Abstract
Macrophages are major upstream regulators of the inflammatory response to implanted biomaterials. Sequential functions of distinct macrophage phenotypes are essential to the normal tissue repair process, which ideally results in vascularization and integration of implants. Improper timing of M1 or M2 macrophage activation results in dysfunctional healing in the form of chronic inflammation or fibrous encapsulation of the implant. Thus, biphasic drug delivery systems that modulate macrophage behavior are an appealing approach to promoting implant integration. In this review, we describe the timing and roles of macrophage phenotypes in healing, then highlight current drug delivery systems designed to sequentially modulate macrophage behavior.
Keywords: macrophage, implant integration, angiogenesis
1. Introduction
The importance of understanding and modulating the inflammatory response is becoming increasingly appreciated for biomaterials in regenerative medicine. Biomaterial implantation immediately stimulates the innate immune system to elicit a response that normally occurs in sequential phases, beginning with inflammation and followed by tissue proliferation and maturation [1]. This process may result in failed biomaterial integration via the foreign body response (FBR), in which immune cells encapsulate the implant to permanently isolate it from the surrounding environment (Figure 1). On the other hand, the innate immune response can also promote successful integration by vascularizing the implant, allowing for the delivery of oxygen and nutrients to the regenerating tissue [2]. Therefore, understanding and controlling how immune cells mediate the FBR versus tissue integration is of paramount importance.
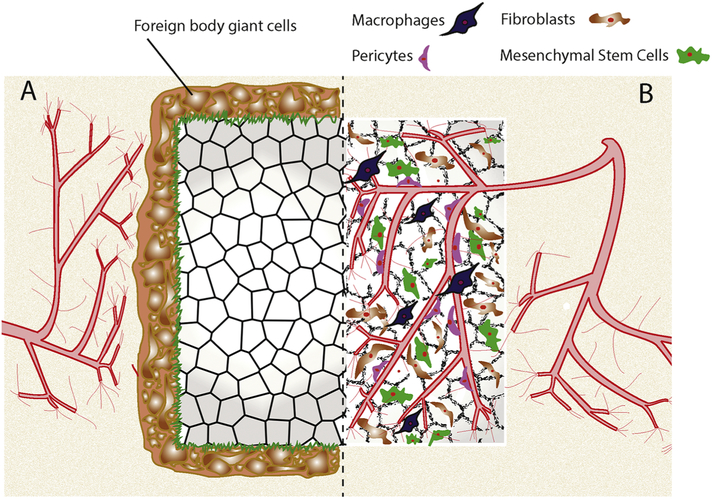
Figure 1: Integration of implanted biomaterials.
(A) Unsuccessful integration results in the fusion of foreign body giant cells, which then secrete extracellular matrix to form a fibrous capsule. This fibrous capsule isolates the biomaterial from the body as part of the foreign body response. (B) Complete integration, largely directed by macrophages, allows for cellular and vascular infiltration to support tissue development. Infiltrating cells may include pericytes to support vascularization, fibroblasts for matrix deposition, and mesenchymal stem cells for longterm stability.
The immune system’s reaction to implants is largely directed by macrophages, which can change their phenotype to promote inflammatory or healing functions. Because of the sequential nature of macrophage activities, as well as their plasticity as regulators of healing, the design of drug delivery systems that can sequentially modulate macrophage phenotype has become a popular approach to promoting implant integration. The advantages of focusing on macrophages are especially apparent when considering the complexities of even normal healing. For example, a study by Kuttappan and colleagues highlighted the limitations of drug delivery strategies that deliver two or even three growth factors in promoting tissue repair [3]. To treat critical-sized calvarial defects in rats, they used a silica scaffold coated with nanohydroxyapatite-gelatin and reinforced with poly(L-lactic acid) to release bone morphogenetic protein-2 (BMP-2) in combination with either fibroblast growth factor-1 (FGF-1) or vascular endothelial growth factor (VEGF). Although FGF-1/BMP-2 release resulted in improved stem cell migration, the VEGF/BMP-2 system was better at promoting neoangiogenesis, suggesting that multiple factors are required to promote the many different aspects of tissue regeneration. Moreover, the dose and timing of the delivered factors are likely to be critical as well. Current drug delivery systems are limited in the number of cytokines and growth factors that can be loaded into a single device. However, the modulation of macrophage phenotype may require fewer components, since macrophages are upstream regulators of healing and secrete many essential growth factors at tightly regulated doses and timing. Therefore, immunomodulatory designs are attractive options for tissue engineers looking to promote more efficacious implant integration. In this review, we will provide an overview of the timing and roles of macrophage phenotypes in tissue repair, then discuss the various engineering approaches that have been proposed to sequentially modulate macrophage phenotype.
1.1. Macrophage phenotypes
Macrophages are major regulators of tissue repair, and observation of normal healing reveals the emergence of distinct phenotypes in sequential phases. The initial stage is marked by the presence of mostly pro-inflammatory macrophages [4, 5], but lasts for only a short time before giving way to the next phase, in which macrophages with a distinct and non-inflammatory phenotype dominate [6]. Although it has been suggested that the latter phenotype is recruited to the site of injury [7], a growing body of evidence shows that pro-inflammatory macrophages are able to repolarize into this less-inflammatory population, suggesting a potential for in situ transition [8-10].
A variety of terminologies, each presenting unique advantages and disadvantages, have been used to describe these two contrasting phenotypes (for more extensive reviews of this topic, see Murray et al. [11] and Spiller and Koh [12]). Most commonly, pro-inflammatory macrophages are referred to as M1, while macrophages that dominate later stages of wound healing are called M2, following the Th1/Th2 nomenclature. However, current research shows that macrophages can exhibit a wide range of phenotypes, with very context-dependent functions [13]. For instance, several M2 subtypes have been identified, including M2a, M2b, and M2c, each with distinct behaviors and biological markers [14-16].
These phenotypes can be modeled in vitro through the addition of various stimuli. Macrophage colony-stimulating factor (MCSF) differentiates monocytes into macrophages (M0) [17]. Interferon-γ (IFNγ), a cytokine produced by natural killer and T helper 1 cells, is primarily used with or without lipopolysaccharide (LPS) to polarize macrophages to the M1 phenotype [13]. Once activated, M1 macrophages produce pro-inflammatory and microbicidal cytokines such as interleukin-1-beta (IL-1β), IL-6, tumor necrosis factor-α (TNFα), and nitric oxide [13, 18, 19]. M2 macrophages are polarized via the cytokines IL-4 and IL-13 (M2a) [19], or IL10 (M2c) [20]. M2a macrophages secrete factors associated with later stages of wound healing, such as platelet-derived growth factor-BB (PDGF-BB), tissue inhibitor of metalloproteinase-3 (TIMP3), and transforming growth factor-beta 1 (TGF-β1) [21, 22]. M2c macrophages secrete high levels of MMPs and may be involved in early stages of wound healing [20], although the lack of distinguishing markers for the different M2 subtypes in murine macrophages has hindered investigations into their timing.
The precise roles of each population of macrophages in tissue repair are poorly understood, but it is clear that dysfunctional regulation of macrophage phenotype can hinder healing (Figure 2). The early presence of M1 macrophages indicates their function in the initiation of tissue regeneration, but chronic M1 activity can delay or prevent total repair [23]. For instance, Sindrilaru et al. demonstrated in a murine model that persistent M1 macrophage activation with iron results in failure to completely transition to the M2 phenotype as well as impaired wound healing [6]. Uncontrolled M2 activation, on the other hand, is thought to lead to fibrosis [24-26], though some studies have suggested a potential role for M1 macrophages in fibrosis as well [27]. Importantly, this fibrotic activity may lead to fibrous encapsulation of biomedical implants, which prevents complete integration. Therefore, systems that attempt to direct macrophages to promote healing must tightly control temporal activation.
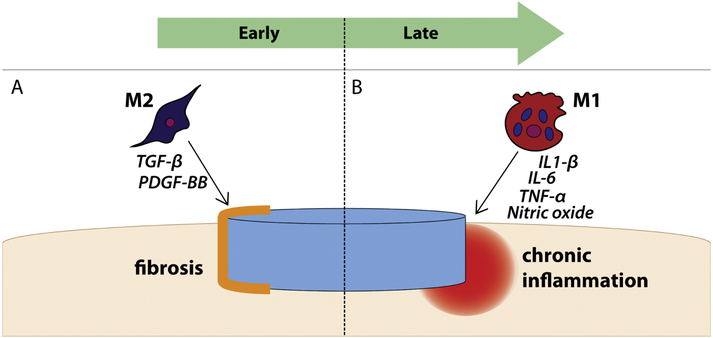
Figure 2: Dysfunctional timing of macrophage phenotypes in failed implant integration.
