Abstract
Osteoarthritis (OA) is a chronic progressive, painful disease of synovial joints, characterized by cartilage degradation, subchondral bone remodeling, osteophyte formation, and synovitis. It is now widely appreciated that the innate immune system, and in particular Toll-like receptors (TLRs), contribute to pathological changes in OA joint tissues. Furthermore, it is now also increasingly recognized that TLR signaling plays a key role in initiating and maintaining pain. Here, we reviewed the literature of the past 5 years with a focus on how TLRs may contribute to joint damage and pain in OA. We discuss biological effects of specific damage-associated molecular patterns (DAMPs) which act as TLR ligands in vitro, including direct effects on pain-sensing neurons. We then discuss the phenotype of transgenic mice that target TLR pathways, and provide evidence for a complex balance between pro- and anti-inflammatory signaling pathways activated by OA DAMPs. Finally, we summarize clinical evidence implicating TLRs in OA pathogenesis, including polymorphisms and surrogate markers of disease activity. Our review of the literature led us to propose a model where multi-directional crosstalk between connective tissue cells (chondrocytes, fibroblasts), innate immune cells, and sensory neurons in the affected joint may promote OA pathology and pain.
Keywords: TLR, innate immunity, osteoarthritis, pain, DAMPs, alarmins
Introduction
Osteoarthritis (OA) is a chronic progressive, painful disease of synovial joints, affecting mainly the knees, hips, hands, and small joints in the spine. OA is the most common form of arthritis, and one of the major causes of chronic pain worldwide [1]. It results in substantial socioeconomic costs due to both healthcare utilization and work loss [2]. In individuals with knee or hip OA, severity of disability is associated with a significant increase in all-cause mortality [3]. Joint pain is the main symptom that drives OA patients to seek medical help, but current strategies for pain management are temporarily palliative at best, inadequate or contraindicated for many patients, and associated with substantial adverse effects. In addition, no available treatments slow or halt the progressive degeneration of the joint characteristic of OA (https://www.oarsi.org/education/oarsi-resources/oarsi-white-paper-oa-serious-disease). Ultimately, patients with knee and hip OA often end up undergoing total joint replacements, and OA is the most common indication for joint replacement.
Age, previous joint injury, and obesity are the strongest risk factors for development of OA. As the population ages the public health burden of OA is increasing, and the need for Disease Modifying OA Drugs (DMOADs) as well as novel efficacious and safe analgesics is urgent. However, OA also occurs in younger patients and incidence may be increasing due to obesity and joint injuries [4, 5]. Joint replacement, albeit effective, is often not an option for older patients with significant medical comorbidities, and is reserved for advanced disease. In younger patients, it is associated with higher risk of complications and revision surgery [6]. Thus, the slowly progressive nature of OA leaves many patients suffering for years without adequate treatment, compounding the urgent need for novel therapeutic approaches.
Osteoarthritis: A disease characterized by progressive joint damage and chronic pain
Cartilage degradation, subchondral bone remodeling, and osteophyte formation are the hallmarks of OA, but there is also low-grade synovitis, and involvement of peri-articular structures and - in the knee – the menisci. There is a strong mechanical component in OA pathogenesis, but it is now also widely appreciated that the interplay between mechanical factors and low-grade inflammation is a key driver for progressive joint damage. Several lines of evidence, both in clinical samples and in animal models, suggest that low-grade inflammation contributes to pathological changes in all OA joint tissues, mainly mediated by the innate immune system (macrophages, complement system) (reviewed in [7, 8]).
Progressive joint damage in OA is associated with pain. While a discordance between radiographic OA and the presence of pain can be observed, strong clinical evidence suggests that ongoing peripheral input from the affected joint drives pain in OA [9]. It is not clear which particular joint structures contribute to pain, but there is increasing evidence that bone marrow lesions and synovitis detected by MRI, correlate with pain, while sensitization is associated with synovitis/effusion [10].
In its most elementary form, pain equals nociception, which is mediated by specialized sensory afferents called nociceptors. The cell bodies of these nociceptors reside in the dorsal root ganglia (DRG), and extend one terminal to the peripheral tissues they innervate and one terminal to the dorsal horn of the spinal cord, where the first synapse is made with a second-order neuron [11]. Noxious triggers, which can be chemical, thermal, or mechanical in nature, activate nociceptors. This means that action potentials are generated and transduced to the DRG and further up into the central nervous system (CNS), resulting in sensation of pain. Tissue injury triggers a cascade of neuro-inflammatory reactions, leading to pain while aiding the recovery process [12]. If tissue damage persists, as is the case in OA (as well as in other forms of chronic arthritis), continuous nociceptive input from the periphery leads to profound changes in the DRGs, in the spinal cord and at supraspinal levels of the CNS. The result is transition from acute to chronic pain [12].
