Abstract
Sickle cell trait (SCT) has been associated with hypercoagulability, chronic kidney disease (CKD), and ischemic stroke. Whether concomitant CKD modifies long-term ischemic stroke risk in individuals with SCT is uncertain. We analyzed data from 3602 genotyped black adults (female = 62%, mean baseline age = 54 years) who were followed for a median 26 years by the Atherosclerosis Risk in Communities Study. Ischemic stroke was verified by physician review. Associations between SCT and ischemic stroke were analyzed using repeat-events Cox regression, adjusted for potential confounders. SCT was identified in 236 (7%) participants, who more often had CKD at baseline than noncarriers (18% vs 13%, P = .02). Among those with CKD, elevated factor VII activity was more prevalent with SCT genotype (36% vs 22%; P = .05). From 1987–2017, 555 ischemic strokes occurred in 436 individuals. The overall hazard ratio of ischemic stroke associated with SCT was 1.31 (95% CI: 0.95–1.80) and was stronger in participants with concomitant CKD (HR = 2.18; 95% CI: 1.16–4.12) than those without CKD (HR = 1.09; 95% CI: 0.74–1.61); P for interaction = .04. The hazard ratio of composite ischemic stroke and/or death associated with SCT was 1.20 (95% CI: 1.01–1.42) overall, 1.44 (95% CI: 1.002–2.07) among those with CKD, and 1.15 (95% CI: 0.94–1.39) among those without CKD; P for interaction = .18. The long-term risk of ischemic stroke associated with SCT relative to noncarrier genotype appears to be modified by concomitant CKD.
1 |. INTRODUCTION
Sickle cell trait (SCT), or heterozygosity for the HbS gene, has a presence of 7–9% in African Americans.1 Observational studies have reported heightened coagulation activation in healthy individuals with SCT,2,3 and experimental studies suggest diminished response to exogenous fibrinolytic agents.4 Large scale epidemiologic studies confirm associations of SCT with both elevated biomarkers of coagulation activation5 and heightened risk of venous thromboembolism.6–8 While sickle cell anemia9 and activation of coagulation10 are known risk factors for ischemic stroke, prospective investigations of the association between SCT and ischemic stroke have been conflicting.11,12 Heterogeneity of the reported effect may be influenced by comorbid conditions, such as chronic kidney disease (CKD).
A higher incidence of cerebrovascular events has been reported in patients with CKD, independent of vascular risk factors.13 Potential contributors to the association are thought to be nontraditional factors, such as hypercoagulability and systemic inflammation.14 Elevations in inflammatory and procoagulant biomarkers observed with CKD may be due to increased production, decreased renal clearance, or possibly a combination of both.15 Notably, epidemiologic research suggests that individuals with SCT not only have a greater prevalence of reduced glomerular filtration, but also albuminuria, which is often associated with endothelial dysfunction.16,17 We hypothesized that associations between SCT and ischemic stroke would be amplified by concomitant CKD. To test this theory, we examined data from the Atherosclerosis Risk in Communities Study, which has followed a cohort of black study participants for 30 years, from midlife through advanced age.
2 |. METHODS
2.1 |. The ARIC study
The ARIC study is an ongoing, prospective cohort drawn from four U.S. areas. Study data and materials are publicly available.18 A population-based sample (N = 4266) of black adults aged 45–64 in 1987–1989 was recruited with informed consent, primarily from Jackson, Mississippi and Forsyth County, North Carolina.19 Participation has included six clinical visits, with annual telephone surveys during interim years and ongoing surveillance of acute cardiovascular events. Study participant retention has been excellent across the 30 years of follow-up, with 80% of survivors participating in the annual survey in 2016. All study protocols were approved by local Institutional Review Boards.
2.2 |. Genotyping
Black study participants were genotyped for hemoglobin S (rs334) and hemoglobin C (rs33930165) using functionally tested TaqMan SNP Genotyping Assays (Life Technologies, Grand Island, NY), as previously described.11 First degree relatives were identified by PLINK.20 After random exclusion of one study participant from each first-degree relative pair, principal components of genetic ancestry were quantified. A total of 10 principal components for the black study population were derived, using EIGENSTRAT 5.0.1 (David Reich, open source), with genomic variation characterized by the HumanExome BeadChip v1.0 (Affymetrix, Santa Clara, CA).21
2.3 |. Clinical covariates
Midlife participant characteristics were ascertained at ARIC visit one (1987–1989), or if missing, from visit two (1990–1992). Medical histories and clinical data were acquired by standardized clinical examinations, home interviews, and health questionnaires. Age, sex, race, and current smoking were self-reported. Seated blood pressures were measured by random-zero mercury manometers, with hypertension classified by a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or antihypertensive medication use. Fasting cholesterol, blood glucose, and serum creatinine were analyzed by ARIC central laboratories. Serum creatinine was quantified using the modified kinetic Jaffe method. Hyperlipidemia was considered a total fasting cholesterol ≥240 mg/dL. Diabetes mellitus was defined by a fasting blood glucose level ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, self-reported physician diagnosis of diabetes, or use of diabetic medications. Estimated glomerular filtration rate (eGFR) was calculated with serum creatinine, using the CKD-Epi formula. We defined CKD by an eGFR <60 mL/min/1.73m2.
