the early DMe (both the CH and ChPe) gives rise to Cajal-Retzius (CR) cells, a transient neuronal population that migrates along the outer surface of cortical neuroepithelium and regulates cortical development37,38. In Lmx1a−/−;b−/− mutants, similar to wild-type embryos, CR cells uniformly covered the neocortical primordium, suggesting that cortical gyrification in these mutants does not directly arise from CR cells migratory defects (Supplementary Fig. 13). Thus, to define the mechanism of Lmx1a−/−;b−/− cortical gyrification, we focused on misregulated DMe-derived Wnt signaling.
Lmx1a−/−;b−/− cortical gyrification depends on Wnt signals
Wnt/β-catenin signaling promotes expansion of cortical APs and suppresses the generation of BPs from APs29,31,39. Early constitutive ablation of β-catenin in the developing telencephalon increased the numbers of both bRG and IPs29, the phenotype we also observed in Lmx1a−/−;b−/− mutants. However, in contrast to Lmx1a−/−;b−/− embryos, β-catenin-ablated mice did not show cortical gyrification, and IP expansion in these mice was observed only at the beginning (e12.5) but not in the middle (e14.5) of neurogenesis, likely due to precocious depletion of the AP pool29.
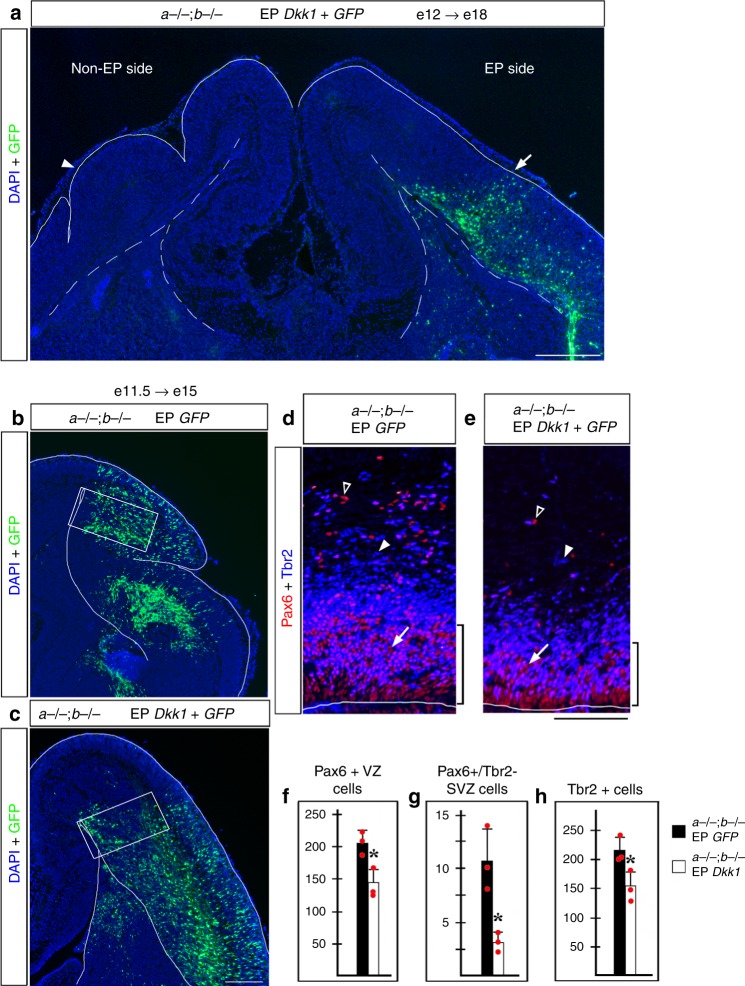
A key difference between β-catenin-ablated mice and our Lmx1a−/−;b−/− mutant embryos is that in contrast to β-catenin-ablated mice, in Lmx1a−/−;b−/− mutants, a dynamic decrease in Wnt-β-catenin signaling followed its transient overactivation and the early overexpansion of APs. To balance early high levels of Wnt signaling in Lmx1a−/−;b−/− neocortex, we electroporated a plasmid encoding Dkk1, a secreted Wnt inhibitor39 into the e12 Lmx1a−/−;b−/− neocortical primordium. This was sufficient to rescue the gyrification phenotype and restore a lissencephalic cortex on the electroporated side, when assessed at e18 (Fig. 4a). Dkk1-electroporated Lmx1a−/−;b−/− hemispheres had significantly lower numbers of APs, bRG, and IPs compared to Lmx1a−/−;b−/− cortices electroporated with GFP alone (Fig. 4b–h). Thus, we conclude that aberrant dynamics of Wnt signaling is a major contributor to neocortical gyrification in Lmx1a−/−;b−/− mice.
Fig. 4.
Electroporation of Dkk1 suppresses Lmx1a−/−;b−/− cortical gyrification. a An Lmx1a−/−;b−/− embryo in utero electroporated with Dkk1+ GFP at e12 and analyzed at e18. Early electroporation of Dkk1 into neocortical primordium restored lissencephaly (arrow), while the non-electroporated side remained gyrencephalic (arrowhead). b–h Lmx1a−/−;b−/− mutants electroporated with Dkk1+ GFP or GFP alone at e11.5 and analyzed at e15. Panels d, e are from sections adjacent to those shown in b, c and correspond to regions boxed in b, c. d–h In Dkk1-electroporated double mutants, the number of Pax6+ APs (arrows), Pax6+ /Tbr2− bRGs (open arrowheads) and Tbr2+ IPs (arrowheads) was reduced compared to GFP-electroporated double mutants (*p < 0.05, two-tailed t-test, n = 3 Dkk1-electroporated and n = 3 GFP-electroporated Lmx1a−/−;b−/− mutants). Bracket (d, e) indicates thickness of the Pax6+ VZ, which was also reduced in Dkk1-electroporated Lmx1a−/−;b−/−mutants. All data are mean ± s.d. Source data for panels f–h are provided as Source Data File. Scale bars: 400 μm (a); 200 μm (b, c); 100 μm (d, e)
Lmx1a/b regulate the DMe Wnt source via Bmp signaling
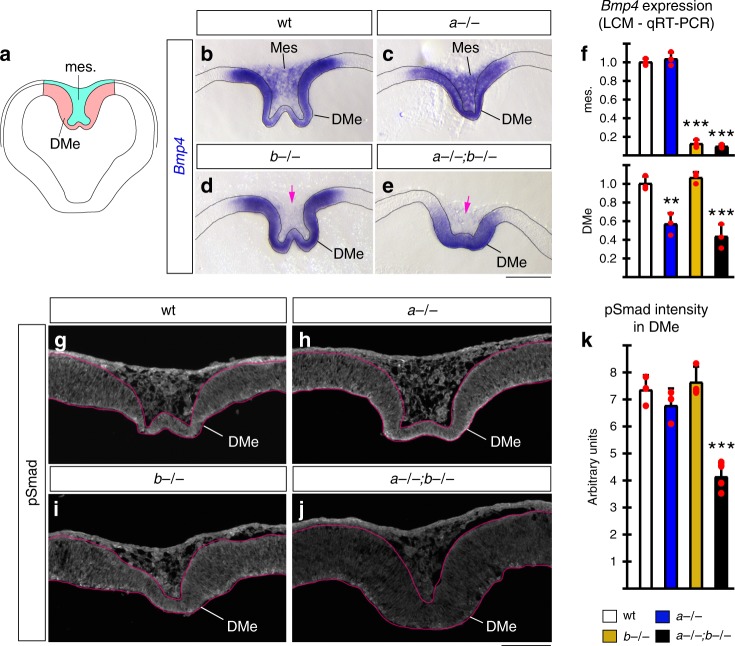
Next, we focused our attention on the early development of the DMe Wnt source. We hypothesized that Lmx1b regulates expression of secreted factors that diffuse from the medial head mesenchyme and cooperate with a Lmx1a-dependent pathway in the DMe to regulate the temporal and spatial patterning of the ChPe/CH. Using a candidate gene approach, in situ hybridization revealed Bmp4 to be expressed in both the Lmx1b (mesenchymal) and Lmx1a (DMe) territory in wild-type embryos (Fig. 5a, b). Loss of Lmx1b reduced Bmp4 expression in the medial mesenchyme but did not alter expression of this gene in the DMe or lateral mesenchyme (Fig. 5b, d, e; Supplementary Fig. 14). Based on in situ hybridization, loss of Lmx1a did not compromise Bmp4 expression in either compartment (Fig. 5b, c, e; Supplementary