Abstract
Triheteromeric NMDA-type glutamate receptors that contain two GluN1 and two different GluN2 subunits contribute to excitatory neurotransmission in the adult central nervous system (CNS). Here, we report properties of the triheteromeric GluN1/2B/2D NMDA receptor subtype that is expressed in distinct neuronal populations throughout the CNS. We show that neither GluN2B nor GluN2D dominate the functional properties of GluN1/2B/2D receptors, since agonist potencies, open probability, and glutamate deactivation time course of GluN1/2B/2D receptors are intermediate to those of diheteromeric GluN1/2B and GluN1/2D receptors. Furthermore, channel block of GluN1/2B/2D by extracellular Mg2+ is intermediate compared to GluN1/2B and GluN1/2D, but GluN1/2B/2D is more sensitive to block by ketamine and memantine compared to GluN1/2B in the presence of physiological Mg2+. Subunit-selective allosteric modulators have distinct activity at GluN1/2B/2D receptors, including GluN2B-selective antagonists, ifenprodil, EVT-101, and CP-101–606, that inhibit with similar potencies, but with different efficacies at GluN1/2B/2D (~65% inhibition) compared to GluN1/2B (~95% inhibition). Furthermore, the GluN2B-selective positive allosteric modulator spermine enhances responses from GluN1/2B/2D, but not GluN1/2A/2B receptors. We show that these key features of allosteric modulation of recombinant GluN1/2B/2D receptors are also observed for NMDA receptors in hippocampal interneurons, but not CA1 pyramidal cells, consistent with expression of GluN1/2B/2D receptors in interneurons and GluN1/2A/2B receptors in pyramidal cells. Altogether, we uncover previously unknown functional and pharmacological properties of triheteromeric GluN1/2B/2D receptors that can facilitate advances in our understanding of their physiological roles in neural circuit function and therapeutic drug actions.
Introduction
NMDA receptors are critically involved in neuronal development, sensory processing, and synaptic plasticity, but are also implicated in numerous diseases in the central nervous system (CNS) (Traynelis et al., 2010). NMDA receptors are tetrameric assemblies of subunits, usually containing two GluN1 subunits that bind glycine (or D-serine) and two GluN2 subunits that bind glutamate (Hansen et al., 2017). One GluN1 subunit has been identified with eight different splice variants (Hansen et al., 2017), and there are four different GluN2 subunits (GluN2A-D) that exhibit striking variation in their developmental and regional expression patterns (Monyer et al., 1994). The composition of GluN2 subunits in NMDA receptors imparts distinct functional properties, and the GluN2 subunits dictate the physiological roles of NMDA receptor subtypes (Monyer et al., 1994; Vicini et al., 1998; Gielen et al., 2009; Yuan et al., 2009; Hansen et al., 2017).
Triheteromeric NMDA receptor subtypes (e.g. GluN1/2A/2B, GluN1/2A/2C, and GluN1/2B/2D), which contain two GluN1 and two different GluN2 subunits, are abundantly expressed in many neuronal populations (Sheng et al., 1994; Takahashi et al., 1996; Chazot & Stephenson, 1997; Luo et al., 1997; Dunah et al., 1998; Rumbaugh & Vicini, 1999; Cathala et al., 2000; Pina-Crespo & Gibb, 2002; Brickley et al., 2003; Jones & Gibb, 2005; Brothwell et al., 2008; Gray et al., 2011; Rauner & Kohr, 2011; Hildebrand et al., 2014; Huang & Gibb, 2014; Swanger et al., 2015; Perszyk et al., 2016; Bhattacharya et al., 2018a; Swanger et al., 2018). Past studies of recombinant NMDA receptors almost exclusively focused on diheteromeric receptors, but recently, properties of recombinant triheteromeric GluN1/2A/2B and GluN1/2A/2C receptors were described (Hansen et al., 2014; Stroebel et al., 2014; Sun et al., 2017; Bhattacharya et al., 2018a). Surprisingly, these studies revealed functional and pharmacological features of triheteromeric receptors that are not predicted from the average of the respective diheteromeric NMDA receptor properties. For example, the GluN2A subunit accelerate deactivation of the GluN2B subunit in GluN1/2A/2B receptors, resulting in deactivation kinetics more similar to GluN1/2A receptors (Hansen et al., 2014; Sun et al., 2017). These dominant allosteric interactions by GluN2A are consistent with cryo-EM structures of GluN1/2A/2B and reduced inhibition by GluN2B-selective antagonists (Hansen et al., 2014; Stroebel et al., 2014; Cheriyan et al., 2016; Lu et al., 2017). By contrast, GluN2A is not governing the function of GluN1/2A/2C receptors that display deactivation kinetics intermediate to those of GluN1/2A and GluN1/2C receptors (Bhattacharya et al., 2018a). However, the GluN2C-selective positive allosteric modulators PYD-106 only potentiate diheteromeric GluN1/2C receptors, but not triheteromeric GluN1/2A/2C receptors (Khatri et al., 2014; Bhattacharya et al., 2018a; Kaiser et al., 2018).
Triheteromeric GluN1/2B/2D receptors have been suggested to participate in neuronal signaling in subthalamic nucleus (Swanger et al., 2015), hippocampal interneurons (von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018), hippocampal granule cells (Pina-Crespo & Gibb, 2002), striatal medium spiny neurons (Logan et al., 2007), substantia nigra pars compacta (Jones & Gibb, 2005; Brothwell et al., 2008; Huang & Gibb, 2014; Bhattacharya et al., 2018b), cerebellar Golgi cells (Brickley et al., 2003), and spinal cord (Hildebrand et al., 2014) based on biochemical data and observations of neuronal NMDA receptors with functional and pharmacological properties that appear intermediate to those of diheteromeric GluN1/2B and GluN1/2D receptors. Despite their prevalence in the CNS, there is a lack of quantitative data describing important properties of triheteromeric GluN1/2B/2D receptors, such as deactivation time course, block by extracellular Mg2+, and subunit-selective allosteric modulation (Stroebel et al., 2018). Here, we describe these properties of recombinant GluN1/2B/2D receptors and demonstrate that key pharmacological features of the receptor are conserved in neuronal NMDA receptors, thereby providing new understanding relevant to neuronal circuit function and the selective pharmacological targeting of NMDA receptor subtypes in disease.
Methods
Ethical approval.
Animal anesthesia and euthanasia procedures were approved by the University of Montana Institutional Animal Care and Use Committee, and performed in accordance with state and federal Animal Welfare Acts and the policies of the Public Health Service. Adult C57Bl/6J mice (aged P21–28) of both sexes were used in this study and mice were given access to food and water at libitum. All efforts were made to minimize animal pain and suffering and to reduce the number of animals used in this study. The authors understand the ethical principles under which The Journal of Physiology operates and the present work complies with the animal ethics checklist outlined by the journal.
DNA constructs and ligands.
Rat cDNAs for GluN1–1a (GenBank accession number U08261), GluN1–1b (U08263), GluN2A (D13211), GluN2B (U11419) and GluN2D (L31611) were provided by S. Heinemann (Salk Institute, La Jolla, CA), S. Nakanishi (Osaka Bioscience Institute, Osaka), and P. Seeburg (University of Heidelberg, Heidelberg). DNA constructs for expression of GluN1/2BC1/2DC2 receptors were designed similar to previously described constructs for GluN1/2AC1/2BC2 (Hansen et al., 2014). Briefly, the peptide tags C1 and C2 were inserted in place of the stop codon in the open reading frame of GluN2A to generate 2AC1 and 2AC2. The entire CTDs of GluN2B or GluN2D were then replaced by the CTD of 2AC1 and 2AC2 to generate the subunits denoted 2BC1, 2BC2, 2DC1, and 2DC2. DL-APV (Hello Bio, Princeton, NJ), ifenprodil (Abcam Biochemicals, Cambridge, MA, and Hello Bio), CP-101,606 (Axon Medchem, Reston, VA), gabazine (Hello Bio), QNZ-46 (Tocris Bioscience, Minneapolis, MN), spermine (Hello Bio), memantine (Hello Bio), and CIQ (Hello Bio) were obtained from commercial sources. MPX-004 was synthesized as previously described (Volkmann et al., 2016) and provided by Luc Therapeutics. DQP-1105 and NAB-14 were synthesized essentially as previously described (Acker et al., 2013; Swanger et al., 2018) and kindly provided by Dr. Dennis Liotta (Emory University). EVT-101 was kindly provided by H. Lundbeck (Denmark). All other ligands were purchased from Sigma-Aldrich (St. Louis, MO).
Two-electrode voltage-clamp recordings.
Oocytes were purchased from Rob Weymouth (Xenopus 1, Dexter, MI) and injected with in vitro-transcribed cRNA encoding NMDA receptor subunits. Expression of triheteromeric receptors in oocytes was performed as previously described (Hansen et al., 2014). Briefly, the ratios of injected cRNAs encoding GluN1 and GluN2 subunits were optimized to produce minimal escape currents from diheteromeric receptors (Fig. 1). The injected oocytes were incubated 2–4 days at 19 °C before electrophysiological recordings were performed at room temperature (23 °C) as previously described (Hansen et al., 2014). Unless otherwise stated, the oocytes were voltage-clamped at −40 mV and the extracellular recording solution contained (in mM) 90 NaCl, 1 KCl, 10 HEPES, 0.5 BaCl2, and 0.01 EDTA (pH 7.4 with NaOH). Oocytes expressing GluN2A- or GluN2B-containing NMDA receptors, including triheteromeric GluN1/2BC1/2DC2 receptors, were injected with 20 nl of 50 mM BAPTA approximately 10–30 min before recordings to minimize activity-dependent increases of response amplitudes (Williams, 1993). On the day of experiments with triheteromeric receptors, the fraction of total current response mediated by escaped receptors (i.e. escape current) was always determined and subsequent experiments were only performed if this fraction was <10% (Fig. 1).