2.4 |. Biomarkers
Select biomarkers of coagulation (factor VII activity, factor VIII activity, fibrinogen) and endothelial function (von Willebrand factor) were measured at ARIC visit one by the Hemostasis Central Laboratory.22 Factor VII and factor VIII activity were measured by one-stage clotting assays. Fibrinogen was quantified by the modified thrombin time method. Von Willebrand factor antigen was quantified by ELISA. Repeat-testing reliability coefficients were 0.78 for factor VII activity, 0.86 for factor VIII activity, 0.72 for fibrinogen, and 0.68 for von Willebrand factor.23 Factor VII activity, factor VIII activity, and von Willebrand factor were considered elevated when exceeding 150% of the laboratory reference. Elevated fibrinogen was defined by a concentration exceeding 400 mg/dL.
2.5 |. Acute ischemic stroke surveillance
Incidence of stroke over the course of follow up was determined by surveillance of hospitalized events, as previously described.24 Hospitalizations for study participants with neurological deficits ≥24 hours and ICD discharge codes 430–438 or 160.x - 167.x were fully abstracted. Stroke diagnosis was ascertained by physician review of the medical record. Diagnoses were considered definite when based on neurological symptoms, imaging studies, and lumbar puncture results (for subarachnoid hemorrhage). When the clinical picture suggested stroke, but either lumbar puncture was not performed (for subarachnoid hemorrhage), or imaging studies were inconclusive, stroke diagnoses were considered probable. Based on the clinical and neuroimaging data, stroke was classified as ischemic or hemorrhagic, with ischemic strokes further classified as either embolic or thrombotic.25 Definite thrombotic stroke was considered lacunar when lesions measured ≤2 cm and were located in the basal ganglia, brain stem, thalamus, internal capsule, or cerebral white matter. For quality assurance, stroke diagnosis was also determined by a computer algorithm. Disagreements between the physician diagnosis and computer algorithm were adjudicated by a second physician reviewer. Agreement rates between the physician reviewer and computer algorithm were 78%.24 In the majority of discordant diagnoses (65%), the physician adjudicator agreed with the physician reviewer, rather than the computer algorithm.24 For the purposes of this analysis, each hospitalization for acute ischemic stroke per participant was counted as an outcome. Hospitalizations for hemorrhagic stroke were not included. The first occurring ischemic stroke hospitalization captured by active surveillance was considered the “index” hospitalization. Hospital transfers were defined by relocation to another acute care hospital or relocation to a rehabilitation unit of the same hospital generating a separate admission. Transfer hospitalizations with admission dates on the day of discharge from a previous acute ischemic stroke hospitalization were excluded from the analysis.
2.6 |. Final study population
A total of 4110 black study participants was successfully genotyped for hemoglobin S and hemoglobin C. Of these, 243 were missing principal components of genomic ancestry due to identification of first-degree relative pairs. After excluding study participants with missing genomic ancestry and those identified with hemoglobin SC disease (N = 5), or sickle cell anemia (N = 3), 3859 remained. A total of 199 participants were missing either creatinine or risk factors common to both ischemic stroke and CKD (hypertension, body mass index, diabetes mellitus, total cholesterol, or current smoking)14 at cohort visit one (1987–1989). However, 169 had available data at visit two (1990–1992), which was used to infer baseline values. None of the 169 participants suffered an ischemic stroke during the visit one to visit two interim. A total of 227 were missing biomarkers of coagulation and endothelial activation at visit one and excluded. The final population was composed of 3602 individuals. The selection flow-chart is presented in Supplemental Figure 1.
