Abstract
Autophagy is a lysosomal degradation pathway that degrades cytoplasmic proteins and organelles. Absence of autophagy in hepatocytes has been linked to promoting liver injury and tumorigenesis, however the mechanisms behind why a lack of autophagy induces these complications is not fully understood. The role of mammalian target of rapamycin (mTOR) in impaired autophagy-induced liver pathogenesis and tumorigenesis was investigated by using liver-specific Atg5 knockout (L-Atg5 KO) mice, L-Atg5/mTOR, and L-Atg5/Raptor double knockout (DKO) mice. At two months of age, we found that deletion of mTOR or Raptor in L-Atg5 KO mice attenuated hepatomegaly, cell death and inflammation but not fibrosis. Surprisingly, at six months of age, L-Atg5/mTOR DKO and L-Atg5/Raptor DKO mice also had increased hepatic inflammation, fibrosis, and liver injury, similar to the L-Atg5 KO mice. Moreover, more than 50% of L-Atg5/mTOR DKO and L-Atg5/Raptor DKO mice already developed spontaneous tumors but none of the L-Atg5 KO mice had developed any tumors at six months of age. At nine months of age, all L-Atg5/mTOR DKO and L-Atg5/Raptor DKO had developed liver tumors. Mechanistically, L-Atg5/mTOR DKO and L-Atg5/Raptor DKO mice had deceased levels of hepatic ubiquitinated proteins and persistent Nrf2 activation but had increased Akt activation compared with L-Atg5 KO mice. In conclusion, loss of mTOR signaling attenuates the liver pathogenesis in mice with impaired-hepatic autophagy but paradoxically promotes tumorigenesis in mice at a relatively young age. Therefore, the balance of mTOR is critical in regulating the liver pathogenesis and tumorigenesis in mice with impaired hepatic autophagy.
Keywords: Atg5, ductular reaction, Nrf2, inflammation, fibrosis, Raptor
INTRODUCTION
Hepatocellular carcinoma (HCC), a liver cancer, has accounted for more than 600,000 deaths per year worldwide (1). A more recent study indicates that in the U.S., HCC mortality rate has increased by 43% between 2000 and 2016 (2). Chronic liver injury due to conditions such as alcohol exposure, hepatitis B or C viruses, hepatotoxins and obesity have led to or been associated with hepatocyte death, inflammatory response, fibrosis, cirrhosis, compensatory hepatocyte proliferation and eventual progression to liver tumorigenesis (3–5). Interestingly, the mentioned conditions that lead to chronic liver injury are often associated with impaired hepatic autophagy, a lysosomal degradation pathway, that plays an important role in regulating the homeostasis of cellular proteins, lipid and organelles in the liver (6, 7). These observations suggest that autophagy may be an important protective mechanism against chronic liver injury and tumorigenesis. Indeed, liver-specific Atg7 (L-Atg7) or L-Atg5 knockout (KO) mice exhibit increased liver injury, severe hepatomegaly, inflammation, fibrosis and eventually develop spontaneous liver tumors (8–12). We and others have previously shown that persistent activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) due to the accumulation of p62/SQSTM1 (hereafter referred to as p62) is the major cause of liver injury and tumorigenesis in autophagy-deficient livers (10, 13). Nrf2 is a transcription factor that regulates the expression of cellular important antioxidant genes, drug metabolism enzymes and transporters, which protects against oxidative and electrophilic stresses (14, 15). However, the mechanisms by which activation of Nrf2 paradoxically promotes liver injury in autophagy-deficient livers are unknown. Intriguingly, persistent activation of Nrf2 alone is not sufficient to cause liver injury because L-Keap1 KO mice have little basal liver injury (16). It seems that persistent activation of Nrf2 needs to be concurrently coupled with defective hepatic autophagy to induce liver injury. It is possible that the robust newly synthesized proteins (an anabolic process) due to persistent activation of Nrf2 are not effectively cleared in autophagy-defective livers (impaired catabolic process), which leads to the accumulation of hepatic proteins, increased hepatic proteotoxicity, and subsequently hepatocyte death.
The cellular biosynthetic pathways for proteins and other macromolecules are mainly regulated by the mammalian target of rapamycin complex 1 (mTORC1). In response to nutrients and growth factors, mTORC1 is activated and responsible for the increased synthesis of macromolecules and building blocks that are required for cell growth and proliferation (17). More than 50% human cancers, including HCC, have aberrant mTORC1 activation (18), which can mostly be attributed to the loss of function mutations of phosphatase and tensin homolog (PTEN) or tuberous sclerosis complex 1 (TSC1) or TSC2 (19, 20). Therefore, liver-specific ablation of PTEN or TSC1 in mice leads to chronic activation of mTORC1, hepatomegaly, and spontaneous liver tumorigenesis (21). Targeting mTOR has become a popular topic in studying various cancer treatments, however, thus far clinical trials using rapamycin and its pharmacological analogs (rapalogs) have shown minimal to modest beneficial effects (22, 23). One of the molecular events upon mTOR inhibition is the induction of autophagy, which may confer cell protective effects by governing proteostasis, redox balance, and bioenergetic homeostasis. This study investigated the molecular relationship and biological relevance of the two interconnected pathways in the liver by generating genetically engineered mice that are deficient in autophagy (Atg5) and/or mTOR (mTOR or Raptor).
MATERIALS AND METHODS
Animal experiments
Atg5 Flox/Flox (Atg5 F/F) mice (C57BL/6/129) were generated by Dr. N. Mizushima (The University of Tokyo) and were backcrossed with C57BL/6J for another 10 generations as described previously (10). mTOR F/F, Raptor F/F (C57BL/6J) and Albumin Cre mice were obtained from Jackson Laboratory. All animals received humane care, and all procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. Alb Cre+ mice and Alb Cre-matched littermates were used and sacrificed at 2, 6, 9 and 12 months. Atg5 F/F Alb Cre+ (L-Atg5 KO) mice were further crossed with mTOR F/F or Raptor F/F mice to generate Atg5 F/F and mTOR F/F Alb Cre+ double knockout (DKO) (L-Atg5/mTOR DKO) or L-Atg5/Raptor DKO mice. Constitutive androstane receptor (CAR) KO mice on the C57BL/6 background were described previously (24), and were crossed with L-Atg5 KO mice to generate L-Atg5/CAR DKO mice.
Liver injury analysis
Liver injury was assessed by the determination of serum alanine aminotransferase (ALT) levels and hematoxylin and eosin (H&E) staining of liver sections as we described previously [1]. Total liver lysates were prepared using RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl [lauryl] sulfate) supplemented with protease inhibitors.
