Abstract
Symptoms of ADHD and anxiety are common during adolescence and frequently co-occur. However, the genetic and environmental influences that underlie this co-occurrence are understudied. Using a large twin sample (N = 1017), we examined cross-sectional genetic and environmental influences on ADHD and anxiety symptoms during childhood. We also explored whether these influences were shared with attentional control, a putative mechanism for symptom comorbidity. We found evidence for common genetic and non-shared environmental influences on the covariation among attentional control, ADHD, and anxiety symptoms, supporting the putative role of attentional control as a mechanism by which comorbid problems may develop. Genetic factors also accounted for symptom co-occurrence after controlling for covariation with attentional control, suggesting the presence of additional unmeasured mechanisms.
Keywords: ADHD, GAD, Attention, Anxiety, Genetic Influences
Worldwide, attention-deficit/hyperactivity disorder (ADHD) and anxiety disorders1 are two of the most highly prevalent clinical conditions in individuals under 18 (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015). Both conditions are heterogeneous. An ADHD diagnosis comprises distinct subtypes reflecting primarily inattentive symptoms (e.g., lack of focus or attention to detail, easily distracted, etc.), primarily hyperactive/impulsive symptoms (e.g., fidgets, talks excessively, doesn’t wait for turn, etc.), or a combined diagnosis (i.e, both inattentive and hyperactive/impulsive). In contrast, many anxiety diagnoses include elements of worry or withdrawal, though some also include problems with attention (e.g., fixating on a source of worry or difficulty concentrating). Despite differences in symptoms for ADHD and anxiety diagnoses, comorbidity rates are high; roughly one third of children with an ADHD diagnosis are also diagnosed with an anxiety disorder during early life (Adler, Barkley, Newcorn, Spencer, & Weiss, 2007; Merikangas et al., 2010). ADHD shows particularly high rates of comorbidity with generalized anxiety disorder (GAD; 15.5%−34.9%) and social phobia (3.2%) relative to other anxiety diagnoses (e.g., Specific Phobia [2.3%], NOS [7.7%]; Anastopoulos et al., 2016; Tsang et al., 2012), though the mechanisms underlying such associations are unclear. To better understand the etiology of ADHD-anxiety comorbidity, we examined shared and unique genetic and environmental influences on adolescent symptoms of ADHD and anxiety (GAD, Social Phobia) and tested attentional control as a plausible mechanism linking these conditions. We investigated these questions during adolescence, a period of development during which symptoms for both conditions will already be visible and during which the brain is rapidly changing, making it a putatively critical period for understanding the development of psychopathology and intervening to enhance long-term outcomes (Cicchetti & Rogosch, 2002).
The individual etiologies of ADHD, GAD, and social phobia are clearer than for comorbid ADHD and anxiety problems. ADHD, GAD, and social phobia can each be partially traced to genetic factors during development. Twin studies have suggested heritability estimates ranging from 0.60 to 0.90 for ADHD (Jarrett & Ollendick, 2008), 0.55–0.71 for social phobia (Ollendick & Hirshfeld-Becker, 2002), and 0.32 for GAD (Hettema, Neale, & Kendler, 2001), though samples often included broad age ranges, from early childhood through adulthood. Family studies have shown that persistent attention problems are more likely in children and adolescents of chronically anxious mothers than in offspring of non-anxious mothers (Clavarino et al., 2009), suggesting that similar genetic factors may contribute to both ADHD and anxiety symptoms. Molecular genetic approaches indicate potential pleiotropy, or the possibility that the same genes may contribute to behaviors in both ADHD and anxiety disorders. For example, serotonin (Lau et al., 2009; Manor et al., 2001) and dopamine (Bellgrove, Hawi, Kirley, Gill, & Robertson, 2005; Rowe et al., 1998) transporter genes have been associated with both ADHD and anxious traits, though this work has not consistently replicated. Genome-wide association studies (GWAS) support polygenic effects on anxiety (Meier et al., 2018), internalizing (Benke et al., 2014), and ADHD symptoms (Demontis et al., 2019), though such work has not yet addressed comorbidity.
Evidence for independent transmission of ADHD and anxiety disorders, indicated as distinct, non-overlapping influences, has also been reported. At least one study has reported a virtual non-overlap and independent segregation of ADHD and anxiety symptoms in children and adolescents (Perrin & Last, 1996). Importantly, evidence for independent transmission does not necessarily contradict evidence for common heritable factors, but rather suggests complex etiological pathways from which ADHD and anxiety symptoms manifest. Specifically, ADHD and anxiety symptoms are each likely influenced by numerous genetic factors, some of which are shared and some of which are specific. Moreover, overlap between symptoms may be more or less visible across different developmental periods, such as a period of significant overlap in symptoms preceding a period of relative independence (Overgaard, Aase, Torgersen, & Zeiner, 2012). Such complexities can lead to discrepancies in conclusions about shared etiology across samples. Adolescence may be a particularly important period of study, as ADHD-anxiety comorbidity is substantial by this period (Merikangas et al., 2010), but is not clearly foreshadowed by overlapping symptoms earlier in life.
Evidence for genetic underpinnings does not preclude environmental effects; both biological and environmental factors are known to contribute, to varying degrees, to most complex behaviors. For example, both ADHD and anxiety symptoms are precipitated by early life stressors and low socioeconomic status. During childhood and early adolescence, both individuals with ADHD and individuals with anxiety problems experience more familial stress (Ginsburg, Grover, & Ialongo, 2005; Shelton et al., 1998) and have parents who show more problematic, less skilled parenting behaviors (DuPaul, McGoey, Eckert, & VanBrakle, 2001; Ginsburg et al., 2005) relative to controls. Although a full review of social, cultural, and other environmental factors associated with risk for ADHD or anxiety problems is beyond the scope of this paper, these two examples support our hypotheses for common mechanisms by offering evidence of common environmental factors contributing to ADHD and anxiety problems in adolescents.
In addition to advancing basic knowledge about the nature of ADHD, identifying putative behavioral mechanisms that may underlie comorbid ADHD and anxiety symptoms is important because specific behaviors frequently serve as targets for clinical intervention and treatment. Here, we build upon the position that overlap between ADHD and anxiety problems stems from the presence of intrusive (i.e., task-irrelevant) thoughts, a hallmark of GAD and social phobia, but which may also present as inattention (Jarrett & Ollendick, 2008), the first feature of ADHD listed in the DSM-5. This premise implicates attentional control, namely the ability to appropriately shift attention to and focus on task-relevant information (Rothbart & Bates, 1998), as a likely mechanism of comorbidity.
Attention shifting and focusing are aspects of attentional control observable by six to nine months of age (Rothbart, 1981) that follow a protracted period of development through adolescence (Rothbart & Bates, 1998). Critically for our questions, poorer attentional control predicts symptoms of ADHD by the early adolescent years (Muris, Meesters, & Rompelberg, 2007), making it a feasible target as a mechanism of comorbidity by this time. Attention shifting and focusing, the hallmarks of attention control, are also associated with anxiety symptoms, although the direction of this association is unclear. Self-report and behavioral measures of attention shifting and focusing have been negatively associated with anxious symptoms between ages 8–12 (Muris, van der Pennen, Sigmond, & Mayer, 2008), though other work suggests greater attention shifting and focusing abilities in youth with greater levels of internalizing problems by age 8 (Eisenberg et al., 2001).
