Abstract
Multiple genes have been identified to cause hereditary predispositions to hematologic malignancies, characterized by an increased risk to develop myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), and/or aplastic anemia (AA). Referral algorithms for patients who may be at higher risk have been proposed, with limited data regarding applicability. Our study aimed to evaluate referral criteria on a population of MDS/AML/AA patients. Demographic information and medical history were obtained from 608 patients referred over a 9-month period. Median age at diagnosis was 67 years (56–73), 387 (64%) were male and the majority of individuals (54.9%) had AML. Overall, 406 individuals (66.8%) had insufficient documentation to determine whether certain criteria were met. Two hundred and two (33.2%) individuals met at least one criteria for genetic counseling referral; however, only nine (4.5%) were referred. Increased documentation of personal and family history is necessary to better assess and validate the applicability of these criteria.
Keywords: hereditary hematologic malignancies, clinical detection algorithm, identifying at risk individuals, leukemia, cancer genetics risk assessment
Introduction
Leukemia is one of the top 10 most common malignancies in the United States with an estimated 60,300 new cases diagnosed in 2018 [1]. Approximately 30,000 of those individuals are diagnosed with myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) and aplastic anemia (AA) [2]. Over the past decade, clinical investigations into families with multiple relatives with leukemia have identified more than a dozen genes related to inherited predispositions to MDS, andAML, [3,4]. Approximately 5–10% of MDS, AML, and AA are related to newly-described hereditary predisposition syndromes [3,5–7]. To date, genes associated with inherited MDS/AML syndromes include, CEBPA, DDX41, ETV6, GATA2, RUNX1, SRP72 and TP53. Additional predisposition genes to MDS/AML relate to underlying inherited bone marrow failure syndromes (IBMFS), including Fanconi anemia (FA) caused by mutations in the Fanconi complementation groups (FANCA, BRCA2, PALB2, etc.) and dyskeratosis congenita (DC) caused by mutations in telomere maintenance genes (TERT, TERC, etc). Together, these syndromes are characterized by a significantly increased risk to develop MDS/AML/AA (30–100% lifetime risk) at younger ages (4–40 years on average), with variable phenotypic and laboratory characteristics including peripheral cytopenias, immune dysfunction, skeletal defects, and clinical bleeding in both adults and children [8,9]. Additional associated phenotypes include primary lymphedema, deafness, cutaneous warts, low CD4/CD8 T cell ratio and mycobacterial infections [10–12], which can be seen in patients with germline GATA2 mutations.
Bone marrow failure syndromes FA and DC have more variable phenotypic expressivity than previously described, particularly in adults, with 25–40% of individuals with FA and 10–25% of individuals with DC having no physical characteristics suggestive of these conditions [13,14]. This variability makes IBMFS difficult to diagnose in individuals who do not display the characteristic phenotype. It is also important to note that next-generation sequencing (NGS) is increasingly utilized for prognostication and management of individuals with newly diagnosed hematologic malignancies. However, such somatic panels are often limited by hot spot mutation testing and do not differentiate between somatic and germline attribution. NGS panels designed for germline mutation detection include full sequencing of the genes of interest and copy number variations. Testing for inherited germline mutations must be performed on germline DNA, most oftenextracted from cultured skin fibroblasts. Accurately differentiating somatic versus germline mutations is of upmost importance as timely diagnosis is critical for treatment of MDS/AML/AA. Individualswith IBMFS and certain familial AML/MDS syndromes have potentially poorer outcomes from hematopoietic stem cell transplantation (HSCT) due to use of a related donor with the same germline mutation and/or increased toxicity from standard transplant conditioning regimens [15–17]. With the identification of these predisposition genes, researchers have sought to understand how frequently these germline mutations occur in patients with leukemia. DiNardo et al. identified germline mutations associated with a hereditary cancer syndrome in 18% of patients with hematologic malignancies referred for genetic testing [18]. Another study by Churpek et al. in 2016 illustrated a similar frequency of germline mutations (11–24%) in patients with hematologic malignancies referred for genetic testing [5]. Notably, germline mutations in hematologic malignancies appear to show similar prevalence to germline mutations in solid tumors such as breast and colon cancers for which genetic risk assessment has become standard of care.
