Abstract
Background
Chronic antibiotic-refractory pouchitis (CARP) occurs in up to 15% of patients with ulcerative colitis (UC) following proctocolectomy with ileal pouch-anal anastomosis (IPAA).
Aim
To investigate the effectiveness of ustekinumab in the treatment of CARP.
Methods
This was a retrospective single-center study of UC patients with an IPAA, who subsequently developed CARP and received ustekinumab with standard Crohn’s disease (CD) dosing between 2016 and 2018. Patients with CD of the pouch were excluded. Demographic, clinical, and endoscopic data were collected. Outcomes included a change in the endoscopic subscore of the Pouchitis Disease Activity Index (PDAI), change in the ulcerated surface area, clinical response, and the number of bowel movements per 24 h.
Results
Twenty-four patients with CARP were included for analysis. Median follow-up time was 12.9 months (IQR 7.9–16). Twelve patients (50%) had a clinical response with the median number of bowel movements within 24 h decreasing from 8 (IQR, 5–12) to 6 (IQR, 5–8) P = 0.002. Thirteen patients had pouchoscopies available post-ustekinumab treatment. In these patients, the median endoscopic subscore of the PDAI decreased from 5 (IQR, 3–6) to 4 (IQR, 2–5), P = 0.016. Likewise, among these thirteen patients, nine (69%) had an ulcerated surface area > 10% before ustekinumab treatment; after treatment with ustekinumab, only four patients (31%) still had an ulcerated surface area of > 10%.
Conclusions
This is the largest study of ustekinumab treatment for patients with chronic antibiotic-refractory pouchitis. We found that ustekinumab therapy led to the improvement in clinical and endoscopic endpoints.
Keywords: Pouchitis, Ustekinumab, Ileal pouch-anal anastomosis, PDAI
Introduction
Colectomy is required in up to 30% of patients with ulcerative colitis (UC) due to medically refractory disease or development of dysplasia/cancer [1, 2]. In these situations, restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the usual surgery of choice. However, pouchitis may occur in up to 80% of patients and is associated with a significantly impaired quality of life due to symptoms of urgency, diarrhea, multiple bowel movements per day, and incontinence [3, 4].
A variety of conditions may cause symptoms of diarrhea and urgency after IPAA [4, 5]. Clinical symptoms correlate poorly with endoscopic disease, and thus, it is essential to perform pouchoscopy to establish pouchitis as the cause of symptoms, assess the severity, and rule out other conditions such as cuffitis [5, 6].
The conventional treatment for confirmed pouchitis is antibiotics such as ciprofloxacin and metronidazole [7]. Up to 15% of patients, however, develop chronic pouchitis and either become dependent on antibiotics for symptom relief or have continuous symptoms despite chronic antibiotic therapy [7–9]. With no approved treatments for chronic antibiotic-refractory pouchitis, a significant unmet medical need exists.
Ustekinumab is a human monoclonal antibody against the p40 subunit shared by both IL-12 and IL-23. It was shown to be effective for the treatment of moderate-to-severe Crohn’s disease (CD). [10]. Recent studies have also demonstrated its efficacy in inducing remission in patients with UC [15], but its utility in the treatment of pouchitis remains unclear.
Our study aimed to evaluate the effectiveness and safety of ustekinumab for the treatment of chronic antibiotic-refractory pouchitis.
Methods
Patient Selection
This was a retrospective single-center study including patients with UC who had undergone a total proctocolectomy with IPAA, subsequently developed chronic antibiotic-refractory pouchitis, and received ustekinumab with standard CD dosing (one 90-mg IV loading dose infusion followed by 90-mg injections every eight weeks). Patients with CD of the pouch were excluded. Patients were defined as having chronic antibiotic-refractory pouchitis when pouch inflammation was confirmed on pouchoscopy, and patients had over four weeks of pouch symptoms; such as increased stool frequency, urgency, tenesmus, and fecal seepage, despite standard courses (> 1 month) of antibiotic treatment. At our institution, pouch inflammation is classified as “CD of the pouch” only when satisfying one of the following criteria: 1) discrete ulcerations in the pre-pouch ileum, 2) de novo perianal disease that is not suspected to be a technical complication of pouch creation, or 3) histopathological presence of granulomas in the pouch biopsies. All eligible patients seen at the University of Chicago IBD center between 2016 and 2018 were included in the study if they had a minimal follow-up time of 3 months. Patients had their UC diagnosis confirmed by review of their prior clinical and pathologic records including their colectomy pathology report. Patients were excluded if they had a pre- or postoperative diagnosis of CD.