A spectrum of possible pathways to ADHD-anxiety comorbidity is summarized elsewhere (Jarrett & Ollendick, 2008). Our hypotheses, reflecting one such pathway, are derived from one primary postulate that attentional control in the form of attention focusing and shifting acts as a mechanism linking ADHD with anxiety diagnoses. In this scenario, comorbid problems result when one is unable to shift mental focus away from worrisome thoughts, which ultimately impair the ability to maintain focus on even low-level tasks (inattention) or result in a nervous restlessness that resembles hyperactivity. In this way, inattention becomes the factor that links anxiety problems with primary ADHD symptoms as problems with attentional control increase along with intrusive, worrisome thoughts.
There is mixed evidence in support of this assertion that attentional control plays a mechanistic role in the link between symptoms of ADHD and anxiety. For example, attentional focusing and shifting inconsistently predict internalizing problems, suggesting that other factors may be contributing to the ultimate manifestation of symptoms. At least one study has shown that associations between attentional control and maladaptive outcomes during childhood are moderated by genetic risk for psychopathology (Brooker et al., 2011), underscoring a need to test putative mechanisms within a genetically-informative framework. Heritability estimates of attentional control range from 0.18 in adolescents and adults based on response time measures (Fan, Wu, Fossella, & Posner, 2001) to as high as 0.83 in middle childhood based on observer report (Lemery-Chalfant, Doelger, & Goldsmith, 2008). Attentional control has also been associated with several of the same genetic (Posner, Rothbart, & Sheese, 2007) and environmental factors that appear to impact the development of ADHD and anxiety symptoms (Bernier, Carlson, & Whipple, 2010; Liston, McEwen, Casey, & Posner, 2009). Furthermore, recent GWAS work (Demontis et al., 2017) identified 12 independent loci associated with ADHD, including those believed to play a role in brain-expressed regulatory markers. Such findings are consistent with the notion of attentional control as a mechanism of ADHD risk (and risk for comorbid anxiety problems), though neither attentional control nor anxiety symptoms were examined in this GWAS research.
In a sample that partially overlapped with this study’s sample, additive genetic variance accounted for covariation between slightly different aspects of parent- and experimenter-rated attentional control and parent reports of internalizing or externalizing symptoms in early to middle childhood (Lemery-Chalfant et al., 2008). Previous work also suggested that generalized and social anxiety symptoms shared 17% to 18% of their variance, respectively, with attentional control during adolescence (Gagne, O’Sullivan, Schmidt, Spann, & Goldsmith, 2017). In both of these reports, genetic correlations between attentional control and internalizing problems were negative, suggesting that similar genetic factors may contribute to both impairments in attentional control and anxiety symptoms. In each case, findings are consistent with the idea that similar genetic mechanisms contribute to attention control problems and internalizing and externalizing symptoms, though they do not specifically inform us about ADHD comorbidity with GAD or social phobia. Thus, previous results provide partial support for our assertion that attentional control may function as a mechanism linking symptoms of ADHD with symptoms of GAD and social phobia. However, the possibility that attentional control, ADHD, and anxiety symptoms share genetic and environmental influences remains untested. We addressed this gap in the literature in the current report.
In sum, we expected that attentional control would share genetic and environmental influences with both ADHD and symptoms of GAD and social phobia. We investigated this possibility in a sample of adolescent twins, given that adolescence is a putatively critical period for the development of systems we hypothesize to underlie ADHD and anxiety comorbidity and because both ADHD and anxiety problems are visible by this stage of development. Twin study designs are particularly useful for improving nosology. Particularly as the field of psychology moves toward continuous, rather than categorical, descriptions of psychopathology, twin studies can offer insight into the degree to which observed comorbidities may be due to shared genetic underpinnings. If shared genetic underpinnings are indeed indicated, disorders previously assumed to be discrete could, in fact, be different manifestations of the same underlying heritable condition for which individual differences in environmental conditions determine symptom expression. Twin studies thus offer insight about how to delineate “distinct” disorders and provide a starting point for studies that will investigate specific biological mechanisms (e.g., candidate genes or neural processes) or environmental influences (e.g., parent psychopathology or parenting behaviors).
Method
Participants
The sample was drawn from the Wisconsin Twin Panel, a longitudinal twin study that followed individuals from childhood through adolescence. Participants, identified from state birth records, were invited by mail to participate. A screening interview produced a sample mildly enriched for psychopathology at twin age seven years. Screening procedures did not target ADHD risk specifically, but nonetheless resulted in 4.5% of participants identified as at-risk for ADHD. The sample resulting from this screening was assessed during middle childhood (target age 7) and adolescence (target ages 13 and 15) via telephone and in-home measures of psychopathology, temperament, cognition, cortisol levels, parenting, family stress, and peer relationships (Schmidt et al., 2013).
The current study is focused on the early adolescent assessment (Mage= 13.6; SD = 19.7 months; range: 11.16 – 18.00), given that it had the most available data for our selected measures of anxiety symptoms and attention control (Schmidt et al., 2013). The sample comprised 508 twin pairs (53.1% female; 36.0% monozygotic, 34.1% same-sex dizygotic, and 29.3% male-female dizygotic), from whom data were collected between 1990 and 1999. Sample demographics, summarized in Table 1, were consistent with the demographics of the recruitment area.
Table 1.
Demographics for sample of N = 1,106 twins (503 pairs) during early adolescence
| MZ | DZ | Total | |
|---|---|---|---|
| % female | 61.2% | 48.7% | 53.1% |
| Race | |||
| White | 86.6% | 80.9% | 82.4% |
| Black | 1.6% | 10.9% | 7.9% |
| Multiracial or Other | 11.6% | 8.2% | 9.7% |
| Median Income Category | $70,001-80,000 | $70,001-80,000 | $70,001-80,000 |
| Mean Education in years | |||
| Mother | 14.9 (2.1) | 15.1 (2,4) | 15.0 (2.3) |
| Father | 14.4 (2.2) | 14.3 (2.5) | 14.3 (2.4) |
| Mean age in years (SD mos) | 13.2 (14.8) | 13.0 (14.4) | 13.0 (14.6) |
Measures
Zygosity.
For most twins, zygosity was determined via the Zygosity Questionnaire for Young Twins (Goldsmith, 1991), which yields over 95% agreement with zygosity determined via genotyping (Price et al., 2000). When this did not result in a clear assignment of zygosity, we examined hospital pathology reports on the placenta(e) or genotyped 15 highly polymorphic alleles. Three twin pairs for whom zygosity could not be determined were excluded.
ADHD Symptoms.
Mothers, fathers, and twins reported on twins’ ADHD symptoms (inattention and impulsivity/hyperactivity) using the MacArthur Health and Behavior Questionnaire (HBQ; Armstrong & Goldstein, 2003). Parents rated the degree to which behaviors were characteristic of their child over the past 6 months (0 = rarely, 2 = certainly applies). Twin ratings involved choosing which, of two opposing options (e.g., It’s not hard for me to stay seated when I’m supposed to vs. It’s hard for me to stay seated when I’m supposed to), was most true for them; they then further rated their choice on a scale from 1 (sort of like me) to 3 (really like me). Mother and father reports were highly correlated (r = 0.54, p < .001). Twin ratings were correlated with mother (r = .43, p <.001) and father reports (r = .32, p < .001).
Primary caregivers (97% mothers) and twins also reported on ADHD symptoms using the Diagnostic Interview Schedule for Children, Version IV (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), a highly structured computer-based interview designed to be administered by lay interviewers. Respondents indicated whether symptoms of ADHD were or were not characteristic of their child (caregivers) or themselves (twins). Positive responses were summed to create symptom counts. Caregiver and twin reports were correlated (r = 0.42, p < .001); 51 participants qualified for an ADHD diagnosis based on parent/twin reports.
