Abstract
Objective:
To describe conjugal multiple system atrophy (MSA) in a couple married for 44 years, and to report environmental risk factors possibly contributing to the occurrence.
Methods:
Case description of conjugal MSA with report of shared environmental risk factors and retrospective review of consecutively diagnosed MSA patients between 1998 and 2012 with autonomic reflex screen at Mayo Clinic, Rochester (clinical series). Probability calculation was based on the age-specific point prevalence of MSA.
Results:
A husband and wife both developed MSA symptoms at age 63. The husband’s onset was of imbalance, followed by falls and genitourinary failure; parkinsonism and antecollis was evident on examination. Autonomic testing showed widespread autonomic failure. The patient died 2.25 years after onset. The wife initially developed urinary symptoms progressing to incontinence. Parkinsonism, dysphonia, and falls began within 1 year. Autonomic testing revealed severe autonomic failure. Interview with the surviving wife and son revealed substantial chemical exposure, in particular pesticides. In our clinical series, there were no other cases of conjugal MSA. Assuming an age-specific point prevalence of MSA based on population studies and independence of the two events, the probability of both individuals developing MSA by chance is 6.08 e–9.
Conclusion:
Based on the population point prevalence of MSA, conjugal MSA is rare but possible. We conclude that this case of conjugal MSA likely occurred by chance; however, exposure to shared risk factors (pesticides) may be contributory. Because this is the first reported case of conjugal MSA, to our best knowledge, evidence for transmissibility between spouses is lacking.
Keywords: Multiple system atrophy, Parkinsonism, Ataxia, Prion, Toxic exposures
1. Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder characterized by autonomic failure with predominantly parkinsonism (MSA-P) or cerebellar ataxia (MSA-C) [1]. Neuropathologically, MSA is a synucleinopathy characterized by α-synuclein aggregates as glial cytoplasmic inclusions [2,3]. Evidence in cell culture of self-templating propagation and animal models of transmissibility has led to the hypothesis that MSA may be a prion disease [4,5]. Transmissibility is an accepted feature of prion disease which has implications for the possibility of transmission through sexual or close personal contact [6,7]. Parkinson’s disease in one spouse and MSA in the other has been reported; however, to the best of our knowledge, there are no reported cases of conjugal MSA [8]. Most reports of conjugal parkinsonism conclude, based on prevalence, that the association may be explained by chance or is related to shared environmental risk factors [9,10,11–13]. In Parkinson’s disease, evidence that pesticide exposure increases disease risk is substantial, but the role of individual compounds remains uncertain [14]. In MSA, environmental toxins and occupational exposures have been associated with increased risk of disease [15,16], although the role of pesticides is less certain [17]. We report the first description of conjugal MSA, determine the probability of both individuals developing disease by chance, and report on occupational exposures that may have contributed to the occurrence.
2. Methods
This is a case description of a married couple in which both husband and wife developed MSA. Clinical history and autonomic function testing was reviewed. Composite Autonomic Severity Score (CASS) was assigned based on autonomic testing and was divided into the following subgroups with maximum scores listed: sudomotor (3), cardiovagal (3), adrenergic (4). A maximum total CASS of 10 indicates severe autonomic failure [18]. Percentage anhidrosis was obtained from thermoregulatory sweat test (TST) results and accounted in the CASS sudomotor subscore. Quantitative sudomotor axon reflex testing (QSART) values and TST results were used to determine central, peripheral, or mixed pattern of anhidrosis.
The surviving wife and the patients’ son were interviewed by telephone regarding shared environmental exposures with review of company records for lifetime pesticide and chemical exposure.
To estimate the probability of chance occurrence of conjugal MSA, we reviewed our clinical series of 685 patients consecutively diagnosed with MSA between 1998 and 2012 at the Mayo Clinic, Rochester for evidence of other spousal pairs with MSA [19]. In addition, a probability calculation was performed based on age-specific point prevalence rates of MSA (age ≥40 years) [20,21].