2.7 |. Statistical analysis
All statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC). Continuous variables were assessed for normality and compared using two-sample t tests or Wilcoxon rank sums tests, as appropriate. Categorical variables were compared using χ2 tests or Fisher’s exact test when expected cell counts were <5. Hazard ratios of ischemic stroke for SCT vs noncarrier genotype were modeled using repeat-events Cox regression with robust variance estimators, counting all occurrences of ischemic stroke per participant. ARIC visit one served as the common origin for all events, because risk was considered to start at study enrollment. The robust variance estimators accounted for within-subject correlation inherent with repeat events; an approach sometimes referred to as the “population-averaged method”.26 Observation time was censored at loss to follow up, death, or date of administrative censoring (December 31, 2017). Models were adjusted for risk factors common to both ischemic stroke and CKD (age, diabetes mellitus, hypertension, hyperlipidemia, obesity, and smoking),14 as well as baseline eGFR and factors routinely adjusted for in genetic analyses (sex and principal components of genomic ancestry). All modeling decisions were made a priori. A similar approach was used to model composite outcomes of death and/or ischemic stroke, counting all occurrences of ischemic stroke per participant. Effect modification by concomitant CKD was assessed by stratifying models by CKD presence, and by testing the multiplicative interaction of CKD and SCT. We also explored potential mediation by hypercoagulability and endothelial dysfunction by comparing hazard ratios with and without adjustment for biomarkers of coagulation and endothelial activation. Finally, we conducted a sensitivity analysis limited to patients known not to have history of stroke at study enrollment, and analyzed associations between SCT and incident (first-occurring) ischemic stroke. This was accomplished using standard Cox regression models with follow up time censored following the first ischemic stroke event, death, loss to follow up, or December 31, 2017, whichever came first.
3 |. RESULTS
A total of 3602 black participants met our inclusion criteria. The mean age at baseline was 54 years, and 62% were women. SCT was identified in 236 (7%) participants, which is consistent with national estimates.1 The median follow-up was 26 years (range: 0.4–31 years), with a total of 555 ischemic strokes recorded in 436 individuals. By 2017, half the participants (52%) had died and 9% were lost to follow up.
At midlife, a total of 468 cases of CKD were identified. The prevalence of CKD was significantly higher among participants with SCT than noncarriers (18% vs 13%, P = .02). Hyperlipidemia was marginally more prevalent with SCT (31% vs 26%, P = .07), but smoking was less common (24% vs 30%, P = .05). Study participants with SCT more often had elevated factor VII activity (29% vs 11%, P = .0002) and elevated von Willebrand factor antigen (39% vs 32%, P = .02). Clinical characteristics were otherwise comparable by carrier status (Table 1). CKD severity, assessed by eGFR, was comparable by genotype among the subset with CKD (Table 2). Biomarkers were consistently higher among participants with CKD compared to those without; however, the highest values of factor VII activity, factor VIII activity, and von Willebrand factor were observed among participants with both SCT and CKD (Table 2 and Supplemental Table 1). Elevated factor VII activity was more frequently observed with SCT, in both the subset with CKD (36% vs 22%, P = .05) and in those without CKD (16% vs 10%, P = .007).
TABLE 1.
Baseline characteristics (1987–1989) of black participants in the Atherosclerosis Risk in Communities Study, stratified by sickle cell trait carrier status
| Characteristic | Sickle cell trait N = 236 |
Noncarrier N = 3366 |
P value |
|---|---|---|---|
| Demographics | |||
| Age (mean years ± SD) | 54 ±6 | 54 ± 6 | .6 |
| Women | 136 (58%) | 2088 (62%) | .2 |
| Medical history | |||
| Current smoking | 57 (24%) | 1012 (30%) | .05 |
| Obesity (BMI > 30 kg/m2) | 92 (39%) | 1370 (41%) | .6 |
| Hypertension | 131 (56%) | 1878 (56%) | .9 |
| Diabetes mellitus | 46 (19%) | 653 (19%) | 1.0 |
| Hyperlipidemia (TC >240 mg/dL) | 74 (31%) | 877 (26%) | .07 |
| Chronic kidney disease (eGFR <60 mL/min/1.73 m2) | 42 (18%) | 426 (13%) | .02 |
| Myocardial infarctiona | 8 (3%) | 127 (4%) | .8 |
| Previous strokea | 3 (2%) | 73 (3%) | |
| Biomarkers | |||
| Factor VII activity (%) | 125.7 ± 35.4 | 117.4 ± 30.9 | <.0001 |
| Elevated factor VII activity (>150%) | 46 (19%) | 385 (11%) | .0002 |
| Factor VIII activity (%) | 152.0 ± 52.6 | 147.0 ± 47.1 | .1 |
| Elevated factor VIII activity (>150%) | 104 (43%) | 1375 (40%) | .3 |
| Fibrinogen (mg/dL) | 322.9 ± 77.5 | 320.6 ± 71.8 | .7 |
| Elevated fibrinogen (>400 mg/dL) | 33 (14%) | 437 (13%) | .6 |
| von Willebrand factor antigen (%) | 142.3 ± 61.0 | 133.3 ± 56.7 | .02 |
| Elevated von Willebrand factor antigen (>150%) | 94 (39%) | 1095 (32%) | .02 |
Abbreviations: SD, standard deviation; BMI, body mass index; TC, total cholesterol; eGFR, estimated glomerular filtration rate.