To create an overall index of ADHD symptoms, caregiver- and twin-reported symptoms on the HBQ and DISC were standardized within measure and then mean composited. Compositing scores across raters and measures reduces the possibility that symptom reports and/or symptom overlap were biased by rater or by instrument, as has been suggested for previous studies (Jarrett & Ollendick, 2008). Though symptoms of inattention and impulsivity/hyperactivity were highly correlated (r = 0.74, p < .001), past work suggests at least partially distinct genetic etiologies of the two symptom domains (Willcutt, Pennington, & DeFries, 2000). Thus, we also created separate inattention and impulsivity/hyperactivity composites by combining relevant items from caregiver- and twin-reports. Composite scores were normally distributed.
Anxiety Symptoms.
We focused on symptoms of GAD and Social Phobia given evidence for high rates of comorbidity with ADHD. Parents and twins reported on twins’ symptoms of overanxiousness (i.e., generalized anxiety) and social anxiety via the HBQ. Participant responses were moderately correlated for HBQ overanxiousness (mean r across raters = 0.35; all ps < .001) and social anxiety (mean r across raters = 0.32; all ps < .001). Overanxiousness and social anxiety were correlated within reporters (rs 0.55 – 0.60, all ps < .001).
Primary caregivers and twins also provided reports of GAD and social phobia symptoms during DISC interviews. Parent and twin ratings of GAD and social phobia symptoms were moderately correlated (r = 0.23 and 0.24 respectively; ps < .001). GAD and social phobia were highly correlated within reporter (r = 0.60, p < .001).
Again, to create an overall index of anxiety problems, caregiver and self-reported symptoms on the HBQ and DISC scales were standardized within measure and then mean composited.
Attentional Control.
Mothers, fathers, and adolescents completed the Early Adolescent Temperament Questionnaire - Revised (EATQ-R; Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001). We used the attentional control scale, which defines attentional control as “the capacity to focus attention as well as shift attention when desired.” Respondents rated, on a scale from 1 (almost always untrue) to 5 (almost always true), the degree to which statements were true for them (e.g., It is easy to really concentrate on homework problems, good at keeping track of several different things that are happening around me/him/her”). Respondents’ ratings were significantly correlated (mean r across raters = .43; all ps < 0.001) and so were composited to create an overall index of attentional control.
Control Variables.
Girls scored higher than boys on attentional control (t(756) = 4.2, p < .001) and anxiety (t(1012) = 3.8, p < .001); boys scored higher on ADHD symptoms (t(1012) = 5.8, p < .001), inattention (t(1015) = 4.8, p < .001), and impulsivity/hyperactivity (t(1015) = 6.4, p <.001; Supplemental Table 1). Given that no sex differences were hypothesized for the role of attentional control in the comorbidity of ADHD and anxiety symptoms and because our sample size was too small to conduct robust statistical tests for sex differences in the latent genetic and environmental factors, we regressed sex out of the phenotypic composites prior to analyses. Age was unrelated to composites (rs: −0.06 – +0.05).
Missing Data and Plan for Analyses
Statistical analyses were conducted in Mplus. The two main sources of missing data were missing father reports (26% of families missing father data) and missing attentional control (129 twin pairs did not complete the attentional control measure due to budget constraints). Children from families missing father data were older than the remaining sample (15 years on average) as were children with missing EATQ-Rs (14 years on average). After adjusting for age, children missing EATQ-R data did not differ from those with EATQ-R data on measures of anxiety or ADHD. However, children with missing father data were more anxious (p=.01), more impulsive and inattentive (ps<.001), and were rated lower in attentional control (p<.001) even after adjusting for age differences. Thus, missing data were best characterized as Missing at Random (MAR) and biometric models were fit to raw data using FIML, which uses all available data, to minimize the bias introduced by missingness. FIML is appropriate when the source of missingness is known and the variables that can account for patterns of missingness are included in the estimation procedure (Enders, 2010).
We first examined correlations that may indicate the presence of genetic or environmental effects. Genetic influences can be estimated by comparing behavioral correlations in monozygotic (identical, or MZ) twins, who share 100% of their genes, with the same correlations in dizygotic (fraternal, or DZ) twins, who share –on average – 50% of their genes. Because MZ cotwins share all of their structural (DNA base sequence variation) genetic factors whereas DZ cotwins share only about one-half of their structural genetic variation, higher correlations in MZ relative to DZ twins should reflect genetic influences. Similarly, correlations in MZ twins that are less than 1.0 reflect the presence of non-genetic (i.e., environmental) influences.
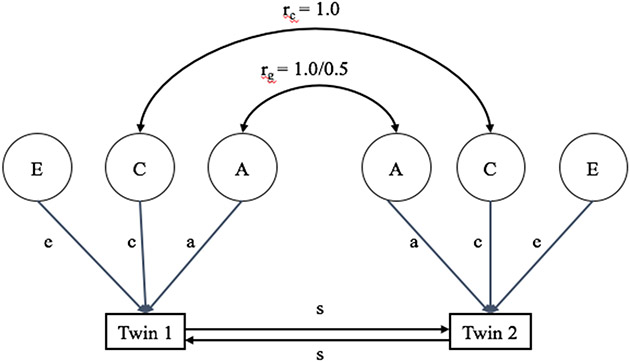
Second, we used a univariate Cholesky decomposition model (Figure 1), which enables the partitioning of the phenotypic variance (i.e., variance of observed behaviors) into additive genetic (A), shared environmental (C), and non-shared environmental (E) influences (Neale & Cardon, 1992). Shared environmental influences are non-genetic influences that make cotwins more similar to each other. Non-shared environmental influences are non-genetic influences that make cotwins different from one another. Statistically, E also includes measurement error and therefore must be included as an underlying source of variation in all models. Consistent with the definitions of C and E, shared environmental influences (C) were assumed to be perfectly correlated between twins, regardless of zygosity, while non-shared environmental influences (E) were assumed to be uncorrelated. We derived univariate Cholesky decompositions for attentional control, ADHD symptoms, and anxiety symptoms, which allowed us to estimate the extent to which A, C, and E contributed to variability in each phenotype.
Figure 1.
Illustration of a univariate ACE twin model with sibling interaction effects. A refers to latent genetic influences on phenotype of interest; C refers to latent shared environmental influences on phenotype of interest, and E refers to latent non-shared environmental influences on phenotype of interest; a, c and e represent the portion of observed variance accounted for by A, C, and E, respectively; rc is the shared environmental correlation within twin pairs and is fixed at 1.0 regardless of zygosity; rg = the genetic correlation within twin pairs and is set to 1.0 for MZ pairs and 0.5 for DZ pairs; s represents the sibling interaction effect.
Third, we planned to test the main study hypothesis by extending the Cholesky model to include multiple phenotypes, allowing for the estimation of the extent to which genetic (A), shared environmental (C), and non-shared environmental (E) influences may be common to multiple phenotypes or unique to a single phenotype (Neale & Cardon, 1992). Because there were three phenotypes of interest, we planned to fit a trivariate Cholesky model to attentional control, ADHD symptoms, and anxiety symptoms. This model allowed for the estimation of influences that are common to all three phenotypes (attentional control, ADHD symptoms, anxiety symptoms), common to just two (ADHD symptoms and anxiety symptoms), or unique to ADHD symptoms. We chose this order of variables given our hypothesis that attentional control is common to both anxiety and ADHD problems and evidence that anxiety symptoms precede symptoms of inattention (Jarrett & Ollendick, 2008). Notably, however, changing the order of the symptom variables (attentional control, ADHD symptoms, anxiety symptoms) does not change model fit or overall conclusions.
