Abstract
Background/Aims:
While overall rates of colorectal cancer (CRC) have declined in individuals aged above 50 years of age, this decline has not been seen in younger individuals who do not benefit from current screening guidelines. We sought to describe the prevalence of CRC in adults 20-39 years of age without family history of CRC or Inflammatory Bowel Disease (IBD)- Early Onset CRC (EoCRC), evaluate associated signs and symptoms and medical co-morbidities in EoCRC and compare them with individuals aged 20-39 years without CRC (NoCRC). Our secondary aim was to compare EoCRC with individuals aged 40 years and above with CRC (LoCRC).
Methods:
Utilizing a commercial database (Explorys Inc, Cleveland, OH), we identified a cohort of patients aged 20-39 years with first ever diagnosis of CRC between 2013 and 2018 based on the Systematized Nomenclature of Medicine-Clinical Terms. We calculated the overall prevalence rate of EoCRC, described age, race, and gender-based prevalence rates of EoCRC, and identified associated symptoms and medical co-morbidities associated with EoCRC.
Results:
The overall rate of EoCRC was 18.9/100,000. Compared to NoCRC, EoCRC patients were more likely to be Caucasian and female, with predominant symptoms of hematochezia, anemia, and decreased appetite. EoCRC group had higher prevalence rates of medical comorbidities such as diabetes, smoking, and obesity. Compared to LoCRC, EoCRC group presented more frequently with left sided CRC and rectal cancers.
Conclusion:
This is one of the largest studies to date to describe the epidemiology of EoCRC in US. We found EoCRC to occur predominantly in the Caucasian and female population. EoCRC presented more frequently with left sided and rectal CRC. We also identified signs/symptoms as well as comorbidities associated with EoCRC. Patients with these features may benefit from earlier screening.
Keywords: Colon cancer, early-onset colon cancer, cancer epidemiology, cancer screening
BACKGROUND
Colorectal cancer (CRC) is the third most common cause of cancer incidence and death in both men and women in the United States [1]. In 2018, we expect 140,250 Americans to be newly diagnosed with CRC and 50,630 to die of the disease [1]. CRC has a median age of diagnosis of 70 in men and 72 in females, although there is a wide range in age [2]. It is most commonly associated with smoking, obesity, alcohol use, inflammatory bowel disease (IBD), and cancer syndromes [3],[4]. While overall rates of CRC have declined recently, possibly due to better screening protocols, studies have shown that incidence has increased in the younger population [5]–[6]. Family history of CRC and CRC syndromes accounts for about 20% of young onset CRC, but we are seeing increased incidence in patients without any family history [7],[8]. Because of this new information, the American Cancer Society has now recommended average risk patients begin CRC screening at 45 years[9], whereas previously screening for average risk individuals was recommended at age 50 and earlier screening was only recommended in African Americans at age 45 years.
Patients who have an earlier diagnosis of CRC are distinct from their older counterparts. They tend to have more left sided and rectal cancers [10],[11]. They also tend to have a more aggressive disease with a worse prognosis [8].
In this study, we sought to define patients as Early onset colorectal cancer as adults having the diagnosis of CRC aged 20-39 years of age (EoCRC), while patients who develop it at 40 years and above are referred to as later onset CRC (LoCRC). This is based on prior data that use these age ranges as a cut off for young CRC [12]. We used 20 years old as the lower limit so that the study is applicable to adult providers, and also because CRC is exceedingly rare in average risk individuals younger than 20. In addition, recent Surveillance, Epidemiology, and End Results (SEER) Program data shows that rectal cancer incidence has risen most quickly in the 20-39 year subset (3.2% per year from 1974-2013) [10]. The main aim of the study is to understand the difference between EoCRC and individuals 20-39 years of age without CRC (NoCRC), with a secondary aim understanding the differences between EoCRC and LoCRC. This study is the largest study to our knowledge looking at the epidemiology of colon cancer in EoCRC without family history or IBD. These patients are especially susceptible to being missed by our current screening guidelines and represent a vulnerable population.