(A) Premature M2 activation may prevent implant integration via production of fibrotic cytokines such as TGF-β, though M1 macrophages may also play a role in fibrosis. (B) Early M1 macrophages that fail to transition to an M2 phenotype at later timepoints continue to secrete pro-inflammatory cytokines, resulting in chronic inflammation and delayed healing.
1.2. Macrophage functions in the foreign body response
When biomaterials are implanted to treat a serious injury, a fibrous capsule may form around them as part of the FBR. The fibrous capsule is a natural defense mechanism used by the immune system to separate foreign materials from the rest of the body [28]. This separation, however, prevents the biomaterial from properly integrating into the implantation site. Fibrous capsule formation is a problem that has long affected implanted biomaterials, and researchers have tried to elucidate the specific role of macrophages, with inconsistent results. Macrophages have been implicated in both propagating the FBR as well as reducing it, indicating that their role is likely complicated and context-dependent. For instance, Bank et al. depleted macrophages in macrophage fas-induced apoptosis (MaFIA) mice from four days before through seven days after subcutaneously implanting collagen discs [29]. They found that macrophage depletion caused significantly more fibrous capsule formation at day seven compared to non-macrophage-depleted mice, implicating macrophages in inhibition of fibrous capsule formation.
In contrast, Doloff et al. depleted macrophages in mice using clodronate liposomes from three days before through eleven days after intraperitoneal injection of alginate spheres, resulting in significantly reduced fibrous encapsulation at day fourteen compared to untreated mice, suggesting macrophages promote fibrous capsule formation [30]. Similarly, Dondossola et al. used clodronate liposomes to deplete macrophages in mice before implanting calcium-coated polycaprolactone electrospun scaffolds [31]. While they found fibrous capsule formation was greatly reduced in macrophage-depleted mice at day fourteen, blood vessel count was also inhibited, which would be detrimental for proper implant integration.
Together, the results of these studies illustrate the complexity of the role of macrophages in the FBR. In some cases, macrophages help to inhibit fibrous capsule formation, while in others the absence of macrophages reduced capsule thickness. One reason for these conflicting results may lie in the proper timing of macrophage polarization.
2. Early and transient activation of M1 macrophages is essential for implant integration
Though it is likely the most well-known and well-described macrophage phenotype, the role of the M1 macrophage in healing and implant integration remains controversial. Concerns over the involvement of M1 macrophages in chronic inflammation have led many researchers to shy away from strategies that promote this phenotype. However, there is growing evidence that early short-term M1 activation is not only beneficial to healing – it is critical.
2.1. M1 macrophage secretions induce healing
The duties of M1 macrophage secretions are almost certainly not limited to inflammation. Recent studies have shown that the secretions of M1 macrophages may play a role in additional aspects of healing, such as promoting angiogenesis and inhibiting fibrosis.
Nitric oxide is classically known as one of the main antimicrobial and cytotoxic byproducts of M1 macrophages [32]. To investigate its supplemental functions, Cassini-Vieira et al. implanted polyether-polyurethane discs into mice deficient in inducible nitric oxide synthase (iNOS), an isoform of nitric oxide synthase that is upregulated in M1 macrophages [33]. Compared to wild-type mice, iNOS-/- mice displayed decreased hemoglobin, VEGF, and vascularization, suggesting nitric oxide production contributes to angiogenesis. Additionally, the implants in iNOS-/- mice had greater fibrous encapsulation, which may indicate a role for nitric oxide in reducing fibrosis.
TNFα is both a product and potential inducer of the M1 phenotype, making it a cytokine of particular interest. Lemos et al. demonstrated that TNFα is responsible for inducing apoptosis in fibro/adipogenic progenitors (FAPs), which would otherwise differentiate into fibrogenic cells, and that the primary producers of TNFα are M1 macrophages [34]. On the other hand, M2-secreted TGF-β1 prevents FAP apoptosis, leading to matrix deposition. The authors suggest that normal healing exhibits a natural progression starting with TNFα activity, followed by TGF-β1 in the proliferation stage when matrix deposition is necessary. Insufficient TNFα in the early phase of healing, however, may instead lead to pathological fibrosis.
2.2. M1 macrophages positively modulate mesenchymal stem cells
Mesenchymal stem cells (MSCs) are commonly utilized in immunomodulatory designs, due to their capacity for isolation and expansion, ability to differentiate into various cell types, and potential anti-inflammatory properties [35]. Several studies have shown that pro-inflammatory environments mediated by IFNγ are necessary to prime MSCs for immunosuppressive activity [36-38]. Furthermore, M1 macrophages may specifically exert pro-healing influence on MSCs. For example, Wang et al. induced an M1-like phenotype in murine RAW 264.7 macrophages using biphasic calcium phosphate (BCP) ceramic degradation particles, then exposed MSCs to conditioned media from those macrophages [39]. As a result, MSCs cultured with the macrophage-conditioned media exhibited increased migration and osteoblastic differentiation.
In a similar study, Lu et al. co-cultured murine MSCs with murine bone marrow-derived M0, M1, or M2a macrophages to determine downstream effects on osteogenesis [40]. While all cocultures increased osteogenesis compared to MSCs cultured alone, M1-MSC co-cultures showed the greatest extent of bone mineralization, which was linked to increased production of prostaglandin E2.
2.3. M1 macrophages promote angiogenesis
The idea that M1 macrophages contribute to angiogenesis, a vital aspect of successful implant integration, has caused some debate. It was long thought that M1 macrophages were anti-angiogenic, and M2 macrophages pro-angiogenic. Conflicting reports further clouded the answer to this question [21,41, 42].
Tattersall et al. used a bead-based capillary sprouting assay to observe the effects of M1 and M2a macrophages on endothelial cell sprouting [43]. M1 macrophages facilitated the greatest number and longest sprouts by day 4, which the authors attributed to increased Notch signaling. M2a activation did not increase sprouting and appeared to shorten the length of sprouts. In a similar study, Gurevich et al. co-cultured primary human M1 or M2a macrophages with human umbilical vein endothelial cells (HUVECs) on a layer of fibroblasts [44]. The M1 macrophages induced the highest total vessel length in HUVECs compared to the M0 control or M2a groups, due to a significant increase in VEGF production. Both of these findings support an earlier study from Spiller et al., in which M1 macrophages secreted more VEGF and induced more HUVEC sprouting than M2a macrophages [21]. In contrast, Jetten et al. showed that murine endothelial cell tube formation was inhibited by murine M1 macrophages and promoted by M2a and M2c macrophages [42]. The conflicting results from these studies may reflect mouse-human differences, or other unknown experimental differences.
Gurevich et al. also ablated macrophages in zebrafish before and throughout the first few days following needlestick injury, which resulted in impaired neoangiogenesis [44]. Furthermore, early treatment with IFNγ in non-macrophage-depleted injuries improved angiogenesis compared to those treated with M2c-promoting IL-10 or a vehicle control. Together, these recent studies strengthen the argument that M1 macrophages promote healthy angiogenesis through multiple mechanisms.
2.4. Depletion of M1 macrophages impairs healing
The depletion of macrophages through diphtheria toxin receptor (DTR) mice or clodronate-loaded liposomes has proven to be highly useful in probing the temporally specific roles of macrophage phenotypes in healing. Interestingly, when macrophages were ablated before or directly after injury (in which macrophages exhibit a predominantly M1 phenotype), healing outcomes were severely diminished [45, 46]. Sandberg et al. used clodronate liposomes to ablate macrophages in a murine cancellous bone injury model [47]. When administered at one or four days before injury, the liposomes decreased macrophages at day one up to day three post-injury. As a result, the newly formed bone displayed significantly less strength and density compared to controls. Contrastingly, when clodronate was administered on day one or three after injury, the effects on bone healing were not as severe and were statistically insignificant.
Using a murine fat graft model, Cai et al. conducted a study to determine the behavior of macrophages in the initial stages of allogenic graft transfer [48]. Immediately after fat grafting, mice were treated with either clodronate liposomes to deplete macrophages, or MCSF to stimulate macrophages, for one week. At twelve weeks post-transfer the fat grafts were explanted and evaluated. Compared to the untreated control, the grafts in the depletion group weighed the least, displayed the least vascularization, and had the poorest survival. The group that received MCSF stimulation, however, showed significant improvement on all counts.
2.5. Recruitment/promotion of M1 macrophages induces healing
Not only does inhibition of M1 macrophages impair healing, but actively recruiting or promoting the M1 phenotype has been shown to actually promote healing. For example, Hsieh et al. treated hindlimb ischemia in mice with either M1 or M2a exogenously polarized macrophages one day after injury [49]. While early M1 treatment resulted in increased blood flow and accelerated muscle regeneration compared to the saline control, early M2a treatment did not. In fact, early M2a treatment resulted in fibrosis, with increased collagen deposition.