Finally, because ADHD is a heterogeneous condition and its subtypes include putatively distinct problems with attentional control, we explored the possibility that genetic and environmental influences on attentional control and anxiety may overlap to different degrees with ADHD subtypes. Specifically, one might expect more overlap for the inattentive relative to the hyperactive phenotype. Thus, we planned two follow-up tests that replaced the global ADHD measure with each phenotype (one test for the inattentive subtype and one test for the hyperactive subtype).
Results
Table 2 shows that the overall pattern of correlations matched expectations. ADHD symptoms were negatively correlated with attentional control, and this relationship remained true for both inattentive and impulsive/hyperactive symptoms2. Anxiety symptoms were also negatively correlated with attentional control. ADHD and anxiety symptom composites were positively correlated, reflecting comorbidity across the two conditions.
Table 2.
Bivariate and twin intraclass phenotypic correlations among attention and ADHD symptom composites during early adolescence
| Bivariate correlations | Twin intraclass | ||||
|---|---|---|---|---|---|
| among composites | correlations | ||||
| Attentional Control |
Anxiety Symptoms |
MZ n = 182 |
SS-DZ n = 173 |
OS-DZ n = 149 |
|
| Attentional Control | 0.68** | 0.13 | 0.04 | ||
| Anxiety Symptoms | −0.32** | 0.56** | 0.30** | 0.36** | |
| ADHD Symptoms | −0.69** | 0.38** | 0.71** | 0.18** | 0.22** |
| ADHD Inattention | −0.74** | 0.41** | 0.72** | 0.16* | 0.18* |
| ADHD Impulsivity/Hyperactivity | −0.54** | 0.29** | 0.59** | 0.12* | 0.19* |
Note: MZ = monozygotic twins; SS-DZ = same-sex dizygotic twins; OS-DZ = male-female dizygotic twins. Composites residualized on adolescent sex.
p < .05,
p < .01.
Intraclass correlations for MZ and DZ twins are shown in Table 2 and cross-twin, cross-trait correlations are shown in Table 3. Correlations for same-sex and opposite-sex DZ twins are presented separately; however, the same-sex and opposite-sex DZ correlations are very similar, as shown in the last two columns of Table 2. MZ correlations were greater than DZ correlations for all three constructs, suggesting the presence of genetic influences on attentional control, ADHD symptoms and anxiety symptoms. Furthermore, correlations between MZ twins were substantially more than twice the observed correlations between DZ twins for each construct except anxiety symptoms. This pattern of correlations could indicate the presence of non-additive, or dominant, genetic effects (D). Because additive (A) and non-additive genetic effects (D) are highly correlated, they are difficult to disentangle without very large sample sizes or samples that contain additional genetic information (e.g., other genetic relatives in addition to twins). Thus, A and D were not parsed in this study. However, the same pattern of correlations can arise from rater bias, which can be partially controlled for by the use of multi-trait, multi-reporter composites (as we have done here) and by including a test of negative sibling interaction effects (Neale & Cardon, 1992). Negative sibling interactions (S) control for similar biases across raters within a family that serve to differentiate between members of a twin pair3. Parent raters may be unfamiliar with a wide range of typical child behaviors or may use one twin as the standard by which the other twin is rated. This can result in parents’ use of even slight differences in behavior as a way to assign distinct identities to individual members of a twin pair. That is, even small differences in twins may be used by parents as justification for assigning quite different ratings to twin siblings, resulting in an overestimation of the degree to which twins differ on a phenotype. Such effects have been shown in twin studies of ADHD (Rietveld, Posthuma, Dolan, & Boomsma, 2003).
Table 3.
Cross-twin, cross-trait phenotypic correlations for attention and ADHD symptom composites
| Attentional Control |
Anxiety Symptoms |
ADHD Symptoms |
Inattention Symptoms |
Impulsivity/ Hyperactivity |
|
|---|---|---|---|---|---|
| Attentional Control | −0.26 | −0.53 | −0.55 | −0.45 | |
| Anxiety Symptoms | −0.12 | 0.29 | 0.26 | 0.27 | |
| ADHD Symptoms | −0.14 | 0.27 | |||
| Inattention | −0.94 | 0.24 | |||
| Impulsivity/Hyperactivity | −0.18 | 0.27 |
Note: MZ twin correlations are above the diagonal; DZ twin correlations are below the diagonal
In the univariate models that provide the foundation for this work, D and S cannot be tested simultaneously. Moreover, shared environment (C) cannot be detected in the presence of dominance or sibling interaction effects; that is, a test of D or S must replace a test of C. Given that our sample was not optimal for testing non-additive genetic effects, we chose to test hypotheses using the AES model in place of the ACE model described above.
Univariate Modeling
Separate univariate Cholesky models (Figure 1) were fit for attentional control, ADHD symptoms, and anxiety symptoms. ADHD symptoms showed the highest proportion of variance explained by genetic influences (A; 70% [95%CI: 64–77]), followed by anxiety symptoms (61%, [95%CI: 54–68]) and attentional control (60%, [95%CI:52–70]). The remaining variance in each phenotype was explained by non-shared environmental influences (E). Negative sibling interaction effects (S) were present for ADHD symptoms (−0.12, p = .001), but not for anxiety symptoms or attentional control.
Multivariate Modeling
To build the appropriate multivariate model, we tested both a full model (i.e., including genetic (A), nonshared environment (E), and sibling interactions (S), as contributors to observed levels of attentional control, ADHD symptoms, anxiety symptoms and a model that included only A and E. Specifically, the full AES model included the following:
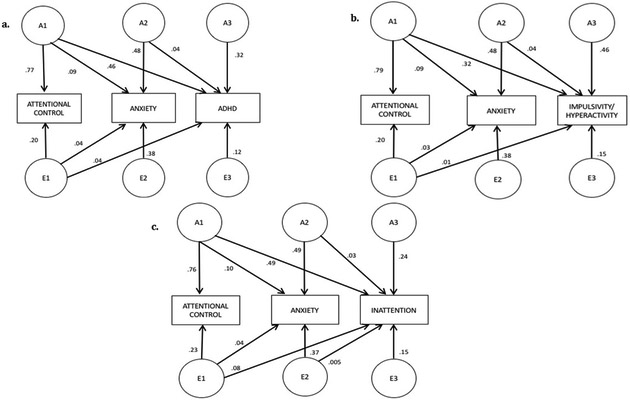
(1) Factors representing genetic (A1 in Figure 2) and non-shared environmental influences (E1 in Figure 2) on attentional control that also influence ADHD and anxiety symptoms;
Figure 2:
Factors representing the proportion of variance explained by genetic (A1) and non-shared environmental (E1) influences on attentional control that also influence anxiety symptoms and ADHD symptoms (or its subcomponents), genetic (A2) and non-shared environmental (E2) influences on anxiety symptoms that also influence ADHD symptoms (or its subcomponents), genetic influences specific to ADHD symptoms (or its subcomponents; A3), and non-shared environmental influences specific to ADHD symptoms (or its subcomponents; E3). Proportions do not sum to 100 due to the presence of sibling interaction effects and rounding error.
(2) factors representing genetic (A2 in Figure 2), and non-shared environmental (E2 in Figure 2) influences that were common only to ADHD symptoms and anxiety symptoms (i.e., were unique from influences on attentional control); and
(3) Factors representing genetic (A3 in Figure 2) and non-shared environmental (E3 in Figure 2) influences specific to ADHD symptoms (i.e., were not shared with attentional control or anxiety symptoms). The full AES model also included separate sibling interaction effects for Attentional Control and ADHD.