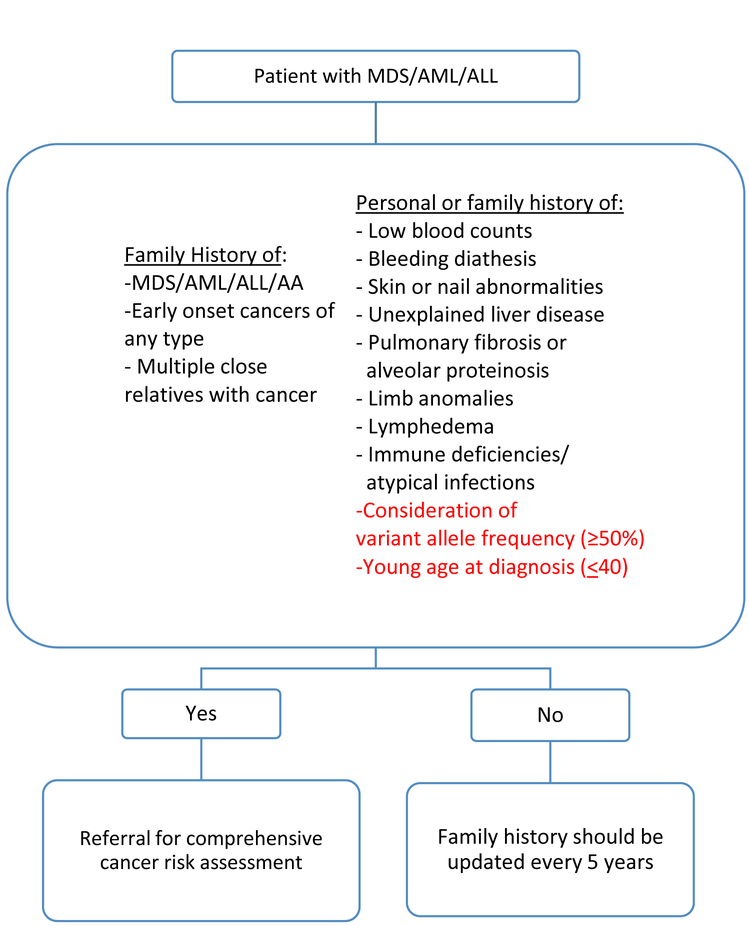
In 2013, Churpek et al. proposed clinical detection and management criteria for patients and family members with familial MDS/AML/AA predisposition syndromes (Figure 1). These criteria assess personal or family history of cytopenias, the presence of skin, nail, or limb abnormalities, unexplained liver disease, pulmonary fibrosis/alveolar proteinosis, lymphedema and/or immune deficiencies/atypical infections. Additionally, the algorithm assesses family history of acute lymphocytic leukemia (ALL)/MDS/AML/AA and other malignancies. Individuals meeting any of these criteria warrant referral to genetic counseling for comprehensive cancer risk assessment [8].
Figure 1.
Proposed algorithm and additional considerations for identifying individuals who warrant comprehensive cancer risk assessment
To the best of our knowledge, no studies have sought to evaluate the clinical applicability of these criteria to an unselected adult population of leukemia patients. We aim to evaluate the proposed algorithm for genetic counseling referral against a large unselected cohort of patients with MDS/AML/AA to determine if the characteristics needed to identify these patients for genetic counseling and risk assessment were reported in the medical record, and whether patients with these identified risk factors were referred for genetic testing. Additionally, this assessment may generate preliminary data regarding whether additional patient/tumor characteristics could enhance recommended referral guidelines for individuals with hematologic malignancies who may benefit from a cancer genetics evaluation. Identifying these individuals is of utmost importance for personalized treatment strategies to maximize positive outcomes and minimize toxicity.