Peripheral sensitization is a hallmark of inflammation
Sensitization means that the threshold for nociceptors to fire action potentials is lowered, resulting in hypersensitivity. This occurs in inflammatory conditions when mediators including cytokines, chemokines, nerve growth factor (NGF), ATP, prostaglandins and protons act on receptors expressed by sensory neurons, leading to increased sensitivity to noxious (hyperalgesia) and even non-noxious (allodynia) stimuli. Immune regulation is an essential component of peripheral sensitization (reviewed in [13]). Crosstalk between nociceptors and the immune system occurs at the site of inflamed tissues, when inflammatory cells (macrophages, mast cells, neutrophils) secrete mediators that act on cognate receptors on nerve endings (for example, innate immune cells can secrete NGF which binds TrkA on nociceptors, leading to transactivation of TRPV1 and lowering the threshold of firing [14]). Prolonged input from the periphery leads to an increase in innate and adaptive immune cells in the DRG, and crosstalk between these cells and DRG neurons results in long-term changes in the neurons (for example, release of the pro-algesic chemokine, CCL2 [15]). These DRG changes have been demonstrated in models of neuropathic and purely inflammatory pain, as well as in a chronic progressive mouse model of OA, induced by destabilization of the medial meniscus (DMM) [16].
The OA research community is increasingly reporting evidence that the innate immune system, and in particular Toll-like receptors (TLRs), plays a key role in driving OA joint damage [7, 17]. Separately, in the pain research field, evidence is accumulating that TLRs contribute to persistent pain, and this both in the periphery and in the CNS (recently reviewed in [18]). Therefore, we prepared a narrative review of the literature from the past 5 years focusing on TLRs in OA joint damage and in OA pain. We searched PubMed for the following terms “osteoarthritis”, “pain”, “TLR”, “animal models”, as well as combinations of these terms with individual TLR ligands, “innate immunity” and “pattern-recognition receptors”. We briefly discuss TLRs and their ligands, and the in vitro biological effects that may contribute to joint damage, peripheral sensitization, and pain in the context of OA. Then, we provide an overview of findings in genetically modified mice, followed by clinical evidence implicating TLRs in OA pathogenesis. Our review of the literature led us to propose a model where crosstalk between connective tissue cells (chondrocytes, fibroblasts), innate immune cells, and sensory neurons in the affected joint may contribute to OA pathogenesis, including pain.
Toll-like receptors: part of the first line defense against danger
The innate immune system provides a first line of defense against pathogens and tissue injury, through orchestration of the inflammatory response. To ensure this function, innate immune cells are equipped with Pattern Recognition Receptors (PRRs), which recognize conserved structural moieties in pathogens like bacteria, microbial products, viruses (“Pathogen Associated Molecular Patterns”, PAMPs), as well as molecular patterns that are associated with tissue damage, cell stress and cell death (termed “Damage Associated Molecular Patterns”, DAMPs). A class of PRR known as TLRs recognize multiple DAMPs, and ligand binding results in activation of inflammatory signaling pathways including nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) and type I interferon pathways, with subsequent release of cytokines, chemokines, and proteases.
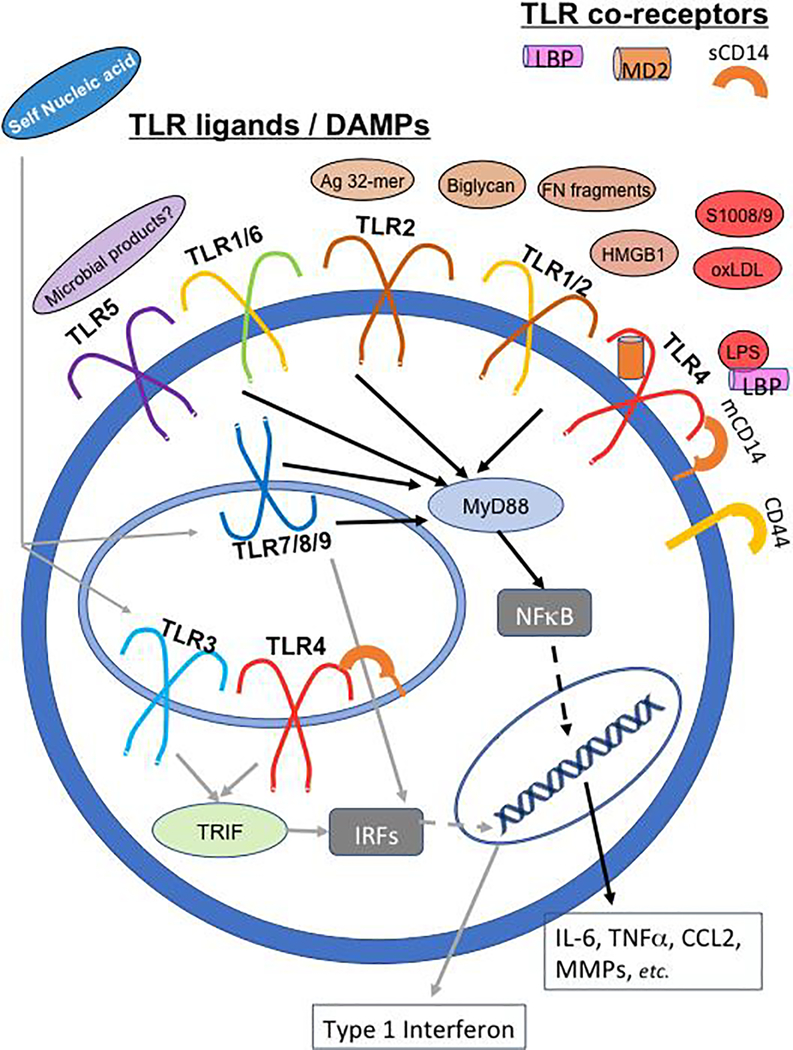
TLRs are type I transmembrane receptors that reside both on the cell surface (in humans, TLR1, 2, 4, 5 and 6) and in endosomes (TLR3, 7, 8, 9) (reviewed in [19]). Cell surface TLRs are largely dependent on the cytoplasmic adaptor, MyD88, to activate inflammatory transcriptional pathways via NFκB. The endosomal TLRs can activate NFκB and Interferon-regulator factors (IRFs). TLR3 signaling is independent of MyD88 but utilizes the adaptor TRIF to activate IRFs. Finally, TLR4 activates MyD88-dependent signaling at the cell surface, and then is internalized to the endosomal compartment where it activates TRIF-dependent pathways as well. TLR localization, signaling pathways, and inflammatory responses are summarized in Figure 1. Also shown are DAMPs and co-receptors reported to modulate TLR activation, as summarized in the next section.