The model fits for the full (AES) and AE-only trivariate Cholesky models are shown in Table 4A. A significant difference in model fit between models would indicate that the full model is required to adequately explain the data. A nonsignificant difference would indicate that the more parsimonious model is sufficient. The AE-only model fit the data significantly worse than the AES model (χdiff= 36.5, df = 3, p < .001), providing evidence consistent with the univariate analyses that negative sibling interaction effects contribute to variance in ADHD. Thus, all estimates reported below are from the AES model. The magnitude of the sibling interaction effects (see Table 5) was very similar across measures, ranging from −.12 to −.16. Note that the negative sibling interaction effect on attentional control was not significant in the univariate model, but it reached significance in the trivariate Cholesky. The negative sibling interaction effect on anxiety was not significant in any model.
Table 4.
Model fit statistics for trivariate Cholesky models testing overlap in underlying genetic and environmental influences on attentional control, anxiety, and ADHD symptoms in adolescent twins
| A. Primary model including attentional control, anxiety, and ADHD symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model | Factors | χ2(df) | p | CFI | TLI | RMSEA | Δχ2 (df) | p |
| 1 | AES | 84.30 (37) | <.001 | .974 | .976 | 0.042 | ||
| 2 | AE | 118.8 (39) | <.001 | .942 | 0.948 | 0.062 | 36.3 (2) | <.001 |
| B. Follow-up model-fitting, with ADHD replaced with symptoms of impulsivity/hyperactivity (models 3-4) and inattention (models 5-6) | ||||||||
| Model | Factors | χ2(df) | p | CFI | TLI | RMSEA | Δχ2 (df) | p |
| 3 | AES | 75.08 (37) | <.001 | .937 | .949 | 0.064 | ||
| 4 | AE | 111.1 (39) | <.001 | .881 | .908 | 0.085 | 41.0 (2) | <.001 |
| 5 | AES | 79.2 (37) | <.001 | .951 | .960 | 0.067 | ||
| 6 | AE | 98.1 (39) | <.001 | .931 | .947 | 0.077 | 18.6 (2) | <.001 |
Note: A = additive genetics, E = non-shared environment, S = Sibling interaction; In each case, the AES model was the most parsimonious, best fitting model (i.e., Models 1, 3, and 5).
Table 5.
Standardized estimatesa and confidence intervals from best fitting models testing overlap in underlying genetic and environmental influences on attentional control, anxiety symptom and either ADHD (Model 1), Impulsivity/hyperactivity (Model 3), or Inattention (Model 5), also depicted in Figure 2.
| A1 | A2 | A3 | E1 | E2 | E3 | s | |
|---|---|---|---|---|---|---|---|
| Model 1. | |||||||
| Attentional Control | .88 (.91 .96) | .45 (.39 .53) | −.15 | ||||
| Anxiety | −.30 (−.39 −.21) | .70 (.63 .77) | −.20 (−.30 −.10) | .62 (.56 .67) | |||
| ADHD | −.68 (−.76 −.61) | .20 (.11 .28) | .57 (.50 .64) | −.20 (−.27 −.13) | .03 (−.03 .09) | .34 (.29 .40) | −.15 |
| Model 3. | |||||||
| Attentional Control | .89 (.81 .96) | .45 (.38 .52) | −.16 | ||||
| Anxiety | −.30 (−.39 −.21) | .69 (.62 .71) | −.18 (−.28 −.08) | .62 (.54 .71) | |||
| Impulsivity/hyperactivity | −.57 (−.66 −.49) | .20 (.10 .30) | .68 (.60 .76) | −.10 (−.16 −.03) | −.02 (−.04 .07) | .39 (.32 .45) | −.16 |
| Model 5. | |||||||
| Attentional Control | .87 (.80 .95) | .48 (.40 .55) | −.12 | ||||
| Anxiety | −.31 (−.40 −.22) | .70 (.62 .77) | −.19 (−.30 −.09) | .61 (.54 .67) | |||
| Inattention | −.70 (−.78 −.62) | .16 (.09 .23) | .49 (.42 .56) | −.28 (−.36 −.19) | .07 (.004 .14) | .39 (.32 .45) | −.13 |
Note:
Estimates are not squared in order to show direction of effects
A1 = genetic influences on attentional control that also influence anxiety symptoms and ADHD symptoms (or its subcomponents)
A2 = genetic influences on anxiety symptoms that also influence ADHD symptoms (or its subcomponents)
A3 = genetic influences specific to ADHD symptoms (or its subcomponents)
E1-3 = non-shared environmental counterpart to A1-3
s = sibling interaction parameter; sibling interaction effects are significant at p<.001).
Standardized, unsquared path estimates with 95% confidence intervals from the best-fitting models are shown in Table 5 and illustrate the directionality of the relations between the underlying genetic and environmental influences on the phenotype. In the AES model, the negative sibling interaction effect contributes to the estimate of the total phenotypic covariation independently of the estimated underlying genetic and non-shared environmental covariation.4 The squared estimates, shown in Figure 2a, reflect the proportion of total variance that is attributable to each underlying factor.
We found genetic influences common to attentional control, ADHD symptoms and anxiety symptoms; genetic influences common to ADHD symptoms and anxiety symptoms (but not attentional control); and genetic influences specific to ADHD symptoms (Figure 2a). These combined genetic factors (A1, A2, and A3) accounted for 82% of the total observed variance in ADHD symptoms. Forty-six percent of the variance in ADHD symptoms was accounted for by genetic influences shared with attentional control and anxiety symptoms (A1 in Figure 2a). Another 4% was accounted for by genetic influences shared just with anxiety symptoms (A2 in Figure 2a) and 32% of the variance was due to genetic factors independent of attentional control and anxiety (A3 in Figure 2a). In contrast, 57% of the total variance in anxiety symptoms was explained by genetic factors; 9% was accounted for by genetic factors shared with attentional control and ADHD symptoms (A1 in Figure 2a). Half (50.5%) of the total covariance between anxiety and ADHD could be accounted for by genetic factors that both shared with attentional control. A further 10.2% of the covariance was explained by non-shared environmental factors related to attentional control.
As can also be seen in Figure 2a, there were non-shared environmental influences specific to anxiety symptoms and specific to ADHD symptoms. Most importantly, we also found non-shared environmental influences common to attentional control, anxiety symptoms, and ADHD symptoms such that 4% of the variance in anxiety symptoms and 4% of the variance in ADHD symptoms was due to non-shared environmental influences common to all three constructs.
Follow-up Model-fitting
To probe the relationship between attentional control, anxiety symptoms and ADHD symptoms more fully, we analyzed the constituent components of ADHD by substituting either impulsivity/hyperactivity or inattention symptoms for ADHD symptoms in the AES model, with attentional control and anxiety symptoms remaining as the first and second variables.
Model fits for these follow-up models are shown in Table 4b, and path estimates are presented in Figure 2b-c. Genetic influences common to all three constructs remained present for both impulsivity/hyperactivity (32%) and inattention (49%). Similarly, the presence of non-shared environmental influences common to all three constructs was present for both impulsivity/hyperactivity (1%) and for inattention (5%). This suggests that the pattern of findings is consistent for ADHD symptoms overall and also for symptoms of impulsivity/hyperactivity and for inattention.
Discussion
Consistent with previous work, symptoms of ADHD, GAD, and social phobia were influenced by both genetic and non-shared environmental factors during adolescence. As predicted, we found evidence for similar genetic influences on association between ADHD and anxiety (GAD and social phobia) at approximately age 13. This finding suggests that at least some overlap in the genetic mechanisms underlying both types of symptoms by this age. Moreover, a proportion of the variance in ADHD and anxiety symptoms showed common genetic influences with our measure of attentional control. This shared genetic covariance supports our hypothesis that the same genetic factors that precipitate problems with attentional control may also be associated with the development of comorbid ADHD and anxiety problems during the adolescent years. Results are also consistent with the possibility that attentional control plays a mechanistic role in the manifestation of comorbid ADHD and anxiety problems.