Monocyte chemoattractant protein-1 (MCP-1) is a powerful chemokine responsible for recruiting circulating monocytes to the site of injury, especially during the inflammatory stage [50]. Hoh et al. loaded MCP-1 in PLGA coils to enhance recruitment of macrophages in mice with aneurysms [51]. In a prior study, these coils displayed sustained release of MCP-1 over the course of three weeks [52]. MCP-1-loaded coils displayed increased M1 recruitment in the first week compared to PLGA-only coils, though both treatments demonstrated an early spike followed by a decline in M1 macrophages [51]. MCP-1 coils also recruited significantly more M2 macrophages in weeks two and three, and resulted in greater tissue ingrowth, compared to PLGA-only coils.
Diabetic wounds are a problem of particular interest for the field of tissue regeneration. These wounds are characterized by low levels of chronic inflammation, so treatment via promotion of pro-inflammatory macrophages seems counterintuitive. However, the results of studies in which diabetic wounds were administrated pro-inflammatory treatments seem to suggest that promoting the M1 phenotype could be a viable strategy. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) treatment of diabetic wounds in mice increased IL-6 and MCP-1 production, which led to increased angiogenesis and enhanced wound healing [53]. Interestingly, non-diabetic wounds did not receive the same benefits from GM-CSF.
Wood et al. used a similar approach by administering MCP-1 to diabetic wounds [54]. They showed that in diabetic wounds, inflammatory macrophages (as measured using histomorphometric analysis) were delayed and fewer in number compared to normal wounds. They attributed this behavior to low MCP-1 expression, and were able to recover a normal healing response with MCP-1 treatment, with increased inflammatory macrophage infiltration and enhanced wound closure. Additionally, inflammation in diabetic wounds treated with MCP-1 was resolved by day ten, whereas untreated wounds displayed chronic inflammation.
Substance P (SP) is a neuropeptide that is used to promote wound healing, and several studies have tested it in diabetic wounds. In one such study, SP administration caused a spike in proinflammatory cytokines by day three post-treatment in murine diabetic wounds [55]. At day ten, this inflammation had resolved, and wound healing was enhanced compared to untreated controls.
Treatment of chronic wounds with direct administration of M1 macrophages has also been shown to induce healing. In rats with diabetic wounds, co-administration of TNFα and exogenous M1 macrophages showed marked improvements in healing compared to treatment with M0 macrophages [56]. The authors attributed this effect partially to increased VEGF production, which is deficient in diabetic wounds. Similarly, Zuloff-Shani et al. activated macrophages via hypo-osmotic shock, producing an M1-like phenotype [57], and administered them to the pressure ulcers of elderly patients [58]. Compared to wounds treated with standard of care, the macrophage-treated wounds, including those of diabetic patients, exhibited significantly greater wound closure.
2.6. Ml macrophages exhibit phenotypic switching to M2
Expedient transition from an inflammatory M1 environment to M2 is essential for proper implant integration. This switching behavior is normal in typical healing, suggesting that promoting an early M1 phenotype would not hinder M2 activity later on. Bencze et al. injected either M1 or IL-10-polarized M2c human macrophages, along with human myoblasts, into cryodamaged murine muscle tissue [59]. Not only did M1 macrophages enhance myoblast engraftment compared to the M2c and control M0 macrophages, but they also transitioned into an M2-like anti-inflammatory phenotype by day five. In zebrafish, M1 macrophages displayed similar behavior: TNFα-expressing M1 macrophages induced neoangiogenesis and sprouting, but later downregulated TNFα to transition to an M2-like phenotype and promote connections between newly formed blood vessels [44].
It has been shown that pro-inflammatory macrophages produce high amounts of pro-angiogenic VEGF [21, 60-62], but its action may not be limited to angiogenesis. Wheeler et al. showed that VEGF stimulates macrophage migration and the upregulation of the M2 markers CD206, CD163, and CCL17 in THP-1 cells [63]. This finding suggests a possible mechanism for the natural progression of the M1-to-M2 transition.
3. Delayed M2 activation complements early inflammatory functions and improves healing outcomes
Proper timing of macrophage-targeted strategies in activating M1 macrophages has been shown to be critical to prevent chronic inflammation and increase vascularization of wounds and scaffolds. Correct timing of M2 macrophages is also necessary; if M2-promoting treatments are introduced to a recovering wound before inflammation has initiated angiogenesis and healing, then wound closure, tissue function and blood vessel reperfusion may be reduced.
3.1. Early treatment of wounds with M2a macrophages inhibits healing
Despite the fact that M2a macrophages are commonly considered pro-healing, they may actually inhibit healing and vascularization at early timepoints. For instance, Jetten et al. used a cutaneous wound model to test the effects of macrophage administration immediately following injury in both wild type and diabetic mice [64]. After making a full thickness wound with a 4mm biopsy punch, exogenously polarized M0, M2a, or M2c macrophages were injected subcutaneously around the wound site. They found that none of the macrophage treatments improved wound closure compared to a saline control in the wild type mouse. Interestingly, they found that immediate injection of M2a and M2c macrophages actually inhibited wound closure in diabetic mice. Upon histological investigation, they found that complete re-epithelialization occurred in the wounds treated with saline or M0 macrophages, but not in those treated with M2a or M2c macrophages. Although these results were not compared to groups in which M2 macrophages were administered at later time points following injury, the results do suggest that the presence of M2 macrophages at early time points after injury can have inhibitory effects on wound healing.
Another study by Duan et al. in mice examined the effects of early M2a administration on healing [65]. They first induced endometriosis, an inflammatory disorder of the uterine tissue lining, by intraperitoneal injection of endometrial fragments. Three days later, they depleted DTR-CD11b mice of macrophages via diphtheria toxin administration. One day following macrophage depletion, ex vivo-polarized macrophages (M0, M1, M2a, and M2c) were injected intravenously and the effects on lesion growth were observed. Compared to the M0 control, the M2a group significantly increased the extent of fibrosis in the lesions, as determined by lesion weight, fibrous tissue formation, and collagen staining, but the M1 and M2c groups did not.
In addition to their possible fibrotic nature, M2a macrophages produce more soluble vascular endothelial growth factor receptor 1 (sVEGFR1) than the other commonly studied phenotypes [66]. sVEGFR1 is a scavenger protein receptor that binds to VEGF to prevent its function [67, 68], which can inhibit the proliferation of endothelial cells downstream [69]. While this inhibition is detrimental to early formation of blood vessels, sVEGFR1 is necessary during later stages to guide their growth [70]. As previously mentioned, M2a macrophages also secrete other factors involved in later stages of healing, including PDGFBB, which is critical for stabilizing VEGF-initiated angiogenesis [71, 72]. Indeed, drug delivery systems that sequentially deliver VEGF and PDGFBB have been shown to enhance biomaterial vascularization [73-77]. Together, these studies suggest that M2a macrophages must activate later in the integration response so they can support the vascularization initiated by M1 macrophages, but their early presence may hinder angiogenesis.
3.2. Delayed anti-inflammatory treatment aids in healing
Several studies have examined the effects of delivering anti-inflammatory compounds after a delay. In this strategy, the M1 macrophages in the inflammatory stage are free to initiate healing and angiogenesis, then the anti-inflammatory treatment can effect a timely switch to an environment more favorable for M2 macrophages. For example, Virchenko et al. evaluated the effects of early or delayed administration of parecoxib, an anti-inflammatory COX-2 inhibitor, on transected Achilles tendons in rats [78]. Daily intramuscular injection of parecoxib for the first five days post-injury led to significant decreases by day eight in tendon stiffness, maximum stress, and force at failure, compared to saline controls. When treatment was delayed until days six through fourteen, however, there was no difference in tendon stiffness or force at failure compared to control, and maximum stress was significantly increased.
Using a similar model, Blomgran et al. transected rat tendons and then subcutaneously injected dexamethasone (Dex) or saline control daily on days 0-4 or days 5-9 post-surgery [79]. While early administration of Dex significantly reduced stiffness and peak force withstood by the tendon compared to the saline control, late administration of Dex had the opposite effect, in that the tendon’s stiffness and ability to withstand force were increased compared to the control. This positive effect of the delayed Dex group was attributed to better collagen organization.
In a model of cerebral ischemia, Brifault et al. tested delayed delivery of embryonic stem (ES) cells that were genetically modified to produce anti-inflammatory pituitary adenylate cyclaseactivating polypeptide (PACAP) [80]. They found that when delivered three days after injury, PACAP dampened gene expression of proinflammatory mediators and increased the expression of the M2 marker Arg-1 in murine microglia/macrophages. Along with the increased M2 population, the healing outcomes of neurological severity score and motor coordination improved in the groups treated with PACAP-producing ES cells compared to non-modified ES cells.