Data Collection
Patients’ demographic, clinical, and endoscopic data were collected by a comprehensive review of their electronic medical records. The following baseline characteristics were collected: age at inclusion, gender, disease duration, UC extent based on the Montreal classification, and smoking status. Pouchoscopies performed before and following ustekinumab initiation were recorded. At our institution, pouchoscopies are performed using a standard operating protocol. These reports include detailed descriptions of the mucosa as well as high-definition images of the different areas of the pouch—specifically, the pre-pouch ileum, the pouch inlet, forward view of the pouch, and a retroverted view of the pouch. Based on these images and descriptions, the reviewer computed the PDAI and ulcer location and area to capture endoscopic data in a standardized manner. Due to the nature of the histologic reports and clinical data available to us, we could not give clinical and histologic subscores of the PDAI. For clinical data, we used bowel movements (BM) over 24 h which was captured in clinic visits and physician global assessment for the outcome of clinical improvement, both of which were collected at the last clinic visit before initiation of ustekinumab and at the first clinic visit following the loading dose of ustekinumab. Exposure to other treatments (antibiotics, mesalamines, immunomodulators, steroids, and biologics) before and after surgery was also recorded. Previous infections including clostridium difficile infection (CDI) and cytomegalovirus infection were recorded as well. All patients gave informed consent to receive ustekinumab. The institutional ethics review board approved the study.
Outcomes
The change in the endoscopic subscore of the PDAI[11], as well as the change in the ulcerated surface area after treatment with ustekinumab, was assessed in patients with follow-up pouchoscopies. The ulcerated surface area of the pouch (including the inlet) was calculated based on the endoscopic image. Patients were divided into three categories (patients who had < 10% of their pouch ulcerated, patients who had 10–30% of the pouch ulcerated, and patients who had > 30% of their pouch ulcerated).
Physician global assessment and the number of BM per 24 h, before and after treatment with ustekinumab, were reported for all patients.
Statistical Analysis
Descriptive statistics for demographic and clinical characteristics include median (IQR) for continuous variables and frequency distributions for categorical data. Nonparametric testing was performed with the Wilcoxon matched-pair signed-rank test. A two-sided p value of < 0.05 was determined to be statistically significant. All analyses were performed with GraphPad Prism version 8.00 for Windows (GraphPad Software, La Jolla, California, USA).
Results
Patient Characteristics
A total of 24 patients met the inclusion criteria. The median age was 35.6 years (IQR 26.6–41.5), 10 (42%) of which were females. Twenty-one (87.5%) of patients had never smoked, and two patients (8.3%) had a concurrent diagnosis of primary sclerosing cholangitis (PSC). Before colectomy, 21 patients (87.5%) had extensive colitis. Fourteen patients (58.3%) and 16 patients (66.7%) were preoperatively treated with biologics and immunomodulators, respectively. Previous treatment for pouchitis included antibiotics (ciprofloxacin or metronidazole) in all patients, biologics other than ustekinumab in 12 patients (50%), and immunomodulators in six patients (25%). Median time from the start of ustekinumab treatment to pouchoscopy was 7.4 months (IQR 4.6–10.6). Median follow-up duration was 12.9 months (IQR 7.9–16). Over the follow-up period, five patients stopped treatment with ustekinumab, two patients had their pouch excised, and three patients were switched to other therapies. Twenty patients were still on ustekinumab at the end of the follow-up period. The baseline demographic and clinical characteristics of the whole cohort and of the subgroup with follow-up endoscopic data are presented in Table 1.
Table 1.