Materials and Methods
Patient Selection
We identified 613 individuals seen as new patients in the Department of Leukemia at The University of Texas M.D. Anderson Cancer Center between March 1, 2014 to December 31, 2014 with a diagnosis of MDS, AML, or AA. This timeline also coincides with the beginning of the Hereditary Hematologic Malignancies clinic (HHMC). Five individuals were excluded either due to age (<18 years old), no confirmed leukemia diagnosis, or no medical records available for review, with 608 individuals remaining for analysis. Medical history, laboratory and pathology results were obtained from a prospectively-maintained clinical database within the Department of Leukemia. The presence of personal and family history, genetic testing performed, and referral criteria from the Churpek et al. algorithm was assessed by chart review within the electronic medical record (EMR). A 28-gene myeloid prognostication panel was performed for all 608 patients, which included the mutation status of CEBPA, GATA2 and RUNX1, three genes which can be mutated in the germline leading to inherited myeloid predisposition syndromes. Germline versus somatic mutation status for this panel is not possible as no normal control sample was obtained. To ensure adequate data capture of all applicable personal and medical history, chart review procedure included assessment of at least the first and last five leukemia department providers’ notes, and any leukemia department providers’ history and physical notes (H&P).
Statistical Analyses
Patient demographics, clinicopathologic characteristics, and personal and family history variables were analyzed with descriptive statistics Statistically significant differences between groups were determined using the chi-squared test and Fisher’s exact test for categorical variables, Wilcoxon rank sum test for continuous variables and nonparametric test for trend for ordered variables. All analyses were performed in STATA (v.13.1, College Station, TX). P-value <0.05 was set for statistical significance. This study was approved by the Institutional Review Board of The University of Texas M.D. Anderson Cancer Center and by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston. A waiver of informed consent was obtained.
Results
Demographics
Clinical data and demographic information from 608 individuals diagnosed with AML/MDS/AA seen at our institution from March 1, 2014-December 31, 2014 are presented in Table 1. The median age at diagnosis was 67 years, 387 (64%) were male, and at the time of data collection, 315 (51.81%) individuals were still living. The majority of individuals (78.6%) were non-Hispanic white. The majority of patients had AML (n=334, 54.9%), and 278 (45.7%) individuals had intermediate cytogenetics.
Table 1.
Patient Demographics (n=608)
| Clinical Characteristics | n (%) |
|---|---|
| Median (IQR), age at diagnosis, years | 67 (56–73) |
| Male sex | 387 (64) |
| Vital status, living | 315 (51.8) |
| Race/Ethnicity | |
| White | 478 (78.6) |
| Hispanic | 47 (7.7) |
| Black | 38 (6.3) |
| Unknown | 22 (3.6) |
| Asian | 18 (3) |
| Other | 5 (0.8) |
| Leukemia Diagnosis | |
| AML | 334 (54.9) |
| MDS | 199 (32.7) |
| MDS/MPD | 59 (9.7) |
| AA | 16 (2.7) |
| Personal History of Cancer | |
| None | 410 (67.4) |
| Prostate | 37 (6.1) |
| Breast | 32 (5.3) |
| Basal cell carcinoma | 32 (5.3) |
| Lymphoma | 29 (4.7) |
| Colon | 12 (2.0) |
| Other | 56 (9.2) |
| Family History of Cancer* | |
| ≥1 FDR | 304 (50%) |
| ≥1 SDR) | 121 (19.9%) |
| None | 183 (30.1%) |
IQR: Inter Quartile Range
AML: Acute Myeloid Leukemia; MDS: Myelodysplastic syndrome; MPD: Myeloproliferative disorder;
AA: Aplastic anemia
FDR: First-degree relative; SDR: Second-degree relative
Family history of cancer does not include family history of hematologic malignancies
One hundred and ninety-eight (32.6%) individuals had a personal history of an additional primary cancer prior to their leukemia diagnosis. Breast cancer, prostate cancer, and basal cell carcinoma of the skin were the three most common, comprising 51.1% of reported prior primary malignancies.
