Abstract
Background
Gastrointestinal (GI) motility dysfunction is the most common non-motor symptom of Parkinson’s disease (PD). Studies have indicated that GI motility functions are impaired before the onset of PD.
Aims
To investigate the underlying mechanism of PD-induced GI dysmotility in MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine)-induced animal model.
Methods
C57BL/6 mice were administered with or without a selective dopamine neurotoxin, MPTP, to induce parkinsonian symptoms. In addition to in vivo studies, in vitro experiments were also conducted in colon specimens using l-methyl-4-phenylpyridinium (MPP+), a metabolic product of MPTP. Gastric emptying, colon motility, nitrergic relaxation, and western blot experiments were performed as reported.
Results
MPTP-induced PD mice showed decreased expression of nuclear factor erythroid 2-related factor (Nrf2) and its target phase II genes in gastric and colon neuromuscular tissues. Decreased levels of tetrahydrobiopterin (BH4, a critical cofactor for nNOS dimerization) associated with uncoupling of nNOS in gastric and colon tissues exposed to MPTP. Impaired enteric nitrergic system led to delayed gastric emptying and slower colonic motility compared to the control mice. In vitro results in colon specimens confirm that activation of Nrf2 restored MPP+-induced suppression of alpha-synuclein, tyrosine hydroxylase (TH), Nrf2, and heme oxygenase-1. In vitro exposure to L-NAME [N(w)-nitro-l-arginine methyl ester], a NOS synthase inhibitor, reduced protein expression of TH in colon tissue homogenates.
Conclusions
Loss of Nrf2/BH4/nNOS expression in PD impairs antioxidant gene expression, which deregulates NO synthesis, thereby contributing to the development of GI dysmotility and constipation. Nitric oxide appears to be important to maintain dopamine synthesis in the colon.
Keywords: Gastrointestinal motility, Parkinson’s disease, Nrf2, Antioxidants, Tetrahydrobiopterin, Nitric oxide synthase, Tyrosine hydroxylase, α-Synuclein
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor impairment that occurs due to loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), formation of Lewy bodies (LB), oxidative stress, and chronic low-grade infiammation, followed by non-motor symptoms. Recent studies have shown that during the initial stages of PD, the extrinsic and intrinsic innervation of the gastrointestinal (GI) tract, the dorsal motor nucleus of the vagus (DMV), and the enteric nervous system (ENS) were first affected before substantia nigra [1]. Thus, non-motor symptoms, the most familiar of which is GI dysfunction, occur earlier than the motor symptoms of PD [2, 3]. GI dysfunction characterized by faster or easy satiety, weight loss from delayed gastric emptying (gastroparesis), and impaired colonic transit contributes directly to the pathogenesis of PD and complicates the disease’s clinical management [4, 5].
GI dysfunction is observed in almost all PD patients during progression of the illness and impairs gastric or colonic motility [6]. GI motility is controlled by the complex neural network of intrinsic enteric nervous system (ENS). ENS consists of a deep myenteric and superficial submucosal plexus acting as the “second brain,” providing a link between the stomach and the brain through the vagus nerve [7, 8]. It has been reported that the aggregation of the protein, namely alpha-synuclein (α-synuclein or α-S) found in LB and Lewy neurites (LN), spreads from neuron to neuron within the gut wall, reaches the vagal projections, and enters the central nervous system, thereby leading to manifestation of motor symptoms [1, 9]. Previous studies in GI biopsies of PD patients have shown that aggregated α-S is accumulated prior to the onset of motor symptoms. Furthermore, these studies demonstrate that α-S may spread also from the GI system to the brain [10–12].
Results from our laboratory and others have shown that GI motility dysfunction occurs due to diminished neuronal nitric oxide synthase (nNOS) activity, which catalyzes the formation of nitric oxide (NO), initiating smooth muscle relaxation [13, 14]. In turn, nNOS activity is regulated by tetrahydrobiopterin (BH4), a cofactor for nNOS dimerization and enzyme activity that couples electron flow to NO generation [15]. BH4 is synthesized from guanosine 5′-triphosphate (GTP) de novo by the rate-limiting enzyme GTP cyclohydrolase 1 (GCH-1) or from a salvage pathway via dihydrofolate reductase (DHFR) using arginine as a substrate [16]. Although previous studies have demonstrated that loss of dopaminergic neurons and/or impaired nitrergic relaxation could lead to GI dysfunction in MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine)-induced PD rodent models, the mechanism of nitrergic-mediated GI motility has not been fully investigated [4].
NOS uncoupling results in superoxide generation. Oxidative stress plays an important role in PD patients who exhibit increased levels of oxidized lipids, decreased levels of reduced glutathione (GSH), mutations of proteins, and DNA [17, 18]. Mechanisms to handle misfolded proteins more effectively within the cell include modulating endogenous cellular stress response pathways that upregulate the protein degradation machinery or sequester the misfolded proteins into inclusion bodies [19]. Nuclear factor erythroid 2-related factor (Nrf2) coordinates a whole program of gene expression that counters stress at multiple levels [20]. In response to electrophilic and reactive oxygen species-producing agents, Nrf2 binds to the antioxidant responsive element and regulates the expression of detoxification genes and oxidative stress-inducible enzymes [21–23]. However, a growing body of evidence indicates that Nrf2 regulates a much broader gene expression response, including genes involved in protein homeostasis, such as chaperones and proteasome subunits, and thus, it is considered as a potential target to deal with damaged proteins [24–26]. However, the protective role of Nrf2 in enteric neurons is yet to be determined.
Due to the lack of proper treatment available for GI dysmotility among PD patients, it is essential to discover new therapies that allow us to improve not only motor symptoms, but also non-motor symptoms, including cognitive impairment and dysfunction of the autonomic nervous system, and thereby modulate disease progression. PD may be initiated and/or precipitated by environmental or endogenous toxins by a mechanism similar to that of MPTP in genetically predisposed individuals [27]. The MPTP model has been widely accepted as a PD model that shows parkinsonogenic effects [28, 29]. In addition, l-methyl-4-phenylpyridinium (MPP+), the active metabolite of MPTP, is shown to deplete dopaminergic neurons and induce oxidative stress [30]. In addition, a previous study from our group has shown that administration of MPTP to adult C57BL/J6 either short term or over several days leads to destruction of substantia nigra, dopaminergic neurons, and depletion of striatal dopamine [31]. The above studies suggest that exposure of the brain to toxic environmental agents in adult life may be an important causative factor for the development of PD. Hence, the current study aimed to evaluate the underlying mechanisms involved in MPTP-induced GI dysmotility in vivo. In addition, we also have investigated whether in vitro activation of Nrf2 restored MPP+-induced suppression of PD and antioxidant markers in the colon.
Materials and Methods
Experimental Design
Animals
C57BL/6J male mice (at least 10 weeks old) were obtained from Jackson Laboratories (The Jackson Laboratory, Bar Harbor, ME). The animals were maintained in the Meharry Medical College (MMC, Nashville, TN) Animal Care Facility under controlled temperature, humidity, and light–dark cycle (12:12 h), with free access to a purified diet (Purina LabDiet, St. Louis, MO) and water. The Institutional Animal Care and Use Committee at MMC approved all experiments in this study, in accordance with recommendations of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Mice were divided into two groups: The control group (n = 6) received PBS (vehicle) only and the PD-induced group (n = 6) received MPTP. To develop the MPTP-PD model, the adult mice were injected with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, Sigma Chemical, St. Louis, MO), 30 mg/kg body weight; intraperitoneally/day/7 days) [31]. Animals were euthanized 7 days after last vehicle or MPTP treatment. Our previous publications using this model and time regimen for MPTP treatment have been shown to develop parkinsonism [31].
Solid Gastric Emptying (GE)
Solid GE studies were performed according to Gangula et al. [32]. Briefly, after fasting overnight (providing water), known amounts of food were fed to the animals for 3 h. At the end of 3 h, the remaining food was weighed to establish the amount of food intake. Animals were fasted for 2 h without food and water and then killed. Gastric tissue was collected, and the weight of the whole stomach and the empty stomach weight were noted after removing the food contents. The rate of GE was calculated according to the following equation: GE (% in 2 h) = (1 − gastric content/food intake) × 100.
Colonic Transit Time
Colon motility experiments were performed using the bead expulsion method according to Anitha et al. [33]. A 3-mm glass bead was placed 2 cm proximal to the anal opening using a plastic Pasteur pipette lightly lubricated with lubricating jelly. Distal colonic transit time was assessed by measuring the amount of time between bead placement and expulsion of the bead. The bead expulsion test was performed in the last week of the study.