METHODS
Database
We performed a retrospective analysis of a large population-based, commercial database (Explorys Inc., Cleveland, OH). Explorys contains electronic health record (EHR) data from 26 major integrated healthcare systems spread over 50 states in the USA from 1999 to 2018. Explorys contains de-identified patient data from participating institutions and uses a health data gateway (HDG) server behind the firewall of each participating healthcare organization that collects de-identified data from various health information systems EHR using billing inquiries. Data are then standardized and normalized by Explorys. As such, diagnoses, findings, and procedures are mapped into the Systematized Nomenclature Of Medicine-Clinical Terms (SNOMED-CT) hierarchy. Each participating healthcare institution has access to Explorys online (password protected), which provides for browsing of the data from all participating healthcare institutions. Explorys data are automatically updated at least once every 24 hours [13]. Explorys is a Health Insurance Portability and Accountability Act (HIPAA) compliant platform, and thus Institutional Review Board (IRB) approval is not required.
It is important to note that this database was used in the past to describe cancer epidemiology. Chouhan et al. [14] published a study in Digestive Diseases and Sciences regarding the prevalence of colorectal cancer in persons 75 years of age and older. Al-Kindi et al. [15] studied the prevalence of preexisting cardiovascular disease in patients with different types of cancer including hematological and solid tumors such as lung, breast, colon, renal, and head and neck cancers, and Panhwar to describe risk of MI in patients with IBD [16].
Patient Selection
Using the Explorys database, we identified and aggregated three cohorts of eligible patients: EoCRC, LoCRC, and NoCRC. CRC patients were defined as those having a first-ever SNOMED-CT diagnosis of “primary malignant neoplasm of the ascending colon (including cecum), transverse colon, descending colon, sigmoid colon, or rectum,” at any point between July 2013 and July 2018. We stratified the patients by demographic “age”, creating groups of patients with ages 20-39 and 40 or greater. We excluded patients with SNOMED-CT diagnoses of “Crohn’s disease,” “Ulcerative Colitis,” and “family history of colorectal cancer” (including patients with familial colorectal cancer syndromes). These patients were excluded because prior studies have shown that inflammatory bowel disease and family history of CRC are known to greatly increase the risk of early onset CRC and does not add to existing knowledge in the field [17]. LoCRC group was comprised of patients aged 40+ who had the CRC diagnosis with the same exclusion criteria. We also identified all individuals in the database aged 20-39 years without a diagnosis of “primary malignant neoplasm of the ascending colon (including cecum), transverse colon, descending colon, sigmoid colon, or rectum,” with the same exclusion criteria, as the NoCRC group. We did a supplementary analysis outside of the primary aim that did include patients with IBD as well as family history of CRC to confirm findings of previous studies.
Associated Medical Findings and Conditions of Interest
There is a large body of literature that describes presenting signs and symptoms that prompt evaluation for CRC. A majority of EoCRC present with an associated presenting symptom [11]. Dozois et al. [8] found that rectal bleeding, altered bowel habits, abdominal pain, weight loss, and anemia were the most common findings in a similar cohort of EoCRC. In addition to these, we also evaluated for constitutional symptoms such as malaise and fatigue in our study.
We identified multiple medical conditions associated with CRC. Inflammatory bowel disease has a well-studied association with EoCRC, which has been reflected in enhanced screening in these patients [18],[19]. The comorbidities that we included for analysis were gender, race (Caucasian vs non-caucasian), tobacco use, diabetes mellitus (type I and II), obesity, family history of non-CRC malignancy, and alcohol abuse. In addition to literature on IBD, many studies have shown associations between these conditions and CRC [20],[21],[3].
Statistical Analysis
In order to calculate the overall rate of occurrence of CRC for each age group, we first determined the total number of individuals with occurrence of CRC active within the last 5 years (July 2013–July 2018) based on age groups (including both males and females). We then calculated the overall prevalence rate of new CRC cases based on age groups. To calculate the prevalence of the condition within the 20-39 years age group, we divided the number of EoCRC patients by the total number of patients from 20-39 years old active within the last five years in the database. To evaluate EoCRC vs NoCRC, we utililized a multivariate analysis with linear regression including these variables: gender, race, diabetes, smoking, obesity, alcohol use, and family history of non-CRC. . Multicollinearity among variables was tested with the Variance Inflation Factor (VIF), with a VIF > 5 indicating presence of multicollinearity for each. Goodness of fit tested by Hosmer and Lemeshow test, with P > 0.05 indicating good fit. To evaluate symptoms and compare secondary aims of EoCRC vs LoCRC we utilized univariate analysis. The odds ratio (OR), its standard error, and the 95% confidence interval (CI) were calculated according to Altman [22] using the MedCalc Statistical Software with a case-control design [23]
RESULTS
A total of 34,606,650 patients were identified as active between July 2013 and July 2018, out of which 8,873,080 were 20-39 years old. Of these patients, we identified 1680 patients who had a first-ever SNOMED-CT diagnosis of CRC without a family history of CRC or diagnosis of IBD, and constituted the EoCRC group, with an overall prevalence of 18.9/100,000 (Figure 1). We identified 8,871,400 patients in the NoCRC group. In addition we identified 92,260 individuals aged ≥40 years with a SNOMED-CT diagnosis of first-ever CRC without family history of CRC or IBD, and this cohort constituted the LoCRC group, with a prevalence of 473/100,000.