Patient demographics
| n = 24 (%) whole cohort | n = 13 (%) cohort with endoscopic follow-up |
|
|---|---|---|
| Female | 10 (42) | 5 (38) |
| Median age at first ustekinumab dose (IQR) | 35.6 (26.6–41.5) | 31.1 (27.4–39.3) |
| Smoking status | ||
| Current smoker | 1 (4.2) | 1 (8) |
| Ex-smoker | 2 (8.3) | 0 (0) |
| Never | 21 (87.5) | 12 (92) |
| Median BMI (IQR) | 24.4 (23–27) | 25 (23–27) |
| PSC | 2 (8.3) | 2 (15) |
| Caucasian | 20 (83) | 11 (85) |
| Extensive colitis prior to colectomy | 21 (87.5) | 12 (92) |
| Treatment prior to colectomy | ||
| Mesalamine | 12 (50) | 7 (54) |
| Immunomodulator | 16 (66.7) | 11 (85) |
| Azathioprine | 15 (62.5) | 10 (77) |
| MTX | 1(4.2) | 1(8) |
| Biologics | 14(58.3) | 8(62) |
| Infliximab | 14 (58.3) | 8 (62) |
| Adalimumab | 2 (8.3) | 1 (8) |
| Vedolizumab | 1 (4.2) | 0 (0) |
| Antibiotics | 35 (21.3) | 3 (23) |
| Prednisone | 13 (54.1) | 8 (62) |
| Treatment after colectomy | ||
| Mesalamine | 6 (25) | 3 (23) |
| Immunomodulator | 6 (25) | 4 (31) |
| Azathioprine | 6 (25) | 4(31) |
| Biologics | 12 (50) | 6 (46) |
| Infliximab | 6 (25) | 3 (23) |
| Adalimumab | 9 (37.5) | 4 (31) |
| Vedolizumab | 4 (16.7) | 3 (23) |
| Certolizumab pegol | 3 (12.5) | 2 (15) |
| Antibiotics | 24 (100) | 13 (100) |
| Prednisone | 13 (54.2) | 9 (69) |
| Budesonide | 16 (66.7) | 8 (62) |
| Rifaximine | 2 (8.3) | 2 (15) |
| VSL | 6 (25) | 1 (8) |
| Biologic naïve prior to UST infusion | 4 (16) | 3 (23) |
| Median PDAI prior to UST infusion | 5(3–6) | 5 (4–6) |
| Median follow-up period—months (IQR) | 12.6 (5.2–10.76) | 16.9 (15–18.7) |
Endoscopic Outcomes
Detailed pouchoscopy reports were available for all the patients in the study before receiving ustekinumab. At the time of pre-ustekinumab pouchoscopy, 33% of patients had pouch inlet ulcers, and 88% of patients had ulcerations in the pouch. Following the initiation of ustekinumab, pouchoscopies were performed in 13 patients. In these patients, the median endoscopic subscore of the PDAI decreased from 5 (IQR, 4–6) to 4 (IQR, 2–5) post-treatment (P = 0.016) (Fig. 1, Panel A). Likewise, among these thirteen patients, nine (69%) had an ulcerated surface area > 10% before ustekinumab treatment; after treatment with ustekinumab, only four patients (31%) still had an ulcerated surface area of > 10% (Fig. 1, Panel B). No subject achieved a PDAI endoscopic subscore of 0 (complete endoscopic normalization of the pouch).
Fig. 1.
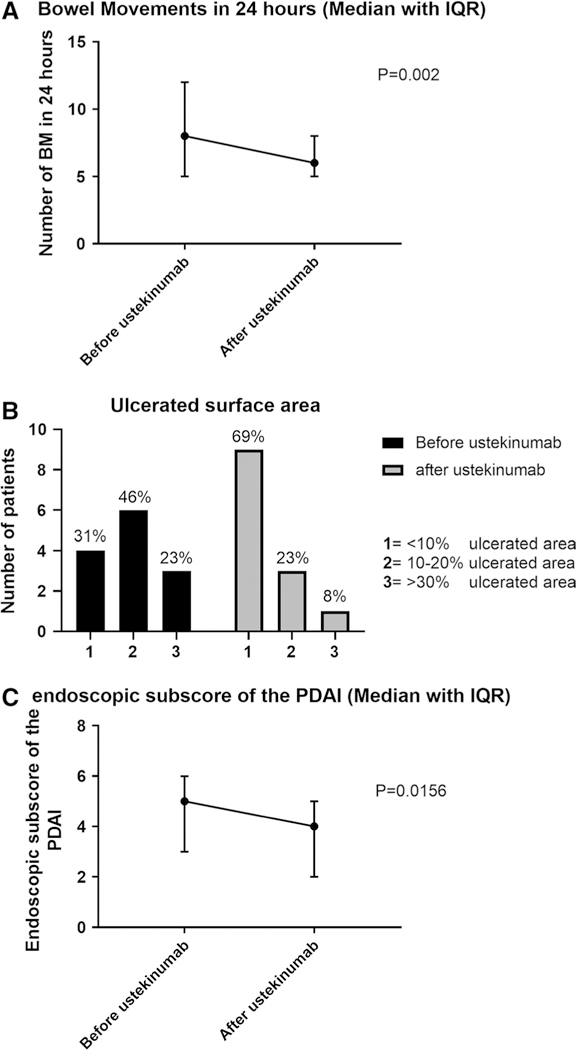
Panel A. Endoscopic subscore of the PDAI prior and post- ustekinumab treatment of pouchitis (median + IQR). Panel B. Ulcerated surface area (%) prior and post-ustekinumab treatment of pouchitis. Panel C. Change in bowel movements in 24 h (Median + IQR)
For the particularly refractory group of patients who had already received treatment for pouchitis with immunomodulatory and biologic drugs (Eight patients), before treatment with ustekinumab and in whom we had follow- up endoscopic data, the endoscopic subscore of the PDAI decreased from a median of 6 to a median of 4. Likewise, four patients had an ulcerated surface area of over 10% before ustekinumab treatment, while only one patient still had an ulcerated surface area of over 10% after treatment with ustekinumab.
Clinical Outcomes