The primary contribution of this work is evidence that the same genetic and non-shared environmental factors are associated with the presence of attentional control deficits, ADHD symptoms, and symptoms of GAD and social phobia. This finding corroborates research linking genetic influences on attentional control with anxiety symptoms (Gagne et al., 2017) and advances attentional control as a putative mechanism in the comorbidity of ADHD and anxiety during the adolescent years, when both types of problems are salient. Our findings also supplement those from other approaches, such as GWAS, which have found relatively little overlap in polygenic risk scores for children’s behavioral problems from early/middle childhood to late childhood/adolescence (Meier et al., 2018). Thus, while each approach has shortcomings, twin study approaches such as ours identify the extent of the genetic overlap across symptom clusters and disorders and provide a foundation of knowledge for further investigation via other approaches (e.g., GWAS, polygenic risk scores).
Nonetheless, three important nuances should be noted in this work. First, associations between attentional control and anxiety symptoms were much smaller than the associations between attentional control and ADHD symptoms. That is, although the same genetic and environmental influences may contribute to both ADHD and anxiety symptoms, they are unlikely to do so in equal proportions. Second, these associations were modest overall, possibly illustrating that different facets of attentional control may be most relevant for ADHD versus anxiety symptoms. For example, an inability to shift attention away from worrisome thoughts or perceived threat may be most relevant for anxiety problems (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), whereas an inability to focus attention may play a larger role in the development of ADHD. Other early-developing attentional processes, such as the ability to ignore irrelevant stimuli, may also play a role in the manifestation of anxiety and ADHD symptoms; however, these other attentional processes are not fully captured in the measures used here. These attentional processes appear to be critical for other aspects of development, such as mitigating impulsive tendencies or sensitivity to reward, that rely on maturation of the prefrontal cortex during adolescence. A more specific understanding of the precise attentional processes that underlie individual facets of risk and pathways to comorbidity will be an important avenue for follow-up work. Third, genetic (but not environmental) influences that were not associated with attentional control were also shared between ADHD and anxiety symptoms, reflecting the presence of other unmeasured genetically-influenced mechanisms that contribute to ADHD-anxiety comorbidity during the adolescent years. These mechanisms will also be important to identify in future research, keeping in mind that heritability estimates can change during the transition from adolescence to adulthood, including for the behaviors assessed here (Bergen, Gardner, & Kendler, 2007).
We also found small but significant non-shared environmental influences common to attentional control, ADHD symptoms, and symptoms of GAD and social phobia. Examples of non-shared environment relevant to attention, anxiety and ADHD during adolescence might include different treatment by family members or peers (Long, Aggen, Gardner, & Kendler, 2015), or differential exposure to biological agents (Lessov-Schlaggar et al., 2013). Further nonshared experiences (e.g., a traumatic classroom experience) may initiate differences between siblings that are then compounded by individual experiences over time (Plomin, 2011).
In addition to overlapping influences from the non-shared environment, we found unique genetic influences on ADHD symptoms, consistent with previous work suggesting the existence of discrete pathways for the development of ADHD and anxiety symptoms. These genetically-influenced mechanisms, which appear to be associated with one set of symptoms or another (but not both), may reflect genetic influences that contribute only to ADHD symptoms or genetic influences that contribute to comorbidity between ADHD and symptoms of disorders other than GAD or social phobia. Although we focused on GAD and social phobia symptoms because of high rates of overlap, ADHD symptoms also overlap with other types of problems that are observable by adolescence (Mash & Barkley, 2014). In our statistical model, which does not include symptoms of other disorders, this type of overlap between ADHD and other symptoms would appear as ADHD-specific effects. The incomplete specification of contributing factors is important to consider when discussing discrete genetic and non-shared environmental influences on ADHD and/or GAD and social phobia symptoms.
We also found that sibling interaction effects contributed to variability in attentional control and symptoms of ADHD. Genetically informative studies of ADHD have previously detected small but significant negative sibling interaction effects (Rietveld et al., 2003). Thus, finding such effects in attentional control given the strong relationship between measures of attentional control and symptoms of inattention was unsurprising. The lack of sibling interaction effects on anxiety symptoms is consistent with current knowledge of the etiology of anxiety problems (Franić, Middeldorp, Dolan, Ligthart, & Boomsma, 2010). Thus, although our results are consistent with findings in the extant literature, ours is the first study to directly test for the presence of sibling interaction effects in attentional control during adolescence.
Finally, this work extends previous knowledge about adolescence as a critical developmental period for understanding the development of psychopathology. Specifically, adolescence represents a period during which the brain, including neural systems underlying attentional control, is rapidly maturing. Because adolescence is also a time during which comorbid ADHD and anxiety conditions become increasingly apparent, focusing our study on this period allows us to identify circumstances that may contribute to compounding psychological problems in young people. Specifically, problems with attention shifting and focusing that are initially noted as ADHD symptoms (as they are easily identified by observers than are internalizing problems) that do not undergo typical age-related improvements may signal an additional marker of risk for comorbid anxiety conditions. Through a developmental psychopathology lens, then, one may view adolescence as offering a unique opportunity to identify transformations in the behavioral manifestation of a common underlying problem with attention. Our work also suggests that intervention programs aimed at improving attentional control during a period of neural plasticity may benefit adolescents in multiple ways by altering a developmental trajectory toward comorbid conditions.
Limitations
Our sample presents potential limitations. First, we used a cross-sectional design to maximize available data that would best capture general processes of attention control and symptoms. This prohibits conclusions about causation and limits the degree to which our findings can be generalized across development. Different developmental periods may be associated with fluctuations in symptom co-occurrence and/or relevant genetic and environmental mechanisms. These fluctuations are important to consider in tandem with typical trajectories of symptom manifestation and diagnoses of comorbid ADHD and anxiety problems. That is, age-related fluctuations in symptoms may indicate changes in the primary mechanisms by which disorders manifest, resulting in questions about the generalizability of attention as a stable mechanism of ADHD-anxiety comorbidity.
Second, because heritability is a population-level parameter estimate, our results are specific to the population from which our sample was drawn. Similarly, although our sample was slightly enriched for symptoms of psychopathology roughly 5 years earlier (ADHD prevalence = 4.5%), this work was conducted in a community sample. Research in clinical, high-risk, or severely impaired populations may not produce the same results, as different mechanisms may operate at the extremes of the distribution. We also acknowledge that the unique nature of this sample and the measures used will make efforts at exact replication difficult. However, given that the effects investigated here are already known to be nuanced in nature, conceptual rather than exact replications may best illuminate the broader nature and boundaries of the effects that we demonstrated.
Third, we note that teacher data, which are important for ultimately diagnosing ADHD in youth, were not available for participants in this study. This may be particularly relevant to the current sample given some question about whether twins are representative in terms of adolescent psychopathology. At least some work suggests that twins do not significantly differ from the general population (i.e., singletons) with regard to characteristics of personality (Johnson, Krueger, Bouchard, & McGue, 2002) or psychopathology (Christensen, Vaupel, Holm, & Yashin, 1995; Kendler & Prescott, 2006), though it not clear whether representativeness may fluctuate as developmental processes unfold.
Parents may contrast behaviors of their twins, leading to exaggerated intrapair differences (Goldsmith, Buss, & Lemery, 1997; Saudino, Cherny, & Plomin, 2000). Combining self-reports with parent reports should mitigate rater contrast effects; however, siblings may engage in a similar calculus when answering questions about their own behavior. Alternatively, parents (and others) may react to siblings in ways that contribute to the differentiation between them (Barrett, Fox, & Farrell, 2005). If one member of the twin pair is truly experiencing more intense symptoms than his or her cotwin, determining the source of sibling interaction effects may point to potential resiliency factors.