This study was approved by the institutional review board of Mayo Clinic, Rochester, MN.
3. Results
3.1. Case descriptions
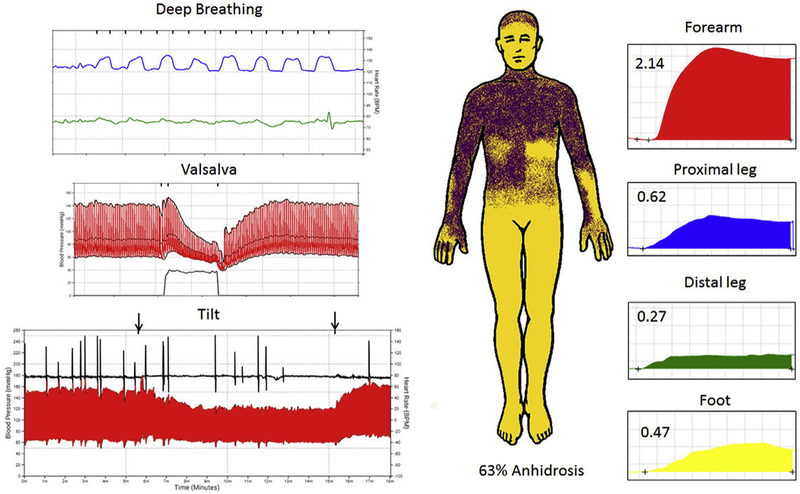
The husband had a history of diabetes, low back pain, and recent gastric bypass surgery, and developed imbalance at the age of 63 years. The patient underwent laminectomy with improvement in back pain but had progressive falls and developed bladder and bowel incontinence requiring intermittent urinary catheterization within 1 year from onset of imbalance. Dream enactment behavior became evident. Parkinsonism was noted on examination and levodopa (with carbidopa) was titrated up to 1,050 mg total daily dose with no improvement. Examination at age 65 revealed antecollis, rigidity, bradykinesia, and freezing on turns but normal cognitive function. Autonomic function testing showed global autonomic failure with a CASS of 9 and widespread anhidrosis in a predominantly central pattern (Fig. 1). The patient died at age 66, 2 years and 3 months following onset of symptoms.
Fig. 1.
Autonomic function testing in the husband.
Legend: Autonomic reflex screen and thermoregulatory sweat testing in the husband is shown. Cardiovagal impairment is shown (top left) with reduced heart rate (green) to deep breathing (blue), adrenergic failure on Valsalva maneuver (middle left) with absent late phase II and phase IV with prolonged blood pressure recovery time and orthostatic hypotension on head-up tilt (bottom left) with absent heart rate response. Thermoregulatory sweat test (middle) demonstrates widespread anhidrosis (yellow) that is predominantly in a central pattern based on quantitative sudomotor axon reflex testing (right panel) showing robust sweat responses at the forearm, proximal leg and foot sites and mild decrease at the distal leg site.