Clinical follow-up data were available for all patients. Half of the patients (12/24) had a significant clinical response based on the physician’s clinic note. Median BM over 24 h decreasing from 8 (IQR, 5–12) to 6 (IQR, 5–8) P = 0.002 (Fig. 1, Panel C). The median time to the clinic visit following ustekinumab initiation was 52 days IQR (34–125).
Discussion
In this retrospective cohort of patients with chronic antibiotic-refractory pouchitis, we observed favorable endoscopic and clinical responses to ustekinumab. More than half of our patients had also been treated with biologics or immunomodulators; thus, ustekinumab demonstrated efficacy in a highly resistant cohort of patients.
A recent multicenter retrospective study reported the effectiveness of ustekinumab for CD of the pouch in 56 patients [12]. The authors of this study concluded that ustekinumab is an effective treatment for chronic pouchitis and CD of the pouch. The study’s strength lies in its large number of patients and its multicenter design. However, the majority (84%) of patients in this study had a diagnosis of CD of the pouch, and only nine patients (16%) had chronic antibiotic-refractory pouchitis. The authors do not specify the criteria they used to diagnose patients as having CD of the pouch. The primary outcome of the study was clinical remission based on physician assessment. When endoscopic data were available, patients were described as being in endoscopic remission or endoscopic response by clinicians performing pouchoscopies in the different institutions. There were no data on the dosing of ustekinumab, and it is probable that the study included patients from 2013 onward (before ustekinumab approval for use in CD) that a variety of dosing regimens was used.
In our single-center study, we reported standardized endoscopic outcome measures, as endoscopic healing of the pouch is considered to be a preferred outcome. Reported measures in our study included the endoscopic subscore of the PDAI, as this is the most widely used metric for reporting endoscopic findings in pouchitis [11]. We also described the ulcerated surface area before and after ustekinumab treatment as this measure was found to have the highest interobserver reliability when grading pouchitis severity in a recent large prospective study and it was recommended that it be part of any future endoscopic score of pouchitis [13]. We believe that using objective endpoints in trials of pouchitis is essential due to the poor correlation between clinical and endoscopic findings in such patients [5].
Ustekinumab has known efficacy in patients with CD[10] and has recently been shown to be effective in inducing remission in patients with UC [15]; therefore, it is plausible that patients with pouchitis will respond to such therapy. However, previous studies have suggested that biologic therapy might be more effective in the treatment of CD of the pouch when compared to chronic antibiotic-refractory pouchitis [14]. Our results using CD dosing showed that ustekinumab is effective in chronic antibiotic-refractory pouchitis without a CD like phenotype as well.
Limitations to our study include its retrospective nature with its inherent risk of bias, and while our results are exploratory due to the small sample size, we believe that our ability to characterize clinical and endoscopic findings is a strength.