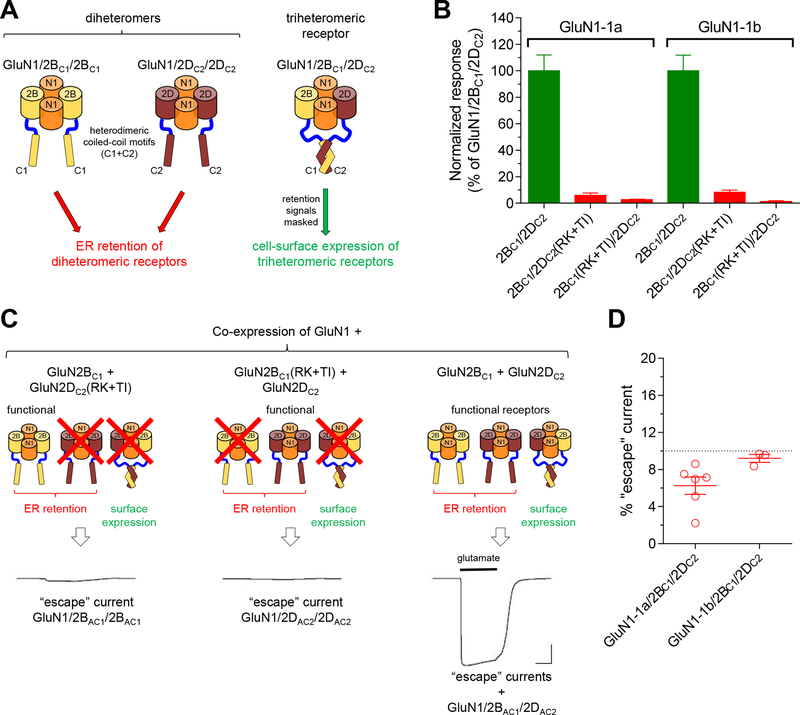
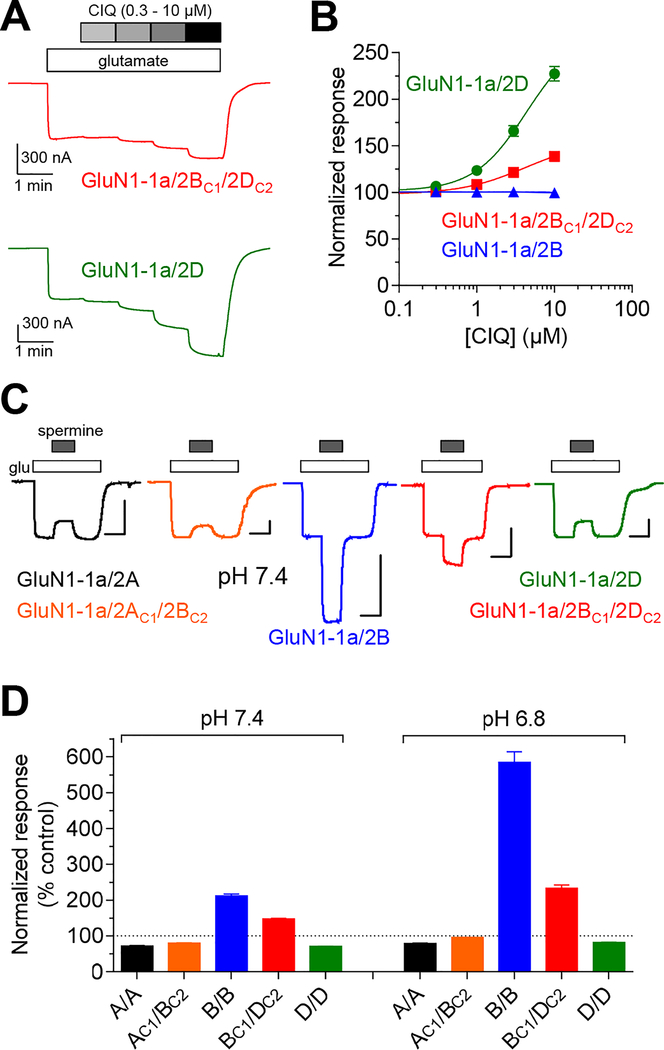
Figure 1. Selective expression of triheteromeric GluN1/2B/2D NMDA receptors.
A) Co-expression of GluN1 with GluN2BC1 and GluN2DC2 produces three populations of functional NMDA receptors, but diheteromeric GluN1/2BC1/2BC1 and GluN1/2DC2/2DC2 are prevented from expressing at the cell surface due to the unmasked dilysine (KKXX) ER retention signals in the intracellular carboxyl-terminal domain. Triheteromeric GluN1/2BC1/2DC2 receptors can traffic to the cell surface following heterodimeric coiled-coil formation between C1 and C2 tags that masks the ER retention signals. B) We co-expressed GluN1, GluN2BC1, and GluN2DC2 subunits in Xenopus oocytes and measured current responses activated by maximal glutamate plus glycine using two-electrode voltage-clamp electrophysiology. These current responses are, in theory, mediated by triheteromeric NMDA receptors composed of two GluN1, one GluN2BC1, and one GluN2DC2 (i.e. GluN1/2BC1/2DC2). However, diheteromeric GluN1/2BC1/2BC1 and GluN1/2DC2/2DC2 receptors may, in practice, escape ER retention and contribute to the measured current responses. To assess the contribution of escaped receptors to the total current response, we used GluN2BC1, and GluN2DC2 subunits with mutations in the agonist binding pocket that abolish glutamate binding and render any receptor containing these subunits non-functional (R519K and T691I mutations in GluN2B and R543K and T715I mutations in GluN2D; indicated as RK+TI). Co-expression of GluN1, GluN2BC1(RK+TI), and GluN2DC2 therefore only generates functional GluN1/2DC2/2DC2 receptors that may escape ER retention and traffic to the cell surface, while co-expression of GluN1, GluN2BC1, and GluN2DC2(RK+TI) only generates functional GluN1/2BC1/2BC1 receptors. The bar graph summarizes responses from 6 oocytes from one batch of oocytes for each receptor. C) The representative two-electrode voltage-clamp recordings show responses from recombinant NMDA receptors activated by 100 μM glutamate plus 100 μM glycine. D) The efficiency of selective expression of triheteromeric NMDA receptors can be assessed by determining the functional contribution of escaped receptors using the RK+TI mutations. The fractional escape current, calculated as the sum of escape currents divided by the total current, were consistently smaller than 10% for GluN1–1a and GluN1–1b isoforms in combination with GluN2BC1 and GluN2DC2 subunits. The data points are for individual batches of oocytes determined as shown in panels B and C.
Outside-out patch-clamp recordings.
NMDA receptor-expressing oocytes were incubated in hyperosmotic solution containing (in mM) 300 NaCl, 20 HEPES, 6 KCl, 1 BaCl2, 0.02 EDTA, and 40 D-mannitol (pH 7.4 with NaOH) for 3–5 min to facilitate removal of the vitelline layer using fine forceps. The oocytes were then transferred to extracellular recording solution containing (in mM) 90 NaCl, 1 KCl, 10 HEPES, 0.5 CaCl2, and 0.01 EDTA (pH 7.4 with NaOH). Outside-out membrane patches were pulled using microelectrodes filled with internal solution containing (in mM) 110 D-gluconic acid, 110 CsOH, 30 CsCl, 5 HEPES, 4 NaCl, 0.5 CaCl2, 2 MgCl2, 5 BAPTA, 2 NaATP, and 0.3 NaGTP (pH 7.35 with CsOH). Recordings were performed using an Axopatch 200B amplifier (Molecular Devices, San Jose, CA) at room temperature (23 °C) as previously described (Hansen et al., 2014). Unless otherwise stated, the patches were voltage-clamped at −100 mV. Rapid solution exchange was achieved using a two-barrel theta-glass pipette controlled by a piezoelectric translator and was 0.4–0.8 ms (10–90% rise time) measured from the open tip junctional potential.
Mouse hippocampal slice preparation.
Adult C57Bl/6J mice (aged P21–28) of both sexes were used in studies of evoked NMDA receptor EPSCs in acute hippocampal slices. Transverse hippocampal slices were cut essentially as previously described (Yi et al., 2014). Briefly, after the deep anesthesia using an overdose of inhaled isoflurane and cardiac perfusion with ice-old oxygenated high sucrose solution containing (in mM): 3 KCl, 24 NaHCO3, 1.25 NaH2PO4, 10 glucose, 230 sucrose, 0.5 CaCl2, and 10 MgSO4, the mouse brain was quickly removed and submerged in ice-cold oxygenated cutting solution containing (in mM): 130 NaCl, 3 KCl, 24 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1 CaCl2, and 3 MgSO4. Transverse hippocampal slices (300 μm) were cut by a vibrating microtome (VT1200S, Leica Microsystems, Buffalo Grove, IL) and then incubated in the cutting solution at room temperature for at least one hour.
Whole-cell patch-clamp recordings of NMDA receptor-mediated EPSCs.
A hippocampal slice was transferred to a recording chamber (RC-26GLP, Warner Instruments, Hamden, CT) mounted on a SliceScope Pro 2000 (Scientifica, Clarksburg, NJ) and continuously perfused at a rate of 2–3 ml/min with oxygenated ACSF solution containing (in mM) 130 NaCl, 3 KCl, 24 NaHCO3, 1.25 NaH2PO4, 10 glucose, 2 CaCl2, and 1 MgSO4, maintained at 32°C using dual in-line and heated platform temperature control (TC-344A, Warner Instruments). Recording electrodes with a tip resistance of 2–4 MΩ were fabricated using a micropipette puller (P-1000, Sutter Instruments, Novato, CA) and filled by internal solution containing (in mM): 120 Cs-methanesulfonate, 15 CsCl, 10 tetraethylammonium chloride, 10 HEPES, 8 NaCl, 3 Mg-ATP, 1.5 MgCl2, 10 QX-314, 0.3 Na-GTP, and 0.2 EGTA, pH 7.3, 295–305 mOsm. Whole-cell recordings were made using a Multiclamp 700B amplifier (Molecular Devices) with filtering at 4 kHz (Bessel) and digitized at 20 kHz using Digidata 1440A with pClamp 10 software (Molecular Devices).
Excitatory postsynaptic currents (EPSCs) from hippocampal CA1 pyramidal cells or interneurons in the CA1 stratum pyramidale (s.p.) and stratum radiatum/lucidum (s.r./s.l.m.) layers, respectively, were recorded at +40 mV by stimulation of Schaffer collaterals with a bipolar electrode (MX211EW(PC3), FHC, Bowdoin, ME). The stimulation (0.1 ms; 20–500 μA) was delivered every 30 s (0.033 Hz) using a Master-8 (A.M.P.I., Jerusalem, Israel) with a stimulus isolator (A365, World Precision Instruments, Sarasota, FL). DNQX (20 μM) and gabazine (10 μM) were included in ACSF solution during recordings to block AMPA/kainate receptors and GABAA receptors, resulting in a DMSO concentration of 0.03%. After 5–10 min stable baseline EPSC recordings, test compounds (CP-101,606, NAB-14, MPX-004, or spermine) were delivered for 10–15 min, followed by 400 μM DL-APV for full inhibition of NMDA receptor-mediated EPSCs. Stock solutions of test compounds were prepared to achieve an added DMSO concentration of 0.01% (i.e. vehicle is 0.01% DMSO) when added to the ACSF recording solution at the concentrations used. Series resistance (typically <20 MΩ) was monitored throughout the experiment and the recording was excluded from analysis if changes of >20% were observed.
Data analysis.
Analyses of concentration-response data from two-electrode voltage-clamp recordings were performed by fitting to the Hill equation as previously described (Hansen et al., 2014). Parameters describing Mg2+ block were determined using a Woodhull model (Woodhull, 1973), in which the current response amplitude in the absence (I0) and presence (IB) of Mg2+ are related by the following relationship: IB = I0 / (1+ [Mg2+]/(K0.5 · exp(zδEF/RT))), where K0.5 is the Mg2+ concentration that produces half-maximal block at a holding potential of 0 mV, δ is the fraction of the membrane electric field sensed by the blocking site, z is the valence of Mg2+, E is the holding potential, and RT/F has its normal thermodynamic definition (25.4 mV at 21°C). In practice, values of ln(IB/(I0-IB)), denoted here as ln(r), plotted as a function of holding potential was fit to the linear relationship defined as: ln(r) = zδEF/RT – ln([Mg2+]/K0.5) (see (Wollmuth et al., 1998). The analysis was limited to the linear region of averaged ln(r) values less than 2.5, at which Mg2+ produces at least 10% inhibition. Analyses of EPSCs peak amplitudes and deactivation time course were performed using Axograph (axograph.com). Data are shown as mean ± SEM. Paired/unpaired t test or one-way ANOVA with Tukey’s post hoc test was used for statistical comparisons as indicated, and P<0.05 was considered significant.
Results
Engineered retention signals enable selective cell-surface expression of triheteromeric GluN1/2B/2D receptors.
