Abstract
Invasive candidiasis is an increasingly frequent cause of serious and often fatal infections in hospitalized and immunosuppressed patients. Mortality rates associated with these infections have risen sharply due to the emergence of multidrug resistant (MDR) strains of C. albicans and other Candida spp., highlighting the urgent need of new antifungal therapies. Rhesus theta (θ) defensin-1 (RTD-1), a natural macrocyclic antimicrobial peptide, was recently shown to be rapidly fungicidal against clinical isolates of MDR C. albicans in vitro. Here we found that RTD-1 was rapidly fungicidal against blastospores of fluconazole/caspofungin resistant C. albicans strains, and was active against established C. albicans biofilms in vitro. In vivo, systemic administration of RTD-1, initiated at the time of infection or 24 h post-infection, promoted long term survival in candidemic mice whether infected with drug-sensitive or MDR strains of C. albicans. RTD-1 induced an early (4 h post treatment) increase in neutrophils in naive and infected mice. In vivo efficacy was associated with fungal clearance, restoration of dysregulated inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-10, and IL-17, and homeostatic reduction in numbers of circulating neutrophils and monocytes. Because these effects occurred using peptide doses that produced maximal plasma concentrations (Cmax) of less than 1% of RTD-1 levels required for in vitro antifungal activity in 50% mouse serum, while inducing a transient neutrophilia, we suggest that RTD-1 mediates its antifungal effects in vivo by host directed mechanisms rather than direct fungicidal activity. Results of this study suggest that θ-defensins represent a new class of host-directed compounds for treatment of disseminated candidiasis.
Subject terms: Immunology, Microbiology
Introduction
Systemic infection caused by MDR fungi is a growing global health concern. It is estimated that approximately 1.5 million cases of disseminated mycoses occur annually and are associated with high mortality rates1–3. Fungal pathogens are a major cause of hospital-acquired infection4, particularly among surgical patients and those with indwelling catheters5. Increased risk of systemic fungal infection is also associated with biologic therapies for treatment of inflammatory or autoimmune diseases6,7.
Among fungal infections, Candida spp. are the most frequent causative organisms worldwide4,8. Over 400,000 cases of candidiasis occur annually9 with increased risk of infection associated with defects in innate immunity, neutropenia, and diabetes10–12. The growing incidence of MDR Candida spp. infections has contributed to the increase in mortality rates associated with systemic candidiasis13–15. A major risk factor for systemic candidiasis is the presence of biofilms that commonly colonize implanted medical devices such as venous catheters. Biofilms are notoriously resistant to antifungal therapy and are the source of blood borne dissemination16–19.
The failure to develop agents that are selective against eukaryotic pathogens has impeded the clinical introduction of new antifungals14. Currently, there are but three classes of antifungal drugs that are relied upon for treatment of invasive fungal infections: polyenes, azoles, and echinocandins20. Echinocandins, introduced nearly 20 years ago, are the most recently approved class of antifungals. Limitations associated with use of currently available agents include limited activity spectra17,21,22, serious adverse side effects23,24, and lack of activity against biofilms25,26. The emergence of MDR fungal pathogens underscores the urgent need for development of new approaches for treatment of fungal infections27–29.
θ-defensins are macrocyclic peptides containing an 18 amino acid cyclic backbone stabilized by a tri-disulfide core30. Numerous naturally occurring θ-defensin isoforms are expressed in Old World monkeys (OWM) such as macaques, baboons, and vervets31–34, but the peptides are absent in New World monkeys, apes and humans30. RTD-1, the prototype rhesus macaque θ-defensin, is effective in preclinical models of polymicrobial and E. coli sepsis35, SARS-coronavirus infection36, a mouse model of P. aeruginosa induced cystic fibrosis37, and endotoxin-induced lung injury38. In a recent study we reported that θ-defensins are potently fungicidal in vitro against MDR C. albicans and non-albicans Candida spp., including the emerging pathogen Candida auris39.
In the current study we evaluated the antifungal activities of RTD-1 against planktonic cells and biofilms of drug sensitive and MDR C. albicans strains in vitro, and found the peptide to be fungicidal against both forms of each strain. We then tested RTD-1 for efficacy in a therapeutic mouse model of systemic candidiasis and analyzed the effects of peptide treatment on survival, fungal clearance, and on inflammatory biomarkers in infections mediated by drug-sensitive and MDR isolates of C. albicans.
Results
RTD-1 is fungicidal against blastospores of MDR C. albicans
Based on the finding that RTD-1 is potently and rapidly fungicidal against several C. albicans and non-albicans Candida spp., including MDR isolates39, we tested for activity of the peptide against blastospores of two caspofungin (Caspo)-resistant C. albicans clinical isolates 43001 and 5326440, as well as the genetically defined, drug-sensitive reference strain SC5314. Minimum inhibitory concentration (MICs) and minimal fungicidal concentration (MFCs) determinations demonstrated that the Caspo-resistant clinical isolates were resistant to fluconazole (Fluco), and were 33-fold (strain 43001) or >133-fold (strain 53264) more resistant to Caspo than drug sensitive SC5314 (Table 1) consistent with a previous study40. RTD-1 was fungicidal against all three strains with MICs/MFCs ranging from 6.25–25 µg/ml (Table 1), and MFCs were 1–2 × the MICs (Table 1). RTD-1 was 5 to >40 times more active than Fluco against the three strains tested under standard conditions (Table 1).
Table 1.
Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) of θ-defensin RTD-1 and clinical antifungalsa.
| RPMI | 50% serum | |||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans # | RTD-1 | Fluco | Caspo | RTD-1 | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| SC5314 | 12.5 | 25 | 64 | >256 | 0.06 | 0.25 | >100 | >100 |
| 43001 | 6.25 | 12.5 | >256 | >256 | 2 | 2 | >100 | >100 |
| 53264 | 12.5 | 12.5 | >256 | >256 | >8 | >8 | >100 | >100 |
aA modified CLSI protocol, described in Methods, was used to determine MICs for RTD-1, fluconazole (Fluco) and caspofungin (Caspo). MFC assays were conducted as described in Material and Methods. MFC values correspond to 99% killing relative to input inoculum. MICs and MFCs for RTD-1 were determined also in 50% serum as described in Material and Methods.
As previously reported, drug sensitive C. albicans SC5314 and MDR C. albicans strains are rapidly and concentration-dependently killed by RTD-1 and related θ-defensin isoforms39. RTD-1 showed similar time and concentration-dependent killing of Caspo-resistant 43001 and 53264, with peptide concentrations as low as 1 µg/ml killing >90% of input blastospores within 10 min of peptide exposure (Fig. 1), and 3 µg/ml sterilized the incubation mixture by 30 min of incubation. Under these conditions, 10 µg/ml Caspo showed very little activity against either strain (Fig. 1).
Figure 1.
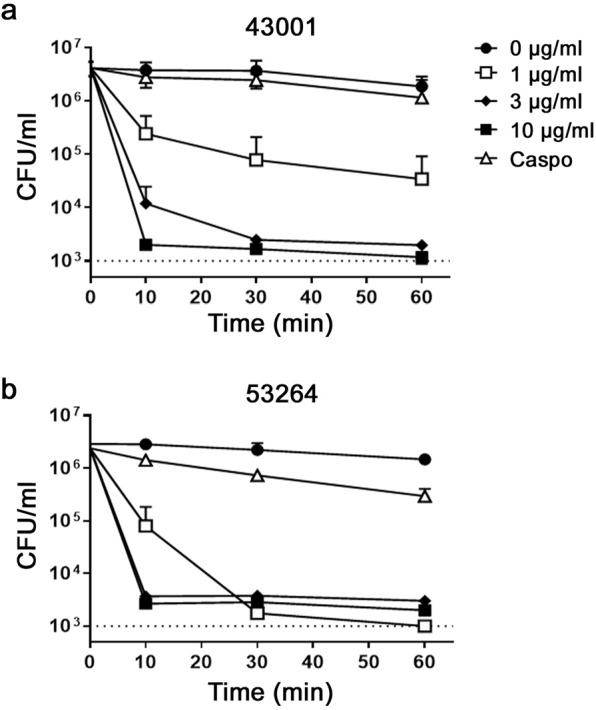
Concentration and time dependence of RTD-1 fungicidal activity. C. albicans clinical isolates 43001 (a) and 53264 (b) were incubated with indicated concentrations of RTD-1 or caspofungin (10 µg/ml) in PIPES-glucose buffer for 0–60 min. Data, plotted as mean CFU/ml +/− S.D, and are representative of experiments performed in triplicate.