Criteria documentation and cancer genetics referral
We evaluated the presence, absence, or unreported documentation of each of the referral criteria proposed by Churpek et al. among the 608 patients [Table 2]. Personal history of blood count abnormalities were unreported ranging from 54.3% (n=300) for anemia to 79.2% (n=482) for neutropenia. Physical characteristics (skin/nail abnormalities, unexplained liver disease, pulmonary fibrosis/alveolar proteinosis, limb anomalies, lymphedema, immune deficiencies/atypical infections) had the highest unreported rate. Family history of leukemia was consistently reported, with 95.4% (n=580) of individuals having the presence or absence of a family history of leukemia documented in their medical record.
Table 2.
Documentation of the evaluated personal and family history criteria (n=608)
| Present n (%) |
Absent n (%) |
Unreported n (%) |
|
|---|---|---|---|
| Personal History Criteria | |||
| Skin/nail abnormalities | 39 (6.4) | 524 (86.2) | 45 (7.4) |
| Anemia | 19 (3.1) | 259 (42.6) | 330 (54.3) |
| Thrombocytopenia | 23 (3.8) | 235 (38.7) | 350 (57.5) |
| Neutropenia | 2 (0.3) | 124 (20.4) | 482 (79.3) |
| Limb anomalies | 7 (1.2) | 70 (11.5) | 531 (87.3) |
| Pulmonary fibrosis/ alveolar proteinosis | 22 (3.6) | 7 (1.2) | 579 (95.2) |
| Unexplained liver disease | 11 (1.8) | 10 (1.7) | 587 (96.5) |
| Lymphedema | 10 (1.6) | 2 (0.3) | 596 (98.1) |
| Immune deficiencies/atypical infections | 5 (0.8) | 3 (0.5) | 600 (98.7) |
| Family History Criteria | |||
| Leukemia | 31 (5.1) | 549 (90.3) | 28 (4.6) |
| Cancer | 364 (59.9) | 216 (35.5) | 28 (4.6) |
| Other Cytopenias | 11 (1.8) | 10 (1.6) | 587 (96.6) |
| Thrombocytopenia | 7 (1.2) | 7 (1.2) | 594 (97.6) |
Family history of MDS/AML/AA
Thirty-one (16.2%) individuals who met referral criteria reported a family history of MDS/AML/AA or acute lymphoblastic leukemia (ALL) [Table 3]. Two individuals reported two first-degree relatives (FDRs) with leukemia, and two individuals reported two second-degree relatives (SDRs) with leukemia, totaling 34 family members with reported hematologic malignancies. The majority of reported family members with leukemia did not have a specified subtype of leukemia documented. Of those specified, five (16.1%) had a first or second-degree relative with AML, four (12.9%) with MDS and two with AA (6.5%).
Table 3.
Family History of Leukemia (n=31)
| Cancer Family History | FDR (n=17) n, % |
SDR (n=17) n, % |
|---|---|---|
| AML | 2 (6.5) | 3 (9.7) |
| MDS | 3 (9.7) | 1 (3.2) |
| ALL | 0 (0) | 1 (3.2) |
| AA | 1 (3.2) | 1 (3.2) |
| Unknown leukemia | 11 (35.5) | 11 (35.5) |
FDR, first-degree relative; SDR, second degree relative
AML: Acute Myeloid Leukemia; MDS: Myelodysplastic syndrome; MPD: Myeloproliferative disorder;
AA: Aplastic anemia
Comparison between individuals who met criteria and those who could not be determined
Overall, 406 (66.8%) individuals had insufficient documentation to determine whether any criteria were met. Two hundred and two (33.2%) individuals met at least one of the proposed criteria for genetic counseling referral. Due to insufficient documentation, it was not possible to classify any individuals as not meeting criteria [Table 4]. There were no significant differences between age at diagnosis or sex and whether they met criteria (p=0.407, p=0.655). However, prior history of breast cancer was associated with whether an individual met criteria for cancer genetics referral (p=0.006). For this study, those who met criteria solely based on their personal history of breast cancer were diagnosed with breast cancer before age 45 as this meets National Comprehensive Cancer Network (NCCN©) Guidelines for genetic risk assessment.