Gastric and Colon Organ Bath Studies
Electric field stimulation (EFS)-induced NANC relaxation was studied in circular gastric and colon neuromuscular strips [34]. Gastric muscle strips were obtained from the antrum region of the stomach. The colon was dissected into three parts—proximal, middle, and distal—and was used for organ bath studies. Strips were exposed to atropine, phentolamine, and propranolol (10 μmol, Sigma-Aldrich, St. Louis, MO) in bath solution for 1 h to block cholinergic and adrenergic responses. 5-Hydroxytryptamine (100 μmol, Sigma-Aldrich, St. Louis, MO) pre-contracted strips were exposed to EFS (90 V, 2 Hz, 1-ms pulse for the duration of 1 min) to elicit NANC relaxation. A relaxation response elicited by low-frequency (2 Hz) stimulus under NANC conditions, as used in this study, was demonstrated as predominantly nitrergic (nNOS) in origin, and this was further confirmed using the NOS inhibitor L-NAME (100 μmol, Sigma-Aldrich, St. Louis, MO) [35]. At the end of the experiment, each muscle strip was blotted dry with filter paper and weighed. Comparisons between groups were made by measuring the area under the curve (AUC/mg of tissue) of the EFS-induced relaxation (AUCR) for 1 min and the baseline for 1 min (AUCB), according to the formula “(AUCR − AUCB)/weight of tissue (mg) = AUC/mg of tissue.”
nNOSα Dimerization in Gastric and Colon Neuromuscular Tissue
Gastric and colon neuromuscular tissue specimens were homogenized using radio-immunoprecipitation assay buffer as reported earlier [36]. Levels of the nNOSα monomer and dimer were quantified by western blot (WB) via LT-PAGE in gastric antrum homogenates as described previously [16]. LT-SDS (sodium dodecyl sulfate)-PAGE was performed on ice. The LT process was used to identify nNOS dimers and monomers in the native state, as LT is known to prevent monomerization of nNOS dimers. For the LT process, 30 μg of protein in the standard Laemmli buffer at 4 °C was used for SDS-PAGE. The mixture was incubated at 0 °C for 30 min before LT loading onto a 6% gel. All gels and buffers were pre-equilibrated to 4 °C prior to electrophoresis, and the buffer tank was placed in an ice bath during electrophoresis to maintain the gel temperature below 15 °C. A polyclonal antibody specific to nNOSα (ab229785, 1: 500, Abcam, Cambridge, MA) and anti-rabbit immunoglobulin G (IgG) conjugated with horseradish peroxidase (31,430, 1:10,000, Thermo Fisher Scientific, Waltham, MA) were used as the primary and secondary antibodies, respectively.
WB Analysis
Tissue lysates containing 30 μg proteins were separated by SDS-PAGE, and the proteins were transferred to a nitrocellulose membrane. The membrane was immunoblotted with polyclonal Nrf2 (sc13032, 1:1000), HO-1 (sc1369601:1000), glutamate–cysteine ligase modifier subunit (GCSm, sc22754, 1:1000), GCH-1 (sc376483, 1:1000), and DHFR (sc33184, 1:1000) as primary antibodies, which were purchased from Santa Cruz (Santa Cruz Biotechnology, Dallas, TX), and anti-rabbit IgG conjugated with horseradish peroxidase (31,430, 1:10,000, Thermo Fisher Scientific, Waltham, MA) as a secondary antibody. Primary nNOSα (1:500, ab229785) antibody, α-synuclein (α-S, ab1903, 1:1000), and tyrosine hydroxylase (TH, ab112, 1:200) were purchased from Abcam (Abcam, Cambridge, MA). P-Glycogen synthase kinase-3beta (p-GSK-3β, 9331s, 1:1000) was from Cell Signaling (Cell Signaling Technology, Danvers, MA). Binding of antibodies to the blots was detected using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ) following the manufacturer’s instructions. Stripped blots were re-probed with β-actin-specific polyclonal antibodies (sc8432, 1:1000, Santa Cruz Biotechnology, Dallas, TX) to enable normalization of signals between samples. Band intensities were analyzed using Bio-Rad Gel Doc (Bio-Rad, Hercules, CA).
Measurement of Gastric Biopterin Levels
Biopterin levels were determined from gastric tissues obtained from controls and PD-induced mice by high-performance liquid chromatography (HPLC) using electrochemical and fluorescent detection methods as described [37]. Following homogenization and centrifugation of the gastric tissues (15 min at 13,000×g at 4 °C), the samples were transferred to new, cooled microtubes and precipitated with an equal volume of a solution of cold phosphoric acid (1 M, Sigma Chemical, St. Louis, MO), trichloroacetic acid (2 M, Sigma Chemical, St. Louis, MO), and dithioerythritol (1 mM, Sigma Chemical, St. Louis, MO). The samples were vigorously mixed and centrifuged again for 15 min at 13,000×g at 4 °C. The supernatants were injected onto an isocratic HPLC system and quantified using sequential electrochemical (Coulochem III, ESA Inc., Mundelein, IL) and fluorescence (Jasco, Easton, MD) detection. HPLC separation was performed using a 250-mm ACE C-18 column (Hichrom, Berkshire, UK) and mobile phase comprised of sodium acetate (50 mM), citric acid (5 mM), EDTA (48 μM), and dithioerythritol (160 μM) (pH 5.2) (all ultrapure electrochemical HPLC grade, Sigma Chemical, St. Louis, MO) at a flow rate of 1.3 mL/min. Background currents of + 500 μA and − 50 μA were used for the detection of BH4 on electrochemical cells E1 and E2, respectively. Biopterin and BH2 were measured using a Jasco FP2020 fluorescence detector. Quantification of BH4, BH2, and biopterin was done by comparison with authentic external standards and normalized to sample protein content [37].
In Vitro Nitrite Measurement in Gastric and Colon Specimens
In vitro nitrite experiments were performed as described previously by Gangula et al. [16]. Briefly, animals from both groups were killed by CO2 asphyxiation, and the whole dissected stomach was transferred to chilled oxygenated Krebs bicarbonate solution of the following composition (in mmol): 118.0 NaCl; 4.7 KCl; 25.0 NaHCO3; 1.5 CaCl2; 1.2 MgSO4; 1.2 KH2PO4; and 11.5 glucose (pH 7.4). All these chemicals were purchased from Sigma Chemical (St. Louis, MO). Gastric and colon neuromuscular tissues were harvested and cut into mucosa-free strips and were cultured for 24 h (37 °C, 5% CO2 in 500 μl of phenol red-free DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with NB27 (2%, Thermo Fisher Scientific, Waltham, MA) and antibiotics (1%, Thermo Fisher Scientific, Waltham, MA). After incubation, DMEM (500 μl) was collected and stored at − 80 °C for analysis of nitrite released in medium during incubation period. Nitrite released in the medium was analyzed as total nitrite (metabolic byproduct of NO) according to the manufacturer protocol supplied with a commercially available kit (EMD Chemicals, Gibbstown, NJ).
In Vitro Organ Culture
Colon neuromuscular strips were organ cultured with MPP+ with or without Nrf2 activators such as CNM (100 μM, Sigma Chemical, St. Louis, MO), CUR (100 μM, Sigma Chemical, St. Louis, MO), and GSK3β inhibitor (SB216763, 30 μM, Tocris, Minneapolis, MN) at 37 °C and 5% CO2 in a humidified chamber [38]. In a separate set of experiment, colon strips were cultured with NOS inhibitor L-NAME (100 μM, Sigma-Aldrich, St. Louis, MO). Full-thickness colon tissues were maintained in DMEM/F12 (Gibco, Waltham, MA) medium supplemented with antibiotics (penicillin/streptomycin, Gibco, Waltham, MA) for 48 h, respectively. WB methods were used to determine changes in targeted proteins.
Statistical Analysis
Data were presented as the mean ± standard error (SE). Statistical comparisons between groups were made by the Student’s t test or Tukey’s test after one-way analysis of variance using the GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). A p-value of less than 0.05 was considered statistically significant.
Results
Our previous study has shown that treatment with MPTP for 7 days produces parkinsonism in a mouse model [31]. The current study focused on whether MPTP-induced PD alters GI motility, and if so, investigated the possible mechanisms involved.