Antifungal activity of RTD-1 against fungal blastospores was also evaluated in the presence of 50% mouse serum. In the presence of serum, RTD-1 was inactive against C. albicans SC5314, 43001 and 53264 at peptide concentrations up to 100 µg/ml (Table 1). The significance of this finding is discussed further below.
RTD-1 is active against C. albicans biofilms
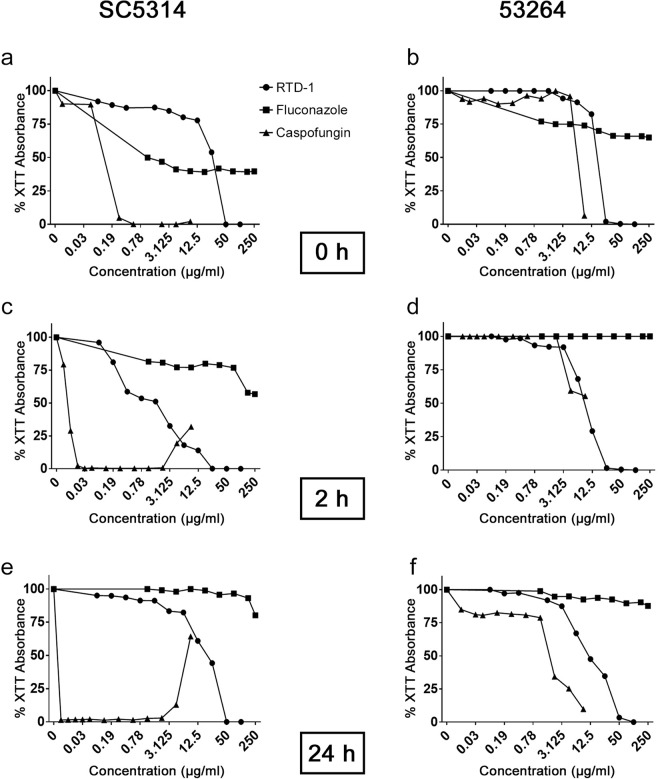
Since fungal biofilms are generally resistant to conventional antifungals, we analyzed the activity of RTD-1, Fluco and Caspo against biofilms of C. albicans SC5314 and MDR isolate 53264. Each agent was tested for its effect on biofilm formation by incubating fungal cell preparations with each agent prior to adhesion, 2 h after adhesion to plastic, or after mature (24 h) biofilms were established (Fig. 2). RTD-1 inhibited biofilms of drug sensitive SC5314 at each time point, but less effectively than Caspo. Under pre-adhesion conditions, Fluco was more effective than RTD-1 at concentrations below 50 µg/ml. However, RTD-1 was much more effective than Fluco in the 2- and 24 h assays (Fig. 2). Of note, the antifungal effect of Caspo against 2- and 24 h SC5314 biofilms was reduced at concentrations >2 µg/ml, consistent with the so-called “paradoxical” or “eagle” effect previously described41,42.
Figure 2.
RTD-1 efficacy against biofilms. RTD-1 was tested for the ability to prevent biofilm formation of C. albicans SC5314 and 53264 (a,b), formation of biofilms after 2 h of adhesion (c,d), and disruption of mature biofilms (e,f). In each condition, biofilm metabolism was evaluated by XTT absorbance 24–48 h after addition of the indicated concentration of RTD-1, fluconazole or caspofungin. The highest concentrations tested were 100 μg/ml (48 μM) RTD-1, 250 μg/ml (816.3 μM) fluconazole (Fluco), and 8 μg/ml (7.3 μM) caspofungin (Caspo).
RTD-1 also inhibited biofilm formation of MDR strain 53264. Unlike SC5314, 53264 was highly resistant to Fluco under each condition tested. Concentration-dependent 53264 biofilm inhibition by RTD-1 was similar to that of Caspo (Fig. 2). These data demonstrate that RTD-1 is effective against fungal biofilms in vitro with activity approximating that of Caspo against C. albicans 53264, an organism that is Fluco- (this study) and Caspo-resistant40.
Systemic RTD-1 administration enhances survival in systemic candidiasis
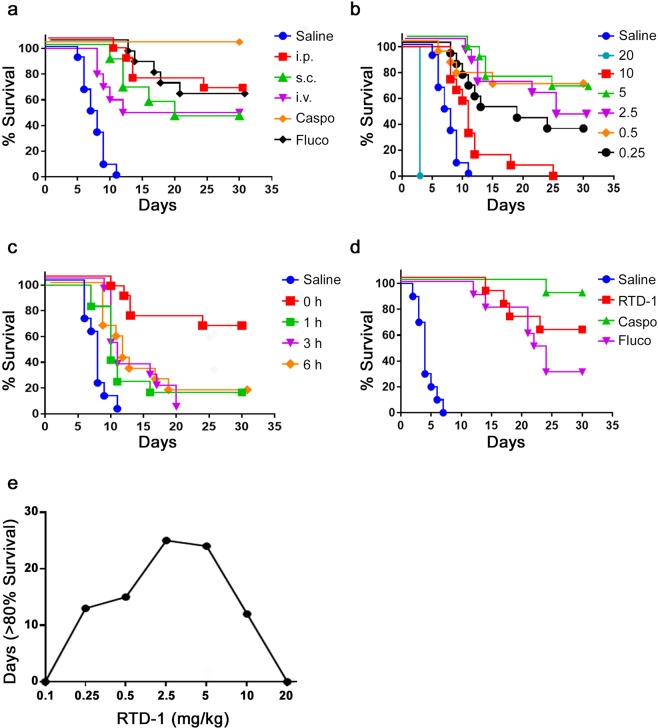
The efficacy of RTD-1 in vivo was evaluated in an established model of systemic candidiasis using C. albicans SC5314. Both immunocompetent and immunosuppressed mouse models are employed in preclinical studies of systemic candidiasis. Since many of our preliminary studies employed the latter model, we conducted efficacy studies with RTD-1 and reference antifungal drugs in immunocompetent BALB/c mice. Mice were challenged i.v. with 3 × 105 blastospores at T = 0, which rapidly produces disseminated candidiasis and colonizes major organs in a manner resembling systemic candidiasis in humans43. Under these conditions, SC5314 typically caused 100% terminal morbidity by day 12 in saline treated controls. We analyzed the effect of a single 5 mg/kg dose of RTD-1 administered i.v., s.c., or i.p. immediately after i.v. fungal challenge, with i.p. saline as vehicle control. Fluco and Caspo, administered i.p. at 5 mg/kg, were used as drug comparators. All saline treated SC5314 challenged mice died or became terminally moribund (requiring euthanasia) in 7–12 days, whereas single dose treatments with Caspo (P < 0.0001) or Fluco (P < 0.0001) were both effective in enhancing survival (Fig. 3a). A single dose of RTD-1 significantly enhanced survival regardless of the routes of administration (P < 0.0001 for i.p. and s.c., and P = 0.0007 for i.v.) and were not significantly different from each other, nor from Fluco treatment (Fig. 3a). Caspo treatment was superior to RTD-1 and Fluco treatment (P ≤ 0.01 in each case; Fig. 3a). In subsequent studies, antifungal agents were administered i.p. to circumvent potentially confounding effects resulting from treatment by the same route as infection, which was i.v. in all experiments.