Table 4.
Comparison between patients who met criteria and could not be determined
| Characteristics | Met Criteria (n=202) n (%) |
Could not be Determined (n=406) n (%) |
p-value |
|---|---|---|---|
| Median (IQR) age at diagnosis, years | 66 (56–73) | 67 (56–73) | 0.407 |
| Male sex | 126 (62.4) | 261 (64.3) | 0.655 |
| Race | 0.475 | ||
| White/Caucasian | 163 (80.7) | 315 (77.6) | |
| Unknown | 21 (10.4) | 48 (11.8) | |
| Black | 12 (5.9) | 26 (6.4) | |
| Asian | 6 (3) | 12 (3) | |
| Middle Eastern | 0 (0) | 5 (1.2) | |
| Diagnosis | 0.250 | ||
| AML | 100 (49.5) | 234 (57.6) | |
| MDS | 76 (37.6) | 123 (30.3) | |
| MDS/MPD | 20 (9.9) | 39 (9.6) | |
| AA | 6 (3) | 10 (2.5) | |
| Personal History of Cancer | |||
| Breast | 18 (8.9) | 14 (3.4) | 0.006 |
| Prostate | 11(5.4) | 26 (6.4) | 0.721 |
| Lymphoma | 11(5.4) | 18 (4.4) | 0.553 |
| Basal Cell Carcinoma | 10 (5.0) | 22 (5.4) | 1.00 |
| Other | 27 (13.4) | 41 (10.1) | |
| None | 125 (61.9) | 285 (70.3) | |
| Family History of Cancer | |||
| BREAST | |||
| FDR | 30 | 39 | 0.058 |
| SDR | 13 | 19 | 0.441 |
| LUNG | |||
| FDR | 25 | 35 | 0.151 |
| SDR | 16 | 17 | 0.060 |
| PROSTATE | |||
| FDR | 24 | 27 | 0.042 |
| SDR | 5 | 8 | 0.768 |
| COLON | |||
| FDR | 18 | 19 | 0.048 |
| SDR | 7 | 15 | 1.000 |
IQR: Inter Quartile Range
AML: Acute Myeloid Leukemia; MDS: Myelodysplastic syndrome; MPD: Myeloproliferative disorder;
AA: Aplastic anemia
FDR: First-degree relative; SDR: Second-degree relative
Family history of cancer does not include family history of hematologic malignancies
Three hundred and four (50%) individuals had at least one documented first-degree relative (FDR) with cancer. One hundred and twenty-one individuals (19.9%) had at least one second-degree relative with cancer documented in their medical record [Table 4]. This included any FDR/SDR with cancer, including leukemia. Age at diagnosis was not available for all FDR and SDR cancer diagnoses. Individuals that had a first- and second-degree relative with the same malignancy were included in both calculations. The most commonly documented malignancies among FDRs were breast, lung, and prostate cancers, comprising 41.5% of total malignancies reported among FDRs (n=180). Additionally, having a FDR with prostate, colon, or ovarian cancer was a significant factor in whether or not an individual met criteria (p=0.042, p=0.048, p=0.000). The most commonly reported malignancies among SDRs were breast, lung, and colon cancers, comprising 50.9% of total malignancies reported in SDRs (n=87).