Figure 1:
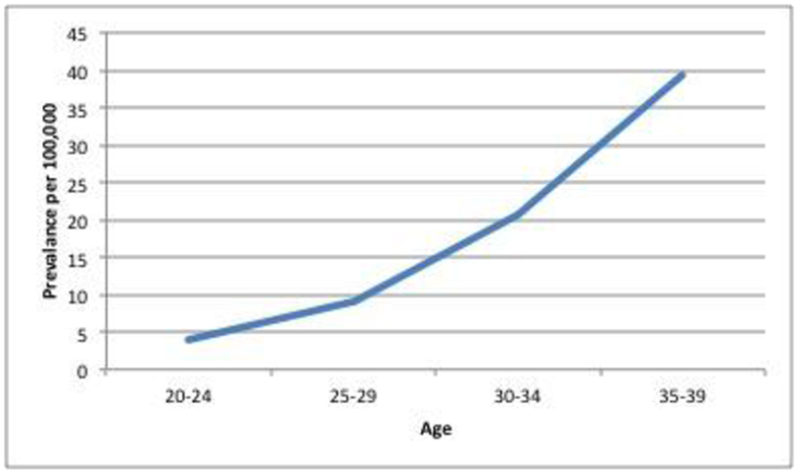
Prevalence of EoCRC as a function of age
EoCRC vs. NoCRC
Out of 29,090,220 patients active in the datebase from July 2013 to July 2018, there were 1,700 patients who fit our definition of EoCRC and 8,904,290 who fit our definition of NoCRC. Their underlying demographic information is displayed in Table 1.
Table 1:
Demographics of EoCRC vs NoCRC
| EOCRC (n,%) | NOCRC (n,%) | OR | 95% CI | Significance | |
|---|---|---|---|---|---|
| Age | |||||
| 20-24 | 80 (5) | 2004560 (23) | |||
| 25-29 | 220 (13) | 2359560 (27) | |||
| 30-34 | 490 (29) | 2299730 (25) | |||
| 35-39 | 910 (54) | 2240430 (23) | |||
| Race | |||||
| Caucasian | 1200 (71) | 5246730 (59) | 1.73 | 1.55-1.92 | p<.0001 |
| African American | 240 (14) | 1178710 (13) | 1.09 | 0.95-1.25 | p=.23 |
| Gender | |||||
| Male | 800 (48) | 3674630 (41) | 1.29 | 1.17-1.41 | p<.0001 |
| Female | 880 (52) | 5196780 (59) | 0.78 | 0.71-0.86 | p<.0001 |
Utilizing a multivariate analysis to compare EoCRC vs NoCRC, EoCRC was independently associated with Caucasian race, women, diabetes, smoking, obesity, and a family history of non-CRC cancer, all p<.0001 (table 2). Of these, diabetes mellitus and family history of non-CRC cancer had highest adjusted odds ratios (19.8 and 7.3, respectively). Alcohol abuse was not significantly associated with EoCRC. Supplementary analysis showed increase risk of EoCRC in patients with IBD or family history of CRC (Table 7).
Table 2:
Multivariate analysis of EoCRC vs NoCRC
| Adjusted OR [95% CI] | P | |
|---|---|---|
| Gender (Male) | 0.272[0.247-0.299] | <0.001 |
| Race (Caucasian) | 2.559[2.43-2.694] | <0.001 |
| DM | 19.797[18.146-21.598] | <0.001 |
| Smoker | 2.675[2.406-2.974] | <0.001 |
| Obesity | 1.819[1.618-2.044] | <0.001 |
| Alcohol | 0.908[0.658-1.254] | 0.56 |
| Fhx | 7.333[6.181-8.699] | <0.001 |
Table 7:
Correlation of IBD and Familial CRC to EoCRC
| EoCRC (n) | NoCRC (n) | OR | 95% CI | Significance | |
|---|---|---|---|---|---|
| Total | 1820 | 8891400 | |||
| Comorbidities | |||||
| Ulcerative Colitis | 40 | 19060 | 10.46 | 7.64-14.31 | p<.0001 |
| Crohn’s Disease | 70 | 33160 | 10.69 | 8.41-13.57 | p<.0001 |
| Family hx of CRC | 200 | 22230 | 49.26 | 42.50-57.08 | p<.0001 |
In terms of associated signs/symptoms, patients with EoCRC were found to have higher rates of abdominal pain, anemia, hematochezia, diarrhea, constipation, malaise and fatigue, weight loss, nausea, and decreased appetite than NoCRC, all p<0.001 (Table 3).