Statistical analysis
Experimental data were subjected to Student t-test or One-Way ANOVA analysis with Scheffé’s post hoc test when appropriate. P<0.05 was considered significant.
Antibodies, Measurement of Caspase-3 activities, Immunohistochemistry, and all others are listed in Supplemental Materials.
RESULTS
Loss of CAR has no effects on hepatomegaly, liver injury and tumorigenesis in L-Atg5 KO mice
Constitutive androstane receptor (CAR) is a nuclear receptor regulating xenobiotic metabolism and the activation of CAR induces hepatomegaly and promotes liver tumorigenesis (24, 25). We found no difference in the ratio of liver/bodyweight and serum ALT levels between L-Atg5 KO and L-Atg5/CAR DKO mice (Supplemental Figure 1A&B). Moreover, while there was a small decrease of tumor incidence in L-Atg5/CAR DKO mice compared with L-Atg5 KO mice at the age of 9 months old (55% vs 83%), all L-Atg5/CAR DKO mice developed tumor at the age of 12 months old (Supplemental Figure 1C & D). These data suggest that CAR does not play a critical role in the liver pathogenesis and tumorigenesis of L-Atg5 KO mice.
Activation of mTOR in L-Atg5 KO mouse livers.
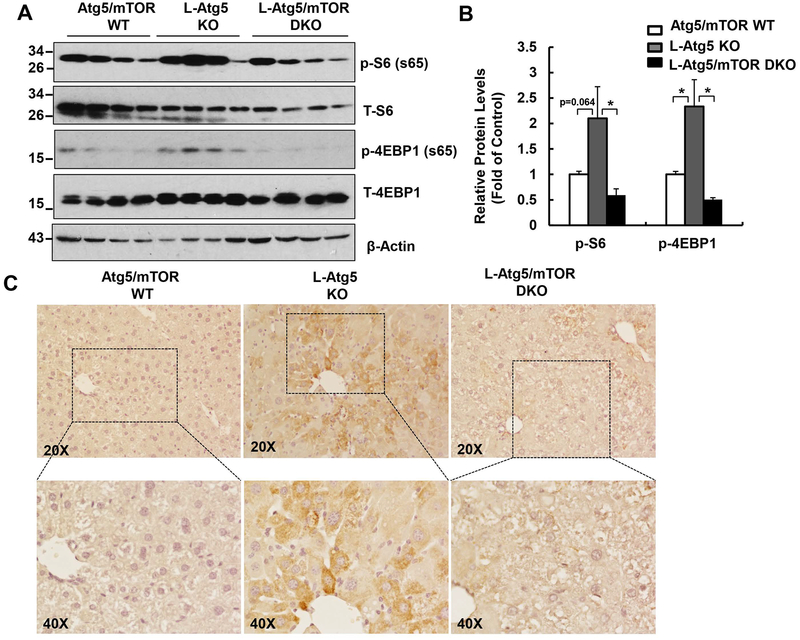
Immunoblotting analysis revealed that the levels of phosphorylated S6 (p-S6) and 4EBP1 (p-4EBP1) were increased in L-Atg5 mouse livers compared with age- and gender-matched WT mice. The levels of hepatic p-S6 and p-4EBP1 were markedly reduced in the L-Atg5/mTOR DKO mice compared with L-Atg5 KO mice (Figure 1 A & B). Immunohistochemistry staining of the liver tissues further confirmed the increased p-S6 staining in L-Atg5 KO mice, which was largely diminished in L-Atg5/mTOR DKO mice (Figure 1C). These data indicate that impaired hepatic autophagy leads to the activation of mTOR, which can be successfully blunted by the genetic ablation of mTOR.
Figure 1. Increased mTOR activation in L-Atg5 KO mice.
(A) Total liver lysates of the indicated genotypes of 2-month-old mice were subjected to western blot analysis. (B) Densitometry analysis of (A). Data are means± SEM (n=4). (C) Representative images of immunostaining of phosphorylated S6 (p-S6) of Atg5/mTOR WT, L-Atg5 KO and L-Atg5/mTOR DKO liver sections.
mTOR ablation attenuates hepatomegaly, liver injury, and inflammation, but not fibrosis in young L-Atg5 KO mice
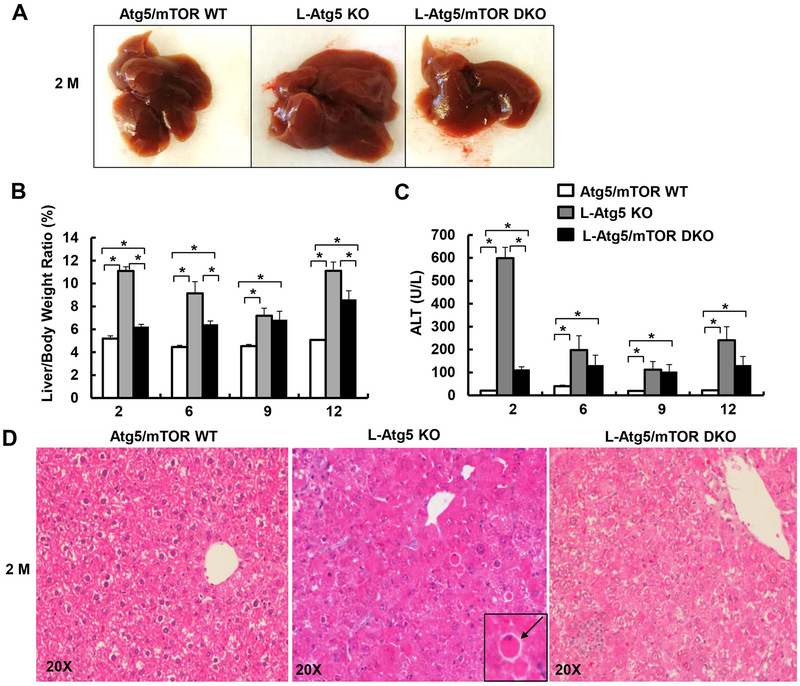
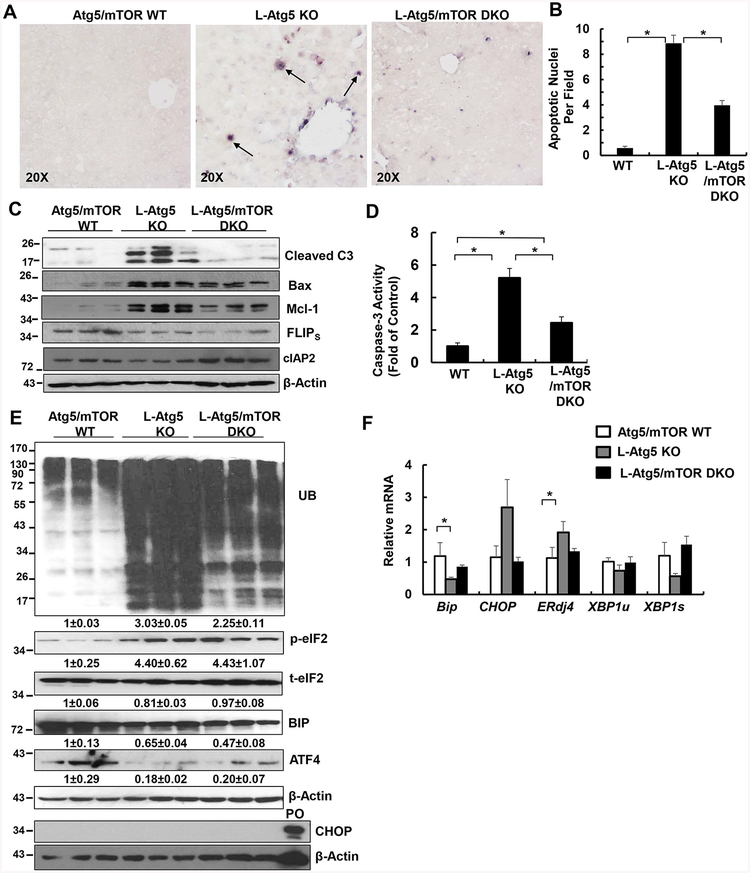
Deletion of mTOR alone in the liver decreased liver/body weight ratio in 2-month old but not 12-month old mice compared with their matched WT littermates. There were no obvious changes in the serum ALT levels and liver histology in the 2-month old and 12-month old L-mTOR KO mice (Supplemental Figure 2). Previous studies have demonstrated that loss of hepatic autophagy caused hepatomegaly, liver injury and fibrosis (8, 10, 12, 13, 26). While this was consistently observed in the L-Atg5 KO mice, ablation of mTOR in L-Atg5 KO mice markedly attenuated hepatomegaly and liver injury at the early ages of 2 and 6 months, but to a lesser extent in the older mice (9 and 12 months) (Figure 2A–C). L-Atg5 KO livers showed increased apoptosis, as demonstrated by H&E and TUNEL staining of liver tissues, as well as increased caspase-3 cleavage and activities in the liver lysates, which were all suppressed in L-Atg5/mTOR DKO mice at the age of 2 months (Figure 2D, Figure 3A–D). L-Atg5 KO mouse livers also had increased levels of pro-apoptotic Bax and anti-apoptotic Mcl1 but had decreased levels of FLIPs and cIAP2, the endogenous apoptosis inhibitors. Deletion of mTOR in L-Atg5 KO mouse livers had little effects on the levels of FLIPs but increased levels of cIAP2, although the levels of Bax and Mcl-1 decreased (Figure 3C). There was no difference in the total protein level per gram of liver among WT, L-Atg5 KO, and DKO mice, but the total protein level per liver increased almost 2-fold in L-Atg5 KO mice compared with WT and DKO mice (Supplemental Figure 3).