PD Induction Results in Delayed Gastric Emptying (GE) and Reduced Intestinal Transit Time
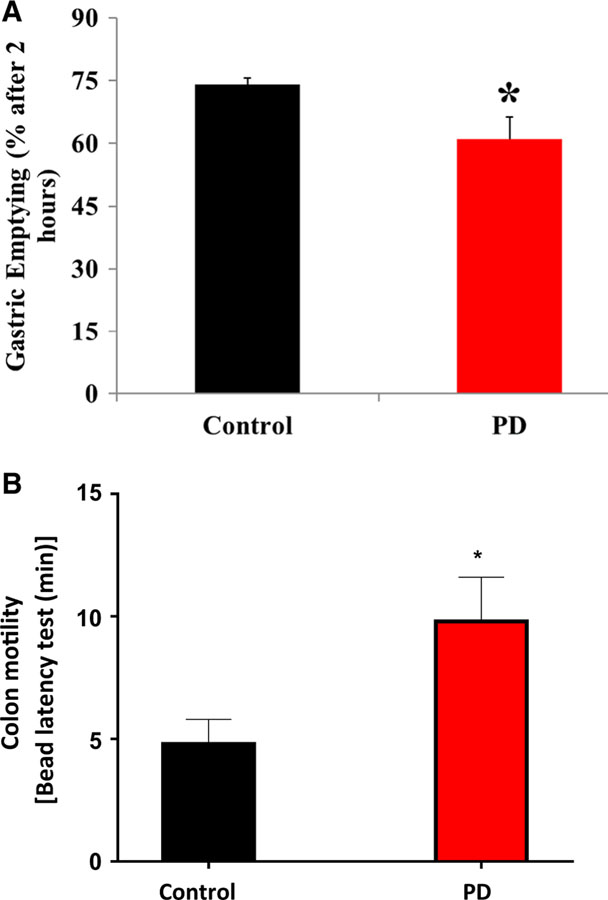
The data depicted in Fig. 1a show that solid GE was significantly (p < 0.05) reduced in PD-induced mice compared with the control group. Colonic motility, as measured by the bead latency test, was also reduced (p < 0.05) in PD-induced mice, where time to expel the bead was longer than the time in the control group (Fig. 1b).
Fig. 1.
Effect of MPTP-induced PD on solid GE and colon motility. a PD-induced mice showed delayed solid GE compared with control group and b bead expulsion time was longer in the PD-induced mice showing colon motility dysfunction. The values are mean ± SE (n = 4) animals. Statistical significance was determined by Student’s t test. *p < 0.05 compared with control group animals. PD Parkinson’s disease
Impairment of Gastric and Colon Nitrergic Relaxation in PD-Induced Mice
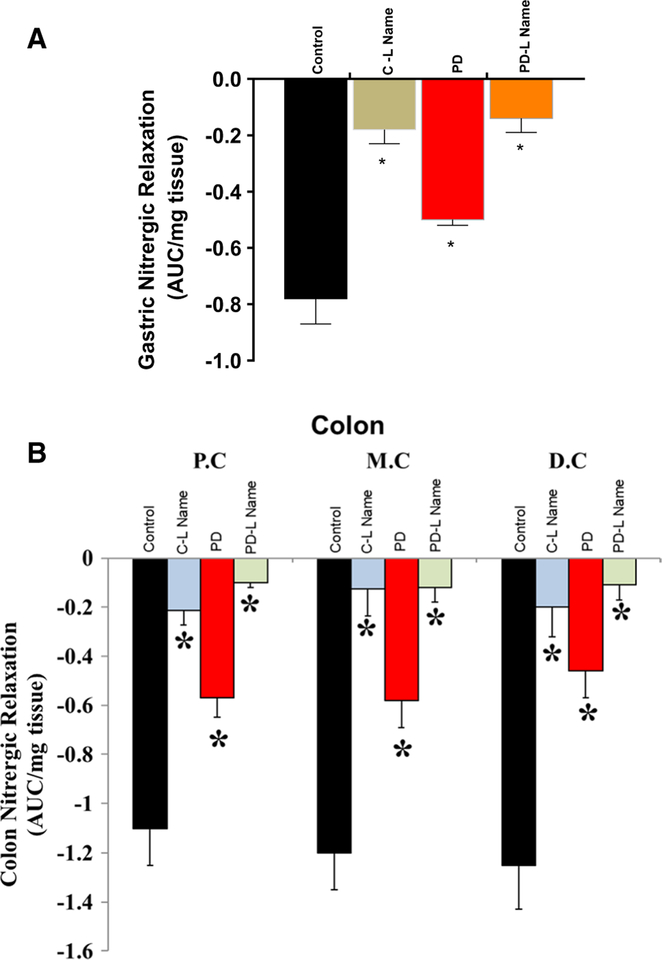
To demonstrate whether delayed GE and reduced colon motility are due to altered nitrergic relaxation, we performed a series of in vitro organ bath experiments to assess electric field stimulation-induced non-adrenergic non-cholinergic (NANC) relaxation at 2 Hz in gastric and colon tissues (proximal, middle, and distal) obtained from control, and PD-induced mice. As shown in Fig. 2, the gastric tissue (a) and colon tissue (b) nitrergic relaxation was reduced significantly (p < 0.05) in the PD-induced mice.
Fig. 2.
Effect of MPTP on nitrergic relaxation at 2 Hz in gastric and colon tissues. Nitrergic relaxation was reduced in PD-induced mice when compared with control group animals. a Nitrergic relaxation in gastric antrum tissues, b intestinal nitrergic relaxation. Values are mean ± SE (n = 4). Statistical significance was determined by Student’s t test. *p < 0.05 compared with wild-type animals. C Control, Cntrl control, PD Parkinson’s disease, P.C proximal colon, M.C mid colon, D.C distal colon
PD Induction Decreases Nrf2 and Phase II Enzyme Protein Levels
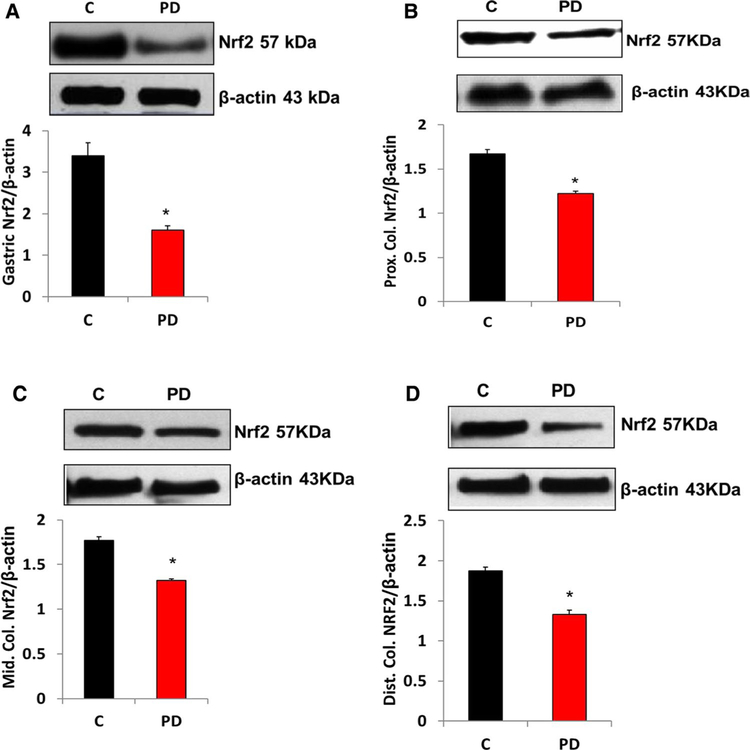
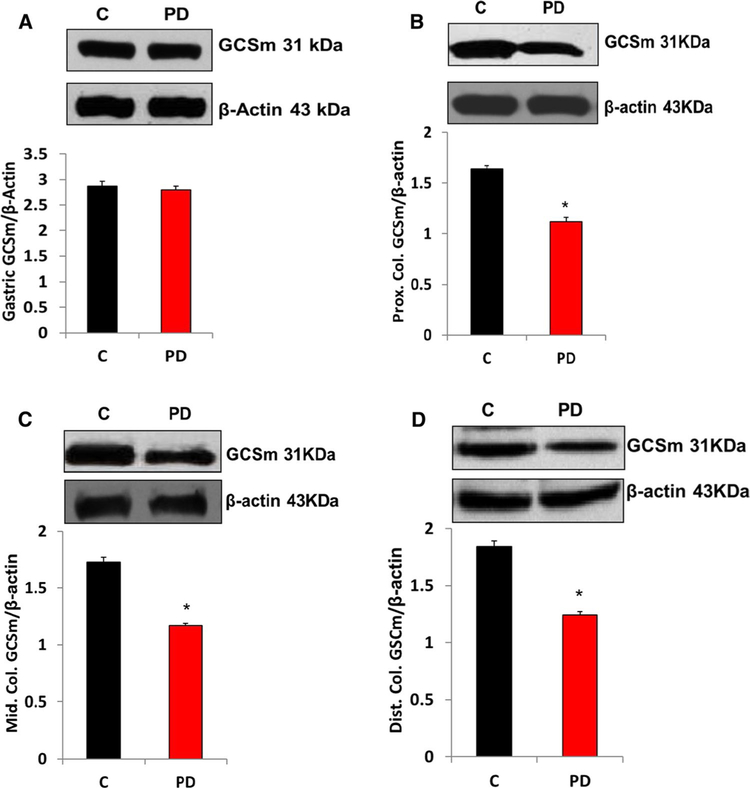
The protein levels of Nrf2 in gastric (Fig. 3a) and all regions of colon (Figs. 3b–d) neuromuscular tissue were significantly (p < 0.05) decreased following PD induction. As shown in Fig. 4, loss of Nrf2 protein expression resulted in the suppression of oxidant detoxifying systems (GcSm) in gastric (Fig. 4a) and all regions of intestinal (Fig. 4b–d) neuromuscular strips.