3.3. Delayed M2 macrophage activation promotes healing
In a murine model of hindlimb ischemia (HLI), Raimondo et al. tested the effects of the M2a-polarizing stimulus IL-4 on skeletal muscle recovery [81]. In the HLI model, the inflammatory period naturally begins to abate about three days after injury in C57BL/6 mice [82, 83], so the authors injected IL-4-conjugated gold nanoparticles at that time. On days nine and fifteen postinjury, the authors confirmed that the macrophages present in the mouse hindlimb were primarily composed of an M2-like phenotype, to a significantly greater extent than the saline control. The mice treated with IL-4 also had significantly increased fiber regeneration, muscle contractile force and reperfusion in the ischemic limb compared to the control.
In another study that employed a murine HLI model, Troidl et al. tested the effects of delivering macrophage-polarizing cytokines to improve healing [84]. On days two, four, and six after femoral artery ligation, they intravenously injected Dex, IL-10, or a combination of IL-4 and IL-13. Although phenotyping of infiltrating macrophages was not conducted, both the IL-4/IL-13-and IL-10-treated groups had significantly increased reperfusion in the ischemic legs compared to the saline control. Interestingly, only the IL-10-treated group had significantly higher amounts of reperfusion compared to the control at two weeks post-injury.
Francos-Quijorna et al. studied pro-inflammatory resident microglia and macrophages and their effects on repair in a spinal cord injury mouse model [85]. They found that the microglia and macrophages failed to switch to an M2a phenotype for up to four weeks after injury, and instead continued to express inflammatory M1 markers. They also found that there was no IL-4 present in the spinal cord at any time. The authors then injected IL-4 into injured spinal cords either immediately or 48 hours after injury to evaluate the effects of delayed IL-4 administration on macrophage behavior. The delayed treatment downregulated pro-inflammatory iNOS and upregulated the M2 markers Arg-1 and CD206, compared to saline control, while the acute treatment only upregulated Arg-1. In addition, delayed IL-4 administration protected against locomotor function loss and caused a significant increase in the myelin sparing at the injury site compared to saline control, though functional assessment of early-treated mice was not conducted. The authors also noted the unexpected appearance of a “resolution-phase” macrophage that was neither M1 nor M2, but expressed resolution-phase markers such as 5-LOX and 15-LOX. Conversely, Mokarram et al. bridged peripheral nerve gaps in rats with agarose hydrogel loaded with IFNγ or IL-4 to compare the effects on regeneration [86]. Although in vitro studies indicated rapid release of the cytokines within the first day, the IL-4-loaded scaffolds induced greater Schwann cell infiltration and axonal regrowth compared to unloaded or IFNγ-loaded scaffolds.
An acute lung injury (ALI) mouse model was used by D’Alessio et al. to investigate the effects of IL-4 on repair [87]. They hypothesized that generating an M2a phenotype would enhance ALI resolution. To test this hypothesis, they induced injury via LPS, then intraperitoneally injected IL-4 days two, three, and four post-injury. The lungs of the IL-4-treated mice displayed improved diffusion and less collagen deposition by day six, compared to control mice treated with saline. Furthermore, the IL-4-treated group also exhibited improved survival and accelerated injury resolution. These benefits were negated by macrophage depletion or inhibition of the M2a macrophage-associated STAT6 pathway, suggesting a specific role for M2a macrophages in injury resolution.
These studies highlight the necessity of avoiding early M2 macrophage stimulation, which could hinder vital M1 activity, and promoting delayed M2 activation. Delayed IL-4 treatment, which is intended to promote the M2a subtype, is a particularly promising strategy to promote healing. Drug delivery systems that can sufficiently delay release of M2-stimulating reagents are imperative in the overall objective of sequentially modulating macrophage behavior.
4. Sequential drug delivery systems to modulate macrophage behavior
The persistent and essential activity of macrophages during implant integration presents a unique opportunity for immunomodulation and tissue engineering, but the importance of the early actions of M1 macrophages and the delayed actions of M2 macrophages necessitates sequential drug delivery strategies (Figure 3). To date, several such designs have been proposed, utilizing various biomaterials to control biphasic delivery of macrophage stimuli (Table 1).
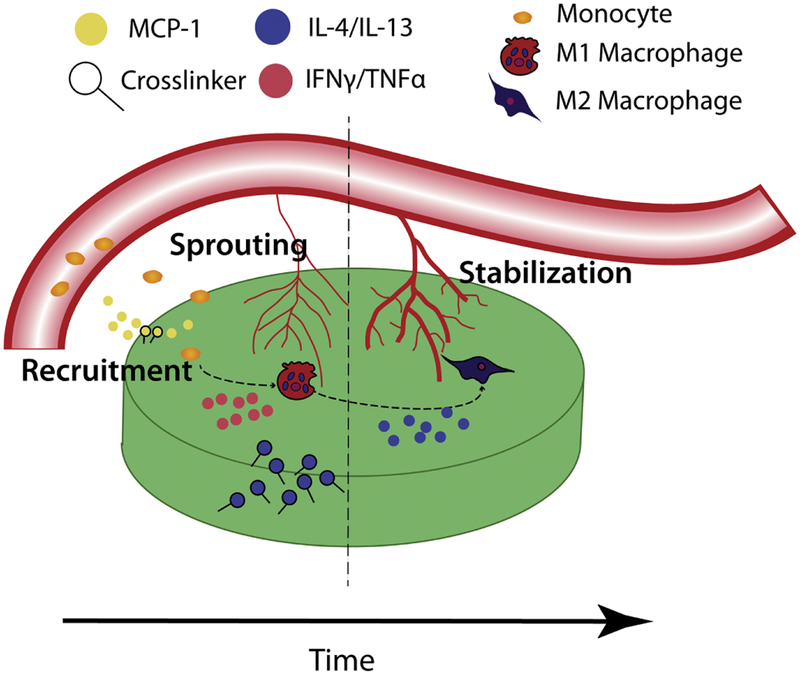
Figure 3: General model of sequential drug delivery system for macrophage modulation and implant integration.
During the initial inflammatory phase, MCP-1 may be incorporated to recruit circulating monocytes to the implant site. Release of IFNγ or TNFα can promote the Ml phenotype in infiltrating macrophages, inducing sprouting of immature blood vessels from the surrounding vasculature. Subsequent release of IL-4 and/or IL-13 would activate switching to the M2(a) phenotype, resulting in the resolution of inflammation and stabilization of newly formed blood vessels.
Table 1:
Sequential drug delivery systems targeting macrophages.
| System Design | Release Profile | Outcome | Reference |
|---|---|---|---|
| Multidomain selfassembling peptide hydrogel | 80% of MCP-1 released over first 2 days; 15–20% of IL-4 released over first six days, 40% by 16 days |
Increased macrophage infiltration; macrophages polarized to an M2a state by day 7 |
Kumar et al. [93] |
| Combined gelatin hydrogels of isoelectric point (IEP)5 and 9, with additional incorporation of IEP 9 micelles | 81% of SDF-1 released over first week in vivo; 51% of SEW2871 released over first week in vivo; 52% of SDF-1 released over first day in vitro; 24% of SEW2871 released over first day in vitro |
Increased M1 recruitment on day 1 and M2 recruitment on day 3; Accelerated wound closure in diabetic mice |
Kim et al. [94] |
| Decellularized bone scaffold with adsorbed IFNg and biotin-streptavidin-conjugated IL-4 | 0.8ng of IFNg released over 2 days; 8ng of IL-4 released over 6 days |
Increased expression of M1 and M2 markers on day 3, M2 markers only on day6; Increased cellular infiltration |
Spiller et al. [8] |
| Titania nanotubes covered with chemically crosslinked hydrogel, then physically crosslinked hydrogel | Most of the loaded IFNg released within3 days; Small burst release of IL-4 within first day followed by steady release over 7 days |
M1 secretions and gene expression increased by day 3; M2 secretions and gene expression increased by day 7 |
Chen et al. [97] (see also Gao et al. [98]) |
| Outer gelatin scaffold surrounding inner ferrogel for release initiated by magnetic stimulation | Loaded MCP-1 or IFNg released within the first day; Slow release of IL-10 or IL-4 over 6 days with significant increases upon stimulation |
Increased macrophage infiltration | Tolouei et al. [99] |
| Biomimetic calcium phosphate coating for phase separation | Most of the adsorbed IFNg released on day 1; Simvastatin releasebegan on day 3 |
Increased M1 gene expression on day 1; Increased M2 gene expression on day 6; Aged human and murine macrophages exhibited phenotype switching |
Alhamdi et al. [100] |
| 5% calcium silicate/B-tricalcium phosphate scaffold | 1.5ng of IFNg released over 5 days; 40ppm of silicon ions released over 7 days |
Increased M1 surface marker and gene expression by day 3 in vitro and in vivo; Increased M2 surface marker and gene expression by day 7 in vitro and in vivo; Increased sequential VEGF and PDGF secretion; Increased scaffold vascularization in vivo |
Li et al. [101] |
4.1. Negative effects of simultaneous M1 and M2 activation
In order to properly modulate macrophage phenotype over time, it is critical that there are distinct periods of activation. Otherwise, overlapping phases may result in a mixed M1-M2a state that may be detrimental for biomaterial integration. For instance, there is some evidence that a mixed M1/M2 phenotype may be responsible for fibrous capsule formation. Several studies of macrophages derived from fibrous capsules have demonstrated that they express both M1 and M2 markers [88, 89]. It should also be noted that simultaneous administration of VEGF and PDGF-BB, growth factors secreted by M1 and M2, respectively [21], has been shown to hinder normal angiogenesis [90-92].