Figure 2. Deletion of mTOR attenuates hepatomegaly and liver injury in young L-Atg5 KO mice.
(A) Representative gross anatomy of livers of indicated genotypes at age of 2 months. Mice with different genotypes and age were analyzed for liver/body weight ratio (B), and serum ALT (C). Data are means± SEM (n=4–10). *p<0.05, One-Way Anova analysis with Scheffé’s post hoc test. (D) Representative H&E staining of mice at age of 2 months. Arrow: apoptotic cell.
Figure 3. Deletion of mTOR attenuates apoptosis and levels of ubiquitinated protein in L-Atg5 KO mice.
(A) Representative photographs of TUNEL staining of mouse livers of Atg5/mTOR WT, L-Atg5 KO and L-Atg5/mTOR DKO at age of 2 months. Arrows: TUNEL positive cells. (B) Quantification of TUNEL positive cells from (A). Data are means± SEM from 3 different mice of at least 5–10 images counted in each mouse. (C) Total liver lysates of the indicated genotypes were subjected to western blot analysis and (D) caspase-3 activity assay. Data are presented as fold of control (means± SEM, n=8–9). * p<0.05 Student t Test. (E) Total liver lysates of the indicated genotypes were subjected to western blot analysis. PO: positive control of CHOP induction from thapsigargin-treated 293Tcells. Densitometry analysis data are presented as fold of control (means± SEM, n=3) (F) Quantitative real-time PCR analysis of the expression of hepatic ER stress genes. Total RNAs were prepared from livers of the indicated genotypes at age of 2 months. Data are presented as means± SEM (n=4). * p<0.05 One-Way Anova analysis with Scheffé’s post hoc test.
L-Atg5 KO mice had increased infiltration of neutrophils and macrophages, as well as increased hepatic expression of inflammation genes, which all decreased in L-Atg5/mTOR DKO mice (Supplemental Figure 4A & B and Supplemental Figure 5A–C). Interestingly, both L-Atg5 KO and L-Atg5/mTOR DKO mice had similarly increased hepatic Sirius Red staining (Supplemental Figure 6A & 4B). The hepatic mRNA levels of fibrotic genes and protein levels of α-SMA increased in L-Atg5 KO mice compared with WT mice, but no differences were found between L-Atg5 KO and L-Atg5/mTOR DKO mice (Supplemental Figure 6C & 6D). L-Atg5 KO mouse livers had increased accumulation of aberrant ER membranes and collagen fibers, which were also readily found in L-Atg5/mTOR DKO mice (Supplemental Figure 7). Moreover, increased focal ductular reaction was found in both L-Atg5 KO and L-Atg5/mTOR DKO mouse livers (Supplemental Figure 8). The levels of ubiquitinated proteins markedly increased in L-Atg5 KO mouse livers, which were blunted in L-Atg5/mTOR DKO mice (Figure 3E). The levels of p-eIF2 increased in L-Atg5 KO mouse livers but the levels of hepatic CHOP were almost undetectable and the levels of BIP and ATF4 decreased in L-Atg5 KO mouse livers. Similarly, the mRNA levels of CHOP and ERdj4 slightly increased but the mRNA levels of BIP and the unspliced form and spliced form of XBP1 even decreased in L-Atg5 KO mouse livers compared with either the matched WT mice and L-Atg5/mTOR DKO mice (Figure 3E & F). Taken together, deletion of mTOR attenuated hepatomegaly, liver injury and inflammation but not fibrosis in L-Atg5 KO mice. ER stress seemed not to be a critical factor in the liver pathogenesis of L-Atg5 KO mice.