Fig. 3.
Effect of MPTP-induced PD on Nrf2 protein expression in gastric and colon tissues. Representative immunoblot and densitometric analysis data for Nrf2 protein. a A significant decrease in Nrf2 was noticed in PD-induced mice compared with the control group. b–d A significant reduction in Nrf2 protein expression was noticed in all parts of colon in MPTP-treated mice. *p < 0.05 compared with control group animals (n = 4). C Control, PD Parkinson’s disease
Fig. 4.
Changes in protein expression of GCSm in MPTP-induced PD mice gastric and colon specimens. A significant decrease in GCSm was noticed in PD-induced mice compared with the control group. a Representative immunoblot and densitometric analysis in gastric antrum tissue, b representative immunoblot and densitometric analysis in proximal colon,c representative immunoblot and densitometric analysis in middle colon, and d representative immunoblot and densitometric analysis in distal colon. *p < 0.05 compared with control group animals (n = 4). C Control, PD Parkinson’s disease
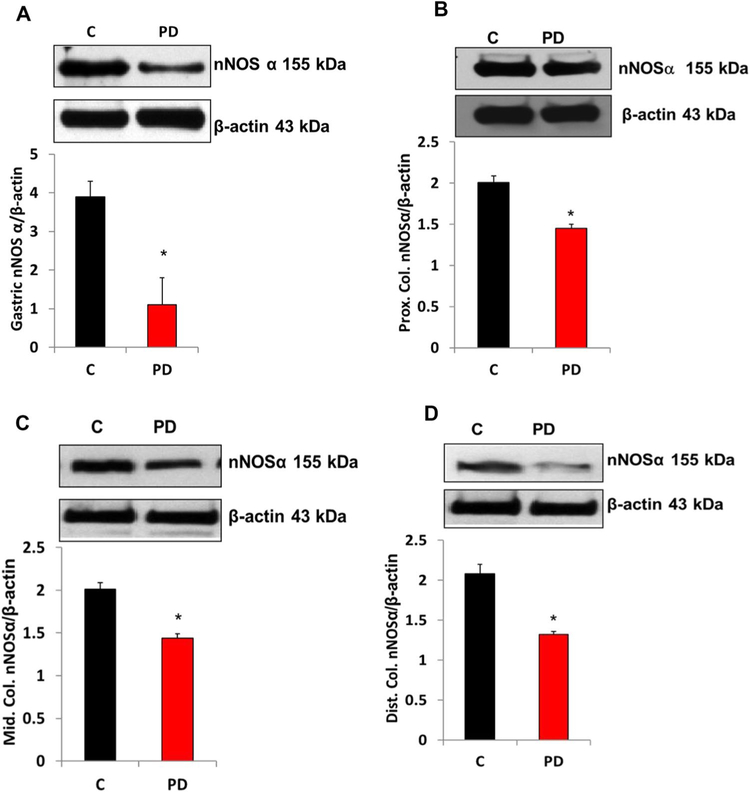
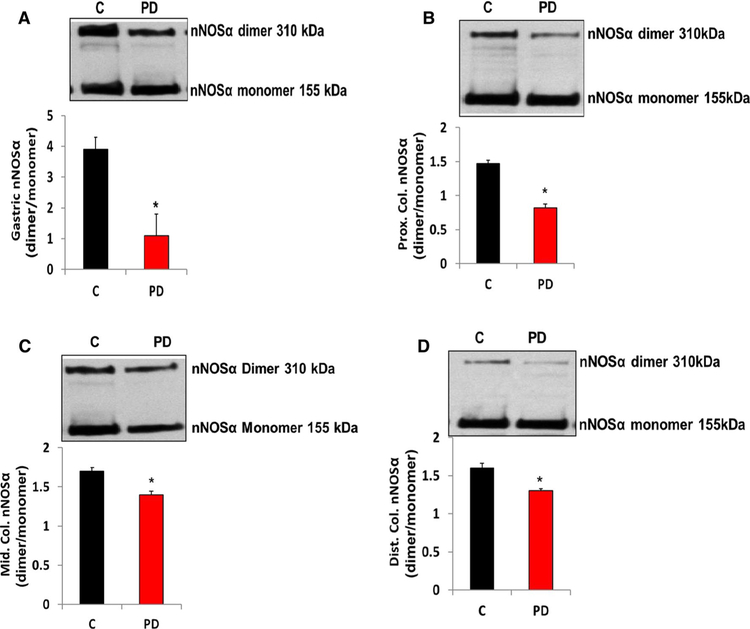
PD-Induced Impairment of Gastric Neuronal Nitric Oxide Synthase alpha (nNOSα) Protein Expression and nNOSα Dimerization
The functional isoform of nNOS (nNOSα) protein expression in gastric (Fig. 5a) and all regions of colon (Fig. 5b–d) neuromuscular tissues was significantly decreased (p < 0.05) following PD induction. Next, dimerization (an indirect measurement of nNOS enzyme activity) studies were performed by low-temperature (LT)-polyacrylamide gel electrophoresis (PAGE) gel to establish whether the decreased nNOSα was the result of altered nNOSα dimer levels in PD-induced mice. As depicted in Fig. 6, a significant decrease in the dimer/monomer ratio of gastric (Fig. 6a) and colon (Fig. 6b–d) nNOSα was noticed in PD-induced mice compared with that of the control group.
Fig. 5.
Changes in protein expression of nNOSα in gastric and colon tissues of control and in MPTP-induced PD mice. nNOSα expression was reduced significantly in PD-induced mice. a Representative immunoblot and densitometric analysis in gastric antrum tissue, b representative immunoblot and densitometric analysis in proximal colon, c representative immunoblot and densitometric analysis in middle colon, and d representative immunoblot and densitometric analysis in distal colon. The values are mean ± SE of four samples in each group. Statistical significance was determined by Student’s t test. *p < 0.05 compared with wild-type animals. C Control, PD Parkinson’s disease
Fig. 6.