Fig. 14). Although in situ hybridization is well suited for spatial distribution of gene expression, it is not strictly quantitative. We, therefore, used qRT-PCR analysis of laser capture microdissected dorsal mesenchyme and DMe to more quantitatively assess expression of Bmp genes in each compartment (Fig. 5a, f; Supplementary Fig. 15). This analysis revealed that loss of Lmx1a decreased expression levels of both Bmp4 and related Bmp2 in the DMe. It also confirmed that loss of Lmx1b alone caused drastic decrease of Bmp4 as well as Bmp2 expression in medial mesenchyme. In double mutants, Bmp4/2 mesenchymal expression levels were similar to those in Lmx1b mutants, while double-mutant DMe Bmp4/2 expression levels were similar to those in Lmx1a mutants (Fig. 5f; Supplementary Fig. 15). A definitive readout of Bmp signal reception, pSmad immunostaining intensity33, was significantly decreased in the DMe of double but not single-gene mutants at e10.5 (Fig. 5g–k).
Fig. 5.
Lmx1a and Lmx1b regulate Bmp signaling by controlling expression of Bmp4. a Schematic of e10.5 coronal telencephalic section showing DMe (red) and medial mesenchyme (green). b–e High magnification images of dorsal midline region of e10.5 telencephalic coronal sections stained with Bmp4 in situ probe. (Low magnification images of the same sections are shown in Supplementary Fig. 14.) b In wild-type telencephalon, Bmp4 was expressed in the DMe and medial mesenchyme adjacent to DMe (mes.). c–e Loss of Lmx1b abolished Bmp4 expression in medial mesenchyme (pink arrows in d, e) but not DMe. Based on in situ, loss of Lmx1a did not obviously reduce Bmp4 expression. f qRT-PCR analysis of DMe (red region in panel a) and medial mesenchyme (green region in panel a) isolated by laser capture microdissection (LCM). Based on qRT-PCR, loss of Lmx1b reduced expression of Bmp4 in medial mesenchyme (mes.) (***p < 0.001 for Lmx1b−/− versus wild type and Lmx1a−/−;b−/− versus wild-type comparisons, one-way ANOVA with Tukey’s post hoc test, n = 3 embryos per genotype). Loss of Lmx1a reduced expression of Bmp4 in the DMe (**p < 0.01 for Lmx1a−/− versus wild type and ***p = 0.001 for Lmx1a−/−;b−/− versus wild-type comparisons, one-way ANOVA with Tukey’s post hoc test, n = 3 embryos per genotype). g–j pSmad immunohistochemistry on e10.5 coronal sections. Only simultaneous, but not individual loss of Lmx1a and 1b significantly reduced pSmad intensity in the DMe (***p ≤ 0.001 for Lmx1a−/−;b−/− versus wild type, Lmx1a−/−;b−/− versus Lmx1a−/−, and Lmx1a−/−;b−/− versus Lmx1b−/− comparisons, one-way ANOVA with Tukey’s post hoc test, n = 3 wild-type embryos, n = 3 Lmx1a−/− embryos, n = 3 Lmx1b−/− embryos, and n = 4 Lmx1a−/−;b−/− embryos). All data are mean ± s.d. Source data for panels f, k are provided as Source Data File. Scale bars: 200 μm (b–e); 100 μm (g–j)
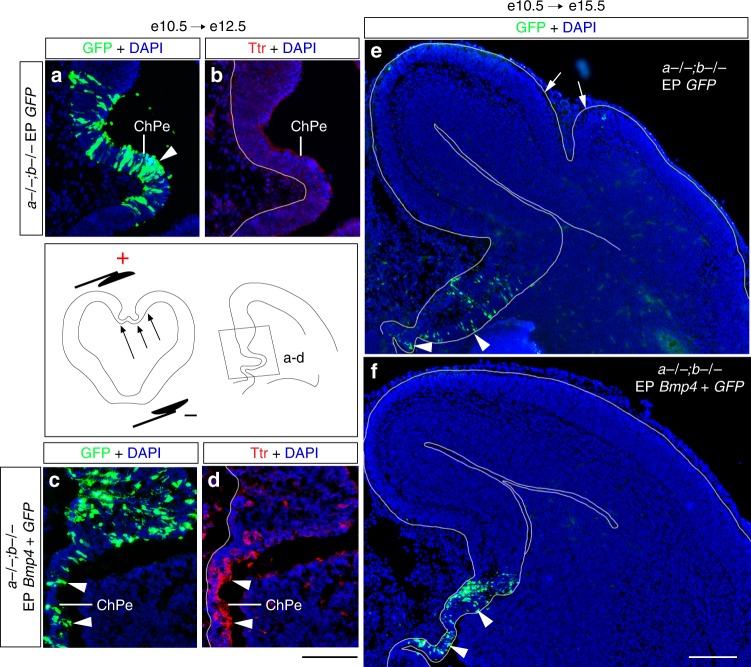
We hypothesized that dual loss of Lmx1a and Lmx1b reduced early Bmp signaling levels below a critical threshold required to pattern and maintain normal DMe and hence subsequent lateral cortical development. We, therefore, selectively electroporated Bmp4 into the DMe territory of Lmx1a−/−;b−/− embryos at e10.5. At e12.5 the double mutant DMe was rescued as demonstrated by the reappearance of Ttr (Fig. 6a–d; Supplementary Fig. 16a, b) and reduction of abnormally high proliferation in the ChPe (Supplementary Fig. 17). Ectopic Wnt3a expression in the double-mutant ChPe was also lost and low levels of Wnt signaling were restored in the distal cortical primordium as revealed by analysis of expression of downstream Wnt-β-catenin target gene Axin2 (Supplementary Fig. 16). At e15.5, the cortical plate of electroporated Lmx1a−/−;b−/− mutants was lissencephalic, with no evidence of gyrification (Fig. 6e, f). Thus, supplementing Bmp at the DMe was sufficient to normalize DMe development and Wnt signaling and suppress gyrification of the distantly located cortex in Lmx1a−/−;b−/− embryos. We conclude that Lmx1a and Lmx1b regulate DMe development via Bmp signaling and that misregulation of the DMe development and its Wnt signaling is central to the Lmx1a−/−;b−/− cortical gyrification phenotype.