Referral to genetic counseling
Of 202 individuals that met ≥1 criteria for cancer genetics referral per the proposed algorithm, 166 (82.2%) met one criterium, 30 (14.8%) met two, five (2.5%) met three, and one patient (0.5%) met four criteria [Table 5]. Only nine patients (4.5%) who met any criteria received a referral for cancer genetics risk assessment. The difference between number of criteria met and the likelihood for subsequent genetics referral was statistically significant (p=0.002) with those meeting more criteria actually being less likely to have received a referral.
Table 5.
Referral criteria and subsequent referral to genetic counseling (n=202)
| Number of criteria met | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Referred to genetics (n=9) | 4 (2.4) | 4 (13.3) | 1 (20) | 0 (0) |
| Not referred to genetics (n=193) | 162 (97.6) | 26 (86.7) | 4 (80) | 1 (100) |
p=0.002
Additional Criteria
In addition to the criteria proposed by Churpek et al., we assessed the mutational profile in these MDS/AML/AA patients. Analysis of somatic mutation status revealed 29 (4.8%) individuals with ≥2 somatic mutations in one of three genes associated with hereditary hematologic malignancies, CEBPA, RUNX1 and GATA2. Of 10 individuals with ≥2 CEBPA mutations, four met the proposed Churpek et al. criteria. One patient with biallelic GATA2 mutations met two criteria. Of the 18 patients with ≥2 RUNX1 mutations, five met one of the referral criteria. We also assessed young age at MDS/AML/AA diagnosis, specifically individuals diagnosed prior to age 40. In our cohort, 66 (10.85%) individuals were diagnosed ≤40 years. Of those, only 17 (25.75%) met at least one of the Churpek criteria.
Discussion
Our study aimed to evaluate the recommended criteria for genetic counseling referral on an unselected population of individuals with MDS/AML/AA. We reviewed the medical records of 608 adultsseen at one institution over a 9-month period to evaluate the criteria proposed by Churpek et al. The median age of diagnosis for our cohort (67 years) is consistent with the median age of diagnosis of leukemia in the general population (66 years). Of note, in the two years since these individuals presented to The University of Texas M.D. Anderson Cancer Center, 51.8% were alive at the time of chart review, reflecting the poor long-term survival of MDS and AML in older patients, which have a 5-year survival rate of 26.9% [1]. The high mortality rate within a short time period illustrates the importance of prompt assessment and referral for genetic counseling and testing in this population. Due to insufficient documentation of the majority of the referral criteria, individuals in this cohort met referral criteria based primarily on personal/family history of malignancies. Those with a personal history of breast cancer, or a FDR diagnosed with prostate, colon or ovarian cancer, were more likely to meet criteria. This is consistent with current national guidelines that recommend genetic counseling referrals based on family history of these specific malignancies (i.e. BRCA1 and BRCA2 evaluation) often regardless of age, such as family history of ovarian cancer at any age[19]. Poor documentation of age at diagnosis in FDR/SDRs makes it difficult to assess the “early-onset” aspect of the Churpek criteria. Family history of non-hematologic malignancies are referenced in the Churpek algorithm with respect to early age at diagnosis. Importantly, family history of certain cancers, such as ovarian cancer, should prompt genetics referral regardless of age. Family history of solid tumors should be assessed according to the ASCO and NCCN guidelines, and followed up bycancer genetics evaluation if appropriate [20].
The criteria proposed by Churpek et al. are some of the only published screening guidelines to help identify individuals at increased risk for inherited leukemia predisposition syndromes. National society guidelines, like those from the National Comprehensive Cancer Network (NCCN©), are lacking. The absence of published guidelines and the relative novelty of these hereditary leukemia syndromes likely contributed to the poor documentation of these criteria in the medical record. As illustrated in Table 2, the vast majority of patients in this cohort have multiple missing variables, including personal or family history characteristics such as prior cytopenias or immune deficiencies/atypical infections. This could partially be due to study design wherein information had to be stated unequivocally (i.e. documentation of pertinent negatives) to be considered present or absent from the medical record. Interestingly, the two most frequently reported criteria were family history of leukemia and personal history of skin/nail abnormalities. Family history and physical exam are integral to an initial medical consultation. Additionally, physical exam documentation is a required component of History & Physical medical record documentation and may have contributed to the increased documentation rate of skin/nail abnormalities.