Loss of mTOR attenuates Atg5-deficiency-induced persistent Nrf2 activation
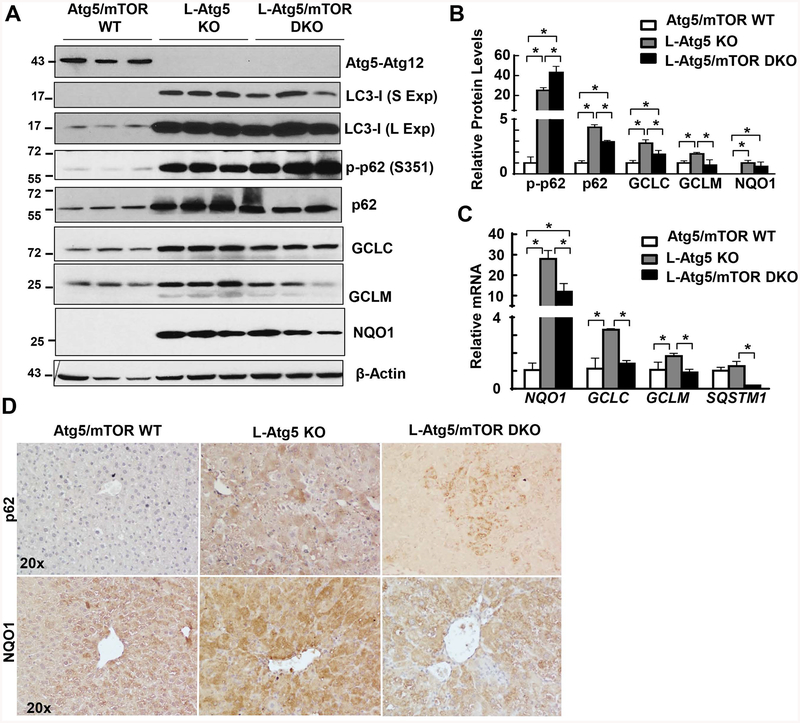
We found markedly increased LC3-I, total p62 and p-p62 protein levels in L-Atg5 KO mouse livers with no detectable Atg5-Atg12 conjugates, confirming impaired autophagy in L-Atg5 KO mouse livers. The protein levels of GCLC, GCLM and NQO1, three target genes of Nrf2, all increased in L-Atg5 KO mouse livers. Deletion of mTOR decreased total p62 without affecting the p-p62 and LC3-I levels. More importantly, deletion of mTOR blunted the expression of GCLC, GCLM and NQO1, both at the protein and mRNA levels in L-Atg5 KO mouse livers (Figure 4A–C). Immunohistochemistry staining analysis confirmed increased hepatic p62 and NQO1 staining in L-Atg5 KO mouse livers, which were attenuated by the deletion of mTOR (Figure 4D). These data suggest that deletion of mTOR attenuates persistent Nrf2 activation in hepatic autophagy defective livers.
Figure 4. Deletion of mTOR attenuates Nrf2 activation in L-Atg5 KO mice.
(A) Total liver lysates of the indicated genotypes were subjected to western blot analysis. (B) Densitometry analysis of (A) (n=3). (C) Quantitative real-time PCR analysis of the expression of hepatic Nrf2-related genes. Total RNAs were prepared from livers of the indicated genotypes at age of 2 months. Data are means± SEM (n=4). * p<0.05 One-Way Anova analysis with Scheffé’s post hoc test. (D) Representative photographs of immunostaining of p62 and NQO1 in mouse livers of Atg5/mTOR WT, L-Atg5 KO and L-Atg5/mTOR DKO at age of 2 months.
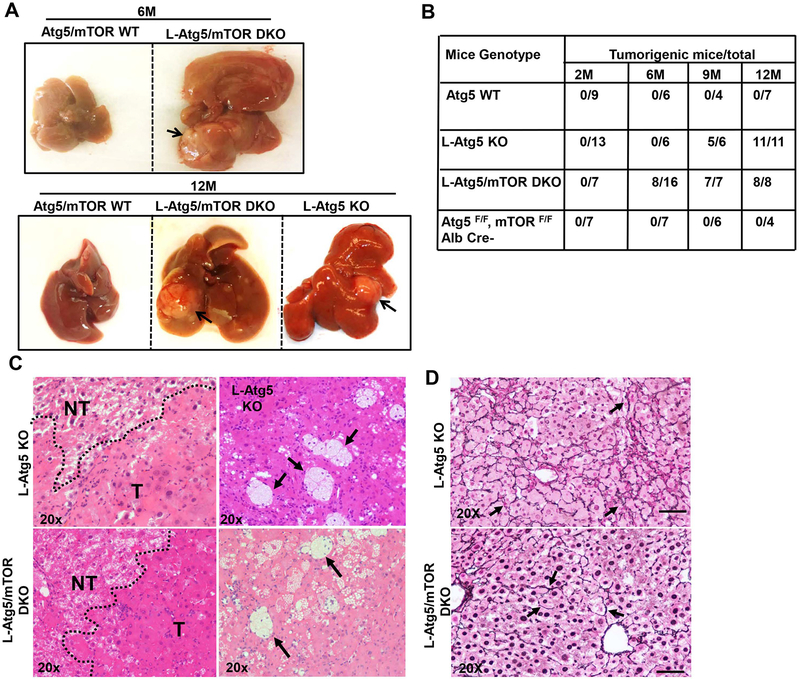
Loss of mTOR promotes early liver tumorigenesis in L-Atg5 KO mice
mTORC1 activation promotes cell growth and proliferation, and is associated with many cancers including HCC (17–20). Surprisingly, deletion of mTOR did not prevent the formation of liver tumors in L-Atg5 KO mice at 9 and 12 month-old. Even more Intriguing was that none of the L-Ag5 KO mice at 6 month-old had developed liver tumors, but eight of sixteen (50%) L-Atg5/mTOR DKO mice had already developed liver tumors (Figure 5A & B). Histological analysis of liver tissues revealed that L-Atg5 KO and L-Atg5/mTOR DKO liver tumors showed similar features and were pathologically diagnosed as inflammatory hepatocellular adenoma as previously reported (27, 28). The tumors were ill-demarcated with benign hepatocytes arranged in regular plates, usually one or two cells in depth. Increased focal ductular reaction was also found in both L-Atg5 KO and L-Atg5/mTOR DKO mouse livers (Supplemental Figure 8). Increased accumulation of foam cells and other inflammatory cells were also observed in various tumors (Figure 5C, arrows), and these tumors also had abundant reticulin staining (Figure 5D, arrows). These phenotypes were distinctively different from the HCC developed in L-TSC1 KO mice in which the tumor cells have a high nuclear: cytoplasmic ratio and were forming pseudoglands within pre-existing liver cell plates, as well as barely detectable reticulin staining (Supplemental Figure 9). Together, the collected data indicate that deletion of mTOR promotes the early development of hepatocellular adenoma in L-Atg5 KO mice.