History of myocardial infarction prior to study enrollment missing for 9 participants, history of stroke prior to study enrollment missing for 769 participants.
TABLE 2.
Baseline characteristics (1987–1989) of black participants in the Atherosclerosis Risk in Communities Study, stratified by chronic kidney disease andickle cell trait carrier status
| Chronic kidney disease |
No chronic kidney disease |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Sickle cell trait N = 42 |
Noncarrier status N = 426 |
P-value | Sickle cell trait N = 194 |
Noncarrier status N = 2940 |
P value |
| Demographics | ||||||
| Age (mean years ± SD) | 56 ±6 | 57 ± 5 | .5 | 53 ± 6 | 53 ± 6 | .6 |
| Women | 31 (74%) | 276 (65%) | .2 | 105 (54%) | 1812 (62%) | .04 |
| Medical history | ||||||
| Glomerular filtration rate (mL/min/1.73 m2) | 51 ± 8 | 51 ± 11 | .8 | 76 ± 11 | 79 ± 12 | .005 |
| Current smoking | 10 (24%) | 103 (24%) | .2 | 47 (24%) | 909 (31%) | .05 |
| Obesity (BMI > 30 kg/m2) | 18 (43%) | 194 (46%) | .7 | 74 (38%) | 1176 (40%) | .6 |
| Hypertension | 27 (64%) | 318 (75%) | .1 | 104 (54%) | 1560 (53%) | .9 |
| Diabetes mellitus | 13 (31%) | 126 (30%) | .9 | 33 (17%) | 527 (18%) | .7 |
| Hyperlipidemia (TC >240 mg/dL) | 23 (55%) | 145 (34%) | .008 | 51 (26%) | 732 (25%) | .7 |
| Biomarkers | ||||||
| Factor VII activity (%) | 137.8 ± 42.6 | 128.1 ± 39.9 | .1 | 123.0 ± 33.2 | 115.8 ± 29.0 | .0009 |
| Elevated factor VII activity (>150%) | 15 (36%) | 94 (22%) | .05 | 31 (16%) | 291 (10%) | .007 |
| Factor VIII activity (%) | 173.5 ± 69.4 | 165.9 ± 57.3 | .4 | 147.4 ± 47.3 | 144.3 ± 44.8 | .4 |
| Elevated factor VIII activity (>150%) | 25 (60%) | 245 (56%) | .7 | 79 (40%) | 1130 (38%) | .5 |
| Fibrinogen (mg/dL) | 336.6 ± 63.8 | 341.7 ± 87.4 | .6 | 320.0 ± 80.0 | 317.5 ± 68.7 | .6 |
| Elevated fibrinogen (>400 mg/dL) | 6 (14%) | 89 (20%) | .3 | 27 (14%) | 348 (12%) | .4 |
| von Willebrand factor (%) | 157.0 ± 62.5 | 149.6 ± 61.6 | .5 | 139.2 ± 60.4 | 130.9 ± 55.6 | .04 |
| Elevated von Willebrand factor (>150%) | 24 (57%) | 192 (44%) | .1 | 70 (35%) | 903 (30%) | .1 |
Abbreviations: SD, standard deviation; BMI, body mass index; TC, total cholesterol.
From 1987–2017, 32 individuals with SCT (14%) and 404 noncarriers (12%) had at least one hospitalization for acute ischemic stroke. The mean age at the index hospitalization did not differ by genotype (68 vs 70 years; P = .4). Presence of atrial fibrillation within four weeks of ischemic stroke and acute symptoms at admission were similar (Supplemental Table 2). Most ischemic strokes at the index hospitalization were classified as thrombotic, both for patients with SCT and noncarriers (81% vs 76%, P = .5). However, among the patients classified with definite thrombotic stroke (N = 276), lacunar stroke was more prevalent with SCT than noncarrier genotype (64% vs 43%, P = .05).