To achieve expression of a homogenous population of triheteromeric GluN1/2B/2D receptors without functional co-expression of diheteromeric GluN1/2B and GluN1/2D receptors, we adopted a previously described trafficking control method for expression of NMDA receptors with a defined subunit composition (Hansen et al., 2014; Yi et al., 2017). The intracellular C-terminal domains (CTD) of GluN2B and GluN2D subunits were exchanged with the GluN2A CTD containing engineered C1 or C2 peptide tags to generate GluN2BC1 and GluN2DC2 (Fig. 1). The engineered C1 and C2 tags are composed of the leucine zipper motifs from GABAB1 and GABAB2 subunits (LZ1 and LZ2, respectively) immediately followed by C-terminal di-lysine (KKXX) endoplasmic reticulum (ER) retention/retrieval signals, which will localize the subunits in the ER unless masked by heterodimeric coiled-coil formation between LZ1 and LZ2 (Kammerer et al., 1999; Zerangue et al., 2001; Brock et al., 2005). We co-expressed GluN1, GluN2BC1, and GluN2DC2 in Xenopus oocytes and evaluated the contribution of escaped receptors to the total current response (i.e. escape current) using two-electrode voltage-clamp recordings and previously described control experiments (Fig. 1) (Hansen et al., 2014; Yi et al., 2017). The estimated escape current was consistently <10% of the total current response, and estimation of escape current was always performed prior to all the subsequent experiments to ensure that triheteromeric GluN1/2BC1/2DC2 receptors mediate >90% of the total current response (Fig. 1). The presence of the GluN2A CTD and the C1 and C2 peptide tags in GluN2B and GluN2D subunits have no discernible effects on the function of GluN1/2B and GluN1/2D receptors (Fig. 2 and Table 1). In summary, a trafficking control method was implemented for expression of triheteromeric GluN1/2B/2D receptors in Xenopus oocytes, thereby enabling quantitative evaluation of their functional and pharmacological properties.
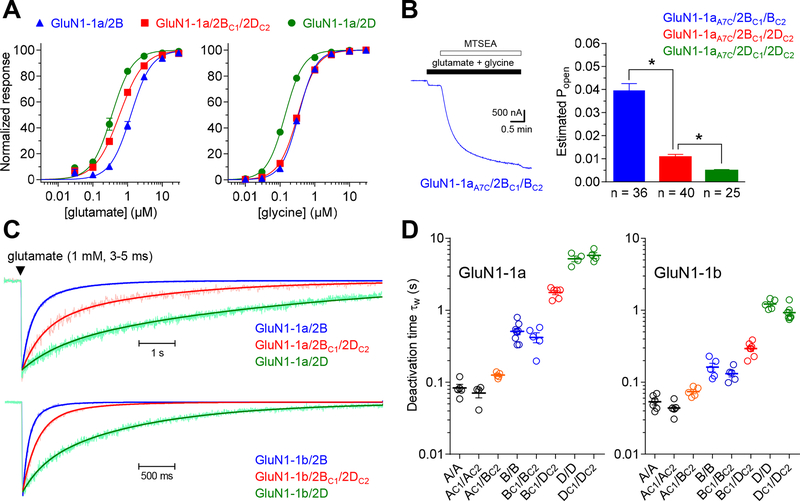
Figure 2. Activation and deactivation of triheteromeric GluN1/2B/2D receptors.
A) Concentration-response data were measured using two-electrode voltage clamp electrophysiology for diheteromeric GluN1–1a/2B and GluN1–1a/2D as well as triheteromeric GluN1–1a/2BC1/2DC2 receptors expressed in Xenopus oocytes. B) The representative two-electrode voltage clamp recording shows activation of NMDA receptors with the GluN1–1a A652C mutation (GluN1–1aA7C), which is locked in the open state by MTSEA following activation by 100 μM glutamate plus 30 μM glycine. The bar graph summarizes the estimated open probability (Popen) of NMDA receptor subtypes calculated using Popen = 0.66 · Iglu/IMTSEA, where Iglu is the response to maximal agonist and IMTSEA is the response following MTSEA exposure as previously described (Yuan et al., 2009). * indicates significantly different (P < 0.05; one-way ANOVA with Tukey’s post hoc test). C) The representative patch-clamp recordings of NMDA receptor responses in outside-out membrane patches pulled from Xenopus oocytes are normalized to the peak amplitude. The responses are averages of >5 sweeps of brief (3–5 ms) exposures to 1 mM glutamate in the continuous presence of 100 μM glycine and the solid lines indicate the sum of two exponential functions fitted to the data. D) The bar graph summarizes the weighted time constant (τw) of glutamate deactivation time course determined using outside-out patch-clamp recordings for NMDA receptor subtypes containing either GluN1–1a or GluN1–1b. See Table 1 for values and statistical tests.
Table 1.
Time course of glutamate-activated NMDA receptor responses.
| GluN1–1a | rise time (ms) | τfast (ms) | τslow (ms) | % fast | τw (ms) | n |
|---|---|---|---|---|---|---|
| A/A | 3.9 ± 0.3 | 87 ± 7 | - | 100a | 87 ± 7#,$ | 7 |
| AC1/AC2 | 4.2 ± 0.9 | 78 ± 13 | - | 100a | 78 ± 13*,#,$ | 7 |
| AC1/BC2 | 10.9 ± 6.2 | 93 ± 27 | 180 ± 62 | 63 ± 19 | 140 ± 10*,#,$ | 5 |
| B/B | 14.9 ± 1.2 | 240 ± 23 | 1020 ± 72 | 59 ± 5 | 548 ± 45*,$ | 12 |
| BC1/BC2 | 15.1 ± 2.7 | 205 ± 35 | 1045 ± 129 | 58 ± 8 | 533 ± 58*,$ | 8 |
| BC1/DC2 | 10.9 ± 2.5 | 516 ± 42 | 3521 ± 446 | 54 ± 6 | 1767 ± 117*,#,$ | 6 |
| D/D | 6.2 ± 0.7 | 832 ± 146 | 7436 ± 890 | 24 ± 3 | 5714 ± 458*,# | 7 |
| DC1/DC2 | 8.6 ± 4.8 | 527 ± 225 | 6912 ± 848 | 17 ± 7 | 5700 ± 561*,# | 4 |
| A/A | 4.8 ± 0.3 | 56 ± 6 | - | 100a | 56 ± 6#,$ | 6 |
| AC1/AC2 | 6.3 ± 2.8 | 53 ± 7 | - | 100a | 53 ± 7#,$ | 5 |
| AC1/BC2 | 3.7 ± 0.5 | 47 ± 4 | 160 ± 25 | 69 ± 12 | 74 ± 5*,#,$ | 5 |
| B/B | 10.3 ± 1.5 | 81 ± 8 | 208 ± 49 | 58 ± 12 | 155 ± 19*,$ | 6 |
| BC1/BC2 | 8.3 ± 1.6 | 101 ± 15 | 360 ± 89 | 84 ± 6 | 138 ± 13*,$ | 7 |
| BC1/DC2 | 9.2 ± 1.3 | 127 ± 31 | 634 ± 132 | 50 ± 15 | 288 ± 22*,#,$ | 8 |
| D/D | 3.5 ± 0.4 | 245 ± 32 | 1609 ± 148 | 40 ± 7 | 992 ± 68*,# | 10 |
| DC1/DC2 | 4.5 ± 0.7 | 256 ± 92 | 1399 ± 141 | 14 ± 4 | 1230 ± 86*,# | 5 |
Responses from NMDA receptors expressed in Xenopus oocytes were measured using outside-out patch-clamp recordings. Naming of subtypes is such that e.g. A/A indicates diheteromeric GluN1/2A/2A receptors and AC1/AC2 indicates diheteromeric GluN1/2AC1/2AC2 receptors that contain the C1 and the C2 tags. Responses were activated by a brief application (3–5 ms) of 1 mM glutamate in the continuous presence of 100 μM glycine. Rise times are for 10–90% of the response amplitude, deactivation time constants were determined using two-exponential fits to obtain τfast, τslow, and % fast is the fitted percentage of the fast component.
indicates that only a single-exponential was fitted to the deactivation time course. Weighted time constants (τw) were calculated as τw = τfast · Ifast/(Ifast + Islow) + τslow · Islow/(Ifast + Islow). Data are mean ± SEM, and n is the number of patches used to generate the data. Deactivation rates (i.e. 1/τw) were used for statistical comparisons using one-way ANOVA with Tukey’s post hoc test.
indicates significantly different from A/A,
indicates significantly different from B/B, and §indicates significantly different from D/D.
Activation and deactivation of GluN1/2B/2D are distinct from GluN1/2B and GluN1/2D.
Evaluation of glutamate and glycine concentration-response data for triheteromeric GluN1/2BC1/2DC2 receptors and diheteromeric GluN1/2B and GluN1/2D receptors using two-electrode voltage-clamp recordings demonstrated that both glutamate and glycine EC50 values for GluN1/2BC1/2DC2 were intermediate to GluN1/2B and GluN1/2D (Fig. 2A and Table 2). Thus, the potencies of endogenous agonists are not prominently altered by the combination of GluN2B and GluN2D in triheteromeric receptors.
Table 2.
Pharmacology of triheteromeric GluN1/2B/2D receptors.
| Ligand | GluN1 isoform | EC50 (μM) | Relative Imax (%) | nH | n | EC50 (μM) | Relative Imax (%) | nH | n | EC50 (μM) | Relative Imax (%) | nH | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glutamate | 1a | 1.09 ± 0.23# | 100a | 1.6 | 6 | 0.49 ± 0.04*,# | 100a | 1.4 | 6 | 0.27 ± 0.01* | 100a | 1.7 | 6 |
| glycine | 1a | 0.34 ± 0.03# | 100a | 1.8 | 6 | 0.30 ± 0.01*,# | 100a | 1.6 | 6 | 0.12 ± 0.01* | 100a | 1.6 | 6 |
| CIQ | 1a | - | 100 ± 2 | - | 8 | - | 139 ± 3 | - | 8 | - | 227 ± 8 | - | 7 |
| at 10 μM | at 10 μM | ||||||||||||
| at 10 μM | |||||||||||||
| CIQ | 1b | - | 91 ± 3 | - | 3 | - | 115 ± 3 | - | 4 | - | 197 ± 8 | - | 5 |
| at 10 μM | |||||||||||||
| at 10 μM | at 10 μM | ||||||||||||
| spermine | 1a | - | 211 ± 6 | - | 13 | - | 150 ± 2 | - | 12 | - | 71 ± 0.4 | - | 14 |
| at 100 μM | |||||||||||||
| at 100 μM | at 100 μM | ||||||||||||
| IC50 (μM) | Relative Imin (%) | nH | n | IC50 (μM) | Relative Imin (%) | nH | n | IC50 (μM) | Relative Imin (%) | nH | n | ||
| ifenprodil | 1a | 0.108 ± 0.005 | 9 ± 1 | 1.1 | 12 | 0.124 ± 0.003* | 33 ± 1* | 1.1 | 17 | - | 97 ± 2 | - | 6 |
| at 10 μM | |||||||||||||
| ifenprodil | 1b | 0.400 ± 0.108 | 12 ± 3 | 1.8 | 6 | 0.272 ± 0.009 | 31 ± 1* | 1.1 | 5 | - | 93 ± 1 | - | 4 |
| at 10 μM | |||||||||||||
| EVT-101 | 1a | 0.009 ± 0.001 | 3 ± 1 | 1.0 | 5 | 0.012 ± 0.001* | 37 ± 2* | 1.1 | 6 | - | 92 ± 2 | - | 6 |
| at 3 μM | |||||||||||||
| CP-101,606 | 1a | 0.049 ± 0.006 | 5 ± 1 | 1.4 | 6 | 0.077 ± 0.009* | 41 ± 1* | 1.4 | 5 | - | 94 ± 1 | - | 4 |
| at 10 μM | |||||||||||||
| DQP-1105 | 1a | - | 58 ± 2 | - | 6 | 13 ± 1# | −8 ± 2# | 0.8 | 6 | 2.2 ± 0.1 | −3 ± 1 | 1.0 | 6 |
| at 30 μM | |||||||||||||
| QNZ-46 | 1a | - | 76 ± 1 | - | 6 | 10 ± 1# | 4 ± 1# | 1.0 | 6 | 2.7 ± 0.1 | −3 ± 1 | 1.0 | 6 |
| at 30 μM | |||||||||||||
| NAB-14 | 1a | - | 84 ± 2 | - | 6 | 3.3 ± 0.1# | 22 ± 4# | 1.0 | 5 | 1.5 ± 0.1 | −3 ± 1 | 1.0 | 6 |
| at 10 μM | |||||||||||||
| NAB-14 + BCD |
1a | - | 86 ± 2 | - | 4 | 6.8 ± 0.3# | 29 ± 2# | 1.0 | 5 | 3.2 ± 0.1 | 0 ± 1 | 1.2 | 6 |
| at 100 μM |
Concentration-response data were measured using two-electrode voltage-clamp recordings. Glutamate data were obtained in the presence of 100 μM glycine, and glycine data were obtained in the presence of 300 μM glutamate. Relative Imax (or Imin) is the fitted maximal (or minimal) response relative to control responses activated by 300 μM glutamate plus 100 μM glycine in the absence of modulator. Limited solubility or channel block (spermine) prevented determination of potencies for some compounds (indicated by -). Concentration-response data for NAB-14 were repeated with 5 mM (2-hydroxypropyl)-β-cyclodextrin (BCD) in the extracellular recording solution to enhance compound solubility. Spermine was evaluated at pH 7.4 and a holding potential of −20 mV. Data are mean ± SEM, nH is the Hill coefficient, and n is the number of oocytes.
indicates that this value was fixed during data analysis. Statistical analyses were preformed using logIC50 values.
indicates significantly different from GluN1/2B (P < 0.05) and
indicates significantly different from GluN1/2D (P < 0.05) using one-way ANOVA with Tukey’s post hoc test.