Figure 3.
RTD-1 promotes survival of mice with disseminated C. albicans infection. (a) RTD-1 efficacy is independent of route of peptide administration. Mice infected i.v. with 3 × 105 C. albicans SC5314 were treated once at T = 0 with 5 mg/kg RTD-1 administered i.p. (n = 13), i.v. (n = 12), or s.c. (n = 12). Cohorts receiving fluconazole (Fluco; n = 12) or caspofungin (Caspo; n = 12) were treated once, 5 mg/kg, by i.p. injection at T = 0. Controls received saline (n = 12) i.p at T = 0, and mice were monitored for 30 days. Relative to saline controls, RTD-1 treated mice had increased survival (P = 3.3 × 10−7, i.p.; P = 1.5 × 10−5, s.c.; P = 6.9 × 10−4, i.v.) as did Caspo and Fluco treated animals (P = 4.3 × 10−7). There was no statistical difference observed among groups treated with RTD-1, or between RTD-1 and Fluco, whereas Caspo was significantly more effective than RTD-1 or Fluco (see Suppl. Table 1). (b) Dose dependent efficacy of RTD-1. Mice (n = 12 or 13 for each cohort) were infected i.v. with SC5314 (as in panel a) and treated with a single i.p. dose (mg/kg shown) of RTD-1 at T = 0 and monitored for 30 d. RTD-1 significantly enhanced survival (P < 0.00001 for all treatment cohorts but the 10 mg/kg cohort for which P = 0.0013, and the 20 mg/kg for which RTD-1 significantly accelerates death, P = 6.3 × 10−5; see Suppl. Table 1 for statistical summary). (c) Effect of infection-treatment interval on single dose RTD-1 efficacy. Mice were infected i.v. (as in panel a) and treated i.p. with 5 mg/kg RTD-1 at intervals following infection: T = 0 (n = 13, data redrawn from Fig. panel a), 1 h (n = 12), 3 h (n = 12), and 6 h (n = 12). Saline (control) vehicle was administered i.p. at T = 0 and all mice were monitored for up to 30 days. While peptide treatment enhanced survival compared to saline control (P < 0.002 for all peptide treated cohort; Suppl. Table 1), delaying treatment reduced single dose efficacy of RTD-1. (d) Multiple RTD-1 dosing is effective in delayed treatment of systemic candidiasis. Mice (n = 10 for each group) were infected i.v. with C. albicans SC5314. Beginning 24 h p.i., mice received daily i.p. injections of 5 mg/kg of RTD-1, 5 mg/kg Fluco, 5 mg/kg Caspo, or saline for 7 days and mice were monitored for up to 30 d. All three agents markedly improved survival (P = 3.4 × 10−6) compared to saline treatment. Efficacy of RTD-1 was not statistically different than that of Fluco or Caspo, but Caspo was superior to Fluco (P = 4.7 × 10−3, Suppl. Table 1). (e) Inverted “U” effects of RTD-1 treatment of systemic candidiasis. Survival rates from Fig. 3b and RTD-1 0.1 mg/kg (not shown) are plotted as number of days showing >80% survival in function of RTD-1 concentration ranging from 0.1 to 20 mg/kg. RTD-1 enhanced survival as dose levels are increased up to 5 mg/kg, but decreased in efficacy with dosing at 10 and 20 mg/kg, resembles an inverted “U” shape.
RTD-1 tolerability/toxicity was evaluated by i.p. dosing of naÏve BALB/c mice with RTD-1 at 5 mg/kg RTD-1 daily for 7 days, 20 mg/kg every other day for 10 days, and once at 50 mg/kg. Mice tolerated RTD-1 dosing at all levels, and all were clinically normal for observation periods of at least 14 days following the last escalating dose.
We next evaluated the administration of single i.p. doses of RTD-1 ranging from 0.1 to 20 mg/kg immediately after i.v. fungal challenge. With the exception of the lowest (0.1 mg/kg; not shown) and the highest dose tested (20 mg/kg, Fig. 3b), RTD-1 treatment enhanced survival significantly (P < 0.002) compared to saline controls (Fig. 3b). Enhanced survival curves following single doses of RTD-1 at 0.25, 0.5, 2.5, and 5.0 mg/kg were statistically equivalent to each other (Fig. 3b) and to administration with 5 mg/kg of Fluco (Fig. 3a,b). Interestingly, dosing with 10 and 20 mg/kg RTD-1 was less effective than Fluco (P < 0.0001) and to RTD-1 doses between 0.25 and 5.0 mg/kg (P ≤ 0.02 in all cases; see Suppl. Table 1).
We then analyzed the effect of delaying RTD-1 treatment following fungal challenge. Compared to RTD-1 dosing at the time of i.v. fungal challenge, a one hour delay reduced survival benefit (Fig. 3c). Nevertheless, even with treatment delays of 1, 3 or 6 h, a single i.p. dose of 5 mg/kg RTD-1 resulted in enhanced survival compared to saline control (P ≤ 0.002; Fig. 3c). However, no survival enhancement was achieved if peptide administration was delayed for 24 h or 72 h (data not shown).
We then determined whether multiple dose administration of RTD-1 would improve survival in mice in the delayed treatment format. Mice challenged i.v. with C. albicans SC5314 were treated i.p. once a day for 7 days with 5 mg/kg RTD-1, Caspo, or Fluco with the first dose of each agent given 24 h after fungal infection. Each agent markedly enhanced survival (P < 0.0002; Fig. 3d), but RTD-1 was more effective in enhancing survival than Fluco, and was nearly equivalent to Caspo. Body weights of long term survivors (≥25 days) of each treatment cohort did not differ statistically from those of uninfected mice 25–30 days post infection and were otherwise clinically normal (Fig. 3 and data not shown).
Pharmacokinetics (PK) of systemically administered RTD-1
To ascertain the systemic levels of RTD-1 found to be effective in vivo, plasma levels of RTD-1 were evaluated over a 24 h period after single 5 mg/kg i.v. or i.p. injections of BALB/c mice (Fig. 4a). A two-compartment model best described the i.v. data. The mean maximum concentration (Cmax) and time to reach the maximum concentration (Tmax) after i.p. injection were 0.67 µg/mL and 4 h, respectively. These data indicate that RTD-1 is slowly absorbed i.p. with 63.4% bioavailability (Suppl. Table 2). Goodness-of-fit plots for the final pharmacokinetic model are shown in Supplementary Fig. 1. RTD-1 plasma levels were also analyzed 60 or 240 min after additional daily 5 mg/kg i.p. doses administered up to 6 days. In each instance, RTD-1 levels were not statistically different from that obtained by a single dose, indicating that RTD-1 did not accumulate in plasma over the course of several injections (Fig. 4b). Of note, i.p. Cmax values were 5–25 fold lower than RTD-1 MICs against C. albicans (Table 1). Moreover, in 50% serum, the MIC for RTD-1 against C. albicans SC5314 was >100 µg/ml, indicating that antifungal efficacy of the peptide in vivo is not a direct antifungal effect.
Figure 4.