Figure 5. Loss of mTOR promotes early liver tumorigenesis in L-Atg5 KO mice.
(A) Representative gross anatomy of livers of Atg5/mTOR WT, L-Atg5 KO and L-Atg5/mTOR DKO mice at the age of 6 and 12 months. (B) summary of tumor formation. (C) Histological analysis of mouse livers with tumors of L-Atg5 KO and L-Atg5/mTOR DKO mice at age of 12 months. NT: non-tumor; T: Tumor. Arrow: foam cells/macrophages. (D) Twelve-month old mice of indicated genotypes were sacrificed and liver tissues were processed for reticulin staining and representative images are shown (20 X).
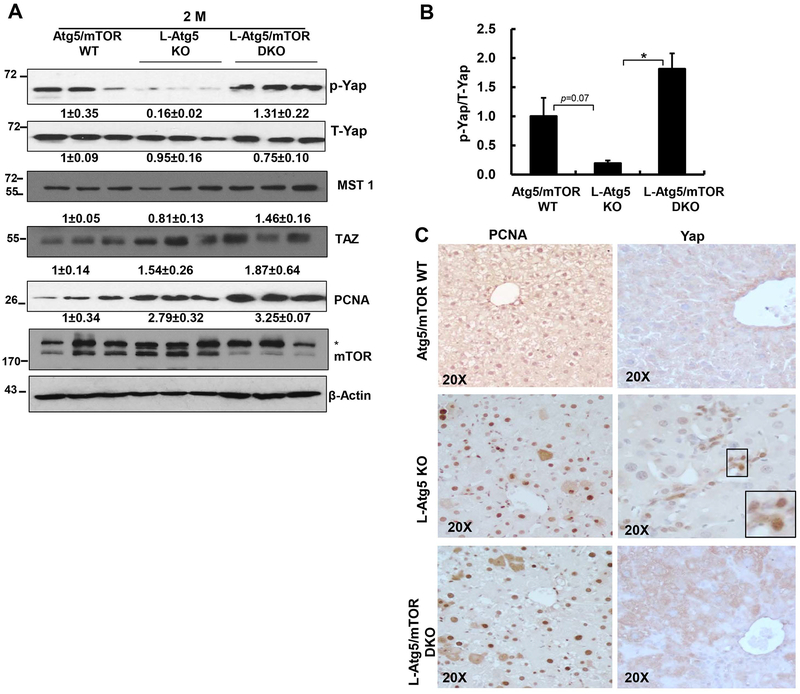
Loss of mTOR attenuates Atg5-deficiency-induced Yap activation but leads to activation of AKT in L-Atg5 KO mice
We found decreased levels of p-Yap and ratio of p-Yap/T-Yap as well as increased nuclear Yap staining but no obvious changes on macrophage stimulating 1 (MST1) and transcriptional coactivator with PDZ-binding motif (TAZ) in 2-month-old L-Atg5 KO mouse livers (Figure 6A–C). This suggests increased hepatic Yap activation in L-Atg5 KO mice, which is consistent with L-Atg7 KO mice (12). L-Atg5/mTOR DKO mouse livers had decreased nuclear Yap staining but increased levels of p-Yap and ratio of p-Yap/T-Yap as well as increased MST1, suggesting Yap inactivation (Figure 6A–C). The levels of hepatic PCNA and the numbers of PCNA positive cells increased in both L-Atg5 KO and L-Atg5/mTOR DKO livers compared with WT mice, but no difference was found between L-Atg5 KO and L-Atg5/mTOR DKO mice (Figure 6A & C). Interestingly, the size of hepatocytes markedly increased in L-Atg5 KO mice, but decreased in L-Atg5/mTOR DKO mice (Supplemental Figure 10A &B), suggesting that the decreased hepatomegaly in L-Atg5/mTOR DKO mice could be due to the decreased hepatocyte hypertrophy.
Figure 6. Loss of mTOR attenuates Atg5-deficiency-induced Yap activation but leads to activation of AKT in L-Atg5 KO mice.
(A) Total liver lysates of the indicated genotypes were subjected to western blot analysis and densitometry analysis. Data are means± SEM (n=3). (B) Ratio of p-Yap/T-Yap. Data are means± SEM (n=3). (C) Representative photographs of PCNA and Yap immunostaining of mouse livers of Atg5/mTOR WT, L-Atg5 KO and L-Atg5/mTOR DKO at age of 2 months.
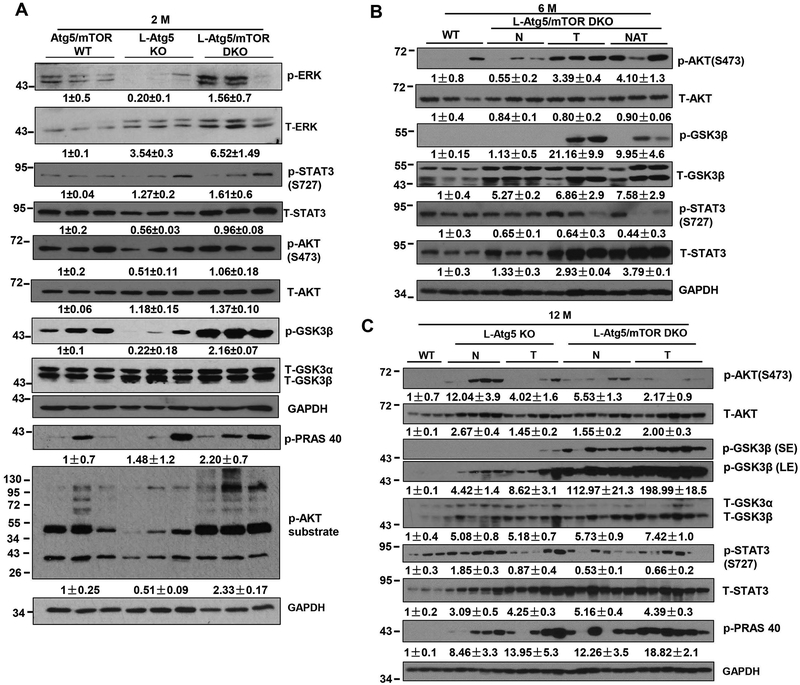
At 2 months of age, the levels of p-ERK and p-STAT3 were found to be elevated in L-Atg5/mTOR DKO livers compared with WT mice. Intriguingly, while the levels of p-AKT (serine 473) remained unchanged, the levels of p-GSK3β, p-PRAS40 and p-AKT substrates increased markedly in L-Atg5/mTOR DKO livers (Figure 7A). IL-6 has been shown to promote activation of oncogenic STAT3 (29), and the hepatic expression levels of IL-6 in L-Atg5/mTOR DKO mice increased more than 3-fold compared with L-Atg5 KO mice (Supplemental Figure 11). At 6 months of age, there were minimal changes in the levels of p-AKT, p-GSK3β and p-STAT3 in these L-Atg5/mTOR DKO mice that had not developed tumors compared with WT livers. However, the levels of total and p-AKT and p-GSK3β dramatically increased in both the tumors and adjacent normal tissues in these L-Atg5/mTOR DKO mice that had developed tumors (Figure 7B). At 12 months of age, all the L-Atg5 KO and L-Atg5/mTOR DKO mice developed spontaneous tumors. We found that the overall levels of p-AKT and p-GSK3β increased in tumors and normal tissues adjacent to tumors in both L-Atg5 KO and L-Atg5/mTOR DKO mice compared with WT mice (Figure 7C). In addition, the levels of total but not the p-STAT3 increased in both the tumors and adjacent normal tissues in the L-Atg5 KO and L-Atg5/mTOR DKO mice of 6 and 12-months of age (Figure 7C). However, it is worthy to point out that there was a heterogeneity nature with the AKT signaling among L-Atg5 KO and L-Atg5/mTOR DKO mouse tumors. Nevertheless, based on the levels of p-GSK3 and p-PRAS40, two substrates of AKT, the levels of AKT activity were much higher in both the tumor and adjacent normal tissues of L-Atg5/mTOR KO mice than in L-Atg5 KO mice. Together, these data collectively suggest that L-Atg5/mTOR DKO mouse livers have generally overall increased AKT activities regardless of their ages compared with WT mice.