In one of the first efforts to sequentially modulate macrophage phenotype, Spiller et al. adsorbed IFNγ to a scaffold for quick release, and attached IL-4 with biotin-streptavidin conjugation for prolonged release [8]. In vitro studies with seeded human macrophages showed that although the rapid release of IFNy did promote M1 polarization of macrophages at early time points, the release of IL-4 promoted M2a polarization at both early and late time points. When implanted subcutaneously in mice, the rapid release of IFNy enhanced blood vessel ingrowth compared to controls, but combination with IL-4 release abrogated these effects. These results were attributed to either the development of a hybrid M1/M2 phenotype that hampered the pro-angiogenic effects of the M1 phenotype, or the anti-angiogenic effects of the M2 phenotype at early time points.
4.2. Sequential delivery of macrophage recruitment agents and M2-promoting stimuli
One approach to biphasic modulation of macrophage behavior involves initial delivery of a recruitment agent to attract circulating monocytes to the site of injury, which tends to result in increased M1 macrophage infiltration [51, 54]. In the second phase, M2-promoting stimuli are delivered to induce phenotype switching. Kumar et al. designed a multidomain self-assembling peptide hydrogel to control the sequential release of MCP-1 and IL-4 [93]. They hypothesized that this design would recruit monocytes to the implant environment to bolster resident M1 activity, then IL-4 would polarize the macrophages to an M2a phenotype. The hydrogels released 80% of loaded MCP-1 in two days, while slower release of IL-4 occurred over all sixteen days. After subcutaneous implantation in mice, the authors showed that the scaffolds were able to increase cellular infiltration, then polarize the infiltrating macrophages to M2. However, there was no evidence of early M1 behavior, compared to unloaded or MCP-1-only controls, suggesting possible interference by quickly-released IL-4.
Kim et al. varied the dual-release profiles of proteins from gelatin hydrogels by changing the hydrogels’ isoelectric points (IEP) [94]. The hydrogels were loaded with micelles containing stromal cell-derived factor-1 (SDF-1) to recruit stem cells and the S1P receptor agonist SEW2871 to recruit macrophages. After implanting hydrogels with different IEP combinations in diabetic mice, the authors identified one hydrogel, termed G5SmG9S, that exhibited quick release of SDF-1 and controlled release of SEW2871. Hydrogels with differing release profiles released the drugs simultaneously or released SEW2871 before SDF-1. When implanted in murine diabetic wounds, G5SmG9s recruited higher numbers of M1 macrophages on day one and M2 macrophages by day three, compared to the other hydrogels. Expression of the M1 marker IL-6 in these macrophages was also higher one day post-implantation, and expression of M2 marker TGF-β was the highest on day three. Finally, the G5SmG9S hydrogel accelerated closure of wounds in diabetic mice compared to other hydrogels with different release profiles. Together, these findings suggest sequential release of SDF-1 and SEW2871 could be a viable way to modulate macrophage behavior. This approach is further supported by a study from Awojoodu et al., which found that S1P receptor agonists can enhance anti-inflammatory monocyte migration towards SDF-1 [95]. Moreover, Awojoodu and colleagues showed in a murine model that delayed delivery of S1P receptor agonist from poly(lactic-co-glycolic acid) films resulted in improved vascularization of the surrounding tissue, compared to acutely delivered S1P receptor agonist.
4.3. Sequential delivery of M1-promoting and M2-promoting stimuli
Biphasic drug delivery methods designed to control macrophage behavior have most commonly utilized sequential release of IFNγ to induce an M1 phenotype, followed by IL-4 to polarize to M2a. After initial studies showed that IFNγ stimulation followed by IL-4 treatment could induce M1-to-M2a switching of macrophages in vitro [8, 96], there have been several other designs aimed at improving temporal control. Chen et al. loaded IL-4 onto titania nanotubes (TNTs), then covered the tubes with a layer of carboxymethyl chitosan (CMCS) gel, which was chemically crosslinked to reduce burst release [97]. IFNγ was added to the system then covered with a layer of chitosan hydrogel, which was crosslinked with β-glycerophosphate disodium to increase hydrophobicity and stability of the system. Rapid release of IFNγ occurred over the first two days, while IL-4 was released slowly over the first week, including the first two days. Compared to controls, in vitro murine RAW264.7 macrophage secretion of the M1 markers TNFα and IL-1β was increased on day three, while secretion of the M2 markers IL-10 and TGF-1ΐ secretion was increased on day seven. Gene expression of M1 and M2 markers followed the same pattern. Gao et al. utilized a similar system, omitting the top hydrogel layer [98]. The system was able to delay release of IL-4 until day 2, and likewise demonstrated phenotype switching in vitro from M1 expression on day three to M2 on day seven.
Rather than using a passive-release design, Tolouei et al. combined an outer gelatin scaffold, loaded with MCP-1 and IFNγ with an inner ferrogel, loaded with IL-4 [99]. After rapid diffusion-mediated release of cytokines from the outer layer, the authors showed they were able to initiate controlled release from the ferrogel via magnetic stimulation. They also demonstrated significant infiltration of macrophages into the scaffold in vitro. Although phenotyping of macrophages was not conducted, this device could be an interesting approach to directly control temporal release of cytokines.
Alhamdi et al. used a biomimetic calcium phosphate coating (bCaP) to separate phases of IFNγ and simvastatin (SIMV) release, the latter of which was intended to promote an M2 phenotype [100]. The authors demonstrated that SIMV was released via macrophage phagocytosis of the bCaP coating, so that release was delayed for around three days. Furthermore, not only did the system induce expression of M1 genes on day one, followed by switching to M2 gene expression by day six, but it was able to do so in macrophages derived from older humans and mice, albeit to a lesser extent, suggesting applicability even for elderly patients, whose immune systems are notoriously compromised.
Aiming to take advantage of the M2-promoting properties of silicon, Li et al. loaded IFNγ onto a 5% calcium silicate/β-tricalcium phosphate scaffold [101]. They hypothesized that rapid discharge of IFNγ would be followed by silicon ion release, resulting in macrophage phenotype switching and improved vascularization. When co-cultured with primary human monocyte-derived macrophages, the scaffolds induced increased surface and gene expression of M1 markers on day three and greater M2a polarization on day seven, compared to controls. Additionally, conditioned media from the macrophages cultured on the IFNγ-only or combination scaffold contained significantly more VEGF on days three and seven than silicate-only or unloaded scaffolds. Similarly, PDGFBB secretion was upregulated in macrophages cultured on silicate-only or combination scaffolds on day three, with a dramatic increase on day seven in the combination scaffold. The increased angiogenic secretions from the combination scaffold led to significantly greater tube length and number of branch points when conditioned media was added to a Matrigel assay with HUVECs, compared to all other groups. Similar results were observed when scaffolds were implanted subcutaneously in mice: the sequential delivery of IFNγ and Si ions upregulated M1 marker expression on day three and M2 marker expression on day seven, and increased the average number of infiltrating blood vessels compared to controls.
5. Outlook
Despite the promise of these biphasic drug delivery systems, there is still much progress to be made in the understanding of temporal macrophage behavior. For example, although it has been shown that M1 macrophages can be easily polarized to M2a [8, 102], whether this switching typically occurs in vivo remains controversial. Additionally, the specific roles of the phenotypes in both angiogenesis and fibrous capsule formation must be completely defined in terms of context and timing if one wishes to promote optimal biomaterial integration and tissue regeneration. It is still unknown exactly how long each phenotype should remain active, and few studies have attempted to delineate temporal macrophage functions throughout each phase of the healing process. One such study from Lucas et al. found that in murine wounds, macrophages are necessary for the early inflammatory phase and the secondary tissue formation phase, each of which lasts for a few days, but are less important in the final stage involving tissue maturation [46]. However, more research is needed to determine a precise timeline of macrophage phenotypes in humans. Furthermore, the exact required doses of M1 - or M2a-promoting drugs have not been studied, and may vary depending on the patient. For example, older patients are known to exhibit a diminished inflammatory response, and thus may require a higher dose of M1-promoting drugs [103-105]. Additional insight into these parameters will be crucial for engineers to optimize the release profiles of either M1 - or M2a-activating materials from sequential drug delivery systems.