To estimate receptor open probability (Popen), we introduced the A652C mutation in GluN1–1a (GluN1–1aA7C) to enable locking of the ion channel in the open state following activation by glutamate and glycine (Jones et al., 2002; Yuan et al., 2005). The GluN1–1a A652C mutation is located at position 7 of the conserved SYTANLAAF gating motif in the M3 transmembrane helix and reaction of this introduced cysteine residue with 2-aminoethyl methanethiosulfonate (MTSEA) locks the channel in the open state (i.e. Popen of 1) (Jones et al., 2002; Yuan et al., 2005). Thus, normalization of the current response activated by saturating agonist to the response following exposure to MTSEA provides an estimate of receptor Popen. This approach was used to compare Popen of triheteromeric GluN1–1aA7C/2BC1/2DC2 to those of diheteromeric receptors using two-electrode voltage-clamp electrophysiology. Estimated Popen values were 0.039 ± 0.003 (n = 36) for diheteromeric GluN1–1aA7C/2BC1/2BC2 and 0.005 ± 0.001 (n = 25) for diheteromeric GluN1–1aA7C/2DC1/2DC2 (Fig. 2B), consistent with previous results using the same approach to estimate Popen of GluN1/2B and GluN1/2D (Yuan et al., 2009). Estimated Popen was 0.011 ± 0.001 (n = 40) for triheteromeric GluN1–1aA7C/2BC1/2DC2, which is intermediate to the diheteromeric receptors.
We pulled outside-out membrane patches from Xenopus oocytes expressing NMDA receptors and measured macroscopic responses activated by synaptic-like exposures (3–5 ms) to 1 mM glutamate in the continuous presence of 100 μM glycine using fast-application patch-clamp electrophysiology. Response rise times (10–90%) were measured and glutamate deactivation time courses were fitted with two exponentials (Fig. 2C,D and Table 1). The time course of responses from wild type receptor subtypes were not significantly different from responses mediated by corresponding receptors with the CTD from GluN2A and C1 and C2 peptide tags (e.g. compare GluN1/2B and GluN1/2BC1/2BC2) (Fig. 2C,D and Table 1). The response rise time was 10.9 ± 2.5 ms (n = 6) for triheteromeric GluN1–1a/2BC1/2DC2 receptors, which is intermediate to both GluN1–1a/2BC1/2BC2 (15.1 ± 2.7 ms; n = 8) and GluN1–1a/2DC1/2DC2 (8.6 ± 4.8 ms; n = 4) (Table 1). Similarly, the weighted deactivation time constant (τw) was 1770 ± 120 ms (n = 6) for triheteromeric GluN1–1a/2BC1/2DC2 receptors, which is also intermediate to both GluN1–1a/2BC1/2BC2 (530 ± 60 ms; n = 8) and GluN1–1a/2DC1/2DC2 (5700 ± 560 ms; n = 4) (Fig. 2C,D and Table 1).
In summary, agonist concentration-response relationships, estimated Popen values, and macroscopic response time courses demonstrate that neither GluN2B nor GluN2D dominates the functional properties of triheteromeric GluN1/2B/2D receptors.
GluN1–1b splice variant accelerates deactivation of GluN1/2B/2D.
Diheteromeric GluN1/2B and GluN1/2D receptors containing the GluN1–1b splice variant, which includes 21 residues in the amino-terminal domain encoded by exon 5, have accelerated glutamate deactivation time course compared to receptors containing the GluN1–1a splice variant that lack residues encoded by exon 5 (Prybylowski et al., 2000; Rumbaugh et al., 2000; Vance et al., 2012; Swanger et al., 2015; Yi et al., 2018). To determine if GluN1–1b similarly influences deactivation of triheteromeric GluN1/2B/2D receptors, macroscopic responses activated by synaptic-like exposures (3–5 ms) to 1 mM glutamate were measured from outside-out membrane patches with NMDA receptors containing GluN1–1b. The weighted deactivation time constant (τw) was 290 ± 120 ms (n = 8) for triheteromeric GluN1–1b/2BC1/2DC2 receptors, which is markedly faster than GluN1–1a/2BC1/2DC2 receptors, but intermediate to both GluN1–1b/2BC1/2BC2 (140 ± 10 ms; n = 7) and GluN1–1b/2DC1/2DC2 (1230 ± 90 ms; n = 5) (Fig. 2C,D and Table 1). GluN1–1b containing triheteromeric GluN1/2A/2B and diheteromeric GluN1/2A and GluN1/2B receptors also displayed accelerated glutamate deactivation time course compared to the respective receptor subtypes containing GluN1–1a (Fig. 2C,D and Table 1). These results demonstrate that the influence of the GluN1–1b splice variant on NMDA receptor deactivation is preserved in both triheteromeric GluN1/2A/2B and GluN1/2B/2D receptors. The faster deactivation of NMDA receptors with exon 5-containing GluN1 subunits (e.g. GluN1–1b) is relevant to synaptic transmission, since some neuronal populations expressing both GluN2B and GluN2D also express exon 5-containing GluN1 isoforms (Prybylowski et al., 2000; Rumbaugh et al., 2000; Swanger et al., 2015).
Channel block of GluN1/2B/2D by extracellular Mg2+ is intermediate to GluN1/2B and GluN1/2D.
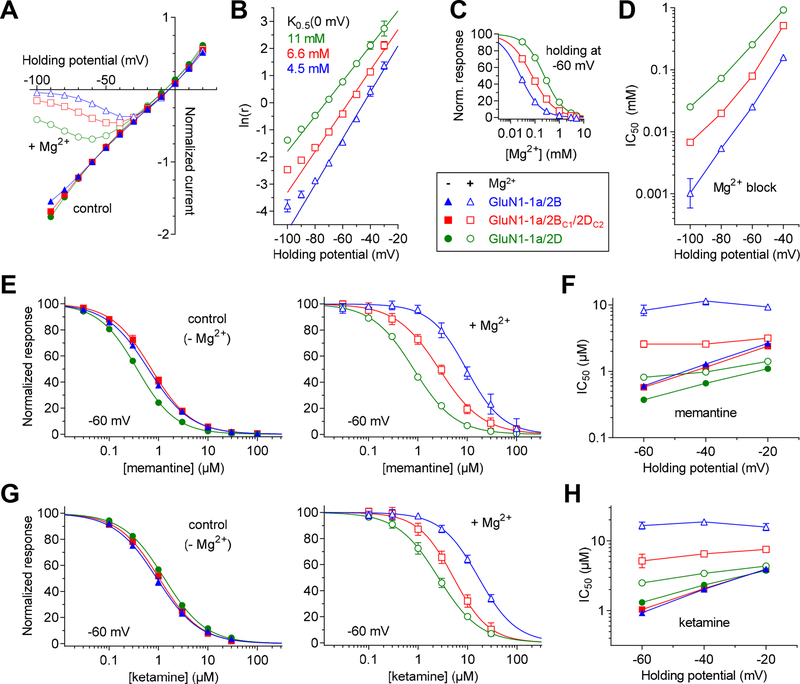
NMDA receptors are blocked by physiological levels of extracellular Mg2+ (~1 mM) in voltage-dependent manner, and GluN1/2A and GluN1/2B are more sensitive to voltage-dependent Mg2+ block compared to GluN1/2C and GluN1/2D receptors (Monyer et al., 1994; Traynelis et al., 2010). We evaluated the current-voltage relationship of NMDA receptor responses in the absence and presence of 0.1 mM Mg2+, and determined the Mg2+ concentration (K0.5) that produces half-maximal block at 0 mV (holding potential) and the fraction of the membrane electric field sensed by the blocking site (δ) (see Methods for details on the analysis) (Fig. 3A,B). In these experimental conditions, the K0.5(0 mV) values were 4.5 mM, 6.6 mM, and 11 mM for GluN1–1a/2B (δ = 1.08), GluN1–1a/2BC1/2DC2 (δ = 0.95), and GluN1–1a/2D (δ = 0.82), respectively, consistent with intermediate Mg2+ block of triheteromeric GluN1–1a/2BC1/2DC2 compared to diheteromeric GluN1–1a/2B and GluN1–1a/2D receptors. To corroborate this observation, we determined IC50 values for Mg2+ block at different holding potentials using two-electrode voltage-clamp recordings (Fig. 3C,D and Table 3). For example, IC50 for Mg2+ block at −60 mV was 80 ± 3 μM (n = 5) for GluN1–1a/2BC1/2DC2, which is increased compared to 26 ± 2 μM (n = 8) for GluN1–1a/2B and decreased compared to 254 ± 11 μM (n = 4) for GluN1–1a/2D (Fig. 3C,D and Table 3). Thus, we demonstrate that co-assembly of GluN2B and GluN2D subunits, which endow NMDA receptors with high and low Mg2+ block, respectively, results in intermediate Mg2+ block for triheteromeric GluN1/2B/2D receptors.
Figure 3. Voltage-dependence of block of NMDA receptors by extracellular Mg2+.
A) Current-voltage relationships for responses activated by 300 μM glutamate plus 100 μM glycine in the absence (control; filled symbols) and presence of 100 μM Mg2+ (open symbols) were measured using two-electrode voltage clamp electrophysiology for diheteromeric GluN1–1a/2B and GluN1–1a/2D as well as triheteromeric GluN1–1a/2BC1/2DC2 receptors. B) To determine Woodhull parameters from the current-voltage relationships shown in A), a linear relationship was fitted to values of ln(IB/(I0-IB)), denoted here as ln(r), plotted against holding potential. The linear region (−80 to −30 mV) and ln(r) values less than 2.5, at which Mg2+ produces at least 10% inhibition, were used for the analysis. The linear fits yields Mg2+ concentration (K0.5) that produces half-maximal block at 0 mV and the fraction of the membrane electric field sensed by the blocking site (δ) (see Methods for details on the analysis). K0.5(0 mV) values were 4.5 mM, 6.6 mM, and 11 mM for GluN1–1a/2B (δ = 1.08), GluN1–1a/2BC1/2DC2 (δ = 0.95), and GluN1–1a/2D (δ = 0.82), respectively. C) Representative concentration-inhibition data for Mg2+ block of responses activated by 100 μM glutamate plus 100 μM glycine measured at −60 mV and fitted to the Hill equation to determine IC50 values for Mg2+ block. D) The IC50 values for Mg2+ block are plotted as a function of holding potential. E, G) Inhibition of responses activated by 100 μM glutamate plus 100 μM glycine by memantine (E) or ketamine (G) was measured in the absence (filled symbols) and presence of 1 mM Mg2+ (open symbols). F, H) The IC50 values in the absence (filled symbols) and presence of 1 mM Mg2+ (open symbols) for memantine (F) or ketamine (H) block are plotted as a function of holding potential. See Table 3 for values and statistical tests.