Fig. 6.
Bmp4 rescues DMe development and cortical gyrification in Lmx1a−/−;b−/− mutants. Bmp4 and/or GFP expressing plasmids were electroporated into DMe of Lmx1a−/−;b−/− embryos at e10.5 and analyzed at e12.5 (a–d) or e15.5 (e, f). To electroporate DMe, the positive electrode was placed against this region, as shown in the schematic below panel a. Negatively charged DNA moves toward the positive electrode (arrows) entering DMe. a–d Show DMe (a region boxed in the diagram below panel b). Immunohistochemistry revealed that Bmp4, but not GFP, induced Ttr in the Lmx1a−/−;b−/− ChPe (arrowheads in d). GFP+ ChPe cells (arrowheads in a, c) confirm successful electroporation of ChPe. e, f While GFP-electroporated Lmx1a−/−;b−/− mutants had cortical gyrification at e15.5 (e, arrows point to cortical gyri), electroporation of Bmp4 into DMe restored cortical lissencephaly (f). Arrowheads point to GFP+ cells in the DMe, showing specific electroporation of this domain. Scale bars: 100 μm (a–d); 200 μm (e, f)
Discussion
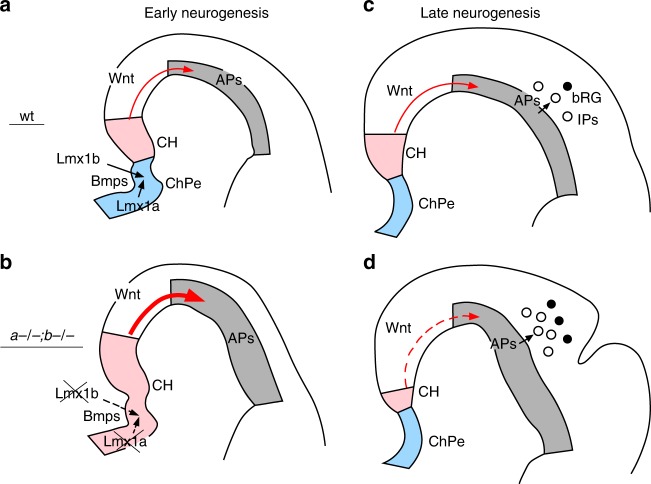
Wnt signaling from medially located CH induces hippocampus and regulates cortical size, while mesenchymal signaling regulates proliferation of cortical APs and differentiation and migration of a subset of cortical neurons40–46. Here we show that simultaneous loss of two transcription factors, Lmx1a, expressed in the telencephalic DMe, and Lmx1b, expressed in head mesenchyme, causes folding of the mouse cerebral cortex, implicating head mesenchyme and DMe in the regulation of cortical gyrification. Our data demonstrate that Lmx1a and Lmx1b normally act to suppress cortical gyrification in the mouse via Bmp-dependent regulation of DMe development and its Wnt signaling dynamics (Fig. 7).
Fig. 7.
Lmx1a/b-dependent dorsomedial tissue interactions regulate cortical gyrification. a In early wild-type embryos, Lmx1a and Lmx1b are expressed in the telencephalic DMe and adjacent mesenchyme, respectively, and promote expression of secreted Bmps within their respective territories. Appropriate level of Bmp signaling specifies ChPe and CH in the DMe. Wnt3a signaling from the CH (red arrow) promotes proliferation of APs in the cortical VZ. Although we show Wnt signaling by arrow extending through neuroepithelium, our data cannot exclude a possibility that Wnt molecules are delivered to neocortical primordium via CSF. b Simultaneous loss of Lmx1a and 1b reduces Bmp expression in both the mesenchyme and DMe, decreasing the level of Bmp signaling in the DMe. This results in DMe overproliferation and mispatterning, creating an enlarged Wnt3a expression domain at the DM in early Lmx1a−/−;b−/− embryos. The excessive secreted Wnt3a (large red arrow) overactivates canonical Wnt-β-catenin signaling in distal cortical primordium, leading to excessive expansion of APs in Lmx1a−/−;b−/− mutants. c At later developmental stages, in wild-type embryos, Wnt-β-catenin signaling (red arrow) limits production of IPs and bRG from APs29. d By e15.5, normal DMe specification is restored in Lmx1a−/−;b−/− embryos, but enhanced apoptosis reduces the size of CH and DM-derived Wnt signaling (dashed red line). Reduced Wnt signaling allows excessive production of IPs and bRG from an earlier expanded AP population, resulting in cortical gyrification
Loss of Lmx1a and 1b reduced expression of genes encoding secreted Bmp4/2 molecules in the DMe and adjacent mesenchyme, respectively, decreasing overall levels of Bmp signaling in the DMe of Lmx1a−/−;b−/− mutants. It has been previously shown that complete loss of Bmp signaling in Bmp receptor 1a and 1b double-mutant mice resulted in failure to induce both the ChPe and CH34. Reduced Bmp signaling in Lmx1a/b double mutant, however, resulted in a qualitatively different phenotype. Specifically, we observed initial mispatterning of the DMe with delayed differentiation of the ChPe and expansion of Wnt3a expression across the prospective ChPe territory. Later during development, contraction of the Wnt3a+ CH domain occurred as apoptosis was elevated and a delayed Ttr+ ChPe developed in the Lmx1a−/−;b−/− DMe (Fig. 7). Exogenous medial Bmp4 rescued DMe patterning in Lmx1a−/−;b−/− embryos, directly implicating downregulated Bmp signaling in the mutant phenotype. Thus, our studies demonstrate that DMe development is particularly sensitive to the level of Bmp signaling in vivo. Previous in vitro analysis revealed that high Bmp levels are needed to induce the ChPe fate47. While low Bmp signaling in Lmx1a−/−;b−/− mutants was sufficient to induce Wnt3a+ CH by e12.5, delayed differentiation of ChPe until later developmental stages potentially reflects a delay in accumulation of threshold levels of Bmp signaling necessary for ChPe development. Regardless, our study revealed that Bmp signaling is required not only for the initial induction of the CH but also regulates its size and, thus, the temporal dynamics of DMe-derived Wnt3a signaling throughout development. Additionally, we identify that Bmp proteins produced both locally in the DMe and in the adjacent mesenchyme participate redundantly in DMe development.