Changes in protein expression of nNOSα dimerization in gastric and colon tissues of control and in MPTP-induced PD mice. a nNOSα dimerization in gastric antrum tissue, b representative immunoblot and densitometric analysis in proximal colon, c nNOSα dimerization in middle colon, and d nNOSα dimerization in distal colon. The values are mean ± SE of four samples in each group. Statistical significance was determined by Student’s t test. *p < 0.05 compared with wild-type animals. C Control, PD Parkinson’s disease
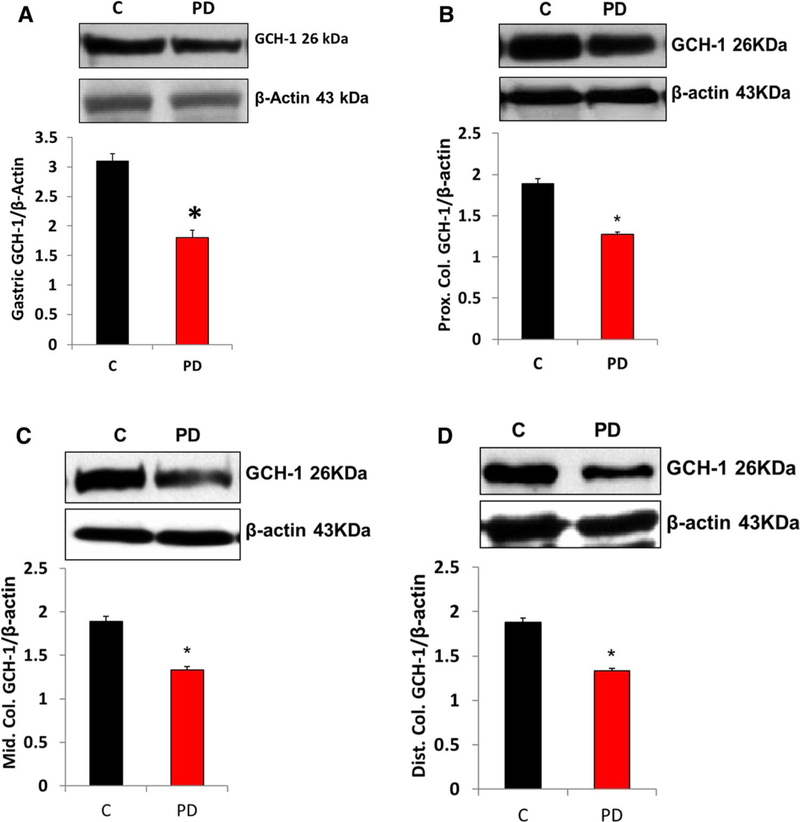
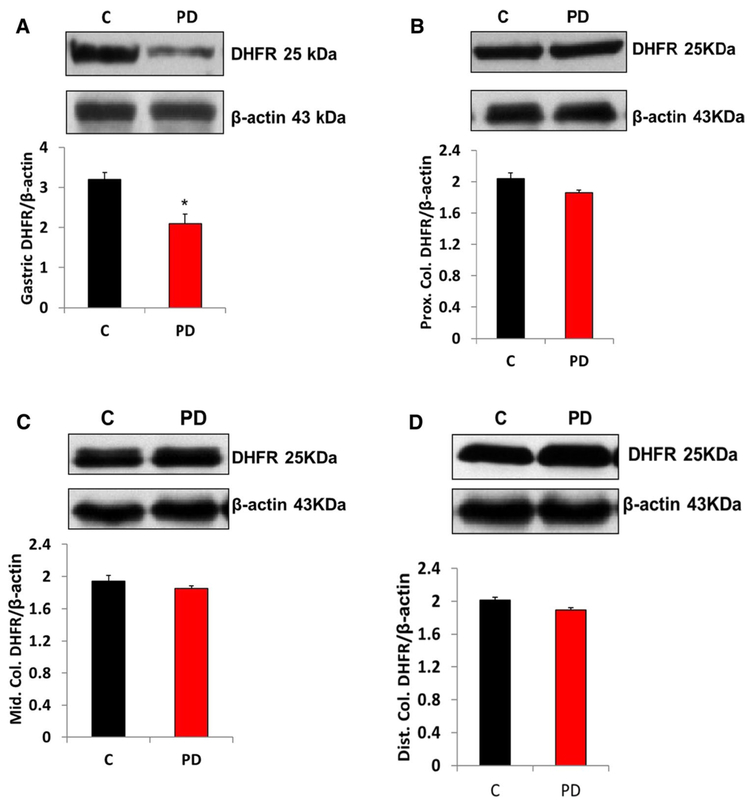
PD Induction Decreased Gastric and Colon Dihydrofolate Reductase (DHFR) Protein Expression and Biopterin Levels
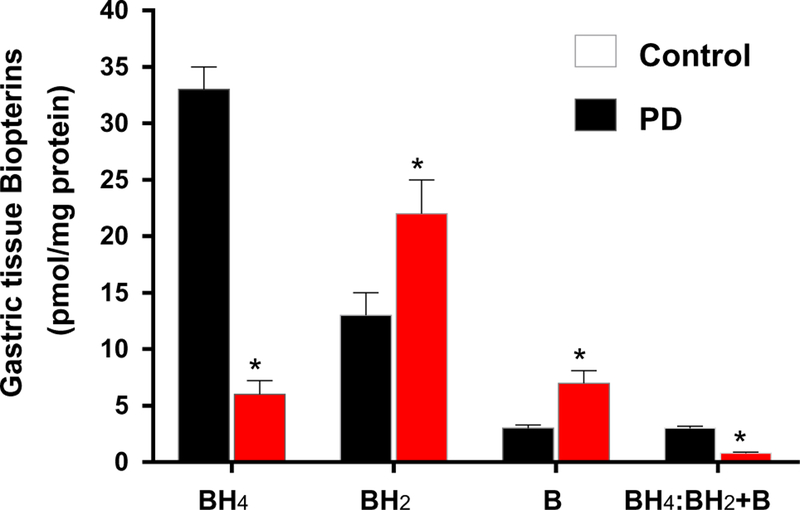
Several cofactors are known to be important for nNOS activity, including tetrahydrobiopterin (BH4). The biosynthesis of bioactive BH4 is dependent on the tissue availability of GCH-1 (de novo) and DHFR (salvage). Our data presented in Figs. 7 and 8 show that induction of PD reduced the protein expression of GCH-1 and DHFR in both gastric neuromuscular tissues. However, DHFR does not show any changes in colon neuromuscular tissues. Next, we quantified the levels of BH4 and its oxidized metabolites in control and MPTP-induced PD mice gastric tissues. Our data demonstrated that MPTP treatment significantly reduced gastric BH4 (reduced and active form that promotes nNOS enzyme activity) availability, while oxidized biopterins BH2 and B (7,8-dihydrobiopterin and biopterin, respectively) were elevated (Fig. 9).
Fig. 7.
Changes in protein expression of GCH-1 in PD mice gastric and colon tissues. a Representative immunoblot and densitometric analysis in gastric antrum tissue, b representative immunoblot and densitometric analysis in proximal colon, c representative immunoblot and densitometric analysis in middle colon, and d representative immunoblot and densitometric analysis in distal colon. *p < 0.05 compared with control (n = 4). C Control, PD Parkinson’s disease
Fig. 8.
DHFR protein expression in MPTP-induced PD in gastric and colon tissues. a Representative immunoblot and densitometric analysis of DHFR in gastric antrum tissue, b representative immunoblot and densitometric analysis of DHFR in proximal colon, c representative immunoblot and densitometric analysis of DHFR in middle colon, and d representative immunoblot and densitometric analysis of DHFR in distal colon. *p < 0.05 compared with control (n = 4). C Control, PD Parkinson’s disease
Fig. 9.
Effect of MPTP treatment in reduced (BH4, biologically active) and oxidized (BH2 and B, biologically inactive) biopterins in gastric muscular tissues. *p < 0.05 vs. controls. p < 0.05 vs. PD-induced group. C Control, BH4 tetrahydrobiopterin, BH2 oxidized form dihydrobiopterin, B biopterin
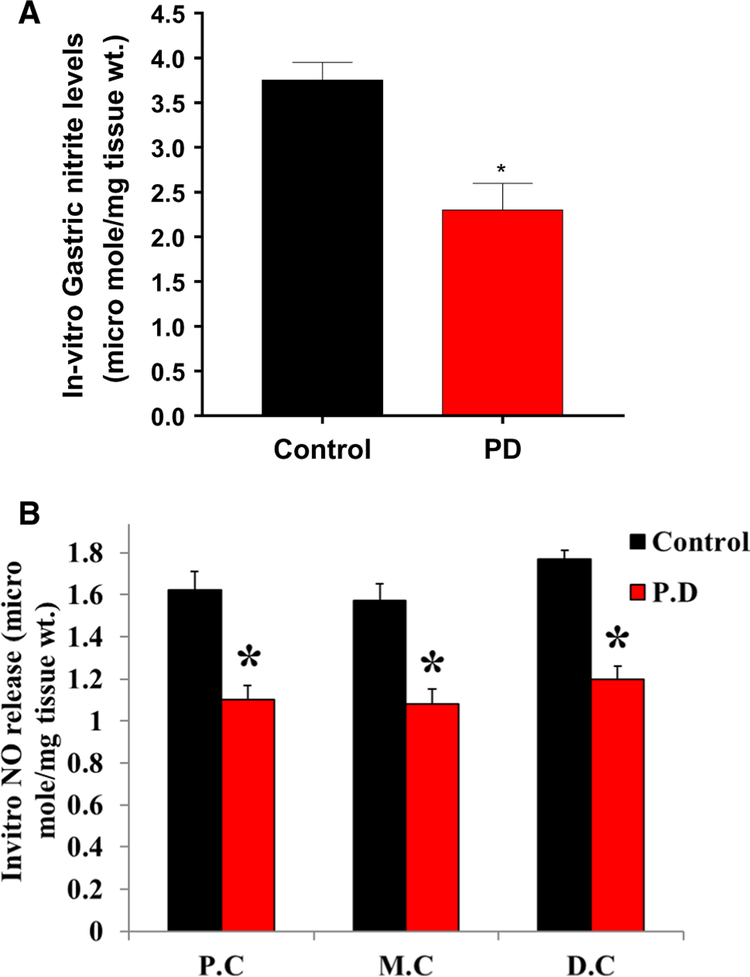
As PD induction reduced protein expression and dimerization of nNOSα, we examined whether nitrite (end product of NO) levels are altered in PD-induced GI specimens. As shown in Fig. 10, nitrite levels were significantly (p < 0.05) reduced in PD-induced gastric (A) and colon (B) specimens compared with control groups.
Fig. 10.