Table 3.
Channel block of diheteromeric and triheteromeric NMDA receptor subtypes.
| GluN1–1a/2B | GluN1–1a/2BC1/2DC2 | GluN1–1a/2D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ligand | Holding potential (mV) | IC50 (μM) or IC50 ratio | nH | n | IC50 (μM) or IC50 ratio | nH | n | IC50 (μM) or IC50 ratio | nH | n |
| Mg2+ | −40 | 161.1 ± 11.8 | 0.9 | 8 | 518.4 ± 14.3*,# | 0.8 | 5 | 936.7 ± 85.9 | 1.0 | 4 |
| Mg2+ | −60 | 25.5 ± 1.5 | 1.0 | 8 | 79.2 ± 3.0*,# | 0.9 | 5 | 253.7 ± 10.9 | 1.0 | 4 |
| Mg2+ | −80 | 5.5 ± 0.4 | 1.0 | 8 | 19.9 ± 0.8*,# | 0.9 | 5 | 72.5 ± 1.8 | 1.0 | 4 |
| Mg2+ | −100 | 1.6 ± 0.3 | 0.9 | 8 | 6.8 ± 0.5*,# | 0.9 | 5 | 25.1 ± 0.4 | 1.0 | 4 |
| memantine (control) | −20 | 2.7 ± 0.2 | 0.9 | 6 | 2.5 ± 0.4# | 1.1 | 4 | 1.1 ± 0.1 | 1.1 | 6 |
| memantine (+ Mg2+) | −20 | 9.5 ± 0.6 | 1.1 | 6 | 3.2 ± 0.1*,# | 1.0 | 6 | 1.4 ± 0.1 | 1.0 | 6 |
| IC50 ratio (Mg2+/control) | −20 | 3.5 | 1.3 | 1.3 | ||||||
| memantine (control) | −40 | 1.3 ± 0.1 | 1.0 | 6 | 1.2 ± 0.1# | 1.0 | 4 | 0.7 ± 0.0 | 1.1 | 6 |
| memantine (+ Mg2+) | −40 | 12.0 ± 2.0 | 1.0 | 6 | 2.6 ± 0.2*,# | 0.9 | 6 | 1.0 ± 0.0 | 1.0 | 6 |
| IC50 ratio (Mg2+/control) | −40 | 9.2 | 2.2 | 1.4 | ||||||
| memantine (control) | −60 | 0.6 ± 0.0 | 1.0 | 6 | 0.6 ± 0.0# | 1.0 | 4 | 0.4 ± 0.0 | 1.1 | 6 |
| memantine (+ Mg2+) | −60 | 8.9 ± 1.5 | 1.1 | 5 | 2.6 ± 0.3*,# | 0.9 | 4 | 0.8 ± 0.0 | 1.0 | 6 |
| IC50 ratio (Mg2+/control) | −60 | 14.8 | 4.3 | 2.0 | ||||||
| ketamine (control) | −20 | 4.2 ± 0.7 | 1.0 | 6 | 3.9 ± 0.2 | 1.0 | 6 | 3.8 ± 0.2 | 1.0 | 6 |
| ketamine (+ Mg2+) | −20 | 16.3 ± 1.9 | 1.2 | 6 | 7.6 ± 0.5*,# | 1.1 | 6 | 4.5 ± 0.5 | 1.0 | 6 |
| IC50 ratio (Mg2+/control) | −20 | 3.9 | 1.9 | 1.2 | ||||||
| ketamine (control) | −40 | 2.1 ± 0.3 | 1.0 | 6 | 2.1 ± 0.1 | 1.0 | 6 | 2.4 ± 0.2 | 1.0 | 6 |
| ketamine (+ Mg2+) | −40 | 19.2 ± 2.0 | 1.3 | 6 | 6.7 ± 0.6*,# | 1.1 | 6 | 3.5 ± 0.5 | 1.0 | 6 |
| IC50 ratio (Mg2+/control) | −40 | 9.1 | 3.2 | 1.5 | ||||||
| ketamine (control) | −60 | 1.0 ± 0.1 | 1.0 | 6 | 1.1 ± 0.1 | 1.1 | 6 | 1.3 ± 0.1 | 1.0 | 6 |
| ketamine (+ Mg2+) | −60 | 17.0 ± 2.0 | 1.1 | 5 | 5.7 ± 0.8*,# | 1.2 | 6 | 2.6 ± 0.4 | 1.1 | 6 |
| IC50 ratio (Mg2+/control) | −60 | 17.0 | 5.2 | 2.0 | ||||||
Channel block of NMDA receptor responses to 300 μM glutamate plus 100 μM glycine was measured using two-electrode voltage-clamp recordings of responses from NMDA receptors. Memantine and ketamine block was evaluated in the absence (control) and presence of 1 mM Mg2+. Maximal inhibition was fixed to 100% during data analysis. Data are mean ± SEM, and n is the number of oocytes used to generate the data. Statistical analyses were performed using logIC50 values.
indicates significantly different from GluN1/2B (P < 0.05) and
indicates significantly different from GluN1/2D (P < 0.05) using one-way ANOVA with Tukey’s post hoc test.
Extracellular Mg2+ renders GluN1/2B/2D more sensitive to ketamine and memantine compared to GluN1/2B.
Ketamine and memantine are NMDA receptor channel blockers with therapeutic potential in the treatment of several CNS disorders (Johnson et al., 2015; Zanos et al., 2018). Ketamine and memantine presumably compete with Mg2+ for binding in the channel pore and their block is attenuated in the presence of physiological Mg2+ concentrations (Kotermanski & Johnson, 2009; Glasgow et al., 2018). In the presence of extracellular Mg2+, the more potent Mg2+ block of GluN1/2A and GluN1/2B receptors therefore results in preferential inhibition of GluN1/2C and GluN1/2D receptors by ketamine and memantine (Kotermanski & Johnson, 2009). Both GluN2B and GluN2D subunits are expressed by GABAergic interneurons in the mature cortex and hippocampus (Monyer et al., 1994; Standaert et al., 1996; von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018). Preferential inhibition of GluN2D-containing NMDA receptors in these inhibitory interneurons have been suggested to mediate disinhibition of pyramidal cells; a mechanism that has been proposed to account for therapeutically relevant actions of both ketamine and memantine (Johnson et al., 2015; Duman et al., 2016; Miller et al., 2016; Chowdhury et al., 2017; Zanos et al., 2018; Krystal et al., 2019). We evaluated if the presence of Mg2+ reduces block of triheteromeric GluN1/2B/2D receptors by ketamine and memantine (Fig. 3E–H and Table 3). In the absence of Mg2+, the potencies of ketamine and memantine for inhibition of GluN1–1a/2B and GluN1–1a/2D were similar to previously published values (Dravid et al., 2007). Ketamine inhibited triheteromeric GluN1–1a/2BC1/2DC2 receptors with a potency similar to those of GluN1–1a/2B and GluN1–1a/2D, whereas block by memantine was similar to that of GluN1–1a/2B, but different from GluN1–1a/2D (Fig. 3E–H and Table 3). In the presence of 1 mM extracellular Mg2+ at a holding potential of −60 mV, memantine IC50 values increased 14.8-fold at GluN1–1a/2B and 2.0-fold at GluN1–1a/2D, whereas the increase was only 4.3-fold at triheteromeric GluN1–1a/2BC1/2DC2 receptors (Fig. 3E–H and Table 3). Similarly, ketamine IC50 values increased 17.0-fold at GluN1–1a/2B and 2.0-fold at GluN1–1a/2D, while the increase was only 5.2-fold at triheteromeric GluN1–1a/2BC1/2DC2 receptors. Extracellular Mg2+ is therefore less effective at attenuating channel block by ketamine and memantine at triheteromeric GluN1/2B/2D receptors compared to GluN1/2B (Fig. 3E–H and Table 3). These results demonstrate that triheteromeric GluN1/2B/2D receptors are more sensitive to channel block compared to GluN1/2B in the presence of physiological Mg2+ concentrations, consistent with the hypothesis that ketamine and memantine can preferentially inhibit native GluN2D-containing NMDA receptors expressed by interneurons (Johnson et al., 2015; Zanos et al., 2018).
Allosteric modulators have distinct activity at GluN1/2B/2D compared to GluN1/2B and GluN1/2D.
The co-expression of GluN2B and GluN2D subunits in distinct neuronal populations is indicated by the sensitivity of NMDA receptor responses to both GluN2B-selective and GluN2C/D-selective allosteric modulators. The activity of these subunit-selective allosteric modulators at GluN1/2B and GluN1/2D receptors is well described, but there is a lack of quantitative data describing modulation of triheteromeric GluN1/2B/2D receptors. We therefore generated concentration-response data for a diverse range of allosteric modulators (Table 2).
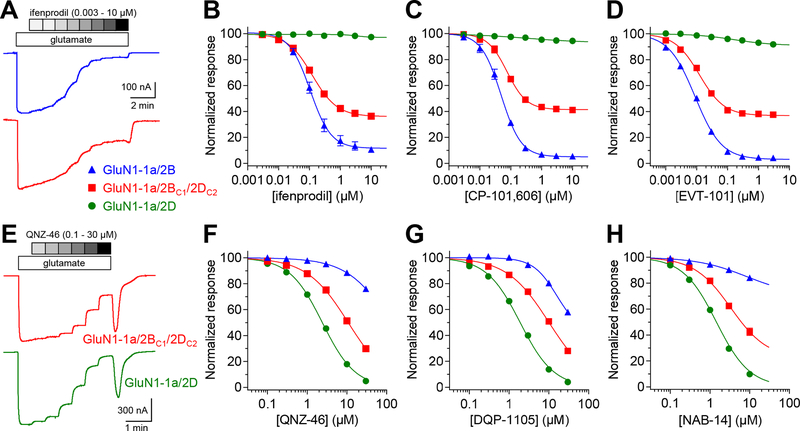
GluN2B-selective antagonists, ifenprodil, EVT-101, and CP-101–606 (Hansen et al., 2010; Stroebel et al., 2016), inhibited GluN1–1a/2B and GluN1–1a/2BC1/2DC2 with similar potencies, but with reduced efficacy at GluN1–1a/2BC1/2DC2 (59–67% maximal inhibition) compared to at GluN1/2B (91–97% maximal inhibition) (Fig. 4A–D and Table 2). This trend was also observed for ifenprodil inhibition of receptors with the GluN1–1b splice variant, albeit with slightly lower potencies at both GluN1–1b/2BC1/2DC2 (69% maximal inhibition) and GluN1–1b/2B (88% maximal inhibition) (Table 2). This finding is different from triheteromeric GluN1–1a/2A/2B receptors that are inhibited by GluN2B-selective antagonists in the absence of extracellular Zn2+ with ~2–6-fold reduced potencies and lower efficacies (~30% maximal inhibition) compared to GluN1/2B (Hatton & Paoletti, 2005; Hansen et al., 2014; Stroebel et al., 2014). Thus, GluN2B-selective antagonists can produce stronger inhibition of triheteromeric GluN1/2B/2D compared to GluN1/2A/2B receptors. This feature is shared with ketamine and memantine, which are also expected to produce stronger inhibition of triheteromeric GluN1/2B/2D compared to GluN1/2A/2B receptors in the presence of extracellular Mg2+.