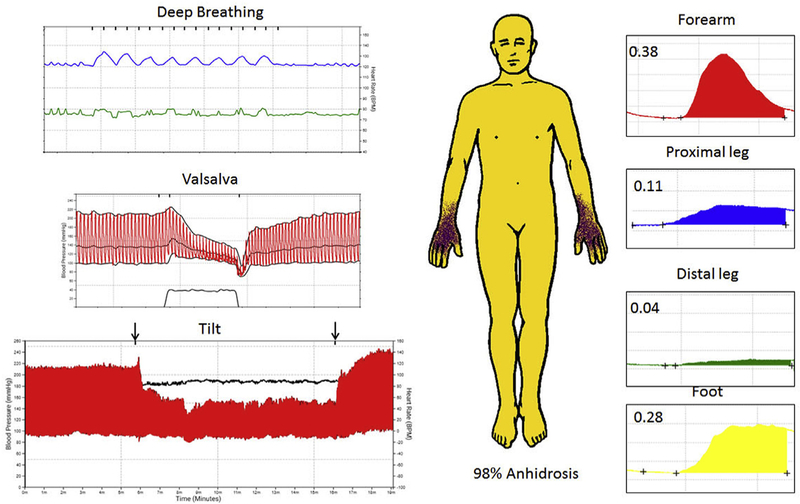
The wife had a history of dream enactment behavior then developed insidious onset of urinary symptoms in the same calendar year as her husband, also at the age of 63 years. Urinary symptoms progressed to incontinence with frequent urinary tract infections. Dysphonia, hypokinetic dysarthria, and micrographia were noted at age 64 years. At the age of 65 years, polysomnography identified central sleep apnea, and adaptive servo-ventilation was begun. Falls began at that time, and the patient began using a walker or a wheel chair for long distance. Parkinsonism was noted on examination with an action tremor and hyperreflexia. Levodopa (with carbidopa) up to 600 mg per day led to initial benefit that later waned. At the age of 67 years, severe stridor was noted and the patient underwent tracheostomy. Autonomic function testing showed autonomic failure, with CASS of 9; thermoregulatory sweat test showed global anhidrosis with acral sparing (Fig. 2), whereas QSART values were reduced at the leg sites, consistent with a mixed, central and peripheral, pattern of sweat loss.
Fig. 2.
Autonomic function testing in the wife.
Legend: Autonomic reflex screen and thermoregulatory sweat test is shown for the wife. Cardiovagal impairment is shown (top left) with reduced heart rate (green) to deep breathing (blue), severe adrenergic failure on Valsalva maneuver (middle left) with absent late phase II and phase IV with prolonged blood pressure recovery time. Head-up tilt demonstrated supine hypertension with orthostatic hypotension (bottom left) and absent heart rate response. Thermoregulatory sweat test (middle) demonstrates global anhidrosis (yellow) with preserved hand sweating. The pattern of sweat loss is mixed (central and peripheral) based on quantitative sudomotor axon reflex testing (right) showing robust sweat response at forearm and foot sites and reduced leg responses.
3.2. Exposure history
The husband and wife were married for 44 years and they owned and ran an animal feed plant company for 40 years. They also used well water for 20 years. The surviving patient and her son disclosed chemical exposures including significant pesticide exposure. Herbicides were used for weed control to maintain the railroad tracks to the plant. Thousands of gallons of glyphosate-based pesticides (Roundup) were used based on the compiled exposure list from company records with concentrated pesticide mixed and sprayed by the husband to maintain an expanse of railroad track. Additionally, the husband also handled liquid nitrogen and urea-ammonia mixture fertilizer as well as the following chemicals: hydrochloric acid, sulfuric acid, diethylhydroxylamine, calcium hydroxide, sodium hydroxide, sodium sulfite, potassium hydroxide, potassium sulfite, magnesium, manganese, selenium, caustic soda lye, and urea. Personal protective equipment was not routinely used with pesticide application until the last 15 years of running the plant. The husband frequently wore his work clothes within the house and the wife was in charge of laundry.
3.3. Retrospective review
Retrospective review of 685 consecutive MSA patients in our Mayo Clinic-Rochester database between in 1998–2012 revealed no additional cases of conjugal MSA.
3.4. Probability estimates
Using a point prevalence rate 7.8:100,000 for MSA at age ≥40 years derived from a population study [20] and assuming independence of the two events, the probability of both individuals developing MSA by chance is 6.08 e–9. With a population estimate for the United States (age group ≥40 years) of 156 million [22], we expect the occurrence of 0.25 conjugal MSA cases due to chance. With a world population estimate of 3.77 billion people ≥40 years, we expect the occurrence of 23 conjugal MSA cases in the world.