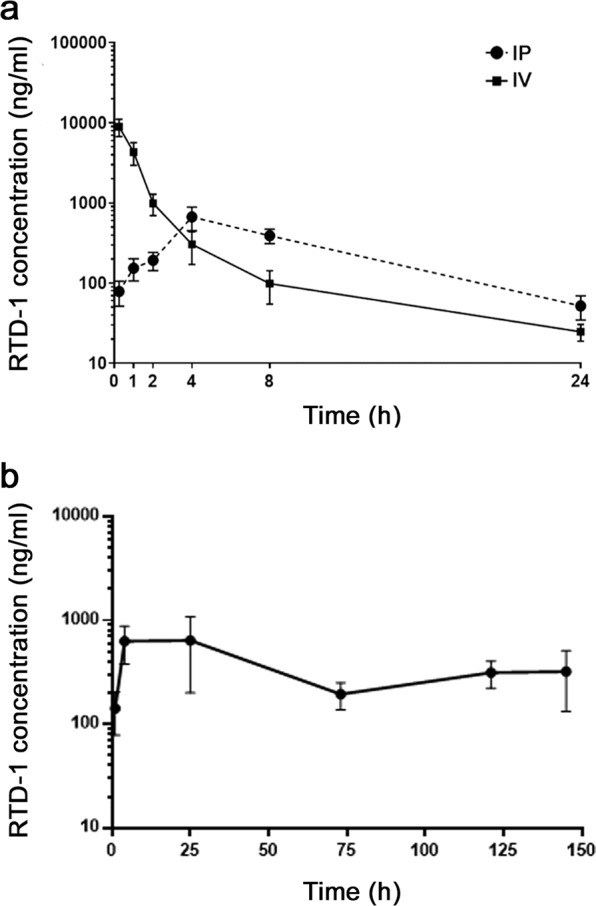
RTD-1 concentration-time profiles following i.v. and i.p. administration. (a) Single. dose PK. Single doses of RTD-1 (5 mg/kg) were administered i.v. or i.p. at T = 0 as described in Methods. Blood was collected at the indicated time points and plasma RTD-1 levels were quantified by LC-MS/MS. Mean plasma levels and SD are plotted at each time point. (b) Multiple dose administration of RTD-1. Mice received 1-7 doses of 5 mg/kg of RTD-1 and plasma levels were determined by LC-MS/MS and plotted versus time and number of injections (Methods). The plot shows the 1 h (n = 12) and 4 h (n = 11) plasma levels of mice receiving 1 injection i.p. at T = 0. All other values are plasma levels (mean +/− S.D.) obtained 1 h after additional daily i.p. doses administered for up to 6 days with times of collection by group: 25 h, n = 4; 73 h, n = 8; 121 h, n = 4; 145 h, n = 7.
RTD-1 treatment promotes fungal clearance and modulates systemic inflammation in candidemic mice
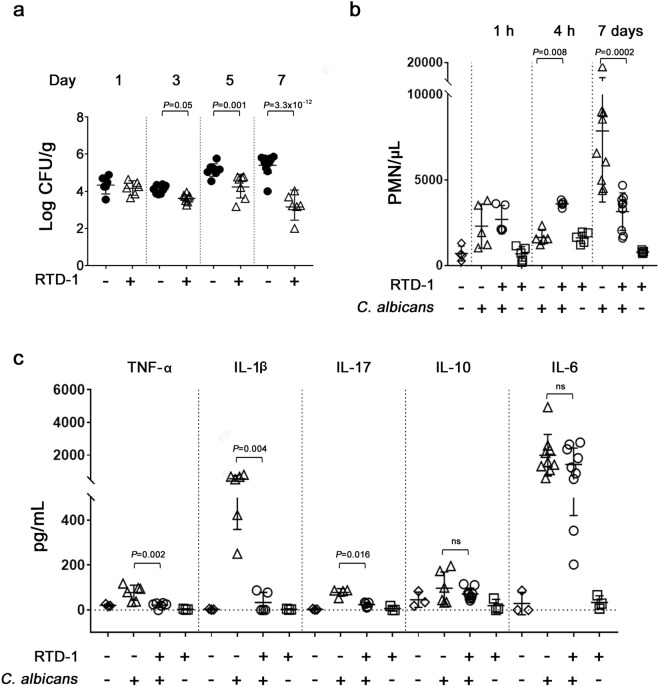
Fungal burdens in RTD-1-treated SC5314 candidemic mice were determined by quantifying CFU in kidney homogenates from animals treated i.p. once with 5 mg/kg of RTD-1 at T = 0. Fungal burden was quantified 1, 3, 5 and 7 days p.i. By day 3 p.i., a single RTD-1 treatment markedly reduced fungal burden compared with saline treated controls wherein fungal burden continued to increase during the 7 days observation period (Fig. 5a).
Figure 5.
Effects of RTD-1 on host response to systemic candidiasis. (a) RTD-1 promotes clearance of C. albicans. Kidney fungal burdens were measured in mice infected with 3 × 105 C. albicans SC5314 and treated i.p. at T = 0 with a single dose of 5 mg/kg RTD-1 or saline vehicle. Mice were euthanized 1, 3, 5 or 7 d after infection and CFU/g of kidney homogenate was determined by plating. Significance of RTD-1 treatment was analyzed by Fisher’s LSD test. (b) RTD-1 treatment reverses neutrophilia in candidemic mice. NaÏve or infected mice were treated with i.p. saline or a single dose of 5 mg/kg RTD-1 administered at the time of i.v. infection (as in Fig. 3a). Blood PMNs were quantified from individual animals 1 h, 4 h, and 7 days in each treatment group (n = 4 - 9 for each treatment cohort) and plotted as mean ± SD. RTD-1 treatment increased PMN levels at 4 h (P = 0.008) in infected mice, while significantly decreased PMNs (P = 0.002) 7 d after infection. (c) RTD-1 normalizes pro-inflammatory cytokines in candidemic mice. Mice (n = 3–9 for each cohort), treated as indicated, were euthanized 7 d after infection. Blood cytokines were quantified and are plotted as means ± SD, RTD-1 treatment effects were analyzed by Mann-Whitney test.
Neutrophils represent the first line of defense in invasive candidiasis, and susceptibility to systemic disease is markedly increased by persistent neutropenia44,45. Systemic disease is also associated with greatly elevated levels of proinflammatory cytokines46. While administration of RTD-1 to uninfected mice had little effect on blood cytokines or leukocytes, peptide treatment of candidemic mice significantly (P = 0.008) increased blood neutrophil counts 4 h p.i. (Fig. 5b). Interestingly, at this same time point, RTD-1 increased blood neutrophils 2.3 fold in naÏve mice (P = 0.32). However, by day 7, when saline treated candidemic mice had marked leukocytosis, neutrophil counts in the RTD-1 treated mouse cohort were significantly (P = 0.0002) lower, and essentially equivalent to that observed in this cohort at the 4 h time point (Fig. 5b). Consistent with these findings, RTD-1 treatment of candidemic mice resulted in a significant reduction of blood proinflammatory cytokines implicated in candida sepsis (TNF, IL-1β, and IL-17) 7 days p.i., while having no effect on IL-10 or IL-6 levels, both of which were elevated in candidemic mice (Fig. 5c). RTD-1 did not alter the levels of these cytokines in naÏve mice.
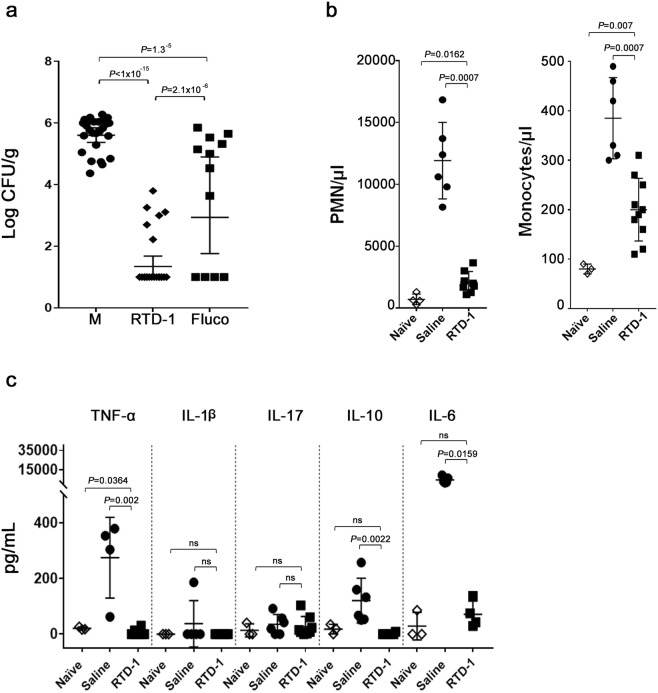
To assess the effect of RTD-1 treatment later in the disease course, we analyzed fungal burden, blood leukocytes, and inflammatory cytokines in RTD-1 treated, SC5314 infected mice at day 30 p.i. and in moribund, saline treated animals euthanized 5–10 p.i. (Fig. 3a,b). RTD-1 treatment markedly reduced kidney fungal burden (P < 1 × 10−15) and peptide treatment was substantially more effective than treatment with Fluco (P = 2.1 × 10−6; Fig. 6a). Of note, no culturable organisms were detected in 50% of the kidneys in RTD-1 treated mice. RTD-1-induced fungal clearance was accompanied by homeostatic normalization of circulating granulocytes which were markedly elevated in moribund saline-treated controls (Fig. 6b). In RTD-1 treated mice, levels of TNF, IL-10, and IL-6 were normalized to those of uninfected controls (Fig. 6c), complementing the homeostatic effects of RTD-1 on TNF, IL-1β, and IL-17 observed at 7 days p.i. (Fig. 5c).