Figure 1: TLRs are transmembrane receptors that facilitate inflammatory signaling in response to PAMPs and DAMPs.
In humans, TLR1, 2, 4, 5 and 6 are found on the cell membrane, while TLR3, 7, 8, 9 are located in endosomes. TLR2 exists as a homodimer, and also as a heterodimer in complex with TLR1 or TLR6. Several DAMPs found in the osteoarthritic joint and discussed in this review can interact with the cell-surface TLRs, including extracellular matrix components (e.g., biglycan, fibronectin (Fn) fragments, and an aggrecan-derived peptide - Ag 32-mer), and molecules produced directly by cells under stress (e.g., HMGB1, S100A8/9). In addition, low levels of the TLR4 ligand lipopolysaccharide (LPS) are found in OA joint fluids. A number of co-receptors or co-factors can modify the nature of TLR/ligand interactions. These include the soluble co-factors LBP and MD2 which facilitate LPS/TLR4 signaling, the receptor CD44 which can modulate the response to TLR2/4 ligands, and CD14 which can modulate the strength/sensitivity of TLR signaling and is active both in membrane and soluble form. Signaling through cell-surface TLRs requires the cytoplasmic adaptor, MyD88, and results in activation of the transcription factor NFκB (black arrows), which in turn leads to production of many inflammatory cytokines, chemokines and proteases. The endosomal TLRs can activate both NFκB and Interferon-regulator factors (IRFs) which promote a type I interferon response (grey arrows). TLR3 signaling is independent of MyD88, but utilizes the adaptor TRIF to activate IRFs. TLR4 can activate MyD88-dependent signaling at the cell surface, and then is internalized to the endosomal compartment where it can activate TRIF-dependent pathways as well. Most findings regarding specific TLR/DAMP interactions in OA models have largely implicated TLR2 and TLR4. However, microbial products of the microbiome have been hypothesized to interact with TLR5, while self-nucleic acids may interact with endosomal TLRs. These potential DAMP/TLR interactions and their relevance to OA need further investigation.
DAMPs in Osteoarthritis
Since OA is characterized by ongoing tissue damage and remodeling, with enzymatic breakdown of articular cartilage molecules, cell stress/death in articular cartilage, and synovitis, a broad variety of DAMPs are generated in the different joint tissues as disease progresses. These DAMPs include extracellular matrix (ECM) fragments, S100 proteins, high-mobility group box protein 1 (HMGB1), as well as histones and nucleic acids (reviewed in [20, 21]). These DAMPs and their respective TLRs are present in cells and tissues throughout the joint, altering the micro-environment, and many can also be detected in OA synovial fluid [20].
Enzymatic cartilage degradation is a hallmark of OA and leads to abundant production of ECM molecules and fragments that can signal through TLR2 and/or TLR4 (Fig. 1). Several detailed reviews have discussed how these matrix-derived proteins bind TLR2 and/or TLR4, including low-molecular weight hyaluronan (LMW-HA), biglycan, fibronectin containing a type III repeat extra domain A (EDA, a splice variant associated with tissue injury), and tenascin-C [20, 22]. In addition to ECM molecules, other DAMPs present in OA joints include plasma proteins, which can enter the joint through a leaky synovial barrier (reviewed in [20]), molecules produced in the setting of cellular stress such as S100A8/9 and HMGB1 (reviewed in [21]), and crystals which can deposit in OA joint tissues (reviewed in [23]).