Another challenge facing immunomodulatory therapies is clearly defining the roles and temporal behavior of other M2 subtypes, especially in terms of the different contexts of tissue repair processes. As one example, IL-10-stimulated M2c macrophages have been implicated in phagocytosis of apoptotic cells and matrix remodeling, and may actually be most active in the early stages of regeneration [6, 20, 106, 107]. It is unknown, however, if there is a critical need for M2c promotion, and whether future designs should attempt to promote M2c concurrently with M1 stimuli, especially considering that the IL-10 would inhibit M1 polarization. Moreover, the potential for other M2 subtypes to polarize to M1 or M2a has not been thoroughly studied, but is important for the design of biomaterials that promote the correct phenotypic transitions of multiple macrophage subtypes.
One major shortcoming of current sequential drug delivery systems is a lack of distinct separation between release phases. Most systems that successfully achieve slow, controlled delivery of M2a-activating reagents do not completely prevent release in the first few days. This early release, even in small doses, could potentially induce a mixed phenotype to the detriment of critical M1 activity. To resolve this issue, researchers may choose to explore more sophisticated methods of delaying IL-4 release, including the design of on-demand release systems.
A complicating factor in the field is the inconsistency in what cytokines are used to polarize macrophages to specific phenotypes. For instance, it has been posited that the complete activation of M1 macrophages requires not only IFNγ, but also TNFα or a Toll-like receptor ligand such as LPS [108]. If this is true, sequential drug delivery systems may need to include an additional payload in the early phase. Similarly, IL-4 and IL-13 are typically used in vitro to polarize macrophages to the M2a phenotype, but the effects of exposure to either cytokine alone versus a combination of both is less well understood. The use of IL-10 in conjunction with IL-4 to produce an M2-like phenotype in studies is also still relatively common, and further confounds interpretation of the outcomes. Both in vitro and in vivo studies utilizing sequential drug delivery systems, especially those that venture outside the realm of cytokine-only stimulation, must ensure that they have the desired effects within the context of injury and implant integration.
Notably, there is a surprising lack of sequential gene delivery systems to modulate macrophage phenotype. Although gene therapy to induce an M2-like phenotype in macrophages has been studied [109, 110], as well as sequential gene therapy to deliver IL-12 then IL-27 encoding DNA for treatment of tumors [111], to our knowledge no one has explored applying these findings to biphasic M1-to-M2 activation. This lack of a macrophage-targeted sequential gene delivery approach may be due to the relative complexity compared to cytokine delivery. As the field of gene therapy continues to advance, however, researchers may want to explore the potential of a gene delivery strategy.
In conclusion, drug delivery systems that can recruit or stimulate the M1 phenotype in the early phase of healing and the M2 phenotype in later stages may be an effective way to promote implant integration. Macrophages are key players in the foreign body response and are responsible for regulating other cell types that react to biomaterial implants. Both M1 and M2 macrophages play crucial roles in the healing process, especially by promoting angiogenesis. However, healing can be delayed or completely prevented by excessive activation of either phenotype, or simultaneous activation of both. It is therefore imperative that sequential drug delivery systems maintain strict temporal control over cytokine release. Deeper understanding of the macrophage response to implanted materials will also lead to improved sequential drug delivery system designs.
Acknowledgments:
The authors gratefully acknowledge funding support from NHLBI (R01 HL130037 to KLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anderson JM, McNally AK, Biocompatibility of implants: lymphocyte/macrophage interactions, Seminars in Immunopathology, 33 (2011) 221–233. [DOI] [PubMed] [Google Scholar]
- [2].Jain RK, Au P, Tam J, Duda DG, Fukumura D, Engineering vascularized tissue, Nature Biotechnology, 23 (2005) 821–823. [DOI] [PubMed] [Google Scholar]
- [3].Kuttappan S, Mathew D, Jo J, Tanaka R, Menon D, Ishimoto T, Nakano T, Nair SV, Nair MB, Tabata Y, Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect, Acta Biomaterialia, (2018). [DOI] [PubMed] [Google Scholar]
- [4].Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE, The phenotype of murine wound macrophages, Journal of Leukocyte Biology, 87 (2010) 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim BS, Tilstam PV, Springenberg-Jung K, Boecker AH, Schmitz C, Heinrichs D, Hwang SS, Stromps JP, Ganse B, Kopp R, Knobe M, Bernhagen J, Pallua N, Bucala R, Characterization of adipose tissue macrophages and adipose-derived stem cells in critical wounds, PeerJ, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice, Journal of Clinical Investigation, 121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haul on S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G, PPARy Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties, Cell Metabolism, 6 (2007) 137–143. [DOI] [PubMed] [Google Scholar]
- [8].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials, 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB, Revision joint replacement, wear particles, and macrophage polarization, Acta Biomaterialia, 8 (2012) 2815–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G, Macrophage activation switching: an asset for the resolution of inflammation, Clinical & Experimental Immunology, 142 (2005) 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA, Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines, Immunity, 41 (2014) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spiller KL, Koh TJ, Macrophage-based therapeutic strategies in regenerative medicine, Advanced Drug Delivery Reviews, 122 (2017) 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nature Reviews Immunology, 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson CF, Mosser DM, A novel phenotype for an activated macrophage: the type 2 activated macrophage, Journal of Leukocyte Biology, 72 (2002) 101–106. [PubMed] [Google Scholar]
- [15].Stein M, Keshav S, Harris N, Gordon S, Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation., Journal of Experimental Medicine, 176 (1992) 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Donnelly RP, Dickensheets H, Finbloom DS, The Interleukin-10 Signal Transduction Pathway and Regulation of Gene Expression in Mononuclear Phagocytes, Journal of Interferon & Cytokine Research, 19 (2004). [DOI] [PubMed] [Google Scholar]
- [17].Becker S, Warren MK, Haskill S, Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures., The Journal of Immunology, 139 (1987)3703–3709. [PubMed] [Google Scholar]
- [18].Menzies FM, Henriquez FL, Alexander J, Roberts CW, Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation, Clinical & Experimental Immunology, 160 (2010) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martinez FO, Gordon S, The M1 and M2 paradigm of macrophage activation: time for reassessment, FlOOOPrime Reports, 6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, Ferraro NM, Rajagopalan R, Sarmady M, Spiller KL, Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing, Immunobiology, 222 (2017) 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials, 35 (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA, Interleukin-13 Induces Tissue Fibrosis by Selectively Stimulating and Activating Transforming Growth Factor β 1, Journal of Experimental Medicine, 194 (2001) 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krzyszczyk P, Schloss R, Palmer A, Berthiaume F, The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes, Frontiers in Physiology, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stahl M, Schupp J, Jager B, Schmid M, Zissel G, Muller-Quernheim J, Prasse A, Lung Collagens Perpetuate Pulmonary Fibrosis via CD204 and M2 Macrophage Activation, PLOS One, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y, Niu J, Wang F, Su L, Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages, PLOS Pathogens, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR, M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure, American Journal of Physiology Heart and Circulatory Physiology, 309 (2015) Pages H906–H917. [DOI] [PubMed] [Google Scholar]
- [27].Witherel CE, Abebayehu D, Barker TH, S. K.L., Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis, Advanced Healthcare Materials, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Klopfleisch R, Jung F, The pathology of the foreign body reaction against biomaterials, J Biomed Mater Res A, 105 (2017) 927–940. [DOI] [PubMed] [Google Scholar]
- [29].Bank RA, Zandstra J, Room H, Petersen AH, van Putten SM, Biomaterial Encapsulation Is Enhanced in the Early Stages of the Foreign Body Reaction During Conditional Macrophage Depletion in Transgenic Macrophage Fas-Induced Apoptosis Mice(), Tissue Eng Part A, 23 (2017) 1078–1087. [DOI] [PubMed] [Google Scholar]