Only 4.5% of patients who met at least one referral criteria received a genetics referral. While not statistically significant, the trends seen in Table 5 illustrate that as the number of criteria increased, so did the percentage of patients referred. The lack of cancer genetics referrals, coupled with the lack of documented personal and family history criteria make it difficult to assess the true applicability of the criteria. This information is critical since understanding germline mutation status has significant treatment and management implications.
One principal researcher reviewed all 608 charts and human error cannot be excluded. Additionally, the data abstraction process included assessment of only the first five and last five leukemia providers’ notes, and H&Ps. It was not possible to review all clinical documents of each patient; thus criteria may have been documented in those charts but not ascertained for this study. It is important to note that over 65% of individuals had insufficient documentation, which further limits the assessment of the proposed criteria. This was especially notable when assessing for family history of leukemia, as over 50% of individuals who reported a FDR or SDR with leukemia did not have a specified subtype of leukemia documented. On the other hand, family history of leukemia, despite unspecified type, was highly documented which is encouraging as it is so pertinent to the evaluation of inherited predisposition. The time interval for data collection coincided with the beginning of the hereditary leukemia clinic. Therefore, it is likely that the presence of this clinic over time has led to better recognition of patients who might benefit from genetic counseling, although whether that would change the documentation practices of leukemia providers is unknown. Additionally, this study was conducted at a singletertiary referral center and may not be representative of patients diagnosed with leukemia across the United States. Additionally the rates of documentation may not be reflective of practices at other institutions. Racial demographics were skewed and not representative of the general population.
In addition to the criteria proposed by Churpek et al., we assessed the somatic mutation profile within this MDS/AML/AA cohort. Current literature suggests that about 7–11% of somatic double mutant CEBPA (dmCEBPA) mutations have a germline mutation [21,22]. This is important when we consider that six individuals in this study with biallelic CEBPA mutations met no additional testing criteria, but warrant evaluation for an inherited susceptibility. Based on previous estimates, it is likely that at least one individual in the cohort had a germline CEBPA mutation. Based on the current, albeit limited data, germline CEBPA mutations are considered nearly 100% penetrant, without signs or prodromal symptoms. We propose that considering ≥2 somatic mutations in CEBPA as an additional referral criterion may be warranted, as it would potentially increase the detection of individuals who otherwise have no additional indications for genetic risk assessment. Additionally, the presence of a variant allele frequency suggestive of germline inheritance in other potentially germline mutations such as GATA2, RUNX1, and DDX41, as well as other genes, is an important question for future analysis regarding h patients at risk for germline mutations [23]. We also assessed young age at MDS/AML/AA diagnosis, as individuals with IBMFS are at significantly increased risk to develop BMF before the age of 40 [16]. Age at diagnosis ≤40 and/or the presence of ≥2 mutations should also be considered as additional criteria when assessing leukemia patients for referral for cancer genetics risk assessment.
Suggestions to increase documentation of criteria in the medical record include integrating criteria assessment into the electronic medical record (EMR), or obtaining information directly from patients via a short intake form/questionnaire.
Future studies may benefit from evaluating the suggested additional criteria including molecular testing results and age of leukemia diagnosis ≤40 years. Further studies are needed to determine which combination of criteria yields the highest association with subsequent positive germline testing results. Improvement of family history data collection by hematologists/oncologists, coupled with studies examining patient attitudes towards genetic testing in this patient population, will likely lead to better referral and clinical evaluation of patients who may benefit from genetic testing. Additionally, national guidelines for referral criteria for inherited leukemia susceptibility syndromes would increase awareness and detection of families with hereditary predispositions to hematologic malignancies.