Expansion of BPs and subsequent production of neurons from these progenitors has been identified as a major driving force of cortical gyrification in naturally gyrencephalic mammals. During development, BPs are generated from APs, and a typical neurogenic sequence in gyrencephalic mammals includes initial expansion of APs followed by an extensive production of bRG and IPs during later peak of neurogenesis3,8–12. Expansion of APs in the VZ, although necessary for BPs production, does not automatically lead to excessive generation of BPs, which need to delaminate from the VZ4,11,48. In the cortical VZ, APs expand via symmetric divisions, while asymmetric divisions of APs result in the production of BPs11,29.
Wnt/β-catenin signaling expands APs by promoting symmetric divisions and inhibiting asymmetric divisions, at least partially by controlling mitotic spindle orientation29. In contrast, reduction of Wnt signaling favors asymmetric cell divisions, activating the production of BPs (both bRG and IPs) from APs, depleting the AP pool29,39. Wnt/β-catenin signaling, however, has not previously been considered a regulator of cortical gyrification because neither constitutive gain-of-function nor loss-of-function Wnt/β-catenin mouse models showed bona fide cortical gyrification3,11,29,31,39. Constitutive overexpression of a transgene encoding stabilized β-catenin in neural precursors produced a pseudo-gyrified cortex with both the ventricular and pial surfaces equally folded31, rather than the asymmetric increase in pial surface as seen in naturally gyrencephalic mammals3. Additionally, there was a profound loss of cortical lamination in these transgenic mice31,49. Further, mice with permanently activated Wnt/β-catenin signaling demonstrated a decrease in the number of BPs, rather than an increase as observed in higher mammals39.
Early constitutive ablation of β-catenin in the developing mouse telencephalon increased the numbers of both bRG and IPs but was insufficient to induce gyrification29,39. Although both bRG and IPs were increased in the number in the telencephalon of β-catenin ablated mice, IPs were expanded only at early stages likely because by the peak of neurogenesis, APs were severely depleted and could not further support the generation of excessive IPs29.
In contrast, in Lmx1a−/−;b−/− mutants, reduction of Wnt/β-catenin signaling occurred only after its transient overactivation and the resultant overexpansion of AP pool at earlier stages. When Wnt signaling was reduced at e15.5, at the peak of cortical neurogenesis, this expanded AP population was apparently sufficient to simultaneously produce enough bRG and IPs to drive cortical gyrification. Consistently with this hypothesis, suppression of the early activation of Wnt/β-catenin signaling by electroporating Wnt inhibitor Dkk1 into the cerebral cortex, reduced the number of cortical APs, bRG and IPs and restored lissencephaly in Lmx1a−/−;b−/− mice, revealing a critical role of Wnt signaling in the development of cortical gyrification in this mutant (Fig. 4).
Interestingly, despite local overexpansion of BPs in the emerging gyri in e15.5 Lmx1a−/−;b−/− embryos, we could not identify differences in progenitor behavior in different cortical regions in e12.5 Lmx1a−/−;b−/− mice. Thus, location of gyri and sulci in Lmx1a−/−;b−/− mice is likely determined by region-specific responses of initially expanded APs to reduced Wnt signaling at later stages, possibly resulting in differential delamination and/or migration of BPs. The molecular basis of differential response of different cortical regions to secreted factors remains unknown. Notably, however, we have recently reported that in another cortical gyrification mouse model, subtle changes in polarity at the apical ventricular surface coincide with location of gyri and are accompanied by localized accumulation of nuclear YAP, which is essential to drive localized proliferation of BPs21.
In the canonical Wnt pathway, Wnt ligands bind to LRP5/Frizzled receptors leading to translocation of β-catenin to the nucleus and activation of transcription of Wnt-β-catenin responsive genes50. In Lmx1a−/−;b−/− mutants, the rate of change of Wnt signaling, as revealed by expression levels of canonical Wnt pathway downstream genes Lef1 and Axin2, was fast: the switch from high to low Wnt signaling happened within 3 days (from e12.5 to e15.5), leading to dramatic changes in cortical progenitor populations. Fast effects of misregulated Wnt signaling have also been described in other experimental systems. For example, activating or reducing activity of Wnt pathway resulted in dramatic biological responses in wild-type chicken spinal cord or mouse neocortex already 24 h after electroporation of expression plasmids39,51 highlighting that tight regulation of Wnt signaling is critical for normal development of the central nervous system. Analysis of Lef1 expression or an Axin2 reporter revealed a Wnt signaling gradient that in the wild-type telencephalon extends through neuroepithelium from the DMe (high) to lateral neocortex (low)40,52 (Supplementary Fig. 11a). The existence of a Wnt response gradient through the cortical neuroepithelium suggests that DMe-derived Wnt ligands diffuse (or are actively transported) via neuroepithelium. In e12.5 Lmx1a−/−b−/− mutants, a medio-lateral Lef1 expression gradient was still observed; however, it was brighter than in comparably staged wild-type controls (Supplementary Fig. 11a, b), as expected by the presence of an enlarged Wnt3a-expressing DMe domain. Previously, several Wnts have been also detected in mouse and human cerebrospinal fluid (CSF)53,54. Thus, we cannot exclude a possibility that in Lmx1a−/−;b−/− mutants, some Wnt molecules reach neocortex via CSF as well.
Importantly, rescue of the DMe patterning in Lmx1a−/−;b−/− mutants by early electroporation of Bmp4 into the DMe was sufficient to normalize activity of the Wnt-β-catenin signaling in the distantly located neocortex to restore lissencephaly. These data confirm that the DMe was the primary source of a gyrification-related Wnt signaling in Lmx1a−/−;b−/− mutants. Our experiments, however, do not exclude a possibility that other signaling molecules, beyond Wnt3a from the DMe, contribute to cortical gyrification in Lmx1a/b double mutants. For example, signaling from lateral mesenchyme located adjacent to the neocortical primordium may directly contribute to cortical developmental abnormalities in Lmx1a/b mutants. However, we consider this an unlikely possibility since cortical gyrification was not detected in Lmx1b−/− mutants. Thus, even if Lmx1b-dependent signaling from lateral mesenchyme contributes to cortical gyrification in Lmx1a−/−;b−/− mutants, it still does so in cooperation with the DMe-derived Wnt signaling, which is dependent on both Lmx1 genes. Therefore, our experiments combining Lmx1a/b mutants analysis with Bmp4/Dkk1 electroporation studies identify misregulation of DMe-derived Wnt signaling as a major contributor to the cortical phenotype of Lmx1a−/−;b−/− mutants, identifying a mesenchymal/DMe Lmx1a/1b-Bmp-Wnt pathway as a regulator of cortical gyrification.