In the past 5 years, novel DAMPs have been identified, and new biological actions of known DAMPs have been described that suggest intriguing new concepts in the context of OA, as discussed below:
DAMPs can promote joint damage but also pain through neuronal TLRs
We reported that specific DAMPs present in OA joints, including α2-macroglobulin and S100A8, can excite sensory neurons through neuronal TLR4 and stimulate them to produce the pro-algesic chemokine, CCL2 [24]. Using ex vivo Ca2+-imaging of intact DRG explants, we also found that exogenously added LPS (the quintessential TLR4 ligand derived from gram-negative bacteria) triggered activation of small-to-medium diameter neurons. These LPS-responsive neurons also responded to capsaicin, suggesting that LPS induces responses specifically in TRPV1-expressing nociceptors, confirming older reports [25]. Consistent with these findings, Guerrero and co-workers reported that intra-articular injection of LPS into the murine tibio-tarsal joint elicited hyperalgesia in wild-type but not Tlr4 null or Myd88 null mice (while Tlr2 null and Trif null mice behaved like wild-types) [26].
One of the most abundant ECM components in cartilage is the proteoglycan, aggrecan. Its enzymatic degradation by the aggrecanases, ADAMTS-4/−5, is a key pathological process in OA [27]. Aggrecanase-mediated cleavage of the aggrecan interglobular domain at E373–374A generates an N-terminal fragment that is retained in the cartilage matrix and subsequently cleaved by MMPs at N341↓342F, releasing a 32-amino acid fragment (“32-mer”, 342F–E373) [28]. This 32-mer can induce pro-inflammatory signaling in cultured chondrocytes, synovial fibroblasts, and peritoneal macrophages, an effect mediated through TLR2 [29]. Unexpectedly, mice that cannot produce the 32-mer (“Chloe mice”, a transgenic line in which the MMP cleavage site (N341↓342F) in the aggrecan interglobular domain is mutated, thus preventing production of the 32-amino acid fragment), developed more pronounced cartilage damage 8 weeks after DMM surgery compared to wild-type mice [30]. This finding was recently confirmed in an independent study where Chloe mice had more severe cartilage damage and osteophytes 4 and 16 weeks after DMM, and more severe subchondral bone sclerosis 4 weeks after DMM compared to wild-types [31]. Recently, we reported that the 32-mer aggrecan fragment can activate cultured nociceptors and trigger the release of CCL2 in DRG cultures specifically through TLR2 [31]. Furthermore, intra-articular injection of 32-mer but not a scrambled peptide elicited knee hyperalgesia in wild-type but not Tlr2 null mice. Intra-articular injection in the knee of the TLR1/2 agonist, Pam3CSK4, also caused immediate hyperalgesia in wild-type but not Tlr2 null mice, suggesting that free nerve endings in the intra-articular space express TLR2 (which has been shown to be present on the cell bodies in the DRG). Remarkably, Chloe mice were protected from knee hyperalgesia after DMM surgery despite exhibiting more severe cartilage damage. These mice still retained the ability to respond to intra-articular administration of exogenous 32-mer. Thus, this single TLR2 ligand, produced by enzymatic degradation of aggrecan (which is the most abundant macromolecule in cartilage), may play a central role in driving knee pain but not joint damage in murine OA.
DAMPs in the OA milieu may exert pro- but also anti-inflammatory actions
Accelerated joint damage in Chloe mice suggests that the expression of DAMPs in the early OA process may be an attempt at promoting tissue repair while creating a pro-inflammatory environment. This balance of pro- and anti-inflammatory signaling has been examined for two other extracellular matrix proteins found in cartilage: biglycan and tenascin-C.
Biglycan is one of several small leucine-rich proteoglycans in cartilage that contribute to cartilage architecture by linking extracellular matrix components. Soluble biglycan, which can be released when cartilage matrix is degraded, can signal through TLR2 and TLR4 along with the TLR co-receptor CD14 on macrophages [32], T-cells [33], and chondrocytes [34, 35], thereby promoting pro-inflammatory signaling. Soluble biglycan has been detected in OA synovial fluid [34], and two recent studies demonstrated that stimulation of human chondrocytes and cartilage explants with soluble biglycan induced a catabolic response through TLR4 [34, 35]. A recent study suggested that biglycan signaling can be pro- or anti-inflammatory, depending on the co-receptor [36]. Indeed, biglycan can also bind the hyaluronan receptor, CD44, which has been shown to act as a co-receptor of TLR4, and depending on the ligand can promote either pro- or anti-inflammatory signaling [37]. In a mouse model of renal ischemia/reperfusion injury, soluble biglycan can signal through either TLR2/CD14, TLR4/CD14 or TLR4/CD44 pathways to regulate macrophage polarization [36]. Macrophage polarization is a dynamic process and is characterized by M1 and M2 macrophages. The M1 phenotype is typically stimulated by TLR ligands and IFN-γ, promoting inflammation, while the M2 phenotype is stimulated by IL-4/IL-13 and promotes resolution of inflammation [38]. In the renal ischemia/reperfusion injury model, overexpression of circulating biglycan increased recruitment of M1-polarized (pro-inflammatory) macrophages to the kidney in the acute injury phase through TLR2/CD14 or TLR4/CD14. Also in the early phase, autophagy was induced in M1 macrophages through soluble biglycan binding TLR4/CD44. This early CD44 signaling event subsequently led to an increased number of M2 polarized (anti-inflammatory) macrophages in the later phase of the model [36]. Future studies will have to investigate exactly how this dual function is regulated, as well as how soluble biglycan and other DAMPs might contribute to macrophage polarization in the context of OA.