Figure 6.
RTD-1 promotes fungal clearance and restores immune homeostasis in long term survivors. (a) RTD-1 mediated fungal clearance. Fungal burdens were quantified in homogenates of individual kidneys of long term survivors treated i.p. at T = 0 with saline, RTD-1 or Fluco (mice from studies presented in Fig. 3a,b). Saline-treated moribund mice (M) were euthanized between 5 and 10 d p.i. CFU/g (geometric means with 95% CI) were plotted for each treatment cohort and analyzed by Fisher’s LSD test. (b) RTD-1 normalizes blood neutrophils and monocytes in long term survivors of systemic candidiasis. Blood PMNs and monocytes were quantified in naÏve mice, moribund saline treated mice, and RTD-1 treated long term survivors (n = 6 per cohort; mice from studies presented in Fig. 3a,b). Effect of RTD-1 treatment was analyzed by Mann-Whitney test. (c) Cytokines normalized by RTD-1 in candidemic long term survivors. In long term survivors, RTD-1 treatment restored pro-inflammatory biomarkers TNF-α, IL-10, and IL-6 to levels equivalent to those of naÏve animals (n = 3-11 in each cohort). Effect of RTD-1 treatment was analyzed by Mann-Whitney test.
RTD-1 is efficacious in candidiasis mediated by MDR C. albicans
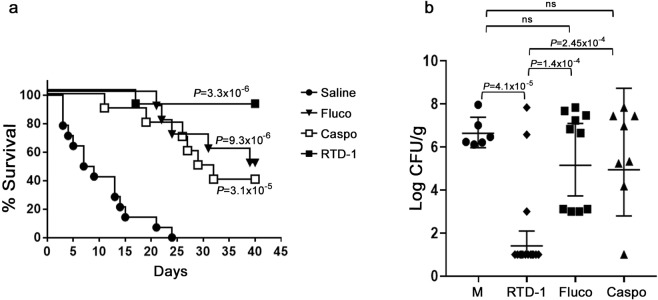
To assess whether the effects of RTD-1 on drug sensitive C. albicans SC5314 extend to drug resistant isolates, CD-1 mice infected i.v. with Caspo-resistant C. albicans 53264 were treated i.p. with 5 mg/kg each of RTD-1, Caspo, or Fluco once per day for 7 days beginning 24 p.i. (as in Fig. 3d). CD-1, rather than BALB/c mice were used in the C. albicans 53264 challenge studies as this mouse strain was employed by Wiederhold et al. in the assessment of caspofungin efficacy against this pathogen40. As shown in Fig. 7A, each agent significantly (P < 0.0001) improved survival compared to saline controls, and RTD-1 was superior to Caspo (P = 0.029) but not Fluco (Fig. 7A). By survival endpoint analysis (χ2) at day 40 p.i, RTD-1 was superior to Fluco (P = 0.01) and Caspo (P = 0.001). Fungal burdens were determined in kidneys of long term survivors in the RTD-1, Caspo, and Fluco-treated cohorts (euthanized at day 40) and from moribund mice from the survival study in Fig. 7a. Of the agents tested, only RTD-1 significantly (P = 4.1 × 10−5) reduced fungal burden relative to moribund saline controls (Fig. 7b).
Figure 7.
RTD-1 promotes survival and fungal clearance in systemic MDR candidiasis. (a) Efficacy of RTD-1 in C. albicans 53264 candidiasis. CD-1 mice infected i.v. with 1.8 × 107 blastospores of C. albicans 53264 were treated i.p. with 5 mg/kg of RTD-1 (n = 10), Fluco (n = 10), Caspo (n = 10), or saline vehicle (n = 14) daily for 7 d beginning 24 h p.i. and monitored for 40 days. Log-rank test analysis demonstrated that each agent enhances survival compared to saline controls, with RTD-1 having the greatest efficacy (see Suppl. Table 1). (b) Clearance of C. albicans in long term survivors. Long term survivors (40 days) from each cohort in panel (a) were euthanized and kidney fungal burdens quantified, as were saline-treated moribund mice (M) euthanized between 5 and 24 d p.i. CFU/g (geometric means with 95% CI) were plotted for each treatment cohort and analyzed by Fisher’s LSD test.
Discussion
Several factors, including indiscriminate and overuse of antifungals, increasing use of immunosuppressive therapies, and nosocomial colonization of hospitalized patients has accelerated the emergence of MDR strains. The severity of this public health threat has gained worldwide attention. While the introduction of echinocandins, nearly two decades ago, provided a valuable alternative to azoles and polyene antibiotics47,48, MDR strains resistant to echinocandins are diagnosed with increasing frequency40,49 highlighting the need for new therapeutic approaches. Studies presented here suggest that RTD-1, a macrocyclic peptide expressed in leukocytes of rhesus macaques, may represent a new therapeutic modality based on its efficacy in systemic candidiasis, including disease mediated by a caspofungin-resistant MDR strain of C. albicans.
RTD-1 is the prototype θ-defensin, the only known backbone cyclized polypeptides in the Animal Kingdom. All natural θ-defensin isoforms are 18-amino acid macrocycles stabilized by a six cysteine, tridisulfide core. Like other host defense peptides (HDPs), θ-defensins were discovered by screening for antimicrobial activities in lysates of rhesus macaque leukocytes which revealed that RTD-1 and closely related isoforms (RTDs 2–5) were broadly bactericidal and fungicidal in vitro against C. albicans and Cryptococcus neoformans31–33,50. As reported here and elsewhere39, RTD-1 kills Candida spp. in vitro by mechanisms that are temporally linked to cell death. For example, RTD-1 rapidly (<10 min) permeabilized C. albicans to propidium iodide, induced ATP release and reactive oxygen species in planktonic cells39. Moreover, θ-defensins are fungicidal against MDR C. albicans and non albicans Candida spp.39.
Here we report for the first time that RTD-1 is effective against both planktonic cells and biofilms of drug sensitive and MDR C. albicans in vitro. However, MICs of RTD-1 were 10–20 higher than Cmax plasma levels obtained following single or multiple dosing regimens in mice. Further, in the presence of 50% mouse serum, MICs were at least 100 fold higher than plasma Cmax values. Therefore, it is highly unlikely that the efficacy of RTD-1 in mouse systemic candidiasis is a direct antifungal effect, but rather is mediated by host-directed mechanisms, producing marked survival enhancement that was superior to that obtained with Fluco, and accompanied by efficient fungal clearance. Consistent with host-directed mechanisms, RTD-1 was highly effective in systemic candidiasis against drug sensitive and MDR strains of C. albicans.