Table 3:
Comparison of Signs and Symptoms and medical comorbidities in EoCRC vs NoCRC
| EOCRC (n) | NoCRC (n) | OR | 95% CI | Significance | |
|---|---|---|---|---|---|
| Total | 1680 | 8871400 | |||
| Signs/symptoms | |||||
| Abdominal pain | 890 | 1787410 | 4.47 | 4.06-4.91 | p<.0001 |
| Hemoglobin low | 620 | 533740 | 9.14 | 8.27-10.09 | p<.0001 |
| Hematochezia | 160 | 67830 | 13.66 | 11.61-16.08 | p<.0001 |
| Diarrhea | 390 | 509210 | 4.96 | 4.43-5.56 | p<.0001 |
| Constipation | 410 | 518220 | 5.65 | 5.05-6.32 | p<.0001 |
| Malaise and fatigue | 280 | 611860 | 2.70 | 2.37-3.07 | p<.0001 |
| Weight loss | 120 | 104190 | 6.47 | 5.38-7.79 | p<.0001 |
| Nausea | 560 | 927820 | 4.28 | 3.87-4.73 | p<.0001 |
| Decreased appetite | 50 | 36110 | 7.51 | 5.66-9.95 | p<.0001 |
EoCRC vs LoCRC
Among all patients who were diagnosed with CRC, compared to LoCRC, patients with EoCRC were less likely to be Caucasian (OR 0.63, 95% CI 0.57-0.71, p<0.0001) and more likely to be African American (OR 1.34, 95% CI 1.16-1.53, p<0.0001) (Figure 2). Women presented with EoCRC more often than men (OR 1.12, 95% CI 1.02-1.24, p=.02) (Table 4, Figure 3).
Figure 2:
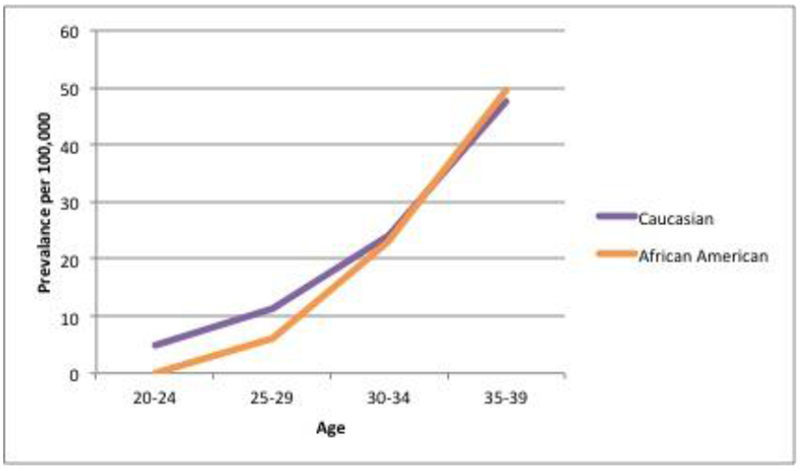
Prevalence of EoCRC based on race
Table 4:
Demographics of patients with EoCRC and LoCRC
| EoCRC (n, %) | LoCRC (n, %) | OR | 95% CI | Significance | |
|---|---|---|---|---|---|
| Race | |||||
| Caucasian | 1200 (71) | 73480 (80) | 0.64 | 0.57-0.71 | p<.0001 |
| African American | 240 (14) | 10230 (11) | 1.34 | 1.16-1.53 | p<.0001 |
| Gender | |||||
| Male | 800 (48) | 46620 (51) | 0.89 | 0.81-0.98 | p=.02 |
| Female | 880 (52) | 45640 (49) | 1.12 | 1.02-1.24 | p=.02 |
Figure 3:
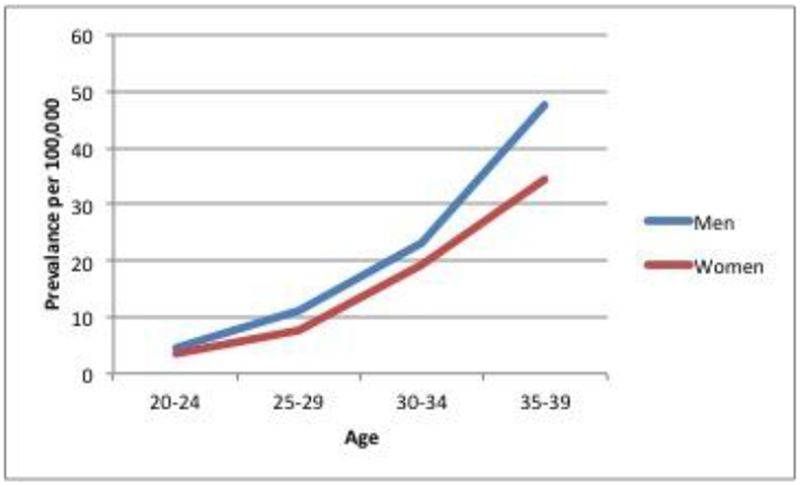
Prevalence of EoCRC based on gender