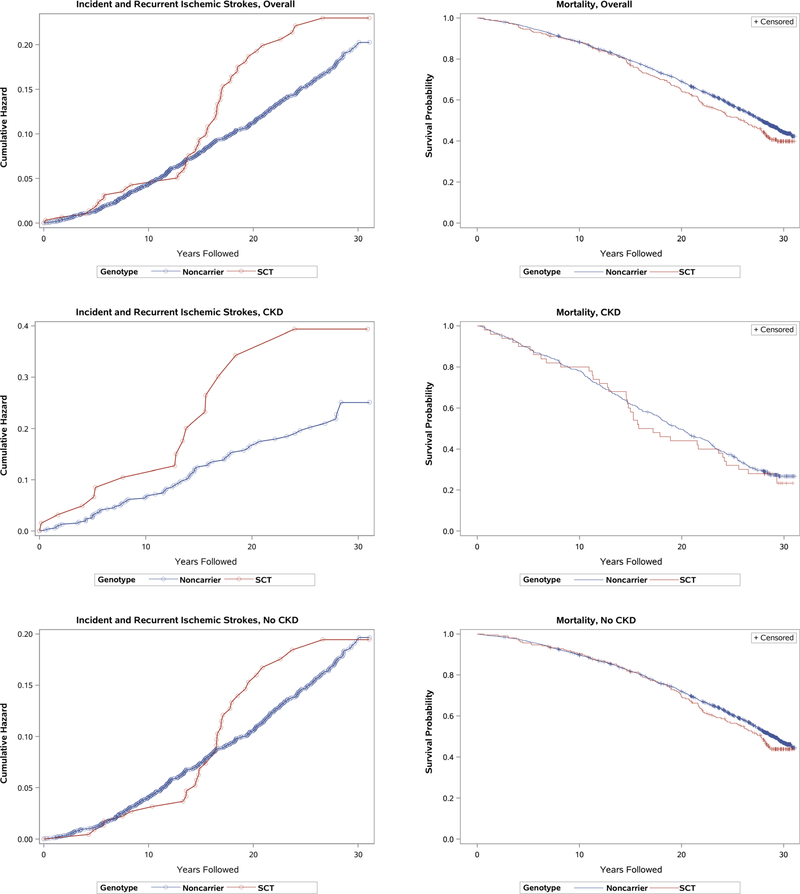
As shown in Supplemental Table 3, the occurrence of at least one ischemic stroke was 26% among participants with SCT and CKD, compared to 14% among noncarriers with CKD. In the absence of CKD, the occurrence of at least one ischemic stroke was similar for both genotypes (11% vs 12%). Death and loss to follow up, both competing risks for ischemic stroke, were comparable by carrier status when stratified by CKD. Recurrent ischemic strokes were uncommon, but ranged from one to five per participant. When analyzed over time, the cumulative hazard of ischemic stroke was similar for participants with SCT vs noncarriers, until diverging at >15 years of follow up (Figure 1). Among the subset with CKD, a separation of the cumulative hazards was observed across all years of follow up. When examining all-cause mortality, survival curves were largely similar for the two genotypes, with late separation observed >15 years of follow up.
FIGURE 1.
Cumulative hazards of incident and recurrent ischemic strokes and Kaplan-Meier survival probabilities for black study participants of the Atherosclerosis Risk in Communities Study, stratified by sickle cell trait carrier status and presence of chronic kidney disease.
CKD, chronic kidney disease; SCT, sickle cell trait. Cumulative hazards of incident and recurrent stroke and survival probabilities are shown on different scales
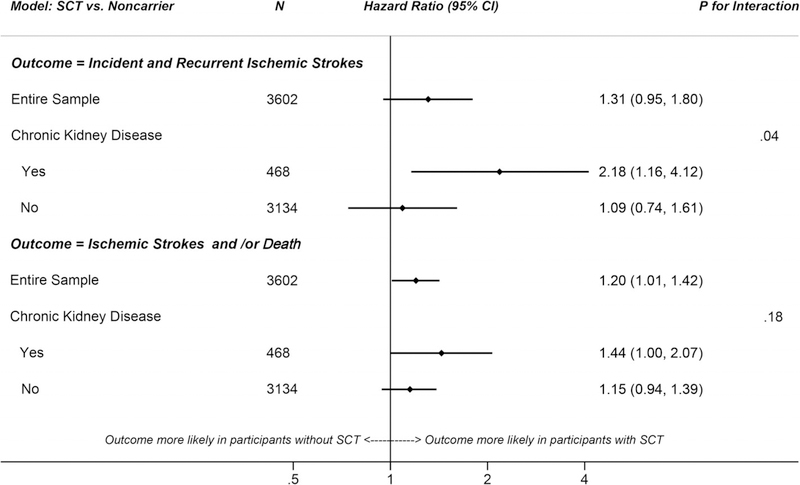
After adjustments, the hazard ratio of ischemic stroke associated with SCT vs noncarrier genotype was 1.31 (95% CI: 0.95–1.80), Figure 2. The association was stronger among those with CKD (HR = 2.18, 95% CI: 1.16–4.12) than those without CKD (HR = 1.09; 95% CI: 0.74–1.61); P for interaction = .04. When analyzing composite outcomes, SCT was associated with a 20% higher hazard of death and/or ischemic stroke relative to noncarrier genotype (HR = 1.20; 95% CI: 1.01–1.42). When stratified, hazard ratios of the composite outcome for SCT vs noncarrier genotype were 1.44 (1.002–2.07) and 1.15 (0.94–1.39) with and without CKD, respectively; P for interaction = .18.