Effect of MPTP treatment in gastric and colon neuromuscular nitrite levels. Nitrite levels at 48 h were measured using the NO assay kit (ELISA). Values are mean ± SE (n = 4). Statistical significance was determined by Student’s t test. *p < 0.05 compared with wild-type animals. C Control, PD Parkinson’s disease, P.C proximal colon, M.C mid colon, D.C distal colon
In Vitro Effects of Nrf2 Activators and GSK3β Inhibitor in the Colon Exposed to MPP+, a Metabolic Product of MPTP
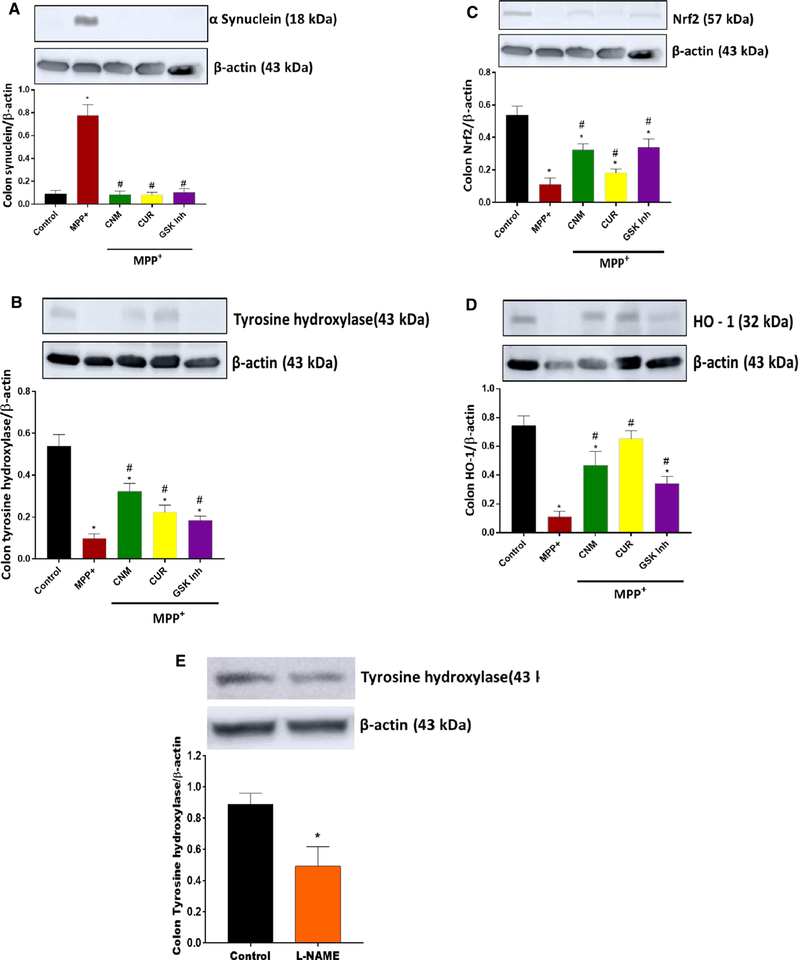
Our data presented in Fig. 11a show that in vitro treatment with MPP+ significantly (p < 0.05) elevated protein expression of alpha-synuclein (α-synuclein or α-S) in colon tissues. Pre-treatment with Nrf2 activators, cinnamaldehyde (CNM, 100 μM), curcumin (CUR, 100 μM), or glycogen synthase kinase 3 beta (GSK3β) inhibitor (30 μM, SB216763) attenuated MPP+-induced elevation of α-S (Fig. 11a). In addition, activation of Nrf2 or inhibition of GSK3β restored MPP+-induced reduction of tyrosine hydroxylase (TH, Fig. 11b), Nrf2, and heme oxygenase-1 (HO-1) protein expressions in colon tissues (Fig. 11c–d). In a separate set of experiment colon tissues exposed to NOS inhibitor L-NAME (100 μM) tyrosine hydroxylase protein expression was reduced (Fig. 11e).
Fig. 11.
Effects of Nrf2 activators cinnamaldehyde (CNM, 100 μM), curcumin (CUR, 100 μM), or GSK3β inhibitor (SB216763, 30 μM) on MPP+-induced alterations of α-synuclein, tyrosine hydroxylase, Nrf2, and HO-1. Effect of L-NAME (100 μM) on tyrosine hydroxylase protein expression in colon tissues. Colon specimens were incubated for 48 h and WB experiments were performed for targeted proteins. a Representative immunoblot and densitometric analysis of α-synuclein in colon tissue, b representative immunoblot and densitometric analysis of tyrosine hydroxylase in colon tissue, c representative immunoblot and densitometric analysis of Nrf2 in colon tissue, d representative immunoblot and densitometric analysis of HO-1 in colon tissue, and e representative immunoblot and densitometric analysis of tyrosine hydroxylase in colon tissue. *p < 0.05 compared with control (n = 4), # p < 0.05 compared to MPP+. C Control, PD Parkinson’s disease, MPP+ (1-methyl-4-phenylpyridinium), MPP++CNM (cinnamaldehyde), MPP++CUR (curcumin), MPP++GSK-3β inhibitor (SB216763)
Discussion
Approximately 30% of PD patients have reported GI dysfunction at all stages of PD. Clinical studies and animal models of PD have shown disturbances in the ENS involving nitrergic nerves [39–41]. Earlier studies have demonstrated that delayed GE, impaired colonic contractility, and stool frequency observed in a rodent model of PD are due to an altered ENS [39]. The results from this study support the previous findings that PD induction led to delayed GE and impaired colon motility. In addition, our study further shows an impairment of nitrergic relaxation due to reduced dimerization of nNOSα and BH4 availability in PD mice. Our data in gastric and colon neuromuscular tissue indicate that Nrf2 and antioxidant protein expression was reduced in PD-induced mice. In addition, in vitro activation of Nrf2 or inhibition of GSK-3β restored MPP+-induced alteration of α-S, TH, Nrf2, and HO-1 protein expression in colon specimens. Collectively, the above findings suggest that an impairment of the Nrf2/BH4/nNOS mechanism could lead to alterations in α-S, TH, delayed GE, and colon dysmotility normally seen in PD patients.
Previous studies have shown that PD induction with MPTP treatment leads to increased oxidative stress in the ileum [9]. Other study reported that loss of Nrf2 increases MPTP sensitivity and affects behavioral, neurochemical, and transcriptional changes in Nrf2 knockout mice, revealing the importance of Nrf2 [42]. A growing body of evidence suggests that Nrf2 has emerged as a pharmacological target for the treatment of PD [43]. In addition, Nrf2 activation showed neuroprotective effects in MPTP mice, and these were associated with a reduction in oxidative damage and neuroinflammation in astrocytes [44, 45]. Nrf2 protects against oxidative stress by activating the antioxidant enzymes, such as GSH synthesis enzymes (GCSc, GCSm), critical for the protection of cells. Loss of Nrf2 exacerbates neuronal degeneration, whereas Nrf2 expression protects against neurodegeneration in genetic and toxin models of PD [21, 39–41]. Likewise, activation of Nrf2 may modify: (1) proteasome and autophagy, (2) oxidative stress (by increasing the expression of phase II enzymes), and (3) neuroinflammation (enhancing antiinflammatory markers) in neurodegenerative brain tissue homogenates [21, 45–49]. Postmortem brain samples from PD patients showed that Nrf2-mediated protein expression of NQO1 and p62 was partly reduced in LB [50]. Previous in vitro and in vivo studies have reported that Nrf2 mitigates neuronal toxicity of rotenone and MPP+ [51]. Skibinski et al. demonstrated that activation of Nrf2 blocks degeneration caused by α-S in neurons in the brain [26]. Earlier studies from our laboratory show that loss of Nrf2 led to reduced nitrergic (nNOS)-mediated gastric motility and delayed GE in Nrf2 knockout mice, suggesting that Nrf2 plays an important role in GI motility function [14]. Recently, we also have reported that in vivo activation of Nrf2 attenuates delayed gastric emptying and improves nitrergic relaxation in high-fat-induced diabetic mice [52]. Our current study demonstrates that PD induction reduced Nrf2 and antioxidant protein expression in gastric and colon tissues. Overall, the above studies suggest that activation of Nrf2 pathway helps GI functions and also protects brain against oxidative damage during the onset of PD.
Glycogen synthase kinase-3beta (GSK-3β) dysregulation played a significant role in the pathogenesis of PD [53]. Further, inhibition of GSK-3β restored PD symptoms [53]; one of the pathways that regulate Nrf2 is GSK3 [54]. Previous studies have shown that both CNM and CUR activate Nrf2 via AKT/JNK and Keap-1 pathways, respectively [55]. Our in vitro results show the activation of Nrf2 or the inhibition of GSK-3β restored MPP+-induced suppression of Nrf2 and HO-1. In addition, our data demonstrate that activation of Nrf2 by CNM, CUR, and GSK-3β inhibitor blocks α-S aggregation in colon tissues.