Figure 4. Negative allosteric modulation of NMDA receptors by subunit-selective ligands.
A) The representative two-electrode voltage-clamp recordings show ifenprodil inhibition of diheteromeric GluN1–1a/2B and triheteromeric GluN1–1a/2BC1/2DC2 receptors. B-D) Concentration-inhibition data for GluN2B-selective antagonists were measured for diheteromeric GluN1–1a/2B and GluN1–1a/2D as well as triheteromeric GluN1–1a/2BC1/2DC2 receptors. Responses were activated by 300 μM glutamate in the continuous presence of 100 μM glycine. E) The representative recordings show QNZ-46 inhibition of diheteromeric GluN1–1a/2D and triheteromeric GluN1–1a/2BC1/2DC2 receptors. F-H) Concentration-inhibition data for GluN2C/D-selective antagonists. See Table 2 for values and statistical tests.
GluN2C/D-selective antagonists, QNZ-46 and DQP-1105 (Acker et al., 2011; Hansen & Traynelis, 2011), inhibited GluN1–1a/2D and GluN1–1a/2BC1/2DC2 with similar efficacies (~100% maximal inhibition), but with reduced potencies at GluN1–1a/2BC1/2DC2 (Fig. 4E–G and Table 2). Another GluN2C/D-selective antagonist, NAB-14 (Swanger et al., 2018), inhibited GluN1–1a/2BC1/2DC2 with reduced potency and efficacy compared to GluN1–1a/2D (Fig. 4H and Table 2). The reduced efficacy of NAB-14 at GluN1–1a/2BC1/2DC2 (i.e. the fitted maximum inhibition) was not due to limited compound solubility, since a similar result was obtained when compound solubility was enhanced by the addition of 5 mM (2-hydroxypropyl)-β-cyclodextrin (BCD) in the extracellular recording solution (Table 2). The variation in activity profiles for GluN2C/D-selective antagonists is presumably due to distinct binding sites and mechanisms of action for NAB-14 compared to QNZ-46 and DQP-1105 (Acker et al., 2011; Hansen & Traynelis, 2011; Swanger et al., 2018). In comparison, the evaluated GluN2B-selective antagonists did not display this variation and they share the same binding site and mechanism of action (Hansen et al., 2010; Stroebel et al., 2016).
Next, we evaluated positive allosteric modulation by GluN2C/D-selective CIQ (Mullasseril et al., 2010) and GluN2B-selective spermine (Traynelis et al., 1995; Rumbaugh et al., 2000). Responses from GluN1–1a/2B to saturating glutamate plus glycine (control) were unaffected by 10 μM CIQ, whereas GluN1–1a/2D and GluN1–1a/2BC1/2DC2 responses were potentiated to 227% and 139% of control, respectively (Fig. 5A,B and Table 2). GluN1–1b/2D and GluN1–1b/2BC1/2DC2 responses were similarly potentiated by 10 μM CIQ to 197% and 115% of control, respectively, while GluN1–1b/2B responses were slightly inhibited to 91% of control (Table 2). Spermine (100 μM) increased GluN1–1a/2B and GluN1–1a/2BC1/2DC2 responses to 211% and 150% of control at physiological pH 7.4, but resulted in inhibition of GluN1–1a/2D responses to 71% of control (Fig. 5C,D and Table 4). Interestingly, the effect of 100 μM spermine at triheteromeric GluN1–1a/2AC1/2BC2 receptors (80% of control) was not markedly different from the effect at GluN1/2A (72% of control) (Fig. 5C,D and Table 4). This activity profile for spermine among all NMDA receptor subtypes was conserved at acidic pH 6.8 (Fig. 5C,D and Table 4). In summary, CIQ (GluN2C/D-selective) and spermine (GluN2B-selective) are both positive allosteric modulators of triheteromeric GluN1/2B/2D receptors.
Figure 5. Subunit-selective potentiation of NMDA receptors by CIQ and spermine.
A) The representative two-electrode voltage-clamp recordings show potentiation of diheteromeric GluN1–1a/2D and triheteromeric GluN1–1a/2BC1/2DC2 receptors by CIQ. B) Concentration-potentiation data for CIQ for NMDA receptors activated by 100 μM glutamate in the continuous presence of 100 μM glycine are normalized to the response in the absence of CIQ. C) The representative two-electrode voltage-clamp recordings show potentiation of diheteromeric and triheteromeric NMDA receptors by 100 μM extracellular spermine at pH 7.4. Responses were activated by 300 μM glutamate in the continuous presence of 100 μM glycine, and oocytes were voltage-clamped at −20 mV to minimize voltage-dependent channel block by spermine. Scale bars indicate 200 nA (vertical) and 0.5 min (horizontal). D) The bar graph summarizes potentiation of NMDA receptor subtypes containing the GluN1–1a subunit by 100 μM spermine at pH 7.4 and 6.8. Data are normalized to control in the absence of spermine (100%). See Table 4 for values.
Table 4.
Modulation of NMDA receptor subtypes by spermine.
| pH 7.4 | pH 6.8 | |||
|---|---|---|---|---|
| Receptor subtype | Spermine modulation (% control) | n | Spermine modulation (% control) | n |
| GluN1–1a/2A | 72 ± 1 | 6 | 79 ± 1 | 6 |
| GluN1–1a/2AC1/2BC2 | 80 ± 1 | 6 | 95 ± 0.1 | 6 |
| GluN1–1a/2B | 211 ± 6 | 13 | 585 ± 29 | 13 |
| GluN1–1a/2BC1/2DC2 | 150 ± 2 | 12 | 233 ± 10 | 12 |
| GluN1–1a/2D | 71 ± 0.4 | 14 | 82 ± 1 | 14 |
Modulation by 100 μM spermine of NMDA receptor responses activated by 300 μM glutamate in the continuous presence of 100 μM glycine was measured using two-electrode voltage-clamp recordings and a holding potential of −20 mV. Spermine modulation is shown as percentage of control responses in the absence of spermine. Data are mean ± SEM, and n is the number of oocytes used to generate the data.
Key features of GluN1/2B/2D allosteric modulation are conserved for NMDA receptors in hippocampal interneurons.
To determine if the pharmacological properties of recombinant GluN1/2B/2D receptors are conserved in neuronal receptors, we evaluated the effects of subunit-selective modulators on NMDA receptor-mediated excitatory postsynaptic currents (NMDAR-EPSCs) in acute hippocampal slices from adult (P21–28) mouse. Specifically, we compared NMDAR-EPSCs from hippocampal CA1 pyramidal cells, which are predominantly expressing triheteromeric GluN1/2A/2B receptors (Gray et al., 2011; Rauner & Kohr, 2011), and GABAergic interneurons in CA1 stratum radiatum/lucidum (s.r./s.l.m.) layers, which are predominantly expressing triheteromeric GluN1/2B/2D receptors (von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018).
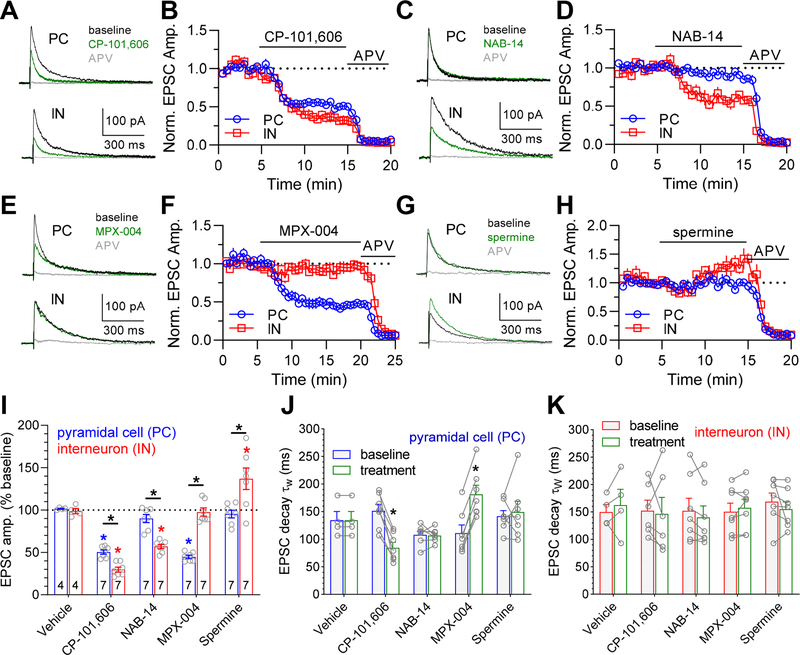
First, we evaluated inhibition of NMDAR-EPSCs by the GluN2B-selective antagonist CP-101,606 (10 μM), which produced a significantly stronger inhibition of NMDAR-EPSCs in interneurons compared to CA1 pyramidal cells (30 ± 3% (n = 7) versus 50 ± 3% (n = 7) of baseline for interneuron versus pyramidal cell; P < 0.05, unpaired t test) (Fig. 6A,B). This result is consistent with stronger inhibition of triheteromeric GluN1/2B/2D compared to GluN1/2A/2B receptors. Second, the expression of GluN2D-containing NMDA receptors in the hippocampal interneurons investigated in this study, but not CA1 pyramidal cells, was corroborated using the GluN2C/D-selective antagonist NAB-14 (Swanger et al., 2018). NAB-14 (10 μM) significantly inhibited NMDAR-EPSCs in interneurons (57 ± 3% of baseline, n = 7, P < 0.05, paired t test), but not in CA1 pyramidal cells (90 ± 5% of baseline, n = 7, P > 0.05, paired t test) (Fig. 6C,D). Third, the expression of GluN2A-containing NMDA receptors in CA1 pyramidal cells, but not the population of interneurons investigated in this study, was demonstrated using the GluN2A-selective antagonist MPX-004 (Volkmann et al., 2016; Yi et al., 2016). MPX-004 (10 μM) significantly inhibited NMDAR-EPSCs in CA1 pyramidal cells (45 ± 2% of baseline, n = 7, P < 0.05, paired t test), but not in interneurons (98 ± 5% of baseline, n = 7, P > 0.05, paired t test) (Fig. 6E,F). Finally, we evaluated potentiation of NMDAR-EPSCs in hippocampal CA1 pyramidal cells and interneurons by the GluN2B-selective positive modulator spermine. Spermine (100 μM) significantly potentiated NMDAR-EPSCs in interneurons to 137 ± 13% of baseline (n = 7, P < 0.05, paired t test), but had no effect on NMDAR-EPSCs in CA1 pyramidal cells (95 ± 5% of baseline, n = 7, P > 0.05, paired t test) (Fig. 6G,H). This result is consistent with the expression of GluN1/2B/2D in interneurons and GluN1/2A/2B in CA1 pyramidal cells, since we find that 100 μM spermine potentiates recombinant GluN1/2B/2D to ~150% of control and inhibits recombinant GluN1/2A/2B to ~80% at pH 7.4 (Fig. 5C,D and Table 4). Vehicle alone (0.01% DMSO) had no effect compared to baseline in either neuron types (Fig. 6I). In conclusion, we demonstrate that key features of the pharmacological properties exhibited by recombinant triheteromeric GluN1/2B/2D receptors, namely spermine potentiation and pronounced inhibition by CP-101,606, are also observed for NMDAR-EPSCs in adult hippocampal interneurons, consistent with expression of triheteromeric GluN1/2B/2D receptors in these neuronal cell types (von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018).
Figure 6. Distinct pharmacology of NMDA receptor-mediated EPSCs between hippocampal CA1 pyramidal cells and interneurons.