Additionally, both genetic dominance and sibling interactions may result in the same pattern of lower-than-expected DZ correlations relative to the MZ correlations. Despite interaction effects in the twin models, tests of overall phenotypic variance differences between MZ and DZ twins were largely non-significant. Both dominance and sibling interaction effects may be simultaneously present, but the classical twin design holds greater power to detect sibling interaction effects (Rietveld et al., 2003). Thus, larger samples are required to determine if dominance effects, sibling interaction effects, or both are responsible for the lower than expected DZ correlations.
Conclusions
We used multi-informant measures to show that attentional control shares genetic and environmental influences with symptoms of ADHD and anxiety (GAD and social phobia). We also found that shared genetic and environmental influences were greater for the inattentive ADHD subtype than for the hyperactive/impulsive ADHD subtype. This work illustrates that similar genetic and environmental factors may be contributing to the development of comorbid ADHD and anxiety diagnoses and offers an initial mechanism – attentional control – by which we can begin to understand ADHD-anxiety comorbidity during adolescence.
Supplementary Material
Acknowledgments
Data collection was supported by R37 MH50560 and R01 MH59785 from the National Institute of Mental Health. The writing of this manuscript was partially supported by T32 MH018931, K01 MH100240, and P50 MH100031 from the National Institute of Mental Health. Infrastructure support was provided by the Waisman Center via P30 HD03352 from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Throughout the manuscript, we refer to disorders, symptoms, and problems (e.g., anxiety disorders, anxiety symptoms, and anxiety problems), which we consider to be related but nonredundant terms. In this context, disorder refers to a clinically-diagnosed condition that meets the criteria set forth by the DSM-V; symptom refers to behavioral criteria for DSM-V disorders, but does not necessarily indicate levels of clinical impairment or diagnosis; problem refers to symptoms and/or behaviors that are related to DSM-V criteria that cause some level of functional impairment.
Notably, the correlation between attentional control and impulsive/hyperactive symptoms became nonsignificant if inattentive symptoms were controlled (r = 0.05) suggesting that attentional control and inattention explained the common variance in impulsive/hyperactive symptoms, potentially offering information about the nature of the overlap between attentional control and ADHD subtypes.
As an additional justification for examining sibling interaction effects, we note that DZ twins tend to be more affected by sibling interactions than MZ twins due to their smaller degree of genetic relatedness. This leads to very low intrapair phenotypic resemblance and higher overall variances for DZ compared to MZ twins (Neale & Cardon, 1992). Indeed, variances appeared greater for DZ than MZ twins for all variables in this sample, though Levene’s test was not significant.
Because the sibling interaction effect on the estimated phenotypic variance is zygosity-dependent, we used the sum of genetic and environmental variation alone as the basis for standardizing path estimates.
References
- Adler L, Barkley RA, Newcorn J, Spencer TJ, & Weiss MD (2007). Managing ADHD in children, adolescents, and adults with comorbid anxiety in primary care. Primary Care Companion to The Journal of Clinical Psychiatry, 9(2), 129–138. [PMC free article] [PubMed] [Google Scholar]
- Anastopoulos AD, DuPaul GJ, Weyandt LL, Morrissey-Kane E, Sommer JL, Rhoads LH, … Gudmundsdottir BG (2016). Rates and patterns of comorbidity among first-year college students with ADHD. Journal of Clinical Child & Adolescent Psychology, 1–12. 10.1080/15374416.2015.1105137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM, & Goldstein LH (2003). Manual for the MacArthur health and behavior questionnaire (HBQ 1.0) MacArthur Foundation Research Network on Psychoapthology and Development, University of Pittsburgh. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Barrett PM, Fox T, & Farrell LJ (2005). Parent—Child Interactions With Anxious Children and With Their Siblings: An Observational Study. Behaviour Change, 22(04), 220–235. 10.1375/bech.22.4.220 [DOI] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, & Robertson IH (2005). Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia, 43(13), 1847–1857. 10.1016/j.neuropsychologia.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Benke KS, Nivard MG, Velders FP, Walters RK, Pappa I, Scheet PA, … Middeldorp CM (2014). A Genome-wide association meta-analysis of preschool internalizing problems. Journal of the American Academy of Child & Adolescent Psychiatry, 53(6), 667–676.e7. 10.1016/j.jaac.2013.12.028 [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, & Kendler KS (2007). Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics, 10(3), 423–433. 10.1375/twin.10.3.423 [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81(1), 326–339. 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Neiderhiser JM, Kiel EJ, Leve LD, Shaw DS, & Reiss D (2011). The association between infants’ attention control and social inhibition is moderated by genetic and environmental risk for anxiety. Infancy, 16(5), 490–507. 10.1111/j.1532-7078.2011.00068.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, & Rothbart MK (1992). Development and validation of an early adolescent temperament measure. The Journal of Early Adolescence, 12(2), 153–173. 10.1177/0272431692012002002 [DOI] [Google Scholar]
- Christensen K, Vaupel JW, Holm NV, & Yashin AI (1995). Mortality among twins after age 6: Fetal origins hypothesis versis twin method. British Medical Journal, 310(6977), 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (2002). A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology, 70(1), 6–20. 10.1037/0022-006X.70.16 [DOI] [PubMed] [Google Scholar]
- Clavarino AM, Mamun AA, O’Callaghan M, Aird R, Bor W, O’Callaghan F, … Alati R (2009). Maternal anxiety and attention problems in children at 5 and 14 years. Journal of Attention Disorders, 13(6), 658–667. 10.1177/1087054709347203 [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … 23andMe Research Team. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1), 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, McGoey KE, Eckert TL, & VanBrakle J (2001). Preschool children with attention-deficit/hyperactivity disorder: Impairments in behavioral, social, and school functioning. Journal of the American Academy of Child & Adolescent Psychiatry, 40(5), 508–515. 10.1097/00004583-200105000-00009 [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, … Guthrie IK (2001). The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development, 72(4), 1112–1134. 10.1111/1467-8624.00337 [DOI] [PubMed] [Google Scholar]
- Ellis LK, & Rothbart MK (2001). Revision of the Early Adolescent Temperament Questionnaire. Poster presented at the Biennial Meeting of the Society for Research in Child Development, Minneapolis, MN. [Google Scholar]
- Enders C (2010). Applied missing data analysis. New York: The Guilford Press. [Google Scholar]
- Fan J, Wu Y, Fossella JA, & Posner MI (2001). Assessing the heritability of attentional networks. BMC Neuroscience, 2, 14–14. 10.1186/1471-2202-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franić S, Middeldorp CM, Dolan CV, Ligthart L, & Boomsma DI (2010). Childhood and Adolescent Anxiety and Depression: Beyond Heritability. Journal of the American Academy of Child & Adolescent Psychiatry, 49(8), 820–829. 10.1016/j.jaac.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Gagne JR, O’Sullivan DL, Schmidt NL, Spann CA, & Goldsmith HH (2017). The shared etiology of attentional control and anxiety: An adolescent twin study. Journal of Research on Adolescence, 27(1), 122–138. 10.1111/jora.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Grover RL, & Ialongo N (2005). Parenting behaviors among anxious and non-anxious mothers: Relation with concurrent and long-term child outcomes. Child & Family Behavior Therapy, 26(4), 23–41. 10.1300/J019v26n04_02 [DOI] [Google Scholar]