- [30].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Aresta-Dasilva S, Griffin M, Jhunjhunwala S, Webber M, Siebert S, Tang K, Chen M, Langan E, Dholokia N, Thakrar R, Qi M, Oberholzer J, Greiner DL, Langer R, Anderson DG, Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates, Nat Mater, 16 (2017) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P, Examination of the foreign body response to biomaterials by nonlinear intravital microscopy, Nat Biomed Eng, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].MacMieking J, Xie Q, Nathan C, Nitric Oxide and Macrophage Function, Annual Review of Immunology, 15 (1997) 323–350. [DOI] [PubMed] [Google Scholar]
- [33].Cassini-Vieira P, Araujo FA, Dias FLC, Russo RC, Andrade SP, Teixeira MM, Barcelos LS, iNOS Activity Modulates Inflammation, Angiogenesis, and Tissue Fibrosis in Polyether-Polyurethane Synthetic Implants, Mediators of Inflammation, 2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FMV, Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors, Nature Medicine, 21 (2015) 786–794. [DOI] [PubMed] [Google Scholar]
- [35].Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, Ji H, Tse H, Fu Q, Lian Q, Mesenchymal stem cells and immunomodulation: current status and future prospects, Cell Death and Disease, 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DelaRosa O, Lombardo E, Beraza A, Mancheno-Corvo P, Ramirez C, Menta R, Rico L, Camarillo E, Garcia L, Abad JL, Trigueros C, Delgado M, Buscher D, Requirement of IFN-γ-Mediated Indoleamine 2,3-Dioxygenase Expression in the Modulation of Lymphocyte Proliferation by Human Adipose-Derived Stem Cells, Tissue Engineering Part A, 15 (2009). [DOI] [PubMed] [Google Scholar]
- [37].Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N, A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1, Cell Research, 18 (2008) 846–857. [DOI] [PubMed] [Google Scholar]
- [38].Pol chert D, Sobinsky J, Douglas GW, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A, IFN- γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease, European Journal of Immunology, 38 (2008)1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang J, Liu D, Guo B, Yang X, Chen X, Zhu X, Fan Y, Zhang X, Role of biphasic calcium phosphate ceramic-mediated secretion of signaling molecules by macrophages in migration and osteoblastic differentiation of MSCs, Acta Biomaterialia, 51 (2017) 447–460. [DOI] [PubMed] [Google Scholar]
- [40].Lu LY, Loi F, Nathan K, Lin T, Pajarinen J, Gibon E, Nabeshima A, Cordova L, Jamsen E, Yao Z, Goodman SB, Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway, Journal of Orthopaedic Research, 35 (2017) 2378–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Aplin AC, Ligresti G, Fogel E, Zorzi P, Smith K, Nicosia RF, Regulation of angiogenesis, mural cell recruitment and adventitial macrophage behavior by Toll-like receptors, Angiogenesis, 17 (2014) 147–161. [DOI] [PubMed] [Google Scholar]
- [42].Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MPJ, Donners MMPC, Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo, Angiogenesis, 17 (2014) 109–118. [DOI] [PubMed] [Google Scholar]
- [43].Tattersall IW, Du J, Cong Z, Cho BS, Klein AM, Dieck CL, Chaudhri RA, Cuervo H, Herts JH, Kitaj ewski J, In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment, Angiogenesis, 19 (2016) 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P, Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression, The EMBO Journal, 37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B, Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis, Journal of Experimental Medicine, 204 (2007) 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA, Differential Roles of Macrophages in Diverse Phases of Skin Repair, The Journal of Immunology, 184 (2010) 3964–3977. [DOI] [PubMed] [Google Scholar]
- [47].Sandberg OH, Tatting L, Bernhardsson ME, Aspenberg P, Temporal role of macrophages in cancellous bone healing, Bone, 101 (2017) 129–133. [DOI] [PubMed] [Google Scholar]
- [48].Cai J, Feng J, Liu K, Zhou S, Lu F, Early Macrophage Infiltration Improves Fat Graft Survival by Inducing Angiogenesis and Hematopoietic Stem Cell Recruitment, Plastic and Reconstructive Surgery, 141 (2018) 376–386. [DOI] [PubMed] [Google Scholar]
- [49].Hsieh PL, Rybalko V, Baker AB, Suggs LJ, Farrar RP, Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia, Journal of Vascular Surgery, 67 (2018) 1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yadav A, Saini V, Arora S, MCP-1: Chemoattractant with a role beyond immunity: A review, Clinica Chimica Acta, 411 (2010) 1570–1579. [DOI] [PubMed] [Google Scholar]
- [51].Hoh BL, Fazal HZ, Hourani S, Li M, Lin L, Hosaka K, Temporal cascade of inflammatory cytokines and cell-type populations in monocyte chemotactic protein-1 (MCP-1)-mediated aneurysm healing, Journal of NeuroInterventional Surgery, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hoh BL, Hosaka K, Downes DP, Nowicki KW, Fernandez CE, Batich CD, Scott EW, Monocyte Chemotactic Protein-1 Promotes Inflammatory Vascular Repair of Murine Carotid Aneurysms via a Macrophage Inflammatory Protein-1α and Macrophage Inflammatory Protein-2-Dependent Pathway, Circulation, 124 (2011) 2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S, Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines, British Journal of Dermatology, 162 (2010) 478–486. [DOI] [PubMed] [Google Scholar]
- [54].Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Qin S, DiPietro LA, Zloza A, Zhang C, Shafikhani SH, Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response, PLOS One, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Leal EC, Carvalho E, Tellechea A, Kafanas A, Tecilazich F, Kearney C, Kuchibhotla S, Auster ME, Kokkotou E, Mooney DJ, LoGerfo FW, Pradhan-Nabzdyk L, Veves A, Substance P Promotes Wound Healing in Diabetes by Modulating Inflammation and Macrophage Phenotype, The American Journal of Pathology, 185 (2015) 1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gu X, Shen S, Huang C, Liu Y, Chen Y, Luo L, Zeng Y, Wang A, Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes, Diabetes Research and Clinical Practice, 102 (2013) 53–59. [DOI] [PubMed] [Google Scholar]
- [57].Frenkel O, Shani E, Ben-Bassat I, Brok-Simoni F, Rozenfeld-Granot G, Kajakaro G, Rechavi G, Amariglio N, Shinar E, Danon D, Activated macrophages for treating skin ulceration: gene expression in human monocytes after hypo-osmotic shock, Clinical & Experimental Immunology, 128 (2002) 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zuloff-Shani A, Adunsky A, Even-Zahav A, Semo H, Orenstein A, Tamir J, Regev E, Shinar E, Danon D, Hard to heal pressure ulcers (stage III-IV): Efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial, Archives of Gerontology and Geriatrics, 51 (2010) 268–272. [DOI] [PubMed] [Google Scholar]
- [59].Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP, Chazaud B, Butler-Browne G, Savino W, Mouly V, Riederer I, Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation, Molecular Therapy, 20 (2012) 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Melton DW, McManus LM, Gelfond JAL, Shireman PK, Temporal phenotypic features distinguish polarized macrophages in vitro, Autoimmunity, 48 (2015) 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cho M, Hunt TK, Hussain MZ, Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release, American Journal of Physiology Heart and Circulatory Physiology, 280 (2001) H2357–H2363. [DOI] [PubMed] [Google Scholar]
- [62].Xiong M, Elson G, Legarda D, Leibovich SJ, Production of Vascular Endothelial Growth Factor by Murine Macrophages: Regulation by Hypoxia, Lactate, and the Inducible Nitric Oxide Synthase Pathway, The American Journal of Pathology, 153 (1998) 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu CD, Wheeler DS, Chen K, Nayak NR, VEGF may contribute to macrophage recruitment and M2 polarization in the decidua, PLOS One, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MP, van der Hulst RR, Xanthoulea S, Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses, PLoS One, 9 (2014) el02994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Duan J, Liu X, Wang H, Guo SW, The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice, Reprod Biomed Online, 37 (2018) 254–268. [DOI] [PubMed] [Google Scholar]
- [66].Melton DW, Lei X, Gelfond JA, Shireman PK, Dynamic macrophage polarization-specific miRNA patterns reveal increased soluble VEGF receptor 1 by miR-125a-5p inhibition, Physiol Genomics, 48 (2016) 345–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Saito T, Takeda N, Amiya E, Nakao T, Abe H, Semba H, Soma K, Koyama K, Hosoya Y, Imai Y, Isagawa T, Watanabe M, Manabe I, Komuro I, Nagai R, Maemura K, VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing, FEBS Lett, 587 (2013) 2179–2185. [DOI] [PubMed] [Google Scholar]
- [68].Yamaguchi S, Iwata K, Shibuya M, Soluble Flt-1 (soluble VEGFR-1), a potent natural antiangiogenic molecule in mammals, is phylogenetically conserved in avians, Biochem Biophys Res Commun, 291 (2002) 554–559. [DOI] [PubMed] [Google Scholar]
- [69].Roeckl W, Hecht D, Sztajer H, Waltenberger J, Yayon A, Weich HA, Differential binding characteristics and cellular inhibition by soluble VEGF receptors 1 and 2, Exp Cell Res, 241 (1998) 161–170. [DOI] [PubMed] [Google Scholar]