Figure 7. Increased AKT signaling in L-Atg5/mTOR DKO mouse livers.
(A) Total liver lysates of the indicated genotypes were subjected to western blot analysis and densitometry analysis. Data are means± SEM (n=3). (B) Total liver lysates of WT mice and normal (N), tumor tissues (T) and normal tissues adjacent to tumors (NAT) of L-Atg5/mTOR DKO mice at the age of 6 months were subjected to western blot analysis. (C) Total liver lysates of WT mice and normal (N) and tumor tissues (T) of L-Atg5/mTOR DKO mice at the age of 12 months were subjected to western blot analysis and densitometry analysis. Data are presented as means± SEM (n=3–6).
We found that the levels of p53 and p-PTEN increased in L-Atg5 KO and L-Atg5/mTOR DKO mouse tumors and adjacent normal liver tissues (Supplemental Figure 12A). Tumors from L-Atg5/mTOR DKO mice also had much higher levels of p27, another tumor suppressor. Increased DNA damage can often lead to increased γ-H2A.X. Interestingly, the hepatic levels of γ-H2A.X and the number of γ-H2A.X positive cells were remarkably higher in L-Atg5 KO mice than in L-Atg5/mTOR DKO mice regardless of tumor or adjacent normal tissues, although the levels of γ-H2A.X were also higher in L-Atg5/mTOR DKO mice compared with WT mice (Supplemental Figure 12A–C). In contrast, tumors from L-Atg5/mTOR DKO mice had lower levels of CD133, a cancer stem cell marker, compared with tumors from L-Atg5 KO mice. These data suggest that both L-Atg5 KO and L-Atg5/mTOR DKO mice have increased tumor suppressor proteins in tumors and adjacent normal liver tissues but L-Atg5/mTOR DKO mice may have decreased hepatic DNA damage and decreased cancer stem cells.
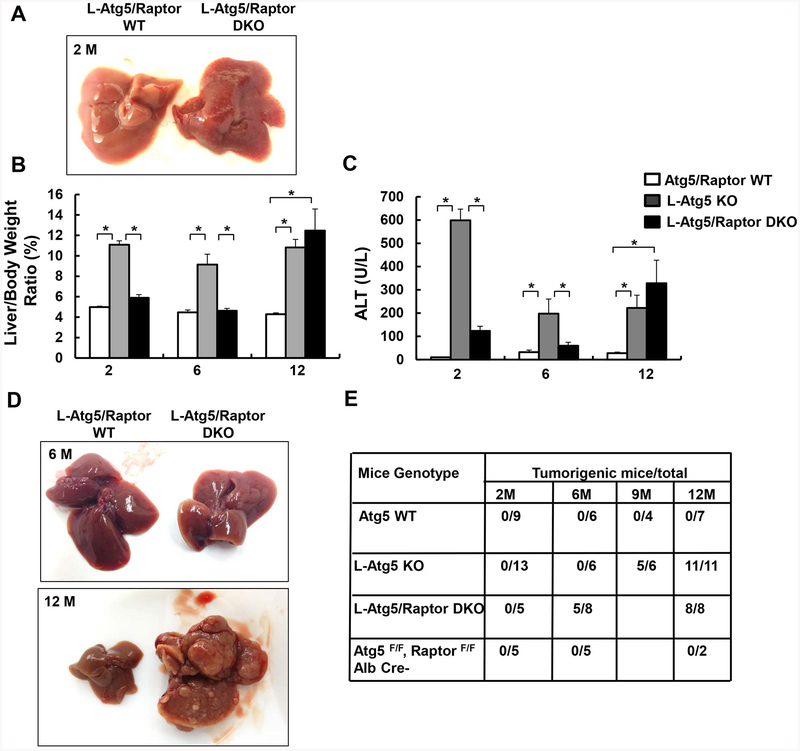
Loss of raptor attenuates hepatomegaly and liver injury in young L-Atg5 KO mice but also promotes early liver tumorigenesis in L-Atg5 KO mice
Similar to the loss of mTOR, loss of Raptor attenuated hepatomegaly and liver injury in L-Atg5 KO mice at age of 2 or 6 months old (Figure 8A–C). The hepatic levels of p62, NQO1, GCLC and GCLM increased in L-Atg5 KO mice, which were all decreased in L-Atg5/Raptor DKO mice (Supplemental Figure 13A), suggesting decreased p62-mediated non-canonical Nrf2 activation in L-Atg5/Raptor DKO mice. Decreased p-Yap in L-Atg5 KO mice was also markedly reversed in L-Atg5/Raptor DKO mice (Supplemental Figure 13A). Furthermore, similar to L-Atg5/mTOR DKO mice, the hepatic levels of p-AKT, p-GSK3β and total STAT3 increased in L-Atg5/Raptor DKO mice (Supplemental Figure 13B). At the age of 6 months old, approximately 60% L-Atg5/Raptor DKO mice developed spontaneous tumors. At the age of 12 months old, all the L-Atg5 KO and L-Atg5/Raptor DKO mice had developed tumors (Figure 8D & E). Histology analysis revealed clear edges between the normal and tumor tissues with increased inflammation (Supplemental Figure 14A&B) and readily detectable foam cells in L-Atg5/Raptor DKO mice (Supplemental Figure 14C, arrow), similar to L-Atg5/mTOR DKO mice (Figure 5C). Increased foam cells may reflect an adaptive immune response to protect the liver damage in these KO mice although their exact roles need to be further elucidated. Together, these results indicate that loss of Raptor attenuates early liver injury but promotes the early tumorigenesis in L-Atg5 KO mice, similar to loss of mTOR in L-Atg5 KO mice.