Finally, tenascin-C is a hexameric glycoprotein that is upregulated during wound healing or inflammation. Tenascin-C is abundant in developing cartilage, its expression is low in healthy adult cartilage, and it is re-expressed in OA cartilage and synovium and can also be detected in OA synovial fluid [39]. In human synovial macrophages and synovial fibroblasts derived from patients with rheumatoid arthritis (RA), tenascin-C promotes pro-inflammatory signaling through TLR4 [40]. Further work from this group has identified 3 sites within the C-terminal fibrinogen-like globe (FBG) domain of tenascin-C that interact with TLR4 [41]. They also demonstrated that the FBG domains of tenascin-R and tenascin-W signaled through TLR4, and two unrelated proteins that contain FBG domains, fibrinogen-γ and ficolin-1, were also able to stimulate cytokine synthesis in human macrophages through TLR4 [41]. However, in a surgical model of OA, tenascin-C knock-out mice developed cartilage damage more rapidly than wild-type mice by 2 weeks after surgery [42]. In the same model, intra-articular injection of tenascin-C slowed down cartilage damage in the early stages through 6 weeks after surgery, after which time cartilage damage appeared similar to wild-type mice [43]. Further work must be performed to determine the mechanism of action of tenascin-C in cartilage protection.
Findings in mice that lack specific Tlr genes: effects on joint damage and associated pain
The effect of genetic ablation of specific Tlr genes has been studied in murine OA models - mostly the effects on joint damage, but increasingly also the effect on pain and sensitization (Table 1). Analyzing the effects of TLR signaling on joint pain is a newer and likely promising approach, since TLRs can contribute to persistent pain through a variety of mechanisms at different levels of the pain pathway [18]. One of the five cardinal signs of inflammation, pain is vital for survival because it acts as a warning and avoidance mechanism against potentially damaging stimuli. As such, the pain pathway and the innate immune system share a critical function of alerting the organism of imminent danger, and TLRs act at the neuro-immune interface of this function. It is now well recognized that cells at every level of the pain neuraxis express TLRs, including sensory afferents in the periphery, CNS neurons, microglial cells and astrocytes [44]. TLRs play a key role in the neuro-immune interaction, either directly through TLRs expressed by neurons, or indirectly through TLRs expressed on innate immune cells, which upon stimulation release pro-inflammatory mediators that then act on sensory neurons (Fig. 2). A recent study employed Myd88 conditional knockout mice, in which Myd88 was deleted in NaV1.8-expressing primary sensory neurons (i.e., nociceptors). While these mice showed normal baseline and acute inflammatory pain responses, late-phase inflammatory pain following complete Freund’s adjuvant (CFA) injection and late-phase neuropathic pain following chronic constriction injury (CCI) were reduced [45]. Furthermore, CCI resulted in increased expression of the pro-algesic chemokine, CCL2, in DRG neurons, as well as macrophage infiltration into the DRG in wild-type but not in Myd88 conditional ko mice.
Table 1.
Preclinical evidence for TLR roles in OA joint damage and pain
| TLR/ Co-receptor/ Adapter protein | Joint Damage | Pain-related behaviors | Reference |
|---|---|---|---|
| TLR1/TLR2/TLR6 |
Tlr1ko, Tlr2ko, or Tlr6 ko mice: • Not protected from cartilage damage or synovial inflammation in the MNX model • Synovial thickness was increased in Tlr1ko mice. |
• Not assessed |
Nasi 2014 |
|
Tlr2ko mice: • Not protected from cartilage damage 16 weeks after DMM surgery |
• Protected from knee hyperalgesia • Not protected from secondary mechanical allodynia of the hind paw in the DMM model |
Miller 2018 | |
| Chloe mice (transgenic mice that cannot produce the 32-mer aggrecan fragment) • Not protected from cartilage damage 8 weeks after DMM surgery • More severe cartilage damage and osteophytes compared to wild-type mice 4 and 16 weeks after DMM surgery |
• Not assessed • Protected from knee hyperalgesia • Not protected from secondary mechanical allodynia of the hind paw in the DMM model |
Little 2007 Miller 2018 |
|
| TLR4 |
Tlr4ko mice: • Not protected from cartilage damage or synovial inflammation in the MNX model |
• Not assessed |