One-third of individuals met at least one criteria for a cancer genetics referral based on the proposed algorithm, while the remainder could not be determined based on insufficient information present in the medical record. Ultimately, only nine individuals received genetic counseling referrals to the leukemia genetics clinic during the study period. Improving the documentation of the proposed criteria in the medical record will increase identification of those at higherrisk for a hereditary predisposition among leukemia patients. Increasing timely and equitable referral, genetics assessment, and identification of hereditary predisposition to MDS/AML/AA is critical as it affects transplant donor selection, treatment, and anticipatory guidance counseling, and increased screening for at-risk family members.
Acknowledgements
All authors contributed to the design of the research study and construction of manuscript. MC performed the chart review. MC and SB analyzed the data. MC and SH performed the statistical analysis.
Footnotes
Disclosure of Interest
The authors report no conflict of interest
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
References
- 1.2016. April SEER Cancer Statistics Review. National Cancer Institute; <http://seer.cancer.gov/csr/1975_2013/>. Accessed 2016 April [Google Scholar]
- 2.2016. December 1, 2016. Surveillance, Epidemiology, and End Results (SEER). National Cancer Institute; <www.seer.cancer.gov>. December 1, 2016. [Google Scholar]
- 3.Zhang MY, Churpek JE, Keel SB, et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet 2015;47:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churpek JE, Godley LA. How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Walsh MF, Wu G, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med 2015;373:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson AEK, Guidugli L, Arndt K, et al. Identification of Genetic Hereditary Predisposition to Hematologic Malignancies By Clinical Next-Generation Sequencing. Blood 2015;126:3854–3854. [Google Scholar]
- 8.Churpek JE, Lorenz R, Nedumgottil S, et al. Proposal for the clinical detection and management of patients and their family members with familial myelodysplastic syndrome/acute leukemia predisposition syndromes. Leuk Lymphoma 2013;54:28–35. [DOI] [PubMed] [Google Scholar]
- 9.Ganly P, Walker LC, Morris CM. Familial mutations of the transcription factor RUNX1 (AML1, CBFA2) predispose to acute myeloid leukemia. Leuk Lymphoma 2004;45:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 2011;118:2656–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011;118:2653–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 2011;43:929–931. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24:101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alter BP. Diagnosis, Genetics, and Management of Inherited Bone Marrow Failure Syndromes. ASH Education Program Book 2007;2007:29–39. [DOI] [PubMed] [Google Scholar]
- 15.Buijs A, Poddighe P, van Wijk R, et al. A novel CBFA2 single-nucleotide mutation in familial platelet disorder with propensity to develop myeloid malignancies. Blood 2001;98:2856–2858. [DOI] [PubMed] [Google Scholar]
- 16.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet 2003;362:1628–1630. [DOI] [PubMed] [Google Scholar]
- 17.Owen CJ, Toze CL, Koochin A, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood 2008;112:4639–4645. [DOI] [PubMed] [Google Scholar]
- 18.DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of Patients and Families With Concern for Predispositions to Hematologic Malignancies Within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk 2016;16:417–428 e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Network NCC. 2017. Genetic/Familial High Risk Assessment: Breast and Ovarian. Accessed 2017.
- 20.Lu KH, Wood ME, Daniels M, et al. American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol 2014;32:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pabst T, Eyholzer M, Haefliger S, Schardt J, Mueller BU. Somatic CEBPA mutations are a frequent second event in families with germline CEBPA mutations and familial acute myeloid leukemia. J Clin Oncol 2008;26:5088–5093. [DOI] [PubMed] [Google Scholar]
- 22.Taskesen E, Bullinger L, Corbacioglu A, et al. Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood 2011;117:2469–2475. [DOI] [PubMed] [Google Scholar]
- 23.Drazer MW, Kadri S, Sukhanova M, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv 2018;2:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]