- [70].Chappell JC, Taylor SM, Ferrara N, Bautch VL, Local guidance of emerging vessel sprouts requires soluble Flt-1, Dev Cell, 17 (2009) 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gianni-Barrera R, Burger M, Wolff T, Heberer M, Schaefer DJ, Gurke L, Mujagic E, Banfi A, Long-term safety and stability of angiogenesis induced by balanced single-vector coexpression of PDGF-BB and VEGF 164 in skeletal muscle, Scientific Reports, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].B. A Gianni-Barrera R, Uccelli A, Certelli A, Valente P, Bartolomeo M, Groppa E, Burger MG, Hlushchuk R, Heberer M, Schaefer DJ, Gurke L, Djonov V, Vollmar B, Banfi A, PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation, Angiogenesis, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Warded E, Brodin LA, Mooney DJ, Sylven C, Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction, Cardiovascular Research, 75 (2007) 178–185. [DOI] [PubMed] [Google Scholar]
- [74].Freeman F, Cohen S, The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization, Biomaterials, 30 (2009) 2122–2131. [DOI] [PubMed] [Google Scholar]
- [75].De la Riva B, Sanchez E, Hernandez A, Reyes R, Tamimi F, Lopez-Cabarcos E, Delgado A, Evora C, Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration, Journal of Controlled Release, 143 (2010) 45–52. [DOI] [PubMed] [Google Scholar]
- [76].Sun Q, Silva EA, Wang A, Fritton JC, Mooney DJ, Schaffler MB, Grossman PM, Rajagopalan S, Sustained Release of Multiple Growth Factors from Injectable Polymeric System as a Novel Therapeutic Approach Towards Angiogenesis, Pharmaceutical Research, 27 (2010) 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhang H, Jia X, Han F, Zhao J, Zhao Y, Fan Y, Yuan X, Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration, Biomaterials, 34 (2013)2202–2212. [DOI] [PubMed] [Google Scholar]
- [78].Virchenko O, Skoglund B, Aspenberg P, Parecoxib Impairs Early Tendon Repair but Improves Later Remodeling, The American Journal of Sports Medicine, 32 (2004) 1743–1747. [DOI] [PubMed] [Google Scholar]
- [79].Blomgran P, Hammerman M, Aspenberg P, Systemic corticosteroids improve tendon healing when given after the early inflammatory phase, Sci Rep, 7 (2017) 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O, Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization, Stroke, 46 (2015) 520–528. [DOI] [PubMed] [Google Scholar]
- [81].Raimondo TM, Mooney DJ, Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice, Proceedings of the National Academy of Sciences, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shireman PK, Contreras-Shannon V, Reyes-Reyna SM, Robinson SC, McManus LM, MCP-1 parallels inflammatory and regenerative responses in ischemic muscle, J Surg Res, 134 (2006) 145–157. [DOI] [PubMed] [Google Scholar]
- [83].Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS, Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development, J Am Coll Cardiol, 43 (2004) 474–482. [DOI] [PubMed] [Google Scholar]
- [84].Troidl C, Jung G, Troidl K, Hoffmann J, Mollmann H, Nef H, Schaper W, Hamm CW, Schmitz-Rixen T, The Temporal and Spatial Distribution of Macrophage Subpopulations During Arteriogenesis, Current Vascular Pharmacology, 11 (2013) 5–12. [PubMed] [Google Scholar]
- [85].Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, Lopez-Vales R, IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury, Glia, 64 (2016) 2079–2092. [DOI] [PubMed] [Google Scholar]
- [86].Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV, Effect of modulating macrophage phenotype on peripheral nerve repair, Biomaterials, 33 (2012) 8793–8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].DAlessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, Fallica J, Tripathi A, Mandke P, Gans JH, Limjunyawong N, Sidhaye VK, Heller NM, Mitzner W, King LS, Aggarwal NR, Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming, Am J Physiol Lung Cell Mol Physiol, 310 (2016) L733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yu T, Wang W, Nassiri S, Kwan T, Dang C, Liu W, Spiller KL, Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels, Journal of Biomaterials Science, Polymer Edition, 27 (2016) 721–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, Rolfe BE, Transcriptional switching in macrophages associated with the peritoneal foreign body response, Immunology & Cell Biology, 92 (2014) 518–526. [DOI] [PubMed] [Google Scholar]
- [90].Benjamin LE, Hemo I, Keshet E, A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF, Development, 125 (1998) 1591–1598. [DOI] [PubMed] [Google Scholar]
- [91].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Polymeric system for dual growth factor delivery, Nature Biotechnology, 19 (2001) 1029–1034. [DOI] [PubMed] [Google Scholar]
- [92].Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ, Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors, Biomaterials, 34 (2013) 9201–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D'Souza RN, Hartgerink JD, Self-assembling multidomain peptides tailor biological responses through biphasic release, Biomaterials, 52 (2015) 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kim YH, Tabata Y, Enhancement of wound closure by modifying dual release patterns of stromal-derived cell factor-1 and a macrophage recruitment agent from gelatin hydrogels, Journal of Tissue Engineering and Regenerative Medicine, 11 (2017). [DOI] [PubMed] [Google Scholar]
- [95].Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E, Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis, PNAS, 110 (2013) 13785–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Reeves ARD, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL, Controlled release of cytokines using silk-biomaterials for macrophage polarization, Biomaterials, 73 (2015) 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen J, Li M, Yang C, Yin X, Duan K, Wang J, Feng B, Macrophage phenotype switch by sequential action of immunomodulatory cytokines from hydrogel layers on titania nanotubes, Colloids and Surfaces B: Biointerfaces, 163 (2018) 336–345. [DOI] [PubMed] [Google Scholar]
- [98].Gao L, Li M, Yin L, Zhao C, Chen J, Zhou J, Duan K, Feng B, Dual-inflammatory cytokines on Ti02 nanotube-coated surfaces used for regulating macrophage polarization in bone implants, Journal of Biomedical Materials Research Part A, 106 (2018) 1878–1886. [DOI] [PubMed] [Google Scholar]
- [99].Tolouei AE, Dulger N, Ghatee R, Kennedy S, A Magnetically Responsive Biomaterial System for Flexibly Regulating the Duration between Pro-and Anti-Inflammatory Cytokine Deliveries, Advanced Healthcare Materials, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Alhamdi JR, Peng T, Al-Naggar IM, Hawley KL, Spiller KL, Kuhn LT, Controlled M1-to-M2 transition of aged macrophages by calcium phosphate coatings, Biomaterials, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Li T, Peng M, Yang Z, Zhou X, Deng Y, Jiang C, Xiao M, Wang J, 3D-printed IFN-γ-loading calcium silicate-P-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone, Acta Biomaterialia, 71 (2018) 96–107. [DOI] [PubMed] [Google Scholar]
- [102].Smith TD, Tse MJ, Read EL, Liu WF, Regulation of macrophage polarization and plasticity by complex activation signals, Integrative Biology, 8 (2016) 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gibon E, Loi F, Cordova LA, Pajarinen J, Lin T, Lu L, Nabeshima A, Yao Z, Goodman SB, Aging Affects Bone Marrow Macrophage Polarization: Relevance to Bone Healing, Regenerative Engineering and Translational Medicine, 2 (2016) 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Swift ME, Bums AL, Gray KL, DiPietro LA, Age-Related Alterations in the Inflammatory Response to Dermal Injury, Journal of Investigative Dermatology, 117 (2001) 1027–1035. [DOI] [PubMed] [Google Scholar]
- [105].Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA, Macrophage hypo-responsiveness to interferon-γ in aged mice is associated with impaired signaling through Jak-STAT, Mechanisms of Ageing and Development, 125 (2004) 137–143. [DOI] [PubMed] [Google Scholar]
- [106].Zizzo G, Hilliard BA, Monestier M, Cohen PL, Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction, The Journal of Immunology, 189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC, Hemoglobin Scavenger Receptor CD163 Mediates Interleukin-10 Release and Heme Oxygenase-1 Synthesis, Circulation Research, 94 (2004) 119–126. [DOI] [PubMed] [Google Scholar]
- [108].Mosser DM, The many faces of macrophage activation, Journal of Leukocyte Biology, 73 (2003)209–212. [DOI] [PubMed] [Google Scholar]
- [109].Kluth DC, Ainslie CV, Pearce WP, Finlay S, Clarke D, Anegon I, Rees AJ, Macrophages Transfected with Adenovirus to Express IL-4 Reduce Inflammation in Experimental Glomerulonephritis, The Journal of Immunology, 166 (2001) 4728–4736. [DOI] [PubMed] [Google Scholar]
- [110].Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC, Inhibition of Macrophage Nuclear Factor-κB Leads to a Dominant Anti-Inflammatory Phenotype that Attenuates Glomerular Inflammation in Vivo, The American Journal of Pathology, 167 (2005) 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhu S, Lee DA, Li S, IL-12 and IL-27 Sequential Gene Therapy via Intramuscular Electroporation Delivery for Eliminating Distal Aggressive Tumors, The Journal of Immunology, 184 (2010) 2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]