4. Discussion
We report MSA in a husband and wife married for 44 years. Both patients had MSA with predominant parkinsonism; the husband had a rapidly progressive course. Although there are reports of conjugal parkinsonism, including a patient with MSA married to a patient with Parkinson’s disease [8], this is the first reported case of conjugal MSA, to the best of our knowledge.
Conjugal MSA has clinical and scientific implications because Prusiner et al. have published a series of articles postulating that MSA is a prion disease [4,5,23]. Misfolded α-synuclein has been shown to self-propagate in neural cell culture, and inoculation of brain homogenates from MSA patients leads to α-synuclein pathology in transgenic mouse models expressing mutant A53T α-synuclein [5,24]. While A53T α-synuclein mutations have never been identified in MSA patients and this mouse model is not considered a valid animal model for MSA, this has led to concern of transmissibility of MSA from person-to-person. However, upon further review of our cases and probability calculations, we suggest that chance or shared environmental risk factors are more plausible explanations for a case of conjugal MSA, although we cannot be certain.
First, probability calculation shows that the probability of two rare events (developing MSA) occurring together is low. However, when extrapolating the probability to the United States population or the world population, the report of a first conjugal pair may well be a chance event.
An alternative explanation is shared exposure to risk factors for developing MSA. Case-control studies investigating risk factors for MSA are limited; however, an association with environmental toxins has been reported [15]. An occupational history of farming was significantly more frequent among patients with MSA, and duration of farming had a dose-response trend [25]. However, the connection between pesticides and MSA is controversial [17]. Studies in Parkinson’s disease have consistently found an association with rural living or farming [14]. Overall, studies have also linked pesticide use to increased risk of Parkinson’s disease. For example, the Agricultural Health Study showed increased risk of Parkinson’s disease in persons with a longer lifetime exposure to pesticides and in persons who applied the pesticides directly [26]. The cumulative amount of pesticide and chemical use was notable in the husband of our conjugal pair. Additionally, the patient did wear his clothing inside the house and the wife laundered his clothing, contributing to a shared exposure in the couple.
There are limitations to this report: post-mortem examination was not performed in the deceased spouse. Recall bias may influence pesticide and chemical exposure estimation; however, attempts were made to mitigate this bias by review of plant records by the patients’ son. Literature review for conjugal MSA cases was performed using the English language or articles translated into English; therefore, we may have missed reports of conjugal MSA from some parts of the world.
In summary, we report a case of conjugal MSA. Although this may elicit concern regarding human-to-human transmission, probability calculation reveals that the event is rare but plausible. Additionally, shared exposure to environmental toxins and chemicals may have contributed to the co-occurrence.
Acknowledgments
Funding sources
Supported in part by NIH (P01NS44233, U54NS065736, K23NS075141, R01 FD004789, R01 NS092625) and Mayo CCaTS (UL1TR000135), and Cure PSP Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Financial disclosure/conflict of interest
There is no support or financial issues from all authors relative to the research covered in the submitted manuscript.