Our study clearly demonstrates that lissencephaly in mice is maintained by redundant function of two Lmx1 genes. Genetic redundancy is believed to be maintained in evolution to stabilize key developmental processes in animal populations to make them less susceptible to various influences such as environmental conditions or genetic background55. Given the important role of DMe for cortical organization and the profound effect of relatively subtle variations of Bmp signaling on DMe development, it seems logical that the development of this particular tissue is stabilized via redundant pathways. Notably, the only two other genes known to maintain lissencephaly in the mouse (or any other lissencephalic species) are Flrt1 and Flrt3 which redundantly regulate neuronal adhesion during migration25. We conclude that lissencephaly is actively regulated during development and protected by redundant mechanisms. Disruption of these mechanisms is sufficient to induce gyrification of normally lissencephalic mouse neocortex, revealing striking plasticity of neocortical development in mice.
Similar to the developing neocortex of naturally gyrencephalic mammals, we observed local expansion of BPs in the emerging gyri but did not detect specific increases in apoptosis in cortical regions developing sulci in e15.5 Lmx1a−/−;b−/− embryos. Taken together, these results suggest localized expansion of BPs as a major mechanism of cortical gyrification in Lmx1a−/−;b−/− mutants, although we cannot exclude a possibility that transient region-specific misregulation of apoptosis, for example at earlier stages, also contributes to the phenotype. In higher mammals, cortical gyrification is a complex process that beyond localized overexpansion of BPs, involves other mechanisms as well, such as region-specific differences in neuronal migration2–4,25. It remains to be investigated whether other cellular mechanisms that regulate cortical gyrification in higher mammals also contribute to cortical folding in Lmx1a−/−;b−/− mutants.
In conclusion, we have identified a redundant Lmx1a/b-Bmp-dependent pathway that shapes the dynamics of dorsal midline Wnt signaling. In mice, this pathway operates to prevent differential expansion of cortical progenitors and suppress gyrification of the distant neocortex. It has been recently demonstrated that similar to the mouse, a Wnt3a-expressing signaling center is also present in the DMe of the naturally gyrencephalic ferret56. Although the mechanisms that regulate DMe development and signaling in gyrencephalic mammals remain unknown, in the developing human neocortex, expression of Wnt receptor FZD8 is higher than in the mouse and even the chimpanzee, potentially making the developing human neocortex particularly sensitive to dynamics of Wnt signaling57. An important next step will be to determine whether modification of the DMe/mesenchymal signaling pathways that we describe here promote gyrification during evolution of naturally gyrencephalic mammals.
Methods
Mice
Lmx1a-null (Lmx1adrJ, The Jackson Laboratory strain #000636)58, Lmx1b-null (Lmx1b−/−)59, Lmx1bLacZ60, Lmx1a-Cre61, and ROSA26-YFP62 (The Jackson Laboratory strain #006148) mice were used in this study. Mice were maintained on a mixed genetic background comprising C57Bl6, 129, FVB, and CD1. Noon of the day of the plug was considered e0.5. Embryos of both sexes were used for experiments. For cell proliferation experiments, pregnant females were intraperitoneally injected with BrdU at 50 mg/kg. All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committees of the University of Tennessee Health Science Center (UTHSC), Seattle Children’s Research Institute or the University of Chicago.
Tissue analysis
Immunohistochemistry was performed using cryosections63. Embryos were collected in cold phosphate-buffered saline (PBS). Whole heads or dissected brains were fixed in freshly prepared 4% paraformaldehyde (PFA) in PBS for 1–12 h., washed three times in PBS (30 min each), equilibrated in 30% sucrose in PBS for 2 h at 4 °C, and embedded in optimum cutting temperature (OCT) compound (Tissue Tek). OCT blocks were sectioned on a cryostat at 12 μm. Sections were collected on Superfrost Plus glass slides (Fisher Scientific) and kept at −20 °C. Then, sections were dried at room temperature for 20 min, washed in PBS, blocked in PBS containing 1% normal goat serum and 0.1% Triton X-100, and incubated with primary antibodies overnight at 4 °C. Secondary antibodies were applied at room temperature for 1 h. Some sections were counterstained with DAPI (Sigma-Aldrich) to visualize tissue. Sections were mounted in Fluorogel (EMS) and coverslipped. For BrdU and Ki67 immunostaining, slides were boiled in 1× target retrieval solution (Dako) for 10 min before the blocking step. For pSmad immunostaining, slides were incubated in the same solution for 7 min at 95 °C. The following primary antibodies were used: anti-Lmx1a (goat, Santa-Cruz, catalog #sc-54273, 1:500, RRID:AB_2136830), anti-Cux1 (rabbit, Santa-Cruz, catalog #sc-13024, 1:200, RRID:AB_2261231), anti-Ctip2 (rat, Abcam, catalog #ab18465, 1:500, RRID:AB_2064130), anti-GFP antibody that recognizes YFP (chicken, catalog #ab13970 Abcam, 1:500, RRID:AB_300798), anti-Reelin (mouse, Chemicon, catalog #Mab5364, 1:500, RRID:AB_2179313), anti-BrdU (rat, Abcam, catalog #ab6326, 1:50, RRID:AB_305426), anti-BrdU (mouse, Rockland, catalog #600-401-c29, 1:300, RRID:AB_10893609), anti-Ki67 (mouse, BD Pharmingem, catalog #556003, 1:250, RRID:AB_396287), anti-Pax6 (rabbit, Covance, catalog #Prb-278p-100, 1:300, RRID:AB_291612), anti-Tbr2 (rat, eBioscience, catalog #14-487582, 1:200, RRID:AB_11042577), anti-β-catenin (mouse, BD Biosciences, catalog #610153, 1:200, RRID:AB_397554), anti-Lef1 (rabbit, Cell Signaling Technology, catalog #2230S, 1:300), anti-pSmad (rabbit, Chemicon, catalog #AB3848, 1:50, RRID:AB_177439), anti-Ttr (rabbit, Dako, catalog #A0002, 1:200, RRID:AB_2335696), anti-activated Caspase 3 (rabbit, Promega, catalog #G7481, 1:250, RRID:AB_430875), and anti-p73 (mouse, Fisher, catalog #MA5-14117, 1:100, RRID:AB_10987160) with species-specific secondary antibodies conjugated with Alexa 350, 488, 568, or 594 fluorophores (Life Technologies).
For Nissl staining, sections were incubated in 0.1% cresyl violet solution for 10 min, dehydrated through an ethanol/xylens series and coverslipped with Permount (Fisher)64. In situ hybridization was performed using digoxigenin-labeled Lmx1a26, Lmx1b26, Bmp465, Ttr66, and Wnt3a32 RNA probes and anti-digoxigenin antibody conjugated with alkaline phosphatase (Roche). Hybridizations were performed in a buffer containing 50% formamide, 4× saline sodium citrate, and 1% sodium dodecyl sulfate at 70 °C overnight. Color reactions were carried out using NBT/BCIP substrates (4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-idolyl phosphate)67. X-gal staining was performed using sections from fresh-frozen embryos68. Slides were fixed in 0.2% glutaraldehyde in PBS for 10 min on ice and after brief rinsing were incubated in X-gal staining solution (0.02% Igepal, 0.01% sodium deoxycholate, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 diluted in 0.1 M phosphate buffer) at 37 °C overnight in the dark. To visualize tissue, X-gal-stained sections were counterstained with Nuclear Fast Red (Sigma).