In regards to signs/symptoms, EoCRC patients presented more often with abdominal pain and nausea while LoCRC presented more with constitutional signs/symptoms such as malaise, anemia, and decreased appetite. Other findings such as hematochezia, diarrhea, and constipation were found in similar frequencies in EoCRC vs. LoCRC (Table 5).
Table 5:
Comparison of Signs and Symptoms and medical comorbidities in EoCRC vs. LoCRC
| EOCRC (n) | LoCRC (n) | OR | 95% CI | Significance | |
|---|---|---|---|---|---|
| Total | 1680 | 92260 | |||
| Signs/Symptoms | |||||
| Abdominal pain | 890 | 37870 | 1.62 | 1.47-1.78 | p<.0001 |
| Hemoglobin low | 620 | 43460 | 0.66 | 0.59-0.73 | p<.0001 |
| Hematochezia | 160 | 7980 | 1.11 | 0.94-1.31 | p=.21 |
| Diarrhea | 390 | 20660 | 1.05 | 0.93-1.17 | p=.42 |
| Constipation | 410 | 21610 | 1.06 | 0.94-1.18 | p=.35 |
| Malaise and fatigue | 280 | 24710 | 0.55 | 0.48-0.62 | p<.0001 |
| Weight loss | 120 | 10100 | 0.63 | 0.52-0.75 | p<.0001 |
| Nausea | 560 | 20850 | 1.71 | 1.55-1.89 | p<.0001 |
| Decreased appetite | 50 | 3730 | 0.73 | 0.55-0.97 | p=.03 |
| Medical Comorbidity | |||||
| Type 1 Diabetes | 30 | 2850 | 0.57 | 0.40-0.82 | p=.002 |
| Obesity | 290 | 21080 | 0.7 | 0.62-0.80 | p<.0001 |
| Smoker | 500 | 24210 | 1.19 | 1.07-1.32 | p=.001 |
| Alcohol abuse | 40 | 3010 | 0.72 | 0.53-0.99 | p=.04 |
| Family hx of Cancer (non-CRC) | 420 | 15470 | 1.65 | 1.48-1.85 | p<.0001 |
| HIV | 50 | 780 | 3.6 | 2.69-4.80 | p<.0001 |
We found that certain medical comorbidites were more frequently associated with EoCRC compared to LoCRC such as smoking, family history of non-CRC malignancy and HIV. Certain medical comorbidities were also significantly less common with EoCRC than LoCRC such as obesity, Type 1 diabetes, and alcohol abuse (Table 6).