In the ENS, nNOS (nitrergic neuron)-derived NO is an important neurotransmitter regulator of motility, vascular tone, blood supply, mucosal secretion, and barrier function [42, 56]. nNOS is shown to be expressed in the ventral tegmental area and SNpc of the brain [57]. The activity of the constitutive NOS isoforms is regulated primarily by calcium fluxes, mediated by calmodulin, and by the availability of BH4. The current study demonstrates that reduced BH4 synthesis is associated with delayed GE, altered colon transit, reduced nitrergic relaxation (nNOS function), and nNOSα dimerization in PD-induced mice. Limited reports are available on possible alterations of gastric nitrergic pathways in the onset of PD [58, 59]. Among multiple pathogenic mechanisms observed in PD, two of the major neuropathological hallmarks are: (1) the loss of dopaminergic neurons from the SNpc and the presence of α-S-containing LB in the surviving neurons, (2) signals from nNOS-NO may influence glutamatergic and dopaminergic neurotransmission, which plays a key role in PD [60, 61]. Our studies further demonstrate that treatment with L-NAME reduced the protein expression of TH in colon. Collectively, the above data suggest that loss of Nrf2–nNOS–NO signaling in the GI system may diminish dopaminergic neurons and thus lead to progression of PD.
Previous studies have demonstrated that low BH4 levels impair the production of NO and lead to increased superoxide radical production due to nNOS uncoupling [62]. BH4 deficiency is associated with both Alzheimer’s disease and PD, implying a contribution to these neurodegenerative diseases [63, 64]. Mainly, two pathways synthesize BH4: de novo and salvage. GCH-1 is the rate-limiting enzyme in the de novo synthetic pathway of BH4. Pyruvoyl-tetrahydropterin synthase and sepiapterin reductase are two additional enzymes required for the final production of BH4 [65]. In the salvage pathway, BH4 that is oxidized to BH2 or B may be recycled back to BH4 by the activity of DHFR [65]. This conversion is particularly crucial, as elevated levels of BH2 may compete with BH4 to bind to nNOS and thus lead to uncoupling of enzyme and superoxide production. In this study, we have observed reduced levels of BH4 and nNOS activity, which in turn affects homeostasis of NO production in the PD-induced mouse model. Levels of BH4 in the brain have been shown to be decreased in PD patients and in the MPP+ model of PD, although the subsequent mechanisms have not been investigated fully [66, 67]. In a clinical study, it has been shown that BH4 treatment for 6 months improved memory and brain activation with neural changes in working memory performance at the 6-month time point [68]. Administration of MPTP in mice did not change BH4 levels in the brain but has significantly decreased tyrosine hydroxylase and dopamine concentrations [69]. Our data show that both GCH-1 and BH4 levels are reduced in the GI specimens. In contrast, DHFR levels are reduced only in the stomach but not in the colon. In addition, previous studies have shown that GCH-1 is regulated by Nrf2 [70]. NO generated from nNOS is known to induce GI smooth muscle relaxation via its downstream signaling pathway, cyclic GMP (cGMP)–protein kinase G (PKG)–phosphodiesterase type 5 (PDE5) [71]. Based on numerous experimental studies, West et al. have suggested that NO-cGMP be recommended as one of the potential therapeutic agents to protect against PD [72]. Collectively, the above studies suggest that an impairment of the Nrf2–BH4–nNOS–NO–cGMP pathway could lead to aggregation of α-S in ENS, GI dysfunction, and subsequent development of PD [73].
In vitro studies using neuroblastoma cells have shown the protective role of sepiapterin (elevates intracellular BH4 via salvage pathway) against MPP+-induced increases in proteosome degradation, oxidative stress, and dopaminergic neuronal degradation [74]. Previous studies from our laboratory demonstrated that in vivo supplementation of BH4/sepiapterin attenuated delayed GE and nNOS function in the onset of diabetes [34, 35]. Nrf2 activators, such as sulforaphane, CDDO-methyl ester (also known as bardoxolone methyl), and DMF, have proven efficacy in normalizing oxidative stress [75]. Our current findings show that in vitro activation of Nrf2 or inhibition of GSK-3β was effective in normalizing dopamine synthesis by enhancing the expression of TH protein in colon tissues after MPP+ treatment. Additional studies are warranted to investigate the mechanistic role of these pathways in both GI motility and parkinsonism.
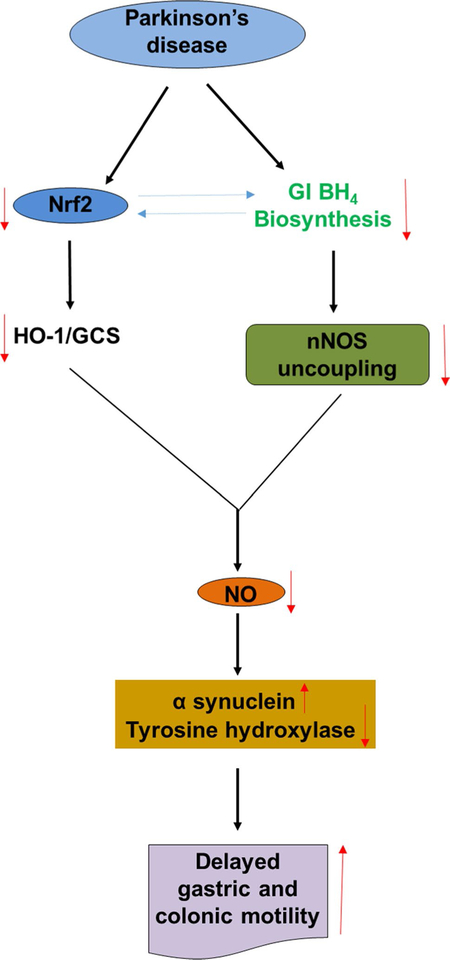
In summary, MPTP is a selective dopaminergic neurotoxin which produces a physiological impairment of ENS inhibitory function causing behavioral symptoms of slow GI motility. Our results in MPTP-induced PD mice show significant alterations in the gastric and colon Nrf2/phase II pathway, reduced gastric and intestinal BH4 biosynthesis enzymes, and nNOS expression and dimerization, leading to an impaired nNOS function (Fig. 12). The changes observed among Nrf2–BH4–nNOS signaling may result in gastroparesis and an altered intestinal transit time, leading to constipation. Finally, our studies suggest that activation of Nrf2 or inhibition of GSK-3β attenuated MPP+-induced alterations in the colon α-S, TH, Nrf2, and HO-1 protein expression in vitro. We suggest that understanding molecular interactions between Nrf2, nNOS, NO-cGMP, dopamine synthesis, and aggregation of α-S in both the GI system and brain is critical for the advancement of proper interventions against the development of PD.
Fig. 12.