Nasi 2014 |
|
Tlr4ko mice: • Not protected from cartilage damage 16 weeks after DMM surgery |
• No protection from secondary mechanical allodynia of the hindpaw in the DMM model |
Miller 2015 | |
|
S100a9 ko mice (which are also functional S100a8 ko mice): • Not protected from joint damage in the DMM model |
• Not assessed |
van Lent 2012; Schelbergen 2016 | |
|
S100a9 ko mice: • Protected from synovitis, cartilage degradation, and osteophyte formation in collagenase-induced OA model |
• Not assessed |
van Lent 2012; Schelbergen 2016 | |
| Tenascin-C ko mice: • Developed cartilage damage more rapidly than wild-type mice after anterior cruciate ligament and medial collateral ligament transection surgery. |
• Not assessed |
Okamura 2010 | |
| CD14 |
Cd14 ko mice: • Protected from cartilage damage and subchondral bone mineral density and trabecular thickness changes in the DMM model |
• Protected from decreases in climbing activity in the DMM model |
Sambamurthy 2018 |
| MyD88 |
Myd88 ko mice: • Not protected from cartilage damage or synovial inflammation in the MNX model |
• Not assessed |
Nasi 2014 |
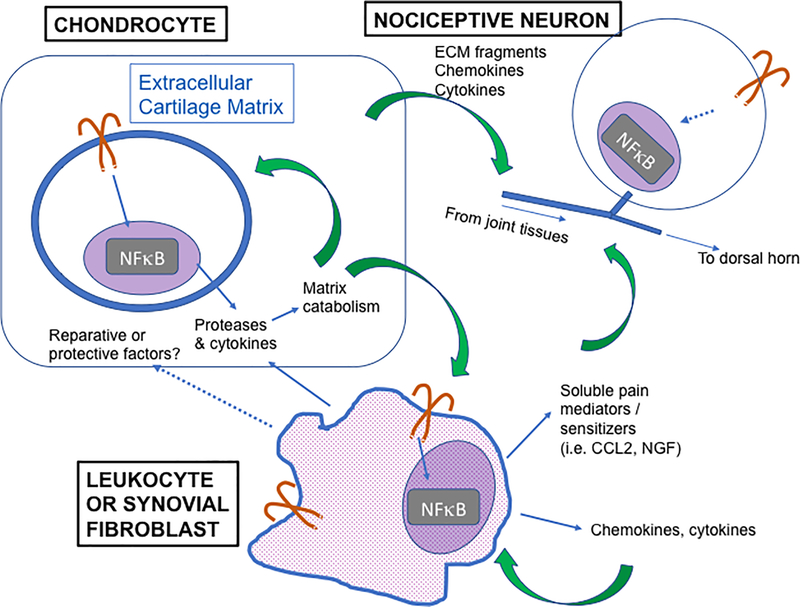
Figure 2: Proposed model of TLR orchestration of the neuro-immune interface in OA.
Chondrocytes (upper left) express a variety of TLRs, and can respond to several DAMPs detected in OA joints with production of catabolic proteases and inflammatory cytokines. These proteases enzymatically cleave ECM components, leading to release of matrix components that can themselves act as DAMPs. DAMPs can also be found in soluble form and interact with cells within the synovial membrane, specifically synovial leukocytes (primarily macrophages) and fibroblasts. TLR activation of these cell types can contribute to inflammatory protease production and articular damage, but can also lead to production of chemokines and cytokines that can draw in other leukocytes, increasing synovial inflammation. In addition, TLR activation of leukocytes can generate soluble pain mediators such as NGF and CCL2 which can sensitize nociceptive nerve endings in the joint, resulting in sensitization and pain. Dotted lines indicate aspects of this axis that are of particular interest for further investigation. For example, TLR activation of myeloid cells can lead to macrophage phenotype polarization, and recent evidence suggests a role in the development of the M2 (reparative) phenotype. In addition, in vivo evidence in OA models suggests that an early macrophage response may play a role in limiting cartilage matrix damage. The specific mechanisms and factors involved in this potential protective effect are not clear. Furthermore, TLRs are also expressed on the cell bodies of nociceptive neurons innervating the joint, and they can be activated directly by DAMPs. These cells also express receptors for pro-inflammatory molecules released by innate immune cells, for example NGF and CCL2, which both have strong pro-algesic effects.
Table 1 summarizes findings in Tlr null mice with experimental OA. In one study, several different individual Tlr knock-out mice were tested in a partial medial meniscectomy model (all female mice) [46]. Eight weeks after surgery, Tlr1, Tlr2, Tlr4, Tlr6, and Myd88 knock-out mice were not protected from cartilage damage. Synovial thickness was also similar among all strains, with the exception of Tlr1 null mice, which had increased synovial thickness. In the 16-week DMM model (all performed in male mice), Tlr2 and Tlr4 null mice developed similar joint damage and associated referred mechanical allodynia in the hindpaw as wild-types [24, 31], but Tlr2 null mice were protected from local knee hyperalgesia [31]. It should be noted that the role of TLR2 in persistent pain has been mostly studied in neuropathic pain models where, by and large, mechanical allodynia is attenuated when Tlr2 is ablated [18]. Likewise, Tlr4 null mice display attenuated mechanical allodynia in a model of neuropathic pain [47], while in the K/BxN serum transfer model of arthritis, Tlr4 null mice showed reversal of mechanical allodynia after resolution of peripheral inflammation, and this through a central mechanism [48]. Divergent findings between models indicate that the relative contribution of TLRs to different components of the pain pathway may depend on the triggers, and underscore the importance of using disease-specific models when interrogating targets in pain pathways.