Table 6:
Location of tumors in EoCRC vs. LoCRC
| Location | EoCRC (n, prevelance/100,000) | LoCRC (n, prevelance/100,000) | OR | 95% CI | Significance |
|---|---|---|---|---|---|
| Right CRC (Cecum to Splenic Flexture) | 900 (10.1/100,000) | 55520 (284.6/100,000) | 0.76 | 0.69-0.84 | p<.0001 |
| Left CRC (Descending and sigmoid colon | 650 (7.3/100,000) | 32800 (168.1/100,000) | 1.14 | 1.04-1.26 | p=.008 |
| Rectal Cancer | 790 (8.9/100,000) | 40640 (208.3/100,000) | 1.13 | 1.02-1.24 | p=.01 |
In terms of anatomical distribution of CRC, we found that patients with EoCRC had a prevalence of right-sided CRC (Cecum to splenic flexure), left-sided CRC (splenic flexure through sigmoid colon), and rectum of 10.1, 7.3, and 8.9/100000, respectively. Patients with LoCRC had a prevalence of right, left, and rectal CRC at 284.6, 168.1, and 208.3/100,000, respectively. EoCRC was significantly more likely to be left-sided Colon cancer (OR 1.14, 95% CI 1.04-1.26, p=.008) and Rectal Cancer (OR 1.13, 95% CI 1.02-1.24, p=.01) and less likely to be right-sided colon cancer (OR 0.76, 95% CI 0.69-0.84, p<.0001) compared to LoCRC (Table 6). Left sided CRC was not statistically significantly associated with EoCRC compared to LoCRC.
DISCUSSION:
Young patients with early onset CRC comprise a unique cohort, separate from their contemporaries and those who develop CRC later in life. By focusing on average risk patients with EoCRC, this study investigates a group of young patients who have traditionally had very poor rates of screening and worse prognosis than older patients with CRC [8],[12]. While overall rates of CRC continue to decrease, patients in the 20-39 age range continue to develop CRC at higher rates annually since the 1980s [24].
In our national cohort, we found that adults aged 20-39 had first-ever CRC at a prevalence of 18.9/100,000. Our data is corroborated by findings in the well-established Surveillance, Epidemiology, and End Results (SEER) Program Statistic review. The most recent version, updated April 2018, showed that patients from age ranges 30-34 and 35-39 were diagnosed with colon cancer with incidence 5.4 and 10/100,000, respectively, which is similar to our findings [25].
In our primary aim, we compared EoCRC to NoCRC and found that patients with EoCRC were more commonly Caucasian and female. In addition, other factors that independently increase the odds ratio for EoCRC included diabetes Mellitus, family history of non-CRC malignancy, obesity, and smoking. These findings are similar to prior studies which have found that these medical comorbidities portend a higher risk of CRC compared to controls [14],[3]. EoCRC patients also had a significantly higher rate of CRC related symptoms than NoCRC, including abdominal pain, anemia, hematochezia, fatigue, nausea, weight loss and constipation. Among those, the strongest association with malignancy was in patients with hematochezia, decreased appetite, and anemia.
Our secondary aim found that compared to LoCRC, EoCRC patients are more likely to be African American. They tend to develop more left-sided and rectal cancers than older patients. In addition, family history of non-CRC malignancy, HIV, and smoking are more commonly associated with EoCRC than LoCRC. EoCRC patients present more commonly with symptoms such as abdominal pain compared to their older counterparts. These findings corroborate most of the data that exists in previous studies of patients with early onset CRC. Many studies, including O’Connell’s [12] review of CRC in the young, have found that rectal cancers were more common in younger patients [26],[8],[10]. In addition, EoCRC patients presented more frequently with abdominal pain and rectal bleeding [12].
The findings that rectal cancer are more common in younger patients can either be due to changes in pathophysiology of the cancers, or may be due to detection [27]. Rectal cancers present more often with rectal bleeding than cancers of the ascending colon, and may prompt more screening in the younger population. In addition, diagnostic accuracy is decreased in cecal tumors, which may contribute to delays in diagnosis and treatment [28].
While there is a large amount of data that shows CRC is overall more common in men, the same has not been true in studies on early onset CRC[12]. In our study, we found that female gender was an independent risk factor to development of CRC in the young. This may be due to younger women being more connected with healthcare at a young age, and thus have higher rates of earlier diagnosis, or may be due to a change in the pathophysiology of the cancers.
Taken together, these data help create a clinical picture of which patients may be at highest risk for EoCRC. In patients 20-39 years old, white women with medical comorbidities and indicative signs/symptoms are most likely to have CRC unrelated to IBD or family history. This study shows that the incidence in this subset is high enough to warrant increased suspicion and possible screening for CRC in these patients. In addition to their medical risk factors, these patients increased rate may related to predispositions to the signet ring subtype of CRC that is more common in EoCRC [6].