FIGURE 2.
Adjusted hazard ratios of incident and recurrent ischemic strokes or composite of incident and recurrent ischemic strokes and/or death among black study participants of the Atherosclerosis Risk in Communities Study, comparing individuals with sickle cell trait to noncarriers
*Models adjusted for risk factors common to both ischemic stroke and CKD (age, diabetes mellitus, hypertension, hyperlipidemia, obesity, and smoking), estimated glomerular filtration rate, and factors routinely adjusted for in genetic analyses (sex and principal components of genomic ancestry)
To evaluate potential mediation by hypercoagulability and endothelial function, we constructed a separate model additionally adjusted factor VII activity, factor VIII activity, fibrinogen, and von Willebrand factor. After adjustments, the hazard ratio of ischemic stroke and/or death for SCT vs noncarrier genotype attenuated modestly among patients with CKD (HR = 1.39; 95% CI: 0.96–2.03).
As a sensitivity analysis, we also analyzed SCT, CKD, and incident ischemic stroke. At the study enrollment, 76 participants had prior history of stroke, while 769 were missing history of stroke. Consequently, our analytic sample was limited to 2757 participants who were known not to have history of stroke. Over the course of follow up, 12% experienced ischemic stroke, 45% died, and 8% were lost to follow-up. The cumulative hazards of incident ischemic stroke for SCT vs noncarrier genotype followed a similar pattern as the primary analysis, with a late divergence >15 years of follow up (Supplemental Figure 2). Hazard ratios of ischemic stroke were analyzed by Cox regression models with identical adjustment as the primary analysis. However, follow-up was censored following the first occurrence of ischemic stroke, rather than counting all ischemic stroke events as the outcome. Among the participants without midlife CKD, no increased hazard of incident ischemic stroke was observed for SCT vs noncarrier genotype (HR = 1.00; 95% CI: 0.59–1.68). However, in the subset with prevalent CKD at midlife, SCT was associated with nearly three times the hazard of incident ischemic stroke than noncarrier genotype (HR = 2.84; 95% CI: 1.37–5.89); P value for interaction = .02.
4 |. DISCUSSION
In this population-based cohort of black adults who were prospectively followed from midlife through advanced age, a higher hazard of ischemic stroke was observed with SCT, primarily among participants with concomitant CKD. Relative to noncarriers, SCT was also associated with an increased hazard of composite death and/or ischemic stroke.
Several factors may contribute to these findings. One possibility is that hemoglobin S polymerizes in the low pH and low oxygen environment of the renal medulla,27 leading to ischemic injury, release of local vasodilators, glomerular hyperfiltration and eventual glomerulosclerosis.17 However, hemoglobin S content within erythrocytes varies widely among individuals with SCT, with concentrations ranging from 25–45%.28 Heterogeneity of the SCT phenotype is known to be influenced by coinheritance of alpha thalassemia,29 with average hemoglobin S concentrations varying from 40% to 35% to 30% with four, three, and two functioning alpha globin genes, respectively.30 A recent investigation from the Jackson Heart Study reported a lower prevalence of CKD and lower levels of circulating D-dimer, a fibrin degradation product, in individuals with SCT and coinherited alpha thalassemia, compared to those without alpha thalassemia.31 Thus, it is plausible that individuals with more severe phenotypes of SCT have not only a greater prevalence of CKD, but also heightened coagulation activation and ischemic stroke risk. Interestingly, the associations that we observed between SCT and ischemic stroke among participants with CKD were not driven by CKD severity, as models were adjusted for eGFR.
Our analysis suggests a synergistic interaction of SCT and CKD on the risk of ischemic stroke. SCT and CKD may work in tandem, amplifying risk of ischemic stroke by elevated production and diminished renal clearance of procoagulant and proinflammatory factors. Uremic toxins are associated with elevated cytokines,32 coagulation factors,15 and circulating microparticles,33 all of which contribute to thrombus formation. Diminished endothelial function in patients with CKD has also been demonstrated by flow-mediated dilation testing.34 Complications of CKD include not only venous thromboembolism,35 but also arterial thrombosis, most notably coronary stent thrombosis.36 As with CKD, hypercoagulability is also observed with SCT. Recent experimental data suggest greater fibrin deposition with injury-induced venous thrombosis in SCT mice, and a diminished response to tissue plasminogen activator in whole blood clots from individuals with SCT.4 In observational research, laboratory assays indicate elevated markers of coagulation activation and circulating microparticles even in healthy individuals with SCT.2,3 Evidence of diminished endothelial function has been demonstrated with SCT as well.37 A recent investigation reported impaired flow mediated dilation and heightened coagulation activation in individuals with SCT and concomitant diabetes mellitus, compared to carriers without diabetes.38 Of note, microalbuminuria was more prevalent among the diabetics, possibly contributing to the observed results.39 In the ARIC cohort, elevations in factor VII activity and von Willebrand factor antigen were more frequently observed in individuals with SCT. As might be expected, all measured markers of coagulation and endothelial activation were amplified in the setting of CKD. However, the greatest prevalence of elevated factor VII activity was observed in participants with SCT and concomitant CKD, suggesting a compounding effect which may potentially influence ischemic stroke risk.