A-H) The representative traces show NMDAR-mediated EPSCs (NMDAR-EPSCs) at a holding potential of +40 mV from hippocampal CA1 pyramidal cells (PC) and interneurons (IN) in acute mouse brain slices (P21–28). Baseline (black) NMDAR-EPSCs were recorded for 5 min before application of allosteric modulators (green) 10 μM CP-101,606 (A), 10 μM NAB-14 (C), 10 μM MPX-004 (E), or 100 μM spermine (G), followed by application of DL-APV (400 μM, gray) to fully block NMDA receptor responses. The averaged data from multiple neuronal recordings show the time course of NMDAR-EPSC peak amplitudes normalized to baseline in CA1 pyramidal cells (PC, blue) and interneurons (IN, red) for perfusion of 10 μM CP-101,606 (B), 10 μM NAB-14 (D), 10 μM MPX-004 (F), or 100 μM spermine (H), followed by 400 μM APV. I) The bar graph summarizes effects of vehicle (0.01% DMSO) and subunit-selective modulators on NMDAR-EPSCs from hippocampal CA1 pyramidal cells (PC) and interneurons (IN). Blue and red asteriscs (*) denote significantly different from baseline (P < 0.05, paired t test) and black asteriscs (*) denote significant difference between PC and IN (P < 0.05, unpaired t test). J-K) The graphs summarize weighted deactivation time constants of NMDAR-EPSCs from hippocampal CA1 pyramidal cells (J) and interneurons (K) before (baseline) and after (treatment) perfusion with allosteric modulator. The average weighted deactivation time constants for baseline NMDAR-EPSCs among all cells were 129 ± 6 ms (n = 32) for pyramidal cells and 155 ± 8 ms (n = 31) for interneurons. Asteriscs (*) denote significantly different from baseline (P < 0.05, paired t test).
Subunit-selective modulation alters decay time of NMDA receptor-mediated EPSCs in CA1 pyramidal cells, but not hippocampal interneurons.
The mean weighted EPSC decay time constant (τW) during the baseline condition (i.e. before addition of vehicle or test compound) was 129 ± 6 ms (n = 32) for CA1 pyramidal cells and 155 ± 8 ms (n = 31) for hippocampal interneurons, which is significantly different (P < 0.05, unpaired t test). The EPSC decay time in CA1 pyramidal cells was significantly accelerated in the presence of the GluN2B-selective antagonist CP-101,606 and significantly slowed in the presence of the GluN2A-selective antagonist MPX-004 (P < 0.05, paired t test) (Fig. 6J). These results suggest that CP-101,606 increases the relative contribution of fast-deactivating GluN2A-containing receptors to EPSCs in CA1 pyramidal cells, whereas MPX-004 increases the relative contribution of slow-deactivating GluN2B-containing receptors. By contrast, the GluN2C/D-selective antagonist NAB-14 and the GluN2B-selective positive modulator spermine had no effects on the EPSC decay time in CA1 pyramidal cells (P < 0.05, paired t test) (Fig 6J), consistent with the lack of effects on the EPSC amplitude by these modulators in CA1 pyramidal cells (Fig. 6I).
By contrast, none of the test compounds produced a change in the EPSC decay time in hippocampal interneurons (Fig. 6K). The weighted deactivation time constant for recombinant GluN1/2B receptors is 6- to 10-fold faster compared to recombinant GluN1/2D receptors, depending on the GluN1 splice variant (Table 1). Thus, if the NMDA receptor-mediated EPSCs in hippocampal interneurons are mediated entirely by mixed populations of diheteromeric GluN1/2B and GluN1/2D receptors, then selective modulation of GluN1/2B with CP-101,606 and spermine or GluN1/2D with NAB-14 would be expected to reshape the deactivation time courses. In fact, although the CP-101,606, NAB-14, and spermine produced significant inhibition or potentiation of EPSC amplitudes in hippocampal interneurons (Fig. 6I), the EPSC decay time was unchanged by these test compounds (P > 0.05, paired t test) (Fig. 6K). These results suggest that the modulators are not markedly changing the relative contribution of different NMDA receptor subtypes to the EPSC.
Discussion
Studies have shown that NMDA receptor responses in neurons that co-express GluN2B and GluN2D subunits are sensitive to both GluN2B- and GluN2C/D-selective modulators, while displaying Mg2+ block and deactivation time course that are different from recombinant GluN1/2B and GluN1/2D receptors (Jones & Gibb, 2005; Logan et al., 2007; Hildebrand et al., 2014; Huang & Gibb, 2014; Swanger et al., 2015; Perszyk et al., 2016; Swanger et al., 2018). These pharmacological and functional properties could be interpreted as evidence for triheteromeric GluN1/2B/2D receptors, but could also occur if mixed populations of diheteromeric GluN1/2B and GluN1/2D receptors are expressed in these neurons. The lack of insight into properties of GluN1/2B/2D receptors has hampered conclusions regarding the prevalence of these triheteromeric NMDA receptors in the CNS. Here, we provide the first quantitative data for GluN1/2B/2D receptors.
In addition to our evaluation of recombinant triheteromeric GluN1/2B/2D receptors, recent studies have also described recombinant triheteromeric GluN1/2A/2B and GluN1/2A/2C receptors (Hansen et al., 2014; Stroebel et al., 2014; Sun et al., 2017; Bhattacharya et al., 2018). However, as our understanding of the functional consequences of NMDA receptor subunit composition is evolving, more complex structure-function relationships are revealed that complicate quantitative determinations of contributions from native diheteromeric and triheteromeric NMDA receptor subtypes to synaptic transmission. For example, the presence of exon 5-containing GluN1 subunits (e.g. GluN1–1b) in NMDA receptors accelerates deactivation time and reduces the activity of certain subunit-selective modulators, including spermine (Traynelis et al., 1995; Prybylowski et al., 2000; Rumbaugh et al., 2000; Vance et al., 2012; Swanger et al., 2015; Yi et al., 2018). The lack of spermine potentiation of NMDA receptor-mediated EPSCs in CA1 pyramidal cells shown in Figure 6 could therefore be due to a complete lack of diheteromeric GluN1/2B receptors or due to preferential expression GluN1–1b splice variants, or a combination thereof. Thus, GluN1 isoforms add another variable to the pharmacology of NMDA receptor EPSCs, which precludes us from drawing conclusions regarding the prevalence of triheteromeric NMDA receptor subtypes in hippocampal CA1 pyramidal cells and interneurons. This shortcoming exposes the need for subtype-selective modulators that can clearly distinguish between diheteromeric and triheteromeric NMDA receptors.
The inability to selectively express recombinant GluN1/2B/2D receptors as a homogenous population has prevented quantitative evaluation of their properties, but a few previous studies have provided important data from native NMDA receptors presumed to be mainly GluN1/2B/2D receptors. Brickley at al. (2003) demonstrated using single-channel recordings that NMDA receptors in outside-out patches from cerebellar Golgi cells, which are thought to be mainly GluN1/2B/2D receptors, are inhibited by ifenprodil with an IC50 of 129 nM and a maximal inhibition of 71.5% (Brickley et al., 2003). These values are similar to those provided in this study for recombinant GluN1/2B/2D receptors (Table 2). Huang and Gibb (2014) described properties of NMDA receptors in neonatal rat SNc dopaminergic neurons, which are presumably mainly GluN1/2B/2D receptors, and found IC50 values for Mg2+ block at different holding potentials that are consistent with IC50 values provided in this study (Table 3) (Huang & Gibb, 2014).
Memantine is well tolerated without abuse potential and is administered for the treatment of Alzheimer’s disease (Wilkinson, 2012). By contrast, ketamine is a dissociative anesthetic that also has promising efficacy as a fast-acting antidepressant at sub-anesthetic doses (Johnson et al., 2015; Duman et al., 2016; Miller et al., 2016; Chowdhury et al., 2017; Zanos et al., 2018; Krystal et al., 2019). However, ketamine has abuse potential and can produce schizophrenia-like symptoms, thereby limiting its potential as an antidepressant (Zanos et al., 2018). The mechanisms that mediate these divergent clinical effects of memantine and ketamine are unresolved and are the topic of intense investigation, driven by the unmet demand for more efficacious antidepressants with a fast onset of action (Johnson et al., 2015; Duman et al., 2016; Miller et al., 2016; Chowdhury et al., 2017; Zanos et al., 2018; Krystal et al., 2019). Here, we demonstrate the both memantine and ketamine exhibit preferential inhibition of recombinant GluN2D-containing NMDA receptors, including GluN1/2B/2D, in the presence of extracellular Mg2+. Future studies are needed to determine if this finding is relevant to the mechanism of action of these channel blockers in the CNS.
It has been hypothesized that the antidepressant action of ketamine is mediated, in part, by preferential inhibition of highly active NMDA receptors in subpopulations of GABAergic interneurons, which control the excitability of pyramidal neurons (Johnson et al., 2015; Duman et al., 2016; Miller et al., 2016; Chowdhury et al., 2017; Zanos et al., 2018; Krystal et al., 2019). Indeed, recent results from adult rat brain slices demonstrate that ketamine and other NMDA receptor antagonists with fast-acting antidepressant actions indeed disinhibit hippocampal microcircuits by preferentially reducing the action potential frequency of GABAergic inhibitory interneurons (Widman & McMahon, 2018). Several subtypes of cortical and hippocampal interneurons express both GluN2B and GluN2D subunits, which can assemble as triheteromeric GluN1/2B/2D receptors (Monyer et al., 1994; Standaert et al., 1996; von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018). In addition, it has been shown for diheteromeric NMDA receptors that ketamine and memantine exhibit preferential inhibition of GluN1/2C and GluN1/2D receptors in the presence of extracellular Mg2+ concentrations (Kotermanski & Johnson, 2009). Here, we demonstrate that ketamine and memantine also preferentially inhibit the physiologically-relevant triheteromeric GluN1/2B/2D receptors, consistent with the prediction that these blockers may more effectively inhibit GluN2B- and GluN2D-expressing interneurons compared to pyramidal neurons, which primarily express GluN2A and GluN2B subunits.
While preferential inhibition of NMDA receptors in GABAergic inhibitory interneurons is an attractive hypothesis to explain the antidepressant effects of ketamine, this mechanism has been difficult to reconcile with the antidepressant effects of GluN2B-selective antagonists, including CP-101,606 (Nagy et al., 2016). We have previously shown that GluN2B-selective antagonists inhibit triheteromeric GluN1/2A/2B receptors, which are expressed in pyramidal neurons in the cortex and hippocampus (Sheng et al., 1994; Chazot & Stephenson, 1997; Luo et al., 1997; Gray et al., 2011; Rauner & Kohr, 2011), with reduced potency and lower efficacy (~30% maximal inhibition) (Hansen et al., 2014). Here, we demonstrate that GluN2B-selective antagonists inhibit recombinant GluN1/2B/2D receptors with higher potency and efficacy (~65% maximal inhibition) compared to recombinant GluN1/2A/2B receptors. Furthermore, we show that the slightly stronger inhibition of NMDA receptor function by CP-101,606 is also observed for hippocampal interneurons compared to CA1 pyramidal cells in acute brain slices. This finding raises the question of whether this apparent point of convergence between ketamine and GluN2B-selective antagonists may account for their similarity in terms of overlapping antidepressant effects.
The promising effects of NMDA receptor antagonists in preclinical models have invigorated drug discovery efforts and strengthened the rationale to develop new therapeutic agents based on NMDA receptor modulation. Here, we describe the functional and pharmacological properties of triheteromeric GluN1/2B/2D receptors, which may be centrally implicated in the beneficial effects of therapeutic agents targeting NMDA receptors. These new findings will help advance our understanding of the physiological roles of triheteromeric GluN1/2B/2D receptors in the CNS and may facilitate the development of more effective treatments for CNS disorders.