While the pharmacologic basis of RTD-1 efficacy in candidemia is yet to be determined, data presented here suggest that peptide administration may promote the mobilization of neutrophils, as blood levels of these cells were significantly elevated in RTD-1 treated candidemic mice (Fig. 5), and we speculate that this early phase neutrophilia contributes to the reduction in systemic fungal burden detected as early as 3 days after a single treatment with RTD-1. As noted above, neutrophils play an important role in host defense against C. albicans infection via phagocytosis and formation of neutrophil extracellular traps51,52.
RTD-1 treatment of candidemic mice also had a profound effect on inflammatory cytokines over the course of infection. By day 7 p.i., RTD-1 treated mice (single dose at T = 0) had marked reductions in TNF, IL-1β, and IL-17 compared to saline treated animals. Additionally, in later stages of the disease course, RTD-1 treatment markedly prolonged survival and in these mice, levels of IL-10 and IL-6 normalized compared to moribund saline-treated controls. This was accompanied by significant reductions in blood neutrophils and monocytes. Thus, RTD-1 treatment of candidemic mice induced transient neutrophilia, followed by profound reductions in fungal burden compared to saline controls (P < 1 × 10−15), and in this regard, RTD-1 treatment was markedly superior to Fluco (Fig. 6). In parallel, peptide treatment homeostatically normalized granulocytes and inflammatory cytokines. The effects of RTD-1 in systemic candidiasis are consistent with results of studies in which the peptide was analyzed in models of E. coli and polymicrobial sepsis35, P. aeruginosa pneumonia37,53, and in murine SARS Coronavirus pneumonitis36. In each of these studies, RTD-1 therapeutically modified the course of disease and efficacy was associated with modulation of pathologic inflammation.
The therapeutic and pharmacologic properties of RTD-1 in systemic candidiasis suggest that the peptide modifies the host damage-response framework articulated by Casadevall54. Consistent with this model, RTD-1 treatment stimulates neutrophilia early in infection but suppresses inflammatory cytokines and promotes homeostatic restoration of blood granulocyte levels and proinflammatory cytokines which are chronically elevated in saline treated candidemic mice. Also consistent with this model is the finding that the dose-effect relationship of RTD-1 is an inverted “U”, as shown by the increasing efficacy as dose levels are increased up to 5 mg/kg, but a marked decrease in efficacy with dosing at 10 and 20 mg/kg (Fig. 3b,e). Of note, i.p. doses of at least 50 mg/kg RTD-1 were well tolerated in uninfected mice (Results), suggesting that higher levels of the peptide are less well tolerated in candidemic mice or that elevated concentrations are overly immunosuppressive. Further studies are required to delineate PK/pharmacodynamic relationships underlying the inverted U effects of RTD-1 treatment of systemic candidiasis.
Despite the remarkable in vitro properties of HDPs, efforts to develop them as therapeutics have met with limited success55. Barriers encountered include lack of peptide stability, toxicity, adverse proinflammatory properties, and poor bioavailability. In the context of antifungal therapeutics, we are aware of only two reports that demonstrate peptide efficacy in systemic candidiasis. In the first, Tavares et al. used plant defensin RsAFP2y in a prophylaxis model to show that that i.v. administration of 14 mg/kg of this peptide, given 1 h prior to i.v. infection with C. albicans strain 78, reduced fungal burden 5 d after infection, but no impact on survival was evaluated for efficacy in a mouse candidiasis model56. More recently, IDR-1018, an analog of bovine bactenecin 2 A, was evaluated in a mouse candidiasis model57. Daily intraperitoneal administration of 10 mg/kg of IDR-1018 beginning 24 h after retroorbital infection with a clinical isolate of C. albicans modestly improved survival up to 8 days p.i. which was accompanied by similarly modest reduction in renal fungal burden57. Of note, several small non-peptidic HDP-mimics, designed by Scott, Diamond and colleagues, were active in vitro against several Fluco-resistant Candida spp., and some were effective in C. albicans SC5314 systemic candidiasis58,59.
Our study is the first to show therapeutic efficacy, e.g., long term survival accompanied by fungal clearance, of a naturally occurring peptide in systemic candidiasis. Findings presented here demonstrate that RTD-1 both promotes fungal clearance and suppresses pathologic inflammation, and these effects augment host responses to infection by drug sensitive and MDR C. albicans strains. Of note, RTD-1 is highly stable in serum and plasma35, to host60 and fungal proteases39, is non-immunogenic61, and non-toxic (Results). Thus, RTD-1 provides a template for design of bioinspired macrocyclic compounds that may contribute an unmet need for new antifungal therapies.
Methods
Ethics
All methods methods were performed in accordance with relevant federal, state, and institutional guidelines. Animal use protocols were approved by The University of Southern California (USC) Institutional Animal Care and Use Committee (IACUC), Protocol #20538.
C. albicans strains
Reference strain C. albicans SC5314 was obtained from American Type Culture Collection. Caspofungin resistant clinical isolates of C. albicans 43001 and 53264 were kindly provided by Dr. Nathan Wiederhold (Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio, TX).
Peptides and antifungal drugs
Highly pure (>98%) RTD-1 hydrochloride was produced by solid phase peptide synthesis as described31,32. Caspofungin and fluconazole were purchased from Sigma-Aldrich (St. Louis, MO). RTD-1 was dissolved at 0.5 mg/ml in 0.01% acetic acid and antifungal drugs were prepared in sterile water. For animal injection, peptides and antifungals drugs were diluted in filter sterilized saline solution.
In vitro antifungal assays
C. albicans strains were tested against RTD-1, fluconazole, and caspofungin in microdilution broth in accordance with Clinical and Laboratory Standards Institute (CLSI) document M27-A2 with the exception that 25 mM MOPS was used in place of 165 mM MOPS, as described previously39. Overnight cultures were harvested by centrifugation, washed twice with Ca2+ and Mg2+-free PBS, pH 7.2, and suspended in test medium (RPMI 1640 medium, 25 mM MOPS, 2 mM L-glutamine) at 104 CFU/ml62. Incubations were conducted in flat bottom, 96-well microtiter plates (Greiner bio-one, Monroe, NC) containing 0.2 ml test medium per well. Duplicate 0.1 ml aliquots of serial two-fold dilutions of peptides and antifungal drugs were dispensed into wells, each of which was inoculated with 0.1 ml of fungal cell suspension containing 1 × 103 CFU. Plates were incubated at 30 °C for 48 hours after which A600 was determined using a SpectraMax M5e plate reader. Minimal inhibitory concentration (MIC) was defined as the lowest agent concentration that completely inhibited growth as determined by A600 absorbance.
Minimal fungicidal concentration (MFC) was determined by plating, on YPD plates, 10 µl from wells in MIC assay without cell growth and the first well with measurable turbidity as described63–66 and corresponding aliquots from the no-agent controls. Under MIC incubation conditions, no filamentation occurred allowing for accurate CFU counts after plating on YPD plates which were incubated for 24 h at 30 °C. MFC is reported as the lowest concentration of peptide or antifungal drug that killed ≥99% of the input organisms.
MICs and MFCs of RTD-1 were also performed in the presence of 50% mouse serum. Serum was prepared from blood collected by cardiac puncture from BALB/c mice. After coagulation at room temperature, serum was collected by centrifugation (200 g for 10 min followed by 23,000 g for 15 min), and diluted with an equal volume of yeast inoculum in MIC test medium described above.
Kinetics of RTD-1 candidacidal activity was determined at peptide concentrations ranging from 0 to 10 µg/ml using a liquid suspension assay described previously39. Overnight cultures of C. albicans 53264 or 43001 were grown to mid-log phase in 5% yeast peptone dextrose broth (YPD) (Difco) at 30 °C. Approximately 2 × 106 CFU/ml were incubated with 0, 0.3, 1, 3 and 10 µg/ml RTD-1 or 10 µg/ml caspofungin, suspended in 10 mM PIPES, pH 7.4, plus 5 mM glucose (PG), in 96-well plates with shaking at 60 RPM at 37 °C. At timed intervals, samples were diluted 1:1000 fold into PG buffer and aliquots plated onto YPD agar plates (TekNova), and surviving organisms quantified by colony counting after incubation for 24 h at 30 °C.