A recent meta-analysis of four cohort studies reported no prospective association between SCT and ischemic stroke; however, CKD was not examined as a potential modifier.12 This investigation involved 19 464 black participants but only 620 ischemic strokes, which is comparable to the 555 ischemic strokes recorded in the ARIC study. Geographic differences may have been a factor, as black participants in the ARIC study were recruited from the “stroke belt” of the Southeastern US, which is well-known for its disproportionately high incidence of stroke. Study population age and duration of follow up may also have contributed to differences in stroke incidence and reported associations with SCT. Median follow-up in the meta-analysis was <10 years for each cohort, in contrast to the 30 years of follow up available in the ARIC study. Interestingly, there was no separation in the cumulative hazards of ischemic stroke for SCT vs noncarrier genotype in the ARIC study until >15 years of follow up. The reason for this is not entirely clear. Although a consistent algorithm was used by the ARIC study to classify ischemic stroke over the 30 years of follow-up, temporal changes in medical imaging technology and access to care may have influenced case ascertainment over time. However, these temporal changes are unlikely to differentially impact stroke identification by genotype. As an alternative explanation, the diverging stroke risk may reflect age-related susceptibility, with an initial low stroke risk for both genotypes at the study onset, which progressively increased as participants aged.
In the subset of index hospitalizations classified with definite thrombotic stroke, a higher prevalence of lacunar infarction was observed with SCT. Although the number of events was too small to analyze comprehensively, one possible explanation may be the higher prevalence of CKD with SCT. Renal disease is associated with glomerular endothelial dysfunction and lipohyalinosis. A similar pathology is observed in the cerebral small vessels in the setting of small vessel disease.14 Whether the increased prevalence of lacunar infarction with SCT is attributable to endothelial activation associated with SCT, CKD, or their synergistic interaction is uncertain.
Our study has some limitations. We were not able to consider SCT severity or hemoglobin S expression as a predictor of cerebrovascular events, nor did we have information on coinherited alpha thalassemia or other genetic modifiers of the SCT phenotype. We limited our analysis to CKD identified at midlife and did not consider duration of CKD prior to study enrollment, incident CKD which may have developed during the interim from enrollment until ischemic stroke occurrence, or the etiologic subtype of CKD. We were unable to consider albuminuria at midlife, because the ARIC study did not measure albumin creatinine ratios until study visit four. Similarly, we did not consider D-dimer, because measurements were not available until ARIC study visit 5. On the other hand, our study has several exceptional and noteworthy strengths. The ARIC cohort is well suited for the analysis of SCT and stroke, due to the large sample of black individuals who were prospectively followed for 30 years from midlife through advanced age. Unlike many other cohorts,12 the ARIC study identified SCT by direct genotyping rather than imputation, providing the best measurement of exposure. Phenotypic data were meticulously collected under extensive quality assurance, and study participant retention was excellent.