Key points:
Triheteromeric NMDA receptors contain two GluN1 and two distinct GluN2 subunits and mediate excitatory neurotransmission in the central nervous system.
Triheteromeric GluN1/2B/2D receptors have functional properties intermediate to those of diheteromeric GluN1/2B and GluN1/2D receptors.
GluN1/2B/2D receptors are more sensitive to channel block by ketamine and memantine compared to GluN1/2B receptors in the presence of physiological Mg2+.
GluN2B-selective antagonists produce robust inhibition of GluN1/2B/2D receptors, and the GluN2B-selective positive allosteric modulator spermine enhances responses from GluN1/2B/2D, but not GluN1/2A/2B receptors.
These insights to the properties of triheteromeric GluN1/2B/2D receptors are necessary to appreciate their physiological roles in neural circuit function and the actions of therapeutic agents targeting NMDA receptors.
Acknowledgements
We thank Dr. Frank S. Menniti for discussion and critical comments, and Gina C. Bullard for excellent technical assistance.
Funding
This work was supported by National Institutes of Health (GM103546 and NS097536 to KBH, and NS036654 and NS065371 to SFT).
Competing interests
KBH is principal investigator on a research grant from Janssen to University of Montana. SFT is principal investigator on a research grant from Allergan to Emory University School of Medicine, is a member of the SAB for Sage Therapeutics, is co-founder of NeurOp Inc, and receives royalties for software. SFT is co-inventor on Emory-owned Intellectual Property that includes allosteric modulators of NMDA receptor function. The remaining authors have no competing interests to declare.
References
- Acker TM, Khatri A, Vance KM, Slabber C, Bacsa J, Snyder JP, Traynelis SF & Liotta DC (2013). Structure-activity relationships and pharmacophore model of a noncompetitive pyrazoline containing class of GluN2C/GluN2D selective antagonists. J Med Chem 56, 6434–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC & Traynelis SF (2011). Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol 80, 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Khatri A, Swanger SA, DiRaddo JO, Yi F, Hansen KB, Yuan H & Traynelis SF (2018a). Triheteromeric GluN1/GluN2A/GluN2C NMDARs with Unique Single-Channel Properties Are the Dominant Receptor Population in Cerebellar Granule Cells. Neuron 99, 315–328 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Ma Y, Dunn AR, Bradner JM, Scimemi A, Miller GW, Traynelis SF & Wichmann T (2018b). NMDA receptor blockade ameliorates abnormalities of spike firing of subthalamic nucleus neurons in a parkinsonian nonhuman primate. J Neurosci Res 96, 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M & Cull-Candy SG (2003). NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23, 4958–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Boudier L, Maurel D, Blahos J & Pin JP (2005). Assembly-dependent surface targeting of the heterodimeric GABAB Receptor is controlled by COPI but not 14-3-3. Mol Biol Cell 16, 5572–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ & Jones S (2008). NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol 586, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Misra C & Cull-Candy S (2000). Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci 20, 5899–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL & Stephenson FA (1997). Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: Direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem 69, 2138–2144. [DOI] [PubMed] [Google Scholar]
- Cheriyan J, Balsara RD, Hansen KB & Castellino FJ (2016). Pharmacology of triheteromeric N-Methyl-D-Aspartate Receptors. Neurosci Lett 617, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL & Sanacora G (2017). Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF & Traynelis SF (2007). Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol 581, 107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G & Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Luo JH, Wang YH, Yasuda RP & Wolfe BB (1998). Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol 53, 429–437. [DOI] [PubMed] [Google Scholar]
- Gielen M, Siegler Retchless B, Mony L, Johnson JW & Paoletti P (2009). Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow NG, Wilcox MR & Johnson JW (2018). Effects of Mg(2+) on recovery of NMDA receptors from inhibition by memantine and ketamine reveal properties of a second site. Neuropharmacology 137, 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K & Nicoll RA (2011). Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron 71, 1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Furukawa H & Traynelis SF (2010). Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol Pharmacol 78, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Yuan H & Traynelis SF (2014). Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB & Traynelis SF (2011). Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J Neurosci 31, 3650–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Menniti FS & Traynelis SF (2017). NMDA Receptors in the Central Nervous System. Methods Mol Biol 1677, 1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ & Paoletti P (2005). Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46, 261–274. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Pitcher GM, Harding EK, Li H, Beggs S & Salter MW (2014). GluN2B and GluN2D NMDARs dominate synaptic responses in the adult spinal cord. Scientific reports 4, 4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z & Gibb AJ (2014). Mg2+ block properties of triheteromeric GluN1-GluN2B-GluN2D NMDA receptors on neonatal rat substantia nigra pars compacta dopaminergic neurones. J Physiol 592, 2059–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Glasgow NG & Povysheva NV (2015). Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol 20, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, VanDongen HMA & VanDongen AMJ (2002). The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci 22, 2044–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S & Gibb AJ (2005). Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J Physiol 569, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser TM, Kell SA, Kusumoto H, Shaulsky G, Bhattacharya S, Epplin MP, Strong KL, Miller EJ, Cox BD, Menaldino DS, Liotta DC, Traynelis SF & Burger PB (2018). The Bioactive Protein-Ligand Conformation of GluN2C-Selective Positive Allosteric Modulators Bound to the NMDA Receptor. Mol Pharmacol 93, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer RA, Frank S, Schulthess T, Landwehr R, Lustig A & Engel J (1999). Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil alpha-helices. Biochemistry 38, 13263–13269. [DOI] [PubMed] [Google Scholar]
- Khatri A, Burger PB, Swanger SA, Hansen KB, Zimmerman S, Karakas E, Liotta DC, Furukawa H, Snyder JP & Traynelis SF (2014). Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator. Mol Pharmacol 86, 548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE & Johnson JW (2009). Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci 29, 2774–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Sanacora G, Charney DS & Duman RS (2019). Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 101, 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Partridge JG, Matta JA, Buonanno A & Vicini S (2007). Long-lasting NMDA receptor-mediated EPSCs in mouse striatal medium spiny neurons. J Neurophysiol 98, 2693–2704. [DOI] [PubMed] [Google Scholar]
- Lu W, Du J, Goehring A & Gouaux E (2017). Cryo-EM structures of the triheteromeric NMDA receptor and its allosteric modulation. Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JH, Wang YH, Yasuda RP, Dunah AW & Wolfe BB (1997). The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51, 79–86. [DOI] [PubMed] [Google Scholar]
- Miller OH, Moran JT & Hall BJ (2016). Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 100, 17–26. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B & Seeburg PH (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540. [DOI] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC & Traynelis SF (2010). A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun 1, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy D, Stoiljkovic M, Menniti FS & Hajos M (2016). Differential Effects of an NR2B NAM and Ketamine on Synaptic Potentiation and Gamma Synchrony: Relevance to Rapid-Onset Antidepressant Efficacy. Neuropsychopharmacology 41, 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ & Traynelis SF (2016). GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity. Mol Pharmacol 90, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina-Crespo JC & Gibb AJ (2002). Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol 541, 41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Rumbaugh G, Wolfe BB & Vicini S (2000). Increased exon 5 expression alters extrasynaptic NMDA receptors in cerebellar neurons. J Neurochem 75, 1140–1146. [DOI] [PubMed] [Google Scholar]
- Rauner C & Kohr G (2011). Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286, 7558–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF & Vicini S (2000). Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol 83, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G & Vicini S (1999). Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci 19, 10603–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN & Jan LY (1994). Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB Jr. & Young AB (1996). Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res 42, 89–102. [DOI] [PubMed] [Google Scholar]
- Stroebel D, Buhl DL, Knafels JD, Chanda PK, Green M, Sciabola S, Mony L, Paoletti P & Pandit J (2016). A Novel Binding Mode Reveals Two Distinct Classes of NMDA Receptor GluN2B-selective Antagonists. Mol Pharmacol 89, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Carvalho S, Grand T, Zhu S & Paoletti P (2014). Controlling NMDA receptor subunit composition using ectopic retention signals. J Neurosci 34, 16630–16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, Casado M & Paoletti P (2018). Triheteromeric NMDA receptors: from structure to synaptic physiology. Curr Opin Physiol 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hansen KB & Jahr CE (2017). Allosteric Interactions between NMDA Receptor Subunits Shape the Developmental Shift in Channel Properties. Neuron 94, 58–64 e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Vance KM, Acker TM, Zimmerman SS, DiRaddo JO, Myers SJ, Bundgaard C, Mosley CA, Summer SL, Menaldino DS, Jensen HS, Liotta DC & Traynelis SF (2018). A Novel Negative Allosteric Modulator Selective for GluN2C/2D-Containing NMDA Receptors Inhibits Synaptic Transmission in Hippocampal Interneurons. ACS Chem Neurosci 9, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y & Traynelis SF (2015). NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus. J Neurosci 35, 15971–15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K & Mishina M (1996). Functional correlation of NMDA receptor epsilon subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. J Neurosci 16, 4376–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M & Heinemann SF (1995). Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268, 873–876. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ & Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Hansen KB & Traynelis SF (2012). GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J Physiol 590, 3857–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JAH, Wolfe BB & Grayson DR (1998). Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol 79, 555–566. [DOI] [PubMed] [Google Scholar]
- Volkmann RA, Fanger CM, Anderson DR, Sirivolu VR, Paschetto K, Gordon E, Virginio C, Gleyzes M, Buisson B, Steidl E, Mierau SB, Fagiolini M & Menniti FS (2016). MPX-004 and MPX-007: New Pharmacological Tools to Study the Physiology of NMDA Receptors Containing the GluN2A Subunit. PLoS ONE 11, e0148129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Bocklisch C, Tonges L, Herb A, Mishina M & Monyer H (2015). GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Frontiers in cellular neuroscience 9, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman AJ & McMahon LL (2018). Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115, E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D (2012). A review of the effects of memantine on clinical progression in Alzheimer’s disease. Int J Geriatr Psychiatry 27, 769–776. [DOI] [PubMed] [Google Scholar]
- Williams K (1993). Ifenprodil Discriminates Subtypes of the N-Methyl-D-Aspartate Receptor - Selectivity and Mechanisms at Recombinant Heteromeric Receptors. Mol Pharmacol 44, 851–859. [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T & Sakmann B (1998). Adjacent asparagines in the NR2-subunit of the NMDA receptor channel control the voltage-dependent block by extracellular Mg2+. J Physiol 506 (Pt 1), 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM (1973). Ionic blockage of sodium channels in nerve. J Gen Physiol 61, 687–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL, Holloway BB, Johnston AD, Nathanson NM, Deisseroth K, Gerber DJ, Tonegawa S & Lawrence JJ (2014). Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol 592, 3463–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Mou TC, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR & Hansen KB (2016). Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors. Neuron 91, 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Traynelis SF & Hansen KB (2017). Selective Cell-Surface Expression of Triheteromeric NMDA Receptors. Methods Mol Biol 1677, 145–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Zachariassen LG, Dorsett KN & Hansen KB (2018). Properties of Triheteromeric N-Methyl-d-Aspartate Receptors Containing Two Distinct GluN1 Isoforms. Mol Pharmacol 93, 453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Erreger K, Dravid SM & Traynelis SF (2005). Conserved structural and functional control of N-methyl-D-aspartate receptor gating by transmembrane domain M3. J Biol Chem 280, 29708–29716. [DOI] [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK & Traynelis SF (2009). Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci 29, 12045–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr. & Gould TD (2018). Convergent Mechanisms Underlying Rapid Antidepressant Action. CNS drugs 32, 197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Malan MJ, Fried SR, Dazin PF, Jan YN, Jan LY & Schwappach B (2001). Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc Natl Acad Sci U S A 98, 2431–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]