Fungicidal activities of RTD-1 and caspofungin were similarly determined in whole blood. Citrate anticoagulated blood was obtained by cardiac puncture from CD-1 mice. Approximately 5 × 104 CFU/ml of C. albicans SC5314 or 53264 were incubated with 0, 1, 3, 10, 30 and 100 µg/ml RTD-1 or caspofungin in 96-well plates with whole blood (85% vol/vol final) with shaking at 60 RPM at 37 °C. After 2 h, samples were diluted 1:10 into 10 mM PIPES, pH 7.4 containing 0.05% YPD and aliquots were plated onto YPD agar plates (Teknova) and CFU were quantified as above.
Biofilm studies
The antifungal activities of RTD-1, caspofungin and fluconazole were evaluated against biofilms of C. albicans SC5314 and 53264 essentially as described by Pierce et al.67. Briefly, biofilms established for 0 h (preadhesion), 2 h (adherent), or 24 h (mature) were developed by adding 1 × 105 yeast cells in 50 or 100 µl of RPMI-1640 to wells of flat-bottom 96-well microtiter plates (Costar, Corning, NY). Fifty or 100 µl aliquots of serial 2-fold dilutions of peptide or antifungal drugs were added to wells in triplicate and incubated for 24 h or 48 h prior to analysis of biofilm metabolic activities. Concentrations of reagents used were as follows: RTD-1 - 0.09 – 100 µg/ml (0.05–48 µM), Fluco – 1–250 µg/ml (3.3–816.3 µM); Caspo- 0.003–8 µg/ml (0.0003–7.3 µM). Analysis of the effect of each agent on Candida biofilms was performed using an XTT colorimetric assay67. One hundred µl of 1 µM XTT/menadione added to each biofilm well, plates were incubated in the dark for 3 h at 37 °C, and 80 µl of supernatant was transferred to a new 96-well flat-bottom microtiter plate (Greiner Bio-one, Monroe, NC). Absorbance was measured at 490 nm on a SpectraMax® i3x (Molecular Devices). Readings were plotted as average of triplicates after subtracting the corresponding values for negative controls (XTT/menadione only).
Systemic candidiasis model
BALB/c female mice (7–8 weeks old) and CD-1 female mice (4–5 weeks old) were obtained from Charles River Laboratories and allocated randomly in groups of five mice per cage. Mice were maintained on a 12 h light/dark cycle in thermostatically controlled rooms for the duration of the experiment. C. albicans inocula were prepared fresh each day from saturated cultures grown overnight in YPD medium, washed in sterile PBS, and fungi were counted with hemocytometer. Stock suspensions were prepared in sterile PBS. BALB/c mice were challenged with 2 × 106 CFU/ml wild-type C. albicans reference strain SC5314, while CD-1 mice were challenged with 1.8 × 107 CFU/ml (0.15–0.2 ml) C. albicans clinical isolate 53264 by i.v. injection into the lateral caudal tail vein using a 28 G 1/2 inch needle. Animals were treated by i.v., s.c., or i.p. routes immediately post-infection (p.i.) or 24 h p.i. with sterile saline (control), RTD-1, fluconazole, or caspofungin administered at the concentrations indicated in a 0.200 ml. Mice were weighted and monitored daily for general clinical condition. Mice were euthanized as approved by the USC IACUC and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were repeated at least three times.
To assess systemic fungal clearance, renal fungal burden was determined. Mice were euthanized at times indicated, and both kidneys were weighed, homogenized in 5 ml of sterile PBS, and serial dilutions of homogenate were plated on YPD agar plates and incubated at 30 °C for 2 days. CFU per gram of kidney was calculated.
RTD-1 pharmacokinetics (PK)
Single dose PK of RTD-1 was evaluated by quantifying plasma peptide concentrations at intervals following 5 mg/kg bolus injections administered i.v. and i.p. to CD-1 mice. At indicated intervals, plasma was prepared from EDTA-anticoagulated blood collected aseptically by terminal cardiac puncture. Plasma was diluted 1:10 or 1:20 into 5% formic/5% acetonitrile. RTD-1 plasma concentrations were determined by reverse-phase liquid chromatography (XBridge phenyl 3.5 µm column, Waters) on an Acquity H-Class UPLC (Waters) with tandem electrospray mass spectroscopy on a Xevo TQ-S running MassLynx V4.1 (Waters). Quantitative mass spectroscopy was performed by multiple-reaction monitoring transition 417.38 > 517.14, with area under the curve determined by TargetLynx (Waters). Pharmacokinetic modeling of intravenous RTD-1 concentration-time data was performed with ADAPT (version 5) software using the naïve pooled data approach. One- and two-compartment models were evaluated. Model selection was based on the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) scores, the likelihood ratio test (LRT), and goodness-of-fit plots. Concentration data below the lower limit of quantitation were treated as missing for pharmacokinetic analysis. Area under the curve (AUC24) was calculated by taking the average of RTD-1 concentrations at each time point and applying the linear trapezoidal method. Bioavailability (F) for i.p. injection was determined using the ratio of AUCs from i.p. and i.v.
Hematology and cytokine analyses
EDTA-anticoagulated blood was collected aseptically by terminal cardiac puncture and analyzed for complete blood cell count using a HemaVet 950 FS hematology analyzer (Drew Scientific). Plasma cytokine levels plasma were quantified using a mouse-specific MILLIPLEX MAP kit (Millipore) as described35.
Statistical analyses
Survival curves were compared using the log rank (Mantel-Cox) test. Blood levels of PMNs and monocytes, and plasma levels of cytokines were compared using Mann-Whitney test. One-way ANOVA was used for analysis of variance of fungal burden data, followed by uncorrected Fisher’s Least Significant Difference (LSD) test. Significance of survival endpoint was calculation by χ2 analysis (Fig. 7 only). All statistical analyses employed GraphPad Prism 8.12.
Supplementary information
Acknowledgements
The present work was supported by grants from National Institute of Allergy and Infectious Diseases grants (RO1 AI22931, R01 AI125141, R44 AI142959), National Institute of Dental and Craniofacial Research grant (R01 DE021341), the Southern California Clinical and Translational Science Institute (UL1 TR000130), and National Cancer Institute (P30 CA014089).
Author contributions
V.B., D.Q.T. and M.E.S. conceived and design the experiments. V.B. and P.T. carried out the experiments. D.N., A.E.C. and Y.E. contributed to sample preparation. V.B., A.P., P.B. and J.S. contributed to the data analysis. V.B., D.Q.T., A.J.O., P.B. and M.E.S. contributed to the interpretation of the results. V.B. and M.E.S. wrote the main manuscript text. V.B. prepared Figs 1–3, 4b, 5–7, Table 1 and Suppl. Table 1-2. A.P. and P.B. prepared Fig. 4a and Suppl. Fig. 1. All authors reviewed the manuscript.
Competing interests
Drs. Selsted (co-founder, chief scientific officer) and D.Q. Tran (scientific director) hold part time appointments with Oryn Therapeutics, LLC which has licensed theta defensin technologies for commercial development. Dr. Selsted receives no income from Oryn Therapeutics but is an equity holder. Dr. Tran is a part time employee of Oryn. The relationship between Oryn Therapeutics and USC is disclosed to and approved by all parties. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53402-z.