In conclusion, the long-term risk of ischemic stroke associated with SCT relative to noncarrier genotype appears to be modified by concomitant CKD. A possible mechanism and potential area of future research may be amplification of coagulation and endothelial activation in individuals with SCT and concomitant CKD.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Caughey conceptualized the study design, analyzed the data, and wrote the manuscript. Drs. Derebail, Key, Reiner, Gottesman, Kshirsagar, and Heiss interpreted the data and revised the manuscript critically. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding information
National Institutes of Health (NIH), Grant/Award Numbers: R01HL132947, R01HL130733, R01HL129132; National Institutes of Health (NIH), Grant/Award Number: 5RC2HL102419; National Institutes of Health (NIH), Grant/Award Number: 5K12HL087097; National Institutes of Health (NIH), Grant/Award Numbers: HHSN268201700004I, HHSN268201700005I, HHSN268201700003I, HHSN268201700002I, HHSN268201700001I
Footnotes
DISCLOSURE OF INTEREST
Dr. Gottesman is Associate Editor for the journal Neurology.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996; 13:501–512. [DOI] [PubMed] [Google Scholar]
- 2.Westerman MP, Green D, Gilman-Sachs A, et al. Coagulation changes in individuals with sickle cell trait. Am J Hematol. 2002;69:89–94. [DOI] [PubMed] [Google Scholar]
- 3.Amin C, Adam S, Mooberry MJ, et al. Coagulation activation in sickle cell trait: an exploratory study. Br J Haematol. 2015;171:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faes C, Ilich A, Sotiaux A, et al. Red blood cells modulate structure and dynamics of venous clot formation in sickle cell disease. Blood. 2019;133:2529–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffield LM, Zakai NA, Duan Q, et al. D-Dimer in African Americans: whole genome sequence analysis and relationship to cardiovascular disease risk in the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2017;37:2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Engl J Med. 1979;300:1001–1005. [DOI] [PubMed] [Google Scholar]
- 7.Austin H, Key NS, Benson JM, et al. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–912. [DOI] [PubMed] [Google Scholar]
- 8.Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91: 288–294. [PubMed] [Google Scholar]
- 10.Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis. 2014; 37:64–75. [DOI] [PubMed] [Google Scholar]
- 11.Caughey MC, Loehr LR, Key NS, et al. Sickle cell trait and incident ischemic stroke in the atherosclerosis risk in communities study. Stroke. 2014;45:2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyacinth HI, Carty CL, Seals SR, et al. Association of sickle cell trait with ischemic stroke among African Americans: a meta-analysis. JAMA Neurol. 2018;75:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 14.Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823–833. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. [DOI] [PubMed] [Google Scholar]
- 16.Derebail VK, Nachman PH, Key NS, Ansede H, Falk RJ, Kshirsagar AV. High prevalence of sickle cell trait in African Americans with ESRD. J Am Soc Nephrol. 2010;21:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312:2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart Lung and Blood Institute, The Biologic Specimen and Data Repository Information Coordinating Center. 2018. [Google Scholar]
- 19.Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grove ML, Yu B, Cochran BJ, et al. Best practices and joint calling of the HumanExome BeadChip: the CHARGE Consortium. PLoS One. 2013;8:e68095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study-I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 23.Chambless LE, McMahon R, Wu K, Folsom A, Finch A, Shen YL. Short-term intraindividual variability in hemostasis factors. The ARIC Study. Atherosclerosis risk in communities intraindividual variability study. Ann Epidemiol. 1992;2:723–733. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 25.Koene RJ, Alraies MC, Norby FL, et al. Relation of the CHA2DS2-VASc score to risk of thrombotic and embolic stroke in community-dwelling individuals without atrial fibrillation (From The Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2019;123:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison P. Survival Analysis Using SAS: A Practical Guide. Vol 324 Cary, NC: SAS Institute; 2010. [Google Scholar]
- 27.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332:647–655. [DOI] [PubMed] [Google Scholar]
- 28.Neel JV, Wells IC, Itano HA. Familial differences in the proportion of abnormal hemoglobin present in the sickle cell trait. J Clin Invest. 1951;30:1120–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg M, Forget B, Higgs D, Nagel R. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management; 2009:1282 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Steinberg MH, Embury SH. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood. 1986;68:985–990. [PubMed] [Google Scholar]
- 31.Raffield LM, Ulirsch JC, Naik RP, et al. Common alpha-globin variants modify hematologic and other clinical phenotypes in sickle cell trait and disease. PLoS Genet. 2018;14:e1007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller C, Katz R, Cushman M, Fried LF, Shlipak M. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Nephrol. 2008;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jalal D, Renner B, Laskowski J, et al. Endothelial microparticles and systemic complement activation in patients with chronic kidney disease. J Am Heart Assoc. 2018;7:e007818 10.1161/JAHA.117.007818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease—a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–451. [DOI] [PubMed] [Google Scholar]
- 35.Cheung KL, Zakai NA, Folsom AR, et al. Measures of kidney disease and the risk of venous thromboembolism in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2017;70:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angiolillo DJ, Bernardo E, Capodanno D, et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. [DOI] [PubMed] [Google Scholar]
- 37.Zawar SD, Vyawahare MA, Nerkar M, Jawahirani AR. Non-invasive detection of endothelial dysfunction in sickle cell disease by Doppler ultrasonography. J Assoc Physicians India. 2005;53:677–680. [PubMed] [Google Scholar]
- 38.Diaw M, Pialoux V, Martin C, et al. Sickle cell trait worsens oxidative stress, abnormal blood rheology, and vascular dysfunction in Type 2 diabetes. Diabetes Care. 2015;38:2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massicotte-Azarniouch D, Bader Eddeen A, Lazo-Langner A, et al. Risk of venous thromboembolism in patients by albuminuria and estimated GFR. Am J Kidney Dis. 2017;70:826–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.