Figure 8. Loss of raptor attenuates hepatomegaly and liver injury in young L-Atg5 KO mice but also promotes early liver tumorigenesis in L-Atg5 KO mice.
(A) Representative gross anatomy of livers of Atg5/Raptor WT and L-Atg5/Raptor DKO mice at age of 2 months. Mice with different genotypes and age were analyzed for liver/body weight ratio (B), and serum ALT (C). Data are means± SEM (n=3–10). *p<0.05, One-Way Anova analysis with Scheffé’s post hoc test. (D) Representative gross anatomy of livers of Atg5/Raptor WT and L-Atg5/Raptor DKO mice at age of 2 and 12 months. (E) Summary of tumor formation in Atg5 WT, L-Atg5 KO, L-Atg5/Raptor DKO and L-Raptor KO mice.
DISCUSSION
Autophagy plays a critical role in regulating liver homeostasis. Genetic disruption of autophagy in mouse livers, such as the liver-specific deletion of Atg5 or Atg7, leads to hepatomegaly, liver injury, inflammation, fibrosis, and eventual hepatocellular adenoma (8, 10–12). Previous studies have demonstrated that the p62-keap1-Nrf2 signaling pathway acts as the central player that contributes to the phenotypes of the mice with defective hepatic autophagy (10, 13). Both L-Atg5 or L-Atg7 KO mice have persistent Nrf2 activation and deletion of p62 attenuates but deletion of Nrf2 completely eliminates hepatomegaly, liver injury and liver tumorigenesis (8, 10, 11). Because p62 can activate Nrf2 by sequestrating keap1, the improvement on the liver pathology by the deletion of p62 is most likely due to the decreased Nrf2 activation in the hepatic autophagy deficient mice. Atg7/p62 DKO mice still have some basal Nrf2 activation, which probably explains why deletion of p62 only partially inhibits liver injury and tumorigenesis in L-Atg7 KO mice (8). These early observations clearly support the notion that persistent Nrf2 activation contributes to the phenotypes in L-Atg5 and Atg7 KO mice. Nrf2 is a transcription factor and Nrf2 activation is generally beneficial for cells against oxidative and electrophilic stresses by upregulating the expression of cellular antioxidant genes, drug metabolism enzymes and transporters (14, 15). L-Keap1 KO mice that have persistent Nrf2 activation have little liver injury and are resistant to drug-induced liver injury (30). Therefore, persistent activation of Nrf2 alone is not sufficient to cause liver pathogenesis but the combination of Nrf2 activation and autophagy deficiency contributes to liver injury and tumorigenesis. It is likely that persistent Nrf2 activation causes continuous gene transcription and protein translation resulting in the overwhelming accumulation of proteins in the absence of autophagy, which ultimately leads to hepatic proteotoxicity and liver injury. In contrast, proteins are timely removed by autophagy in keap1 KO mice resulting in no obvious liver dysfunction despite the persistent Nrf2 activation. The Hippo/Yap signaling pathway is a key regulator of cell proliferation, differentiation, and organ size. Liver injury in L-Atg7 KO mice is improved by deletion of Yap, which is a co-activator for transcription factor TEAD that may also increase overall protein production similar to Nrf2 activation (12). Therefore, it is likely that the liver pathology in autophagy deficient mouse livers could be improved if we can blunt the protein translation or decrease the overall protein production.
mTORC1 is a main nutrient sensing kinase that regulates the anabolic process and activation of mTORC1 increases synthesis of proteins and lipids as well as cellular building blocks for cell proliferation (17). Deletion of mTOR decreased hepatic levels of ubiquitinated proteins and inhibited liver injury and hepatomegaly in young-L-Atg5 KO mice. Interestingly, deletion of mTOR partially decreased Nrf2 activation in -LAtg5 KO mouse livers, supporting the notion that overall increased protein production in L-Atg5 KO mice may indeed contribute to the liver pathogenesis of L-Atg5 KO mice. Increased ubiquitinated proteins often lead to ER stress and subsequent UPR to relieve ER stress. Surprisingly, most UPR markers that we assessed at both the mRNA and protein levels did not increase except the levels of phosphorylated eIF2 in L-Atg5 KO mouse livers, suggesting that ER stress may not be a major player in liver injury of L-Atg5 KO mice. However, increased hepatic expression of TNF-α and other proinflammatory cytokines as well as increased infiltration of neutrophils and macrophages in L-Atg5 KO mouse livers are all inhibited by deletion of mTOR. Increased cytokines in particular TNFα may activate death-receptor-mediated apoptotic pathways resulting in hepatocyte apoptosis as reported previously in Atg7 KO mouse livers (31). L-Atg5/mTOR DKO mice have increased hepatic cIAP2 but have less Bax protein, caspase-3 activation and TUNEL positive cells, suggesting that deletion of mTOR attenuates apoptosis in L-Atg5 KO mouse livers.
Perhaps the most intriguing findings were that L-Atg5/mTOR DKO mice improved hepatomegaly and liver injury in the young mice (age of 2 months), but this benefit was gradually lost at 6 months of age and older. Moreover, while activation of mTOR has been linked to cancer and at least 50% HCC are associated with mTOR activation, we found that deletion of mTOR did not inhibit tumorigenesis but instead tumors occurred even earlier in L-Atg5/mTOR DKO mice. The protection against early hepatomegaly and liver injury and the development of early tumors is likely due to the mTORC1 because L-Atg5/Raptor DKO mice shared almost the same phenotypes as L-Atg5/mTOR DKO mice. While L-TSC1 KO mice have hyperactivation of mTOR and develop spontaneous liver tumors (32), L-Raptor KO mice that have decreased mTOR also have increased liver tumorigenesis when mice were challenged with the hepatic carcinogen Diethylnitrosamine (DEN) or DEN plus a high fat diet (29). These observations together with our findings in the present study suggest that both hyper- and hypo-activation of mTOR may yield unwanted harmful effects to the liver resulting in the development of liver tumors.