The increasing incidence of CRC in Amercians 20-39 years old is not completely understood. However it is likely related to the overall health trends in America. The increasing rates of cancer in this cohort mirror similar trends in obesity, a comorbidity that demonstrated significant correlation to EoCRC in our analysis. Previous studies have shown that a 5-unit increase in BMI correlates to an increase in risk of CRC of 13-18% [6]. From 1999 to 2015 rates of childhood obesity increased from 13.9% to 18.5% [29]. We can expect that the rates of early onset CRC will continue to increase as this population ages. The increased rate of cancer in these patients may be due to several mechanisms, including insulin resistance, chronic inflammation, and altered levels of growth factor hormones.
Our study does have few limitations that must be addressed. It is possible that our determination of CRC prevalence may be overestimated or underestimated due to some patients being mislabeled with the SNOMED-CT diagnosis of colorectal cancer when in fact they did not have the disease or if they were not given the SNOMED-CT diagnosis of colorectal cancer when in fact they actually had the disease. The validation of SNOMED-CT diagnostic codes is not possible in this database for our current study given patient information is de-identified. However, it has to be noted that the International Classification of Diseases-9th Revision (ICD-9) and SNOMED-CT are validated medical terminology systems for diagnoses and SNOMED-CT has been shown to have more concepts to be coded per clinical document than ICD-9 [30], making it more accurate regarding enlisting clinical information.
In addition, certain patients may have been counted multiple times if they received health care at multiple institutions that utilize the Explorys database. Although Explorys does utilize a master patient identifier to match in order to combine information if a patient receives care at different healthcare institutions [31], there is still a possibility that some of this information gets duplicated multiple times for the same patient, leading to potential overestimation of the true prevalence rate.
Another limitation is that we were unable to obtain certain information that is unavailable through the Explorys database. This includes geographic information of our population, socioeconomic status, colonoscopy abnormalities, and pathology reports. The presence of such information may add further value to our analysis of CRC incidence in the young in that we would have more accurate data about cancer diagnosis and what other factors affect CRC incidence.
In summary, a major strength of our study was our utilization of the Explorys database in order to acquire data on a very large source population across many healthcare centers. We were able to identify a subgroup of patients that has a relatively low CRC prevalence rate, specifically patients 20-39 years old, and more specifically, we found that the risk of rectal cancer is higher in younger patients. In addition, another strength of this study is that the database contains administrative data from different healthcare systems, which is more representative of the real world. Thus, our study provides a potential basis for adjusting screening guidelines in this population.
Currently, routine screening colonoscopies are not recommended in individuals aged 45 years and younger. There are few guidelines or education in the physician or general population for evaluating young patients without family history or IBD who present with concerning symptoms. This has led to delay in diagnosis caused by both physician and patient factors [32],[17]. This study hopes to provide more information about patients at highest risk of early onset CRC and the signs/symptoms such as hematochezia and medical comorbidities. Supplemental analysis also supports the continued screening of patients at high baseline risk for EoCRC including patients with IBD and family history of CRC. Further studies should be done to look at effectiveness of early immunochemical or endoscopic screening in patients at high risk of early disease. If these EoCRC are identified, further work can be done to evaluate use of germline mutation testing in this population, as almost 1/10 may harbor clinically silent mutations [7].
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Writing Assistance: There is no writing assistance to disclose.
Conflicts of Interest: Glover, Mansoor, Panhwar, Parasa, and Cooper deny any relevant personal or financial conflicts of interest related to the material in this manuscript
Contributor Information
Michael Glover, MS-4, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106.
Emad Mansoor, Department of Internal Medicine and Division of Gastroenterology and Liver Disease, University Hospitals Cleveland Medical Center, Cleveland OH USA.
Muhammed Panhwar, Department of Internal Medicine, University Hospitals Cleveland Medical Center, Cleveland OH USA.
Sravanthi Parasa, Department of Internal Medicine and Division of Gastroenterology and Liver Disease, University Hospitals Cleveland Medical Center, Cleveland OH USA; Current address: Swedish Medical Center, Seattle WA USA.
Gregory S. Cooper, Department of Internal Medicine and Division of Gastroenterology and Liver Disease, University Hospitals Cleveland Medical Center, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, Ohio 44106, Wearn 244.