We used objective outcomes such as endoscopic disease activity and characterized the severity of pouch inflammation using measures which have been shown to have high interobserver reliability [13]. However, none of the physicians were blinded to the treatment or evaluations, and so, treatment effects and endoscopic objectivity might have been biased. We also applied the standard treatment regimen for CD to our pouchitis patients; further studies are needed to identify the optimal dose and schedule for pouchitis.
In conclusion, we report on the largest experience with ustekinumab treatment of patients with chronic antibiotic-refractory pouchitis and demonstrate that ustekinumab improves both clinical and endoscopic endpoints. Prospective studies are warranted to confirm these findings.
Acknowledgments
Funding This work received no outside funding.
DTR is a consultant and has received grant support from Abbvie, Merck & Co., Janssen, Takeda, and Pfizer. AS has received funding from AbbVie, Celltrion, and Takeda. JP has received grants from Takeda and Abbvie and serves as a consultant for Verastem. He was on the advisory board for Pfizer and Janssen. RDC is a consultant at Abbvie, Celgene, Janssen, Pfizer, Takeda, and UCB Pharma. SD has received grant support from Pfizer. No funding or sponsorship was received for this study or publication of this article.
Footnotes
Compliance with ethical standards
Conflict of interest JEO, RW, KEJ, AI, NKC, and NH have no relevant disclosures.
Publisher's Disclaimer: Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population- based cohort. Am J Gastroenterol. 2012;107:1228–1235. 10.1038/ajg.2012.127. [DOI] [PubMed] [Google Scholar]
- 2.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431–440. 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 3.Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology. 2003;124:1636–1650. PubMed PMID: 12761722. [DOI] [PubMed] [Google Scholar]
- 4.Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-Related Symptoms and Quality of Life in Patients with Ileal Pouch-Anal Anastomosis. Inflamm Bowel Dis. 2017;23:1218–1224. 10.1097/mib.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuschen UA, Allemeyer EH, Hinz U, et al. Diagnosing pouchitis: comparative validation of two scoring systems in routine follow-up. Dis Colon Rectum. 2002;45:776–786. discussion 86–88. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Shimoyama T, Bamba T, Matsumoto K. Consecutive Monitoring of Fecal Calprotectin and Lactoferrin for the Early Diagnosis and Prediction of Pouchitis after Restorative Proctocolectomy for Ulcerative Colitis. Am J Gastroenterol. 2015;110:881–887. 10.1038/ajg.2015.129. [DOI] [PubMed] [Google Scholar]
- 7.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649–670. 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 8.Dalal RL, Shen B, Schwartz DA. Management of Pouchitis and Other Common Complications of the Pouch. Inflamm Bowel Dis. 2018;24:989–996. 10.1093/ibd/izy020. [DOI] [PubMed] [Google Scholar]
- 9.Bar F, Kuhbacher T, Dietrich NA, et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018;47:581–587. 10.1111/apt.14479. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016;375:1946–1960. 10.1056/nejmoa1602773. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409–415. PubMed PMID: 8170189. [DOI] [PubMed] [Google Scholar]
- 12.Weaver KN, Gregory M, Syal G, et al. Ustekinumab Is Effective for the Treatment of Crohn’s Disease of the Pouch in a Multicenter Cohort. Inflamm Bowel Dis. 2018;. 10.1093/ibd/izy302. [DOI] [PubMed] [Google Scholar]
- 13.Samaan MA, Shen B, Mosli MH, et al. Reliability among central readers in the evaluation of endoscopic disease activity in pouchitis. Gastrointest Endosc. 2018;88:360–369 e2 10.1016/j.gie.2018.04.2330. [DOI] [PubMed] [Google Scholar]
- 14.Huguet M, Pereira B, Goutte M, et al. Systematic Review With Meta-Analysis: Anti-TNF Therapy in Refractory Pouchitis and Crohn’s Disease-Like Complications of the Pouch After Ileal Pouch-Anal Anastomosis Following Colectomy for Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:261–268. 10.1093/ibd/izx049. [DOI] [PubMed] [Google Scholar]
- 15.Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, Peyrin-Biroulet L, Van-Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C. Safety and Efficacy of Ustekinumab Induction Therapy in Patients with Moderate to Severe Ulcerative Colitis: Results from the Phase 3 UNIFI Study. Presented at ACG; 2018. [Google Scholar]