As discussed earlier, recent studies have revealed important roles of Yap and HMGB1 in liver pathogenesis of autophagy-deficient liver. However, deletion of HMGB1 only attenuated the ductular reaction and delayed the tumorigenesis but had no improvement on hepatomegaly, liver injury and fibrosis in L-Atg7 KO mice (11). L-Atg7/Yap DKO mice showed a better improvement on hepatomegaly and tumorigenesis but these mice still develop liver tumors when they were aged (12). The nuclear receptor CAR is also dispensable for hepatomegaly and liver tumorigenesis in L-Atg5 KO mice as we demonstrated in the present study. In great contrast, deletion of Nrf2 completely abolished hepatomegaly, liver injury, inflammation and liver tumorigenesis in either L-Atg5 or L-Atg7 KO mice (10). Interestingly, deletion of Nrf2 also attenuated HMGB1 release, Yap and mTOR activation (10–12), which places Nrf2 activation as the upstream regulator of HMGB1 nuclear release, Yap and mTOR activation. It is highly likely that the activation of anti-oxidant response due to the persistent activation of Nrf2 plays a critical role in driving the progression of tumorigenesis by favoring the survival of cancer cells in the later stage of autophagy-deficient livers. However, in addition to Nrf2 activation, the activation of the oncogenic AKT may also play a role in the early development of tumors in L-Atg5/mTOR DKO and L-Atg5/Raptor DKO mice. It should be noted that increased expression of tumor suppressor proteins including p53, PTEN and p27 were found in L-Atg5/mTOR DKO mouse tumors, which are similar to tumors in L-Atg5 KO mice that have been reported (33). These results suggest that although AKT may promote early tumor cell growth, autophagy is required for the tumor progression. The liver phenotypes of mice with genetic deletion of Atg5, mTOR, Raptor and CAR are summarized in Supplemental Table 1 and possible molecular cellular events are illustrated in Supplemental Figure 15.
In conclusion, over-production of proteins due to Nrf2 and mTOR activation in autophagy-deficient mouse livers may contribute to hepatomegaly, inflammation and liver tumorigenesis. Blocking mTOR-mediated protein synthesis attenuates hepatomegaly and liver injury in young mice but promotes early liver tumorigenesis. These findings not only imply that the balance of mTOR activity is critical to maintain the normal physiology of the liver but also raise concerns on the clinical chronic use of mTOR inhibitors for metabolic liver diseases and HCC.
Supplementary Material
ACKNOWLDGEMENTS
We thank Ms. Barbara Fegley (KUMC Electron Microscopy Research Laboratory) for her excellent technical assistance. We thank Dr. Noboru Mizushima (University of Tokyo) for providing Atg5 F/F and Tara McKeen for critical reading of this manuscript. This study was supported in part by the National Institute of Health (NIH) funds R01 AA020518, R01 DK102142, U01 AA024733, R21 AA026904 and P20GM103549 & P30GM118247.
List of Abbreviations:
- Alb
Albumin
- ALT
Alanine aminotransferase
- α-SMA
α-smooth muscle actin
- CAR
Constitutive androstane receptor
- CK 19
cytokeratin 19
- DKO
Double knockout
- EM
Electron microscopy
- FLIP
FLICE-like protein (FLIP) protein
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- HCC
Hepatocellular carcinoma
- Keap1
Kelch-like ECH-associated protein 1
- KO
Knockout
- LC3
Microtubule light chain 3
- LD
Lipid droplets
- Ly6B
lymphocyte antigen B superfamily
- mTOR
mechanistic target of rapamycin
- NQO1
NAD(P)H quinone oxidoreductase
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PCNA
Proliferating cell nuclear antigen
- PTEN
phosphatase and tensin homolog
- ROS
Reactive oxygen species
- TGF-β1
transforming growth factor beta 1
- TNF-α
Tumor necrosis factor alpha
- TSC1
tuberous sclerosis complex
- UPR
unfolded protein response
- WT
wild type
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Xu J Trends in Liver Cancer Mortality Among Adults Aged 25 and Over in the United States, 2000–2016. NCHS Data Brief 2018:1–8. [PubMed] [Google Scholar]
- 3.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012;56:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425–456. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 6.Czaja MJ, Ding WX, Donohue TM Jr., Friedman SL, Kim JS, Komatsu M, Lemasters JJ, et al. Functions of autophagy in normal and diseased liver. Autophagy 2013;9:1131–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology 2008;47:1773–1785. [DOI] [PubMed] [Google Scholar]
- 8.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev 2011;25:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005;169:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, Jaeschke H, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 2014;61:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khambu B, Huda N, Chen XY, Antoine DJ, Li Y, Dai GL, Kohler UA, et al. HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. Journal of Clinical Investigation 2018;128:2419–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YA, Noon LA, Akat KM, Ybanez MD, Lee TF, Berres ML, Fujiwara N, et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nature Communications 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010;12:213–223. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011;16:123–140. [DOI] [PubMed] [Google Scholar]
- 15.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res 2008;58:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, et al. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A 2012;109:13561–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol 2014;60:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003;97:1929–1940. [DOI] [PubMed] [Google Scholar]
- 20.Ho DWH, Chan LK, Chiu YT, Xu IMJ, Poon RTP, Cheung TT, Tang CN, et al. TSC1/2 mutations define a molecular subset of HCC with aggressive behaviour and treatment implication. Gut 2017;66:1496–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenerson HL, Yeh MM, Kazami M, Jiang X, Riehle KJ, McIntyre RL, Park JO, et al. Akt and mTORC1 have different roles during liver tumorigenesis in mice. Gastroenterology 2013;144:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 2007;13:3109–3114. [DOI] [PubMed] [Google Scholar]
- 23.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med 2007;13:433–442. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Ni HM, Guo F, Ding Y, Shi YH, Lahiri P, Frohlich LF, et al. Sequestosome 1/p62 Protein Is Associated with Autophagic Removal of Excess Hepatic Endoplasmic Reticulum in Mice. J Biol Chem 2016;291:18663–18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 2005;19:1646–1653. [DOI] [PubMed] [Google Scholar]
- 26.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 2011;55:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 2011;193:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA. A role for autophagy during hepatic stellate cell activation. J Hepatol 2011;55:1353–1360. [DOI] [PubMed] [Google Scholar]
- 29.Umemura A, Park EJ, Taniguchi K, Lee JH, Shalapour S, Valasek MA, Aghajan M, et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab 2014;20:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochemical and Biophysical Research Communications 2006;339:79–88. [DOI] [PubMed] [Google Scholar]
- 31.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ 2013;20:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, Bronson RT, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal 2012;5:ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Y, Kuo CF, Sir D, Wang L, Govindarajan S, Petrovic LM, Ou JH. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis. Cell Death Differ 2015;22:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.