Images of immunostained sections (except β-catenin-stained sections) were taken using a Zeiss AxioImager A2 microscope equipped with an AxioCam Mrm camera and AxioVision Rel 4.9 software (Zeiss). Images of β-catenin-immunostained sections were taken using a Zeiss LCM 710 laser scanning confocal microscope with Zen software. Brightfield images were taken using a Leica MZFLIII microscope with Leica DFC425 camera and LAS V3.8 software or Olympus SZX microscope with Leica MC170HD camera and LAS V4.5 software. Figure panels were assembled in Photoshop CC 2018 (Adobe).
Mouse in utero electroporation
Plasmids encoding GFP, Dkk1, and Bmp4 (1 μg/μl) were injected using a fine-glass microcapillary into a lateral ventricle and electroporated into either telencephalic DMe or the neocortical primordium using paddle electrodes with BTX ECM830 electroporator69. To target the telencephalic DMe, the positive electrode was placed adjacent to the DMe, as shown in Fig. 6 (in schematic below Fig. 6a). The neocortical primordium was targeted by placing the positive electrode against the neocortical wall. To facilitate injections, Fast Green dye was added to plasmid solutions. The following plasmids were used: pCIG-mouse Dkk139, pCAGGS-mouse Bmp470, and pCAGGS-GFP71. After electroporation, the uterus was returned to abdomen, and the embryos continued development. Upon collection, embryos were evaluated under a fluorescence stereo microscope, and those showing GFP fluorescence in appropriate brain regions were serially sectioned on a cryostat and used for analysis.
Laser capture microdissection (LCM) and qRT-PCR
Embryonic heads were flash-frozen in OCT on dry ice and coronally sectioned on a cryostat at 10 μm. Sections for LCM were mounted on uncharged glass slides (AmLabsTM), dried at room temperature for 30 s, and stained with hematoxylin and eosin to visualize tissue morphology, using HistoGene LCM Frozen Sections Staining kit (Fisher Scientific, Cat #KIT0401)72. LCM was performed using an ArcturusXT laser capture microdissection machine with Arcturus CapSure Macro LCM caps. For Bmp expression analysis, five telencephalic sections separated from each other by 10 μm were used for LCM from each e10.5 embryo. Adjacent sections were immunostained against Lmx1a and used as templates to localize boundaries of the telencephalic DMe. For analysis of mesenchyme, mesenchymal tissue located above the DMe was microdissected (green domain in Fig. 5a). For Axin2 expression analysis in e12.5 and e15.5 embryos, cortical primordium located between the dorsal cortical bend and LGE (gray region in schematics adjacent to Supplementary Fig. 11c and Supplementary Fig. 12b) was microdissected from 4 to 6 non-adjacent telencephalic sections from each embryo. For gene expression analysis in Bmp rescue experiments (Supplementary Fig. 16), sections of pCAGGS-Bmp4 and/or pCAGGS-GFP-electroporated embryos were first evaluated under a fluorescence microscope and embryos that specifically expressed GFP in the DMe (similar to those shown in Fig. 6e, f) were identified. ChPe and neocortical primordium from sections from these embryos were isolated by LCM and used for gene expression analysis.
RNA from microdissected samples was isolated using Pico Pure RNA purification kit (Arcturus KIT#0204). Before eluting RNA, RNAse free DNAse I (Qiagen) was applied to each column and incubated for 15 min at room temperature to remove traces of genomic DNA. cDNA was generated using cDNA synthesis kit (Biorad, cat#1708890). qRT-PCR was performed on Roche LC480 Real-time PCR machine with SYBR Fast qPCR master mix (Kapa Biosystems)72,73. Transcript levels were normalized to Gapdh. All samples were tested in triplicates. At least three embryos per genotype per experimental condition were tested using primers listed in Supplementary Table 1.
Cell counts, measurements, and statistical analysis
For each embryo, the telencephalon was coronally serially sectioned and the same number of sections was placed on each slide to identify the position of each section along the anterior–posterior (A–P) axis of the telencephalon. For consistency, all comparisons between mutant and control embryos were performed using sections taken at similar A–P levels, approximately midway along the A–P axis of the telencephalon. At least three embryos per genotype or experimental condition (typically three non-adjacent sections per embryo) were evaluated in each analysis at each developmental stage.
Cortical parameters (area, regional thickness of cortical neuroepithelium) were measured between the telencephalic dorsal bend (pointed by upper arrow in Supplementary Fig. 1e) and LGE (pallial-subpallial boundary) (lower arrow in Supplementary Fig. 1e)74–76, using ImageJ (NIH). For each embryo, cortical area in left and right hemispheres was added to generate a total cortical area, which was compared between Lmx1a−/−;b−/− and control littermates (Supplementary Fig. 1f). For clarity, cortical area in Lmx1a−/−;b−/− mutants was normalized to that in wild-type controls. To evaluate whether cortical gyri occupy non-random positions or are randomly distributed throughout e18.5 Lmx1a−/−;b−/− neocortex, the cortical ventricular surface (between the dorsal cortical bend and LGE) was divided into 10 equally sized segments (Supplementary Fig. 1e). Cortical thickness was measured in the middle of each segment in different embryos and plotted (Supplementary Fig. 1g, h). Cortical progenitors were counted in 100-μm-wide radial segments (columns) spanning the entire thickness of the neocortex, from ventricular to outer cortical surface. For consistency, in Lmx1a−/−;b−/− embryos, cell counts were performed in dorsal neocortical primordium, which develops a dorsal bulge, the most consistent cortical gyrus in Lmx1a−/−;b−/− embryos (Supplementary Fig. 1b–d, h). In control embryos, cell counts were performed in the same region of the cortex as in Lmx1a−/−;b−/− mutants. IPs were defined as Tbr2+ cells16,29. Originally, bRG cells were defined as Pax6+/Tbr2− progenitors that occupy the SVZ and possess a single basal process8,9. More recently, however, bRG cells with diverse morphology were described, indicating that apical-only process is not a definitive feature of bRG11,77. Thus, similar to Wang et al.16 and Draganova et al.29, we identified bRG as Pax6+/Tbr2− cells located basal to the Tbr2+IP band. Similar to wild-type controls, in Lmx1a−/−;b−/− mutants, these basal Pax6+/Tbr2− cells were Ki67+ and incorporated BrdU after a short BrdU pulse (Supplementary Fig. 3a–e, g–i), indicating that they indeed were proliferating progenitors. To compare distribution of proliferating cells in e15.5 wild type and Lmx1a−/−;b−/− embryos (Supplementary Fig. 3k), a 90 min BrdU pulse was performed and fractions of basally located proliferating (S-phase) cells (the number of BrdU+ cells located beyond the Pax6+VZ divided by the total number of BrdU+ cells) were calculated. To evaluate activity of the Bmp signaling pathway in the telencephalic DMe of e10.5 embryos, intensity of pSmad immunostaining was measured using ImageJ (NIH)33. The boundaries of the DMe were identified using adjacent Lmx1a-immunostained sections as templates. To analyze cell-cycle exit, embryos were harvested 20–24 h after a BrdU pulse and the fraction of cells that exited the cell cycle (the number of BrdU+/Ki67− cells divided by the total number of BrdU+ cells) was calculated in the neocortex78.