References
- [1].Fanciulli A, Wenning GK, Multiple-system atrophy, N. Engl. J. Med 372 (2015) 1375–1376. [DOI] [PubMed] [Google Scholar]
- [2].Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, Alpha-synuclein in lewy bodies, Nature 388 (1997) 839–840. [DOI] [PubMed] [Google Scholar]
- [3].Tu PH, Galvin JE, Baba M, et al. , Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein, Ann. Neurol 44 (1998) 415–422. [DOI] [PubMed] [Google Scholar]
- [4].Prusiner SB, Woerman AL, Mordes DA, et al. , Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Watts JC, Giles K, Oehler A, et al. , Transmission of multiple system atrophy prions to transgenic mice, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brown P, Cervenakova L, McShane L, et al. , Creutzfeldt-Jakob disease in a husband and wife, Neurology 50 (1998) 684–688. [DOI] [PubMed] [Google Scholar]
- [7].Frontzek K, Moos R, Schaper E, et al. , Iatrogenic and sporadic Creutzfeldt-Jakob disease in 2 sisters without mutation in the prion protein gene, Prion 9 (2015) 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miwa H, Kondo T, Conjugal parkinsonism: multiple system atrophy and Parkinson’s disease, Park. Relat. Disord 16 (2010) 232. [DOI] [PubMed] [Google Scholar]
- [9].Rajput AH, Ferguson LW, Robinson CA, Guella I, Farrer MJ, Rajput A, Conjugal parkinsonism - clinical, pathology and genetic study. No evidence of person-to-person transmission, Park. Relat. Disord 31 (2016) 87–90. [DOI] [PubMed] [Google Scholar]
- [10].Ubeda JV, Null hypothesis of husband-wife concordance of Parkinson’s disease in 1,000 married couples over age 50 in Spain, Neuroepidemiology 17 (1998) 90–95. [DOI] [PubMed] [Google Scholar]
- [11].Balint B, Erro R, Brugger F, et al. , Conjugal Parkinson’s disease - real or chance? Park. Relat. Disord 33 (2016) 146–148. [DOI] [PubMed] [Google Scholar]
- [12].Ramani M, Saur DP, Rabin M, Kurlan R, Conjugal and familial Lewy body disorders: a report of one family, Park. Relat. Disord 19 (2013) 498. [DOI] [PubMed] [Google Scholar]
- [13].Willis AW, Sterling C, Racette BA, Conjugal Parkinsonism and Parkinson disease: a case series with environmental risk factor analysis, Park. Relat. Disord 16 (2010) 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ascherio A, Schwarzschild MA, The epidemiology of Parkinson’s disease: risk factors and prevention, Lancet Neurol 15 (2016) 1257–1272. [DOI] [PubMed] [Google Scholar]
- [15].Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ, Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation, Clin. Auton. Res 1 (1991) 9–13. [DOI] [PubMed] [Google Scholar]
- [16].Vanacore N, Bonifati V, Fabbrini G, et al. , Case-control study of multiple system atrophy, Mov. Disord 20 (2005) 158–163. [DOI] [PubMed] [Google Scholar]
- [17].Vidal JS, Vidailhet M, Elbaz A, Derkinderen P, Tzourio C, Alperovitch A, Risk factors of multiple system atrophy: a case-control study in French patients, Mov. Disord 23 (2008) 797–803. [DOI] [PubMed] [Google Scholar]
- [18].Low PA, Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure, Mayo Clin. Proc 68 (1993) 748–752. [DOI] [PubMed] [Google Scholar]
- [19].Coon EA, Sletten DM, Suarez MD, et al. , Clinical features and autonomic testing predict survival in multiple system atrophy, Brain 138 (2015) 3623–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bjornsdottir A, Gudmundsson G, Blondal H, Olafsson E, Incidence and prevalence of multiple system atrophy: a nationwide study in Iceland, J. Neurol. Neurosurg. Psychiatry 84 (2013) 136–140. [DOI] [PubMed] [Google Scholar]
- [21].Bower JH, Maraganore DM, McDonnell SK, Rocca WA, Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990, Neurology 49 (1997) 1284–1288. [DOI] [PubMed] [Google Scholar]
- [22].U.S.C. Bureau, American Community Survey 1-year Estimates, (2017).
- [23].Woerman AL, Stohr J, Aoyagi A, et al. , Propagation of prions causing synucleinopathies in cultured cells, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E4949–E4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Woerman AL, Kazmi SA, Patel S, et al. , MSA prions exhibit remarkable stability and resistance to inactivation, Acta Neuropathol 135 (2018) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vanacore N, Bonifati V, Fabbrini G, et al. , Case-control study of multiple system atrophy, Mov. Disord 20 (2005) 158–163. [DOI] [PubMed] [Google Scholar]
- [26].Kamel F, Tanner C, Umbach D, et al. , Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study, Am. J. Epidemiol 165 (2007) 364–374. [DOI] [PubMed] [Google Scholar]