In contrast to Tlr2 and Tlr4 null mice, Cd14 knock-out mice were protected from cartilage damage 19 weeks after DMM, as well as from changes in subchondral bone mineral density and trabecular thickness at 6 and 19 weeks [49]. These mice were also protected from the decline in spontaneous activity after DMM observed in wild-type mice, most prominently seen in climbing. Post-DMM decline in spontaneous murine locomotor activities has been suggested to be a surrogate measure of pain-related behavior [16, 50]. Since CD14 acts as an adapter protein for multiple TLRs, it is possible that this helps to explain the protection noted in this study compared to other studies where individual TLRs were knocked out.
Findings in Tlr null mice also suggest how these pathways may differentially contribute to OA pathology in models with varying degrees of synovitis. In an abstract presented at the 2012 annual meeting of the Orthopedic Research Society, Tlr2 knock-out mice reportedly developed more severe cartilage damage after 6 weeks in the collagenase-induced model of OA, while in the DMM model, cartilage damage was similar to wild-type mice after 8 weeks, perhaps reflecting the difference in synovial involvement between the two models [51]. This group has demonstrated similar findings in S100a9 knock-out mice (which are also functional S100a8 knock-outs), which were protected from synovitis, cartilage damage, and osteophyte formation in collagenase-induced OA but not in the DMM model [52, 53]. Furthermore, prophylactic treatment with paquinimod, an immunomodulatory compound that prevents S100A9 binding to TLR4, reduced synovitis, cartilage damage, and osteophyte formation in collagenase-induced OA, but only had minor preventative effects on cartilage damage in the DMM model [54].
Clinical correlates
Several lines of evidence from human in vitro and patient cohort studies highlight the importance of TLR-mediated inflammatory activity in OA. Disease-related modulation of TLR expression, adaptor proteins and downstream mediators have been demonstrated in multiple tissues and cell types throughout the joint. Associations between TLR-polymorphisms, epigenetic influences of TLR activity, and disease risk or phenotype have been described. Moreover, recent studies suggest that soluble markers of TLR activation may be associated with specific disease characteristics. Overall, recent work provides support that TLR-pathways may be an important link between inflammatory activity in the joint, OA pain, and disease progression that could point to novel targets for therapy.
Early reports appearing in the mid 2000s documented expression of TLR2 [55, 56] and TLR4 [57] on human chondrocytes, and upregulation in lesional areas of cartilage from patients with knee and hip OA [57]. In addition, Kim et al. demonstrated that stimulation of TLR2 and TLR4 led to enzymatically mediated aggrecan and collagen loss from human cartilage explants [57]. More recent work has demonstrated that TLR activity can suppress chondrocyte anabolic responses. Specifically, a 29kDa fibronectin fragment that interacts with TLR2 [58], suppressed expression of XT-1 (an enzyme which catalyzes GAG synthesis) by human chondrocytes; this enzyme is also suppressed in patients with OA. It is now clear that several TLRs are expressed by human chondrocytes, including the cell-surface TLRs (TLRs 1, 2, 4, 5 and 6) as well as TLR3 [59] and TLR9 [60]. The relevance of chondrocyte TLR responses in OA is further supported by the finding that proportions of chondrocytes expressing TLR-1, 2, 4 and 9 increase with worsening grade of OA [60]. Inflammatory cytokines such as TNFα [60], and DAMPs such as fibronectin fragments [56], upregulate TLR expression, which may lead to a vicious cycle of chondrocyte-intrinsic inflammatory signaling that perpetuates matrix degradation as disease advances. Finally, a recent study suggests that there are joint-specific TLR expression patterns that point to differential mechanisms in large and small joint OA. Barreto and colleagues [61] compared expression of TLRs 1–9 between cartilage taken from the tibial plateau of patients with knee OA, or the first carpometacarpal joint (CMC) of patients with hand OA. All TLRs were expressed in knee OA cartilage, but CMC OA cartilage showed no TLR4 expression. In contrast, TLR4 mRNA and synovial fluid levels of soluble TLR4 (sTLR4) were higher in CMC than knee OA. The authors hypothesized that solubilization of TLR4 could be a negative regulatory loop in response to chronic TLR4 activation, and that the differential findings in hand and knee cartilage suggest joint-specific TLR stimuli or regulation. These data may impact the potential for TLR-targeted therapies in different patient populations.