Statistical analysis was performed using IBM SPSS Statistics (version 25) software. All quantitative data are presented as the mean ± s.d. Statistical analysis between two groups was performed using two-tailed t-test. Multiple groups were analyzed using one-way ANOVA with Tukey’s post hoc test. Bonferroni correction was applied when multiple comparisons were performed. P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgments
We thank R. Johnson (MD Anderson Cancer Center), S. Pleasure (UCSF), S. Ohta and G. Schoenwolf (University of Utah), and B. Nelson (Seattle Children’s) for mice, expression vectors, and plasmids for in situ probes, Y. Mishima (University of Chicago), C. Wang, J. Skibo (Seattle Children’s), S. Krat, L. Mukhametzyanova, and A. Zakharova (UTHSC) for mouse genotyping. This work was supported by NIH grants T32GM07179 supporting A.G.L., R01 NS093009 and R21 NS077163 to V.V.C., R01 NS072441 and R01 NS099027 to K.J.M., and by the Neuroscience Institute, and Center of Integrative and Translational Genomics of the UTHSC.
Author contributions
V.V.C. and K.J.M. designed the study; V.V.C., I.Y.I., E.Y.S., N.F. A.G.L., A.S.S., and A.R. performed experiments and analyzed data; V.V.C., S.T., and K.J.M. supervised the study; V.V.C., A.S.S., A.R., S.T., and K.J.M. wrote the paper.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data for Fig. 1k, n; 2f, i, l, o, r, u; 4f–h; 5f, k and Supplementary Figs. 1f–h; 3k–o, r; 4d; 7e; 9g, l; 11c, d; 12b, c; 15 and 16b, c are provided as a Source data file.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Victor Borrell, Thomas Pratt and Alessandro Sessa for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Victor V. Chizhikov, Email: vchizhik@uthsc.edu
Kathleen J. Millen, Email: kathleen.millen@seattlechildrens.org
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-12913-z.
References
- 1.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrell V. How cells fold the cerebral cortex. J. Neurosci. 2018;38:776–783. doi: 10.1523/JNEUROSCI.1106-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llinares-Benadero C, Borrell V. Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat. Rev. Neurosci. 2019;20:161–176. doi: 10.1038/s41583-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 5.Lewitus E, Kelava I, Kalinka AT, Tomancak P, Huttner WB. An adaptive threshold in mammalian neocortical evolution. PLoS Biol. 2014;12:e1002000. doi: 10.1371/journal.pbio.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Leary MA, et al. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 2013;339:662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- 7.Kelava I, et al. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb. Cortex. 2012;22:469–481. doi: 10.1093/cercor/bhr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 9.Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrell V, Götz M. Role of radial glial cells in cerebral cortex folding. Curr. Opin. Neurobiol. 2014;27:39–46. doi: 10.1016/j.conb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Taverna E, Götz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 12.Fernández V, Llinares-Benadero C, Borrell V. Cerebral cortex expansion and folding: what have we learned? EMBO J. 2016;35:1021–1044. doi: 10.15252/embj.201593701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahl R, et al. Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell. 2013;153:535–549. doi: 10.1016/j.cell.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Florio M, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- 15.Ju XC, et al. The hominoid-specific gene TBC1D3 promotes generation of basal neural progenitors and induces cortical folding in mice. Elife. 2016;5:e18197. doi: 10.7554/eLife.18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Hou S, Han YG. Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat. Neurosci. 2016;19:888–896. doi: 10.1038/nn.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, et al. The primate-specific gene TMEM14B marks outer radial glia cells and promotes cortical expansion and folding. Cell Stem Cell. 2017;21:635–649. doi: 10.1016/j.stem.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Fiddes IT, et al. Human-specific NOTCH2NL genes affect Notch signaling and cortical neurogenesis. Cell. 2018;173:1356–1369. doi: 10.1016/j.cell.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki IK, et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through Delta/Notch regulation. Cell. 2018;173:1370–1384. doi: 10.1016/j.cell.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RS, et al. Sodium channel SCN3A (NaV1.3) regulation of human cerebral cortical folding and oral motor development. Neuron. 2018;99:905–913. doi: 10.1016/j.neuron.2018.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy A, et al. PI3K-Yap activity drives cortical gyrification and hydrocephalus in mice. Elife. 2019;8:e45961. doi: 10.7554/eLife.45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto N, Shinmyo Y, Ichikawa Y, Kawasaki H. Gyrification of the cerebral cortex requires FGF signaling in the mammalian brain. Elife. 2017;6:e29285. doi: 10.7554/eLife.29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rash BG, Tomasi S, Lim HD, Suh CY, Vaccarino FM. Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain. J. Neurosci. 2013;33:10802–10814. doi: 10.1523/JNEUROSCI.3621-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rash BG, Lim HD, Breunig JJ, Vaccarino FM. FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis. J. Neurosci. 2011;31:15604–15617. doi: 10.1523/JNEUROSCI.4439-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Toro D, et al. Regulation of cerebral cortex folding by controlling neuronal migration via FLRT adhesion molecules. Cell. 2017;169:621–635. doi: 10.1016/j.cell.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Mishima Y, Lindgren AG, Chizhikov VV, Johnson RL, Millen KJ. Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J. Neurosci. 2009;29:11377–11384. doi: 10.1523/JNEUROSCI.0969-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doucet-Beaupré H, Ang SL, Lévesque M. Cell fate determination, neuronal maintenance and disease state: the emerging role of transcription factors Lmx1a and Lmx1b. FEBS Lett. 2015;89:3727–3738. doi: 10.1016/j.febslet.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Itoh Y, et al. PDK1-Akt pathway regulates radial neuronal migration and microtubules in the developing mouse neocortex. Proc. Natl. Acad. Sci. USA. 2016;113:E2955–E2964. doi: 10.1073/pnas.1516321113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draganova K, et al. Wnt/β-catenin signaling regulates sequential fate decisions of murine cortical precursor cells. Stem Cells. 2015;33:170–182. doi: 10.1002/stem.1820. [DOI] [PubMed] [Google Scholar]
- 30.Nowakowski TJ, et al. MicroRNA-92b regulates the development of intermediate cortical progenitors in embryonic mouse brain. Proc. Natl. Acad. Sci. USA. 2013;110:7056–7061. doi: 10.1073/pnas.1219385110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 32.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 33.Cheng X, et al. Central roles of the roof plate in telencephalic development and holoprosencephaly. J. Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes M, Gutin G, Alcorn H, McConnell SK, Hébert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- 35.Voss AK, Krebs DL, Thomas T. C3G regulates the size of the cerebral cortex neural precursor population. EMBO J. 2006;25:3652–3663. doi: 10.1038/sj.emboj.7601234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Dev. 2009;4:3. doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber M, Pierani A. Tangential migration of glutamatergic neurons and cortical patterning during development: lessons from Cajal-Retzius cells. Dev. Neurobiol. 2016;76:847–881. doi: 10.1002/dneu.22363. [DOI] [PubMed] [Google Scholar]