REFERENCES:
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Marley AR, Nan H: Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 2016;7:105–114. [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y-X, Hennessy S, Lewis JD: Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2005;3:587–594. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC: Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 1995;87:265–273. [DOI] [PubMed] [Google Scholar]
- 5.Singh KE, Taylor TH, Pan C-JG, Stamos MJ, Zell JA: Colorectal Cancer Incidence Among Young Adults in California. J Adolesc Young Adult Oncol 2014;3:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, et al. : The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc 2014;89:216–224. [DOI] [PubMed] [Google Scholar]
- 7.Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, Osborne J, et al. : Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018;154:897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dozois EJ, Boardman LA, Suwanthanma W, Limburg PJ, Cima RR, Bakken JL, et al. : Young-Onset Colorectal Cancer in Patients With No Known Genetic Predisposition. Medicine (Baltimore) 2008;87:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society Guideline for Colorectal Cancer Screening [cited 2018 Jul 8];Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html
- 10.Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. : Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J Natl Cancer Inst 2017; 109 DOI: 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers EA, Feingold DL, Forde KA, Arnell T, Jang JH, Whelan RL: Colorectal cancer in patients under 50 years of age: A retrospective analysis of two institutions’ experience. World J Gastroenterol WJG 2013;19:5651–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell JB, Maggard MA, Livingston EH, Yo CK: Colorectal cancer in the young. Am J Surg 2004;187:343–348. [DOI] [PubMed] [Google Scholar]
- 13.IBM Explorys - IBM Watson Health. 2017. [cited 2018 Dec 19];Available from: undefined.
- 14.Chouhan V, Mansoor E, Parasa S, Cooper GS: Rates of Prevalent Colorectal Cancer Occurrence in Persons 75 Years of Age and Older: A Population-Based National Study. Dig Dis Sci 2018;63:1929–1936. [DOI] [PubMed] [Google Scholar]
- 15.Al-Kindi SG, Oliveira GH: Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc 2016;91:81–83. [DOI] [PubMed] [Google Scholar]
- 16.Panhwar MS, Mansoor E, Al-Kindi SG, Sinh P, Katz J, Oliveira GH, et al. : Risk of Myocardial Infarction in Inflammatory Bowel Disease: A Population-based National Study. Inflamm Bowel Dis DOI: 10.1093/ibd/izy354 [DOI] [PubMed] [Google Scholar]
- 17.Jarvinen HJ, Turunen MJ: Colorectal carcinoma before 40 years of age: Prognosis and predisposing conditions. Scand J Gastroenterol 1984;19:634–638. [PubMed] [Google Scholar]
- 18.Beaugerie L, Itzkowitz SH: Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441–1452. [DOI] [PubMed] [Google Scholar]
- 19.Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M: Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012; 143:375–381.el; quiz e13-14. [DOI] [PubMed] [Google Scholar]
- 20.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P: Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–2778. [DOI] [PubMed] [Google Scholar]
- 21.Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. : Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst 2012;104:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Practical Statistics for Medical Research. CRC Press 1990. [cited 2018 Jul 10];Available from: https://www.crcpress.com/Practical-Statistics-for-Medical-Research/Altman/p/book/9780412276309
- 23.Schoonjans F: MedCalc’s Odds ratio calculator. MedCalc [cited 2018 Jul 10];Available from: https://www.medcalc.org/calc/odds_ratio.php
- 24.Rabin RC: Colon and Rectal Cancers Rising in Young People. N Y Times 2017. [cited 2019 Apr 21];Available from: https://www.nytimes.com/2017/02/28/well/live/colon-and-rectal-cancers-rising-in-young-people.html
- 25.Cancer Statistics Review, 1975-2015 - SEER Statistics [cited 2018 Jul 11];Available from: https://seer.cancer.gov/csr/1975_2015/
- 26.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY: Rates of colon and rectal cancers are increasing in young adults. Am Surg 2003;69:866–872. [PubMed] [Google Scholar]
- 27.Mik M, Berut M, Dziki L, Trzcinski R, Dziki A: Right- and left-sided colon cancer – clinical and pathological differences of the disease entity in one organ. Arch Med Sci AMS 2017;13:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh H, Nugent Z, Demers AA, Bernstein CN: Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol 2010;105:2588–2596. [DOI] [PubMed] [Google Scholar]
- 29.Adult Obesity Facts | Overweight & Obesity | CDC 2018. [cited 2018 Dec 20];Available from: https://www.cdc.gov/obesity/data/adult.html
- 30.Nadkarni PM, Darer JA: Migrating existing clinical content from ICD-9 to SNOMED. J Am Med Inform Assoc JAMIA 2010;17:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaelber DC, Foster W, Gilder J, Love TE, Jain AK: Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc JAMIA 2012;19:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adkins RB, DeLozier JB, McKnight WG, Waterhouse G: Carcinoma of the colon in patients 35 years of age and younger. Am Surg 1987;53:141–145. [PubMed] [Google Scholar]