- Goldsmith HH (1991). A zygosity questionnaire for young twins: A research note. Behavior Genetics, 21(3), 257–269. 10.1007/BF01065819 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss KA, & Lemery KS (1997). Toddler and childhood temperament: Expanded content, stronger genetic evidence, new evidence for the importance of environment. Developmental Psychology, 33(6), 891–905. 10.1037/0012-1649.33.6.891 [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, & Kendler KS (2001). A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry, 158(10), 1568–1578. 10.1176/appi.ajp.158.10.1568 [DOI] [PubMed] [Google Scholar]
- Jarrett MA, & Ollendick TH (2008). A conceptual review of the comorbidity of attention-deficit/hyperactivity disorder and anxiety: Implications for future research and practice. Clniical Psychology Review, 28, 1266–1280. 10.1016/j.cpr.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF, Bouchard TJ, & McGue M (2002). The personalities of twins: Just ordinary folks. Twin Research, 5(2), 125–131. 10.1375/twin.5.2.125 [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Prescott CA (2006). Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York: The Guilford Press. [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, … Ernst M (2009). Amygdala function and 5-htt gene variants in adolescent anxiety and major depressive disorder. Deep Brain Stimulation for Depression: Emerging Targets and Differing Approaches, 65(4), 349–355. 10.1016/j.biopsych.2008.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Doelger L, & Goldsmith HH (2008). Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant and Child Development, 17(4), 365–385. 10.1002/icd.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov-Schlaggar CN, Lepore RL, Kristjansson SD, Schlaggar BL, Barnes KA, Petersen SE, … Barch DM (2013). Functional neuroimaging study in identical twin pairs discordant for regular cigarette smoking: fMRI smoking study in twins. Addiction Biology, 18(1), 98–108. 10.1111/j.1369-1600.2012.00435.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ, & Posner MI (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106(3), 912–917. 10.2307/40254771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EC, Aggen SH, Gardner C, & Kendler KS (2015). Differential parenting and risk for psychopathology: a monozygotic twin difference approach. Social Psychiatry and Psychiatric Epidemiology, 50(10), 1569–1576. 10.1007/s00127-015-1065-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor I, Eisenberg J, Tyano S, Sever Y, Cohen H, Ebstein RP, & Kotler M (2001). Family-based association study of the serotonin transporter promoter region polymorphism (5-HTTLPR) in attention deficit hyperactivity disorder. American Journal of Medical Genetics, 105(1), 91–95. [DOI] [PubMed] [Google Scholar]
- Mash EJ, & Barkley RA (2014). Child Psychopathology (Third). New York, New York: The Guilford Press. [Google Scholar]
- Meier S, Trontti K, Als TD, Laine M, Pedersen MG, Bybjerg-Grauholm J, … Mors O (2018). Genome-wide association study of anxiety and stress-related disorders in the iPSYCH cohort. BioRxiv, 263855 10.1101/263855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E (2004). Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development, 75(5), 1373–1386. 10.2307/3696489 [DOI] [PubMed] [Google Scholar]
- Muris P, Meesters C, & Rompelberg L (2007). Attention control in middle childhood: Relations to psychopathological symptoms and threat perception distortions. Behaviour Research and Therapy, 45(5), 997–1010. 10.1016/j.brat.2006.07.010 [DOI] [PubMed] [Google Scholar]
- Muris P, van der Pennen E, Sigmond R, & Mayer B (2008). Symptoms of anxiety, depression, and aggression in non-clinical children: Relationships with self-report and performance-based measures of attention and effortful control. Child Psychiatry and Human Development, 39(4), 455–467. 10.1007/s10578-008-0101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M, & Cardon L (1992). Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Ollendick TH, & Hirshfeld-Becker DR (2002). The developmental psychopathology of social anxiety disorder. Social Anxiety: From Laboratory Studies to Clinical Practice, 51(1), 44–58. 10.1016/S0006-3223(01)01305-1 [DOI] [PubMed] [Google Scholar]
- Overgaard KR, Aase H, Torgersen S, & Zeiner P (2012). Co-occurrence of adhd and anxiety in preschool children. Journal of Attention Disorders, 20(7), 573–580. 10.1177/1087054712463063 [DOI] [PubMed] [Google Scholar]
- Perrin S, & Last CG (1996). Relationship between ADHD and anxiety in boys: results from a family study. Journal of the American Academy of Child & Adolescent Psychiatry, 35(8), 988–996. 10.1097/00004583-199608000-00009 [DOI] [PubMed] [Google Scholar]
- Plomin R (2011). Commentary: Why are children in the same family so different? Non-shared environment three decades later. International Journal of Epidemiology, 40(3), 582–592. 10.1093/ije/dyq144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, & Rohde LA (2015). Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry, 56(3), 345–365. 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, & Sheese BE (2007). Attention genes. Developmental Science, 10(1), 24–29. 10.1111/j.1467-7687.2007.00559.x [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, & Plomin R (2000). Infant zygosity can be assigned by parental report questionnaire data. Twin Research and Human Genetics, 3(03), 129–133. 10.1375/twin.3.3.129 [DOI] [PubMed] [Google Scholar]
- Rietveld MJH, Posthuma D, Dolan CV, & Boomsma DI (2003). ADHD: Sibling interaction or dominance: An evaluation of statistical power. Behavior Genetics, 33(3), 247–255. [DOI] [PubMed] [Google Scholar]
- Rothbart Mary K., & Bates JA (1998). Temperament In Eisenberg N (Ed.), Handbook of child psychology (5th ed., Vol. Vol. 3 Social, emotional, and personality development, pp. 105–176). New York: Wiley. [Google Scholar]
- Rothbart Mary Klevjord. (1981). Measurement of temperament in infancy. Child Development, 52(2), 569–578. [Google Scholar]
- Rowe D, Stever C, Gard JC, Cleveland H, Sanders M, Abramowitz A, … Waldman I (1998). The relation of the dopamine transporter gene (DAT1) to symptoms of internalizing disorders in children. Behavior Genetics, 28(3), 215–225. 10.1023/A:1021427314941 [DOI] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, & Boyce WT (2011). Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society, 17(01), 120–132. 10.1017/S1355617710001335 [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Cherny SS, & Plomin R (2000). Parent ratings of temperament in twins: explaining the ‘too low’ DZ correlations. Twin Research, 3(04), 224–233. 10.1375/twin.3.4.224 [DOI] [PubMed] [Google Scholar]
- Schmidt NL, Van Hulle CA, Brooker RJ, Meyer LR, Lemery-Chalfant K, & Goldsmith HH (2013). Wisconsin twin research: Early development, childhood psychopathology, autism, and sensory over-responsivity. Twin Research and Human Genetics, 16(Special Issue 01), 376–384. 10.1017/thg.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Shelton T, Barkley R, Crosswait C, Moorehouse M, Fletcher K, Barrett S, … Metevia L (1998). Psychiatric and psychological morbidity as a function of adaptive disability in preschool children with aggressive and hyperactive-impulsive-inattentive behavior. Journal of Abnormal Child Psychology, 26(6), 475–494. 10.1023/A:1022603902905 [DOI] [PubMed] [Google Scholar]
- Tsang TW, Kohn MR, Efron D, Clarke SD, Clark CR, Lamb C, & Williams LM (2012). Anxiety in young people with ADHD: Clinical and self-report outcomes. Journal of Attention Disorders, 19(1), 18–26. 10.1177/1087054712446830 [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, & DeFries JC (2000). Etiology of inattention and hyperactivity/impulsivity in a community sample of twins with learning difficulties. Journal of Abnormal Child Psychology, 28(2), 149–159. 10.1023/A:1005170730653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.