- 38.Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- 39.Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J. Neurosci. 2011;31:1676–1687. doi: 10.1523/JNEUROSCI.5404-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caronia-Brown G, Yoshida M, Gulden F, Assimacopoulos S, Grove EA. The cortical hem regulates the size and patterning of neocortex. Development. 2014;141:2855–2865. doi: 10.1242/dev.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangale VS, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielen H, Houart C. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev. Neurobiol. 2014;74:772–780. doi: 10.1002/dneu.22168. [DOI] [PubMed] [Google Scholar]
- 43.Borrell V, Marín O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat. Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- 44.Siegenthaler JA, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegenthaler JA, Pleasure SJ. We have got you ‘covered’: how the meninges control brain development. Curr. Opin. Genet. Dev. 2011;21:249–255. doi: 10.1016/j.gde.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choe Y, Siegenthaler JA, Pleasure SJ. A cascade of morphogenic signaling initiated by the meninges controls corpus callosum formation. Neuron. 2012;73:698–712. doi: 10.1016/j.neuron.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe M, et al. BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J. Neurosci. 2012;32:15934–15945. doi: 10.1523/JNEUROSCI.3227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo Ortega G, et al. The centrosome protein AKNA regulates neurogenesis via microtubule organization. Nature. 2019;567:113–117. doi: 10.1038/s41586-019-0962-4. [DOI] [PubMed] [Google Scholar]
- 49.Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb. Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- 50.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 51.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 52.Mutch CA, Schulte JD, Olson E, Chenn A. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS One. 2010;5:e12376. doi: 10.1371/journal.pone.0012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson PA, et al. The transcription factor Otx2 regulates choroid plexus development and function. Development. 2013;140:1055–1066. doi: 10.1242/dev.090860. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser K, et al. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat. Commun. 2019;10:1498. doi: 10.1038/s41467-019-09298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagha M, Bothma JP, Levine M. Mechanisms of transcriptional precision in animal development. Trends Genet. 2012;28:409–416. doi: 10.1016/j.tig.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones WD, Guadiana SM, Grove EA. A model of neocortical area patterning in the lissencephalic mouse may hold for larger gyrencephalic brains. J. Comp. Neurol. 2019;527:1461–1477. doi: 10.1002/cne.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyd JL, et al. Human-chimpanzee differences in a FZD8 enhancer alter cell-cycle dynamics in the developing neocortex. Curr. Biol. 2015;25:772–779. doi: 10.1016/j.cub.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chizhikov V, et al. Molecular definition of an allelic series of mutations disrupting the mouse Lmx1a (dreher) gene. Mamm. Genome. 2006;17:1025–1032. doi: 10.1007/s00335-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 59.Chen H, et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat. Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- 60.Schweizer H, Johnson RL, Brand-Saberi B. Characterization of migration behavior of myogenic precursor cells in the limb bud with respect to Lmx1b expression. Anat. Embryol. 2004;208:7–18. doi: 10.1007/s00429-003-0373-y. [DOI] [PubMed] [Google Scholar]
- 61.Chizhikov VV, et al. Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc. Natl. Acad. Sci. USA. 2010;107:10725–10730. doi: 10.1073/pnas.0910786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iskusnykh IY, Steshina EY, Chizhikov VV. Loss of Ptf1a leads to a widespread cell-fate misspecification in the brainstem, affecting the development of somatosensory and viscerosensory nuclei. J. Neurosci. 2016;36:2691–2710. doi: 10.1523/JNEUROSCI.2526-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy A, et al. Mouse models of human PIK3CA-related brain overgrowth have acutely treatable epilepsy. Elife. 2015;4:e12703. doi: 10.7554/eLife.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones CM, Lyons KM, Hogan BL. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–542. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- 66.Duan W, Cole T, Schreiber G. Cloning and nucleotide sequencing of transthyretin (prealbumin) cDNA from rat choroid plexus and liver. Nucleic Acids Res. 1989;17:3979. doi: 10.1093/nar/17.10.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev. Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- 68.Kridsada K, et al. Roof plate-derived radial glial-like cells support developmental growth of rapidly adapting mechanoreceptor ascending axons. Cell Rep. 2018;23:2928–2941. doi: 10.1016/j.celrep.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsui A, Yoshida AC, Kubota M, Ogawa M, Shimogori T. Mouse in utero electroporation: controlled spatiotemporal gene transfection. J. Vis. Exp. 2011;54:3024. doi: 10.3791/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohta S, Wang B, Mansour SL, Schoenwolf GC. BMP regulates regional gene expression in the dorsal otocyst through canonical and non-canonical intracellular pathways. Development. 2016;143:2228–2237. doi: 10.1242/dev.137133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogawa H, Inouye S, Tsuji FI, Yasuda K, Umesono K. Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc. Natl. Acad. Sci. USA. 1995;92:11899–11903. doi: 10.1073/pnas.92.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iskusnykh IY, Buddington RK, Chizhikov VV. Preterm birth disrupts cerebellar development by affecting granule cell proliferation program and Bergmann glia. Exp. Neurol. 2018;306:209–221. doi: 10.1016/j.expneurol.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoo JY, Mak GK, Goldowitz D. The effect of hemorrhage on the development of the postnatal mouse cerebellum. Exp. Neurol. 2014;252:85–94. doi: 10.1016/j.expneurol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Pulvers JN, Huttner WB. Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. Development. 2009;136:1859–1868. doi: 10.1242/dev.033498. [DOI] [PubMed] [Google Scholar]
- 75.Fei JF, Haffner C, Huttner WB. 3’ UTR-dependent, miR-92-mediated restriction of Tis21 expression maintains asymmetric neural stem cell division to ensure proper neocortex size. Cell Rep. 2014;7:398–411. doi: 10.1016/j.celrep.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q, et al. EphA4 regulates the balance between self-renewal and differentiation of radial glial cells and intermediate neuronal precursors in cooperation with FGF signaling. PLoS One. 2015;10:e0126942. doi: 10.1371/journal.pone.0126942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Namba T, Huttner WB. Neural progenitor cells and their role in the development and evolutionary expansion of the neocortex. WIREs Dev. Biol. 2017;6:e256. doi: 10.1002/wdev.256. [DOI] [PubMed] [Google Scholar]
- 78.Lavado A, et al. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development. 2013;140:3323–3334. doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data for Fig. 1k, n; 2f, i, l, o, r, u; 4f–h; 5f, k and Supplementary Figs. 1f–h; 3k–o, r; 4d; 7e; 9g, l; 11c, d; 12b, c; 15 and 16b, c are provided as a Source data file.