References
- 1.Brown GD, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 3.Slavin M, et al. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21(490):e491–410. doi: 10.1016/j.cmi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006;2:602–610. doi: 10.1038/ncprheum0336. [DOI] [PubMed] [Google Scholar]
- 7.Rosen LB, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190:3959–3966. doi: 10.4049/jimmunol.1202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 9.Pianalto Kaila, Alspaugh J. New Horizons in Antifungal Therapy. Journal of Fungi. 2016;2(4):26. doi: 10.3390/jof2040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerr A, et al. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus-infected women: Risk factors and severity. Obstet Gynecol. 2003;101:548–556. doi: 10.1016/s0029-7844(02)02729-1. [DOI] [PubMed] [Google Scholar]
- 11.Hope W, Morton A, Eisen DP. Increase in prevalence of nosocomial non-Candida albicans candidaemia and the association of Candida krusei with fluconazole use. J Hosp Infect. 2002;50:56–65. doi: 10.1053/jhin.2001.1131. [DOI] [PubMed] [Google Scholar]
- 12.Nucci M, et al. Fungal infections in neutropenic patients. A 8-year prospective study. Rev Inst Med Trop Sao Paulo. 1995;37:397–406. doi: 10.1590/S0036-46651995000500004. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong-James D, Meintjes G, Brown GD. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 2014;22:120–127. doi: 10.1016/j.tim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010;9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 15.Rogers TR. Antifungal drug resistance: limited data, dramatic impact? Int J Antimicrob Agents. 2006;27(Suppl 1):7–11. doi: 10.1016/j.ijantimicag.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Nobile CJ, Johnson AD. Candida albicans Biofilms and Human Disease. Annu Rev Microbiol. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. Pathog Dis. 2016;74:ftw018. doi: 10.1093/femspd/ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra J, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominic RM, Shenoy S, Baliga S. Candida biofilms in medical devices: evolving trends. Kathmandu Univ Med J (KUMJ) 2007;5:431–436. [PubMed] [Google Scholar]
- 20.Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86–96. doi: 10.1016/j.bcp.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams David, Lewis Michael. Pathogenesis and treatment of oral candidosis. Journal of Oral Microbiology. 2011;3(1):5771. doi: 10.3402/jom.v3i0.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tverdek FP, Kofteridis D, Kontoyiannis DP. Antifungal agents and liver toxicity: a complex interaction. Expert Rev Anti Infect Ther. 2016;14:765–776. doi: 10.1080/14787210.2016.1199272. [DOI] [PubMed] [Google Scholar]
- 24.Meletiadis J, Chanock S, Walsh TJ. Defining targets for investigating the pharmacogenomics of adverse drug reactions to antifungal agents. Pharmacogenomics. 2008;9:561–584. doi: 10.2217/14622416.9.5.561. [DOI] [PubMed] [Google Scholar]
- 25.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 26.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science. 2015;347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 28.Perfect JR. “Is there an emerging need for new antifungals?”. Expert Opin Emerg Drugs. 2016;21:129–131. doi: 10.1517/14728214.2016.1155554. [DOI] [PubMed] [Google Scholar]
- 29.Roemer T., Krysan D. J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harbor Perspectives in Medicine. 2014;4(5):a019703–a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehrer RI, Cole AM, Selsted ME. theta-Defensins: cyclic peptides with endless potential. J Biol Chem. 2012;287:27014–27019. doi: 10.1074/jbc.R112.346098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun. 2008;76:5883–5891. doi: 10.1128/IAI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang YQ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 33.Tran D, et al. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem. 2002;277:3079–3084. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Schaal JB, et al. Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS One. 2012;7:e51337. doi: 10.1371/journal.pone.0051337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohlford-Lenane CL, et al. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J Virol. 2009;83:11385–11390. doi: 10.1128/JVI.01363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensman, T. J. et al. Efficacy of Rhesus Theta-Defensin-1 in Experimental Models of Pseudomonas aeruginosa Lung Infection and Inflammation. Antimicrob Agents Chemother61, 10.1128/AAC.00154-17 (2017). [DOI] [PMC free article] [PubMed]
- 38.Jayne JG, et al. Rhesus Theta (theta)-Defensin-1 Attenuates Endotoxin-Induced Acute Lung Injury by Inhibiting Proinflammatory Cytokines and Neutrophil Recruitment. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2016-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basso, V. et al. Fungicidal potency and mechanisms of theta-defensins against multi-drug resistant Candida species. Antimicrob Agents Chemother, 10.1128/AAC.00111-18 (2018). [DOI] [PMC free article] [PubMed]
- 40.Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob Agents Chemother. 2011;55:3254–3260. doi: 10.1128/AAC.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. Paradoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother. 2004;48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005;48:151–161. doi: 10.1111/j.1439-0507.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 44.Fulurija A, Ashman RB, Papadimitriou JM. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology. 1996;142((Pt 12)):3487–3496. doi: 10.1099/13500872-142-12-3487. [DOI] [PubMed] [Google Scholar]
- 45.Kullberg BJ. van ‘t Wout, J. W. & van Furth, R. Role of granulocytes in increased host resistance to Candida albicans induced by recombinant interleukin-1. Infect Immun. 1990;58:3319–3324. doi: 10.1128/iai.58.10.3319-3324.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo EK, MacCallum DM. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol Lett. 2011;320:1–8. doi: 10.1111/j.1574-6968.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 47.Denning DW. Echinocandins and pneumocandins–a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 48.Walsh TJ. Echinocandins–an advance in the primary treatment of invasive candidiasis. N Engl J Med. 2002;347:2070–2072. doi: 10.1056/NEJMe020142. [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer CD, et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol. 2010;48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tongaonkar P, et al. Rhesus macaque theta-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J Leukoc Biol. 2011;89:283–290. doi: 10.1189/jlb.0910535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15:630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 52.Urban CF, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beringer PM, et al. Rhesus theta-defensin-1 (RTD-1) exhibits in vitro and in vivo activity against cystic fibrosis strains of Pseudomonas aeruginosa. J Antimicrob Chemother. 2016;71:181–188. doi: 10.1093/jac/dkv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haney EF, Straus SK, Hancock REW. Reassessing the Host Defense Peptide Landscape. Front Chem. 2019;7:43. doi: 10.3389/fchem.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tavares PM, et al. In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrob Agents Chemother. 2008;52:4522–4525. doi: 10.1128/AAC.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freitas, C. G. et al. An Immunomodulatory Peptide Confers Protection in an Experimental Candidemia Murine Model. Antimicrob Agents Chemother61, 10.1128/AAC.02518-16 (2017). [DOI] [PMC free article] [PubMed]
- 58.Menzel LP, et al. Potent in vitro and in vivo antifungal activity of a small molecule host defense peptide mimic through a membrane-active mechanism. Sci Rep. 2017;7:4353. doi: 10.1038/s41598-017-04462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowdhury Mobaswar, Ryan Lisa, Cherabuddi Kartikeya, Freeman Katie, Weaver Damian, Pelletier Jeffry, Scott Richard, Diamond Gill. Antifungal Potential of Host Defense Peptide Mimetics in a Mouse Model of Disseminated Candidiasis. Journal of Fungi. 2018;4(1):30. doi: 10.3390/jof4010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaal JB, et al. Macrocyclic theta-defensins suppress tumor necrosis factor-alpha (TNF-alpha) shedding by inhibition of TNF-alpha-converting enzyme. J Biol Chem. 2018;293:2725–2734. doi: 10.1074/jbc.RA117.000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaal JB, et al. Suppression and resolution of autoimmune arthritis by rhesus theta-defensin-1, an immunomodulatory macrocyclic peptide. PLoS One. 2017;12:e0187868. doi: 10.1371/journal.pone.0187868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh-Babak SD, et al. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog. 2012;8:e1002718. doi: 10.1371/journal.ppat.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canton E, et al. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn Microbiol Infect Dis. 2003;45:203–206. doi: 10.1016/S0732-8893(02)00525-4. [DOI] [PubMed] [Google Scholar]
- 64.Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J Clin Microbiol. 2002;40:3776–3781. doi: 10.1128/JCM.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfaller MA, Sheehan DJ, Rex JH. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin Microbiol Rev. 2004;17:268–280. doi: 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silveira CP, et al. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J Med Microbiol. 2009;58:1607–1610. doi: 10.1099/jmm.0.007609-0. [DOI] [PubMed] [Google Scholar]
- 67.Pierce CG, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.