Schematic diagram of proposed BH4/Nrf2/nNOS mediated gastrointestinal motility in MPTP-induced PD mice. The solid lanes indicate the sequential events occur through protein–protein interactions that connect the gene promoters to execute transcription of target genes. The dotted connections indicate possible interactions between the proteins. The reversible arrows indicate possible cross talk between the proteins. ↑ and ↓ arrows indicate increase and decrease levels, respectively
Acknowledgment
The National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number SC1GM121282 supported research reported in this publication. Finally, we thank the Meharry Office for Scientific Editing and Publications for editorial support.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lionnet A, Leclair-Visonneau L, Neunlist M, et al. Does Parkinsons disease start in the gut? Acta Neuropathol. 2018;135:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil. 2008;20:741–749. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:10–15. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Noorian AR, Taylor G, et al. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol. 2007;207:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23–32. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. [DOI] [PubMed] [Google Scholar]
- 7.Kujawska M, Jodynis-Liebert J. What is the evidence that Parkinson’s disease is a prion disorder, which originates in the gut? Int J Mol Sci. 2018;19:3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. [DOI] [PubMed] [Google Scholar]
- 9.Ellett LJ, Hung LW, Munckton R, et al. Restoration of intestinal function in an MPTP model of Parkinson’s Disease. Sci Rep. 2016;6:30269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton D, Stephens M, Kirk L, et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–241. [DOI] [PubMed] [Google Scholar]
- 11.Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–715. [DOI] [PubMed] [Google Scholar]
- 12.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–949. [DOI] [PubMed] [Google Scholar]
- 13.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis–a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, Sekhar KR, Hale AB, et al. Loss of NRF2 impairs gastric nitrergic stimulation and function. Free Radic Biol Med. 2011;51:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangula PRR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Liver Physiol. 2010;298:G692–G699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangula PRR, Mukhopadhyay S, Pasricha PJ, Ravella K. Sepiapterin reverses the changes in gastric nNOS dimerization and function in diabetic gastroparesis. Neurogastroenterol Motil. 2010;22:e351–e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puspita L, Chung SY, Shim J-W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain. 2017;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuanalo-Contreras K, Mukherjee A, Soto C. Role of protein mis-folding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney P, Park H, Baumann M, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. [DOI] [PubMed] [Google Scholar]
- 21.Lastres-Becker I, Ulusoy A, Innamorato NG, et al. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet. 2012;21:3173–3192. [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. [DOI] [PubMed] [Google Scholar]
- 23.Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Xu C, Shen G, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6 J mice and C57BL/6 J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. [DOI] [PubMed] [Google Scholar]
- 25.Kwak M-K, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23:8786–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skibinski G, Hwang V, Ando DM, et al. Nrf2 mitigates LRRK2-and α-synuclein-induced neurodegeneration by modulating proteostasis. Proc Natl Acad Sci USA. 2017;114:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthukumaran K, Smith J, Jasra H, et al. Genetic susceptibility model of Parkinson’s disease resulting from exposure of DJ-1 deficient mice to MPTP: evaluation of neuroprotection by Ubisol-Q10. J Parkinsons Dis. 2014;4:523–530. [DOI] [PubMed] [Google Scholar]
- 28.Langston JW. The MPTP Story. J Parkinsons Dis. 2017;7:S11–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm. 2001;108:1263–1282. [DOI] [PubMed] [Google Scholar]
- 30.Lotharius J, O’Malley KL. The Parkinsonism-inducing drug 1-Methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. J Biol Chem. 2000;275:38581–38588. [DOI] [PubMed] [Google Scholar]
- 31.King JM, Muthian G, Mackey V, Smith M, Charlton C. L-Dihydroxyphenylalanine modulates the steady-state expression of mouse striatal tyrosine hydroxylase, aromatic L-amino acid decarboxylase, dopamine and its metabolites in an MPTP mouse model of Parkinson’s disease. Life Sci. 2011;89:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773–e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anitha M, Reichardt F, Tabatabavakili S, et al. Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice. Cell Mol Gastroenterol Hepatol. 2016;2:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangula PR, Challagundla KB, Ravella K, et al. Sepiapterin alleviates impaired gastric nNOS function in spontaneous diabetic female rodents through NRF2 mRNA turnover and miRNA biogenesis pathway. Am J Physiol Liver Physiol. 2018;315:G980–G990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangula PRR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji C, Tang M, Johnson GVW. Assessing the degradation of tau in primary neurons: the role of autophagy. Methods Cell Biol. 2017;141:229–244. [DOI] [PubMed] [Google Scholar]
- 37.Ali ZA, Rinze R, Douglas G, et al. Tetrahydrobiopterin determines vascular remodeling through enhanced endothelial cell survival and regeneration. Circulation. 2013;128:S50–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahan D, Ekman M, Larsson-Callerfelt A-K, et al. Induction of angiotensin-converting enzyme after miR-143/145 deletion is critical for impaired smooth muscle contractility. Am J Physiol Physiol. 2014;307:C1093–C1101. [DOI] [PubMed] [Google Scholar]
- 39.Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol. 2009;218:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, Fornai F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 2010;1355:195–206. [DOI] [PubMed] [Google Scholar]
- 41.Sander LE, Lorentz A, Sellge G, et al. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirst CS, Foong JPP, Stamp LA, et al. Ion channel expression in the developing enteric nervous system. PLoS One. 2015;10:e0123436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker IL. Role of the transcription Factor Nrf2 in Parkinson’s disease: new insights. J Alzheimer’s Dis Park. 2017;07:340. [Google Scholar]
- 44.Williamson TP, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology. 2012;33:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P-C, Vargas MR, Pani AK, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gazaryan IG, Thomas B. The status of Nrf2-based therapeutics: current perspectives and future prospects. Neural Regen Res. 2016;11:1708–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JA, Johnson DA, Kraft AD, et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurode-generation. Ann N Y Acad Sci. 2008;1147:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuadrado A, Manda G, Hassan A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–383. [DOI] [PubMed] [Google Scholar]
- 49.Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–1669. [DOI] [PubMed] [Google Scholar]
- 50.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of -synuclein. J Neurosci. 2011;31:14508–14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Todorovic M, Wood SA, Mellick GD. Nrf2: a modulator of Parkinson’s disease? J Neural Transm. 2016;123:611–619. [DOI] [PubMed] [Google Scholar]
- 52.Sampath C, Sprouse JC, Freeman ML, Gangula PR. Activation of Nrf2 attenuates delayed gastric emptying in obesity induced diabetic (T2DM) female mice. Free Radic Biol Med. 2019;135:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golpich M, Amini E, Hemmati F, et al. Glycogen synthase kinase-3 beta (GSK-3β) signaling: implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26. [DOI] [PubMed] [Google Scholar]
- 54.Cuadrado A, Kügler S, Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paul EJ, Kalk E, Tossell K et al. : nNOS-expressing neurons in the ventral tegmental area and substantia nigra pars compacta. eneuro 2018;5:ENEURO.0381–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czarnecka A, Lenda T, Domin H, Konieczny J, Smiałowska M, Lorenc-Koci E. Alterations in the expression of nNOS in the substantia nigra and subthalamic nucleus of 6-OHDA-lesioned rats: the effects of chronic treatment with l-DOPA and the nitric oxide donor, molsidomine. Brain Res. 2013;1541:92–105. [DOI] [PubMed] [Google Scholar]
- 59.Anitha M, Shahnavaz N, Qayed E, et al. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2010;298:G375–G383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim WS, Kågedal K, Halliday GM. Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther. 2014;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López JM, Lozano D, Morales L, González A. Pattern of nitrergic neuronal system organization in the brain of two holostean fishes (Actinopterygii: Ginglymodi). Brain Behav Evol. 2017;89:117–152. [DOI] [PubMed] [Google Scholar]
- 62.Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO- cycle. Med Hypotheses. 2007;69:821–825. [DOI] [PubMed] [Google Scholar]
- 63.Kuiper MA, Teerlink T, Visser JJ, Bergmans PLM, Scheltens P, Wolters EC. L-Glutamate, l-arginine and L-citrulline levels in cerebrospinal fluid of Parkinson’s disease, multiple system atrophy, and Alzheimer’s disease patients. J Neural Transm. 2000;107:183–189. [DOI] [PubMed] [Google Scholar]
- 64.Foxton RH, Land JM, Heales SJR. Tetrahydrobiopterin availability in Parkinson’s and Alzheimer’s disease; potential pathogenic mechanisms. Neurochem Res. 2007;32:751–756. [DOI] [PubMed] [Google Scholar]
- 65.Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50:1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Paula Martins R, Glaser V, Aguiar AS Jr, et al. De novo tetrahydrobiopterin biosynthesis is impaired in the inflammed striatum of parkin (−/−) mice. Cell Biol Int. 2018;42:725–733. [DOI] [PubMed] [Google Scholar]
- 67.Ryan BJ, Lourenço-Venda LL, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R. α-Synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med. 2014;67:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hölscher C New drug treatments show neuroprotective effects in Alzheimer′s and Parkinson′s diseases. Neural Regen Res. 2014;9:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee K-S, Lee J-K, Kim H-G, Kim HR. Differential effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on motor behavior and dopamine levels at brain regions in three different mouse strains. Korean J Physiol Pharmacol. 2013;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xue J, Yu C, Sheng W, et al. The Nrf2/GCH1/BH4 axis ameliorates radiation-induced skin injury by modulating the ROS cascade. J Invest Dermatol. 2017;137:2059–2068. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi S, Lin H, Geshi N, et al. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol. 2008;586:4209–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West AR, Tseng KY. Nitric oxide–soluble guanylyl cyclase–cyclic GMP signaling in the striatum: new targets for the treatment of parkinson’s disease? Front Syst Neurosci. 2011. 10.3389/fnsys.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116. [DOI] [PubMed] [Google Scholar]
- 74.Shang T, Kotamraju S, Zhao H, Kalivendi SV, Hillard CJ, Kalyanaraman B. Sepiapterin attenuates 1-methyl-4-phenylpyridinium-induced apoptosis in neuroblastoma cells transfected with neuronal NOS: role of tetrahydrobiopterin, nitric oxide, and proteasome activation. Free Radic Biol Med. 2005;39:1059–1074. [DOI] [PubMed] [Google Scholar]
- 75.Lastres-Becker I, García-Yagüe AJ, Scannevin RH, et al. Repur-posing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s Disease. Antioxid Redox Signal. 2016;25:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]