In vitro studies using human joint tissues discussed suggest higher expression and importance of the cell-surface TLRs in OA. Genetic associations with OA risk, however, suggest a potential role for the endosomal TLRs. The first TLR-related genetic associations were reported in a Chinese case-control study [62]. Investigators measured six TLR-related single nucleotide polymorphisms (SNPs) spanning TLR2, TLR4, and TLR9, in two separate populations. No associations with the TLR2 or TLR4 SNPs were found, while the CC variant of the T-1486C SNP in TLR9 was associated with knee OA in both populations. Combining both populations (total 503 OA patients and 428 healthy controls), this SNP conferred an overall risk (adjusted OR) of 2.29, 95% CI 1.39–3.75 (p < 0.001). The same SNP, located in the promoter region of TLR9, was also found to be associated with knee OA in a Turkish population [63], but in this study the TT genotype associated with OA. The reasons for this discrepancy remain to be elucidated, and findings await validation in other populations. As TLR9 is a nucleic acid sensing TLR that resides in the endosomal compartment, the authors of the Chinese study subsequently investigated other endosomal TLRs (TLR3, −7 and −8) in an expanded population (823 OA cases and 594 healthy controls) [64]. Two TLR3 SNPs (rs3775296 and rs3775290) were significantly associated with OA, with overall adjusted OR of 2.11 (1.33–3.33) and 1.86 (1.32–2.62), respectively. Associations between OA and TLR7 and TLR8 SNPs were also found, but only in males; these genes are expressed on the X-chromosome. While these studies require validation in other populations, they provide early support for the clinical relevance of endosomal TLR-mediated pathways and OA. Self dsRNA is produced by damaged chondrocytes in vitro and could potentially be a DAMP for TLR3 [65], but the mechanisms by which these endosomal receptors influence OA risk require further investigation.
The role of numerous DAMPs has been supported by recent in vitro and preclinical studies discussed earlier. In addition, there is also emerging evidence suggesting that PAMPs may be important in OA patients. Low levels of LPS are detectable in serum and SF from OA patients and associated with synovial macrophage inflammation [66]. Intriguingly, SF levels of LPS were also associated with radiographic severity and symptom severity measured by the WOMAC score in this study. Although the specific source of this LPS in OA patients is unclear, based on findings in obesity and other chronic diseases, an alteration in the gut microbiome and disturbance in gut permeability has been hypothesized [67]. Indeed, two recent preclinical studies support these ideas. In one study, aged female Tlr4 null mice subjected to a high-fat diet were protected from articular cartilage damage, despite becoming obese and glucose intolerant [68]. In addition, a study in Tlr5 null mice, which display an altered microbiome and metabolic phenotype, showed that altering the gut microbiome with antibiotics was sufficient to lessen cartilage damage in a load-induced model of OA [69].
The evidence supporting a role for TLR pathways in OA is also leading to identification of surrogate markers of disease severity and pathology. Several co-receptors are required for LPS to optimally activate TLR4-signaling, including LPS binding protein (LBP), MD-2 and CD14. LBP and MD-2 are secreted proteins, while CD14 is found as a GPI-anchored membrane protein and as a soluble form generated during macrophage activation [70]. Our group demonstrated that SF sCD14 levels in OA patients are elevated compared to controls [71]. In addition, the soluble form remains biologically active, as it can augment TLR signaling in endothelial cells [70] and synoviocytes [71]. Daghestani et al. subsequently reported that SF sCD14 levels were associated with density of activated macrophages in the joint capsule/synovium measured semi-quantitatively on SPECT/CT images [72], consistent with its primary cellular source. This study also found that SF sCD14 was associated with several clinical measures of disease severity, including severity of knee pain and joint space narrowing, and was predictive of osteophyte progression in a cohort of patients followed longitudinally with radiographs taken at 3-year follow-up. Most recently, these investigators extended their findings by investigating clinical relationships between plasma levels of LBP, sTLR4, and IL-6 as biomarkers of chronic innate inflammatory activity [73]. IL-6 is a prominent NFκB-induced cytokine mediator downstream from TLR activation. Plasma samples from 431 participants in a previous trial of doxycycline in OA [74] were included, collected at both baseline and 18 month visits. Interestingly, plasma LBP (both at baseline and time-integrated concentrations) correlated with worsening joint space width and narrowing after adjustment for age and BMI. sTLR4 levels were not associated with radiographic progression, but did correlate with urinary CTX-II levels over time, a measure of OA activity reflective of collagen type II turnover. This study only included obese female participants with unilateral knee OA, so it remains to be seen if these markers of innate inflammatory activity, and specifically of TLR-mediated inflammation, have potential to predict structural progression or symptomatic disease in more generalizable populations. Taken together, however, this growing body of work demonstrating correlations between soluble markers reflective of TLR pathway activity and both structural and symptomatic disease outcomes, provides strong support for the clinical relevance of TLR activity in OA disease pathogenesis. The relationships between markers of TLR activity and symptom severity are particularly intriguing in light of the mechanistic links between pain sensitization and TLR pathways described earlier. However, a direct relationship between TLR activation and sensitization remains to be tested.
Future Directions and Conclusions
It is now clearly accepted that, within an OA joint, tissue damage and cellular stress lead to elaboration of various TLR agonists and co-factors within different joint tissues, activating inflammatory and catabolic signaling cascades in chondrocytes, synovial leukocytes and fibroblasts. This results in a vicious cycle that perpetuates progressive joint damage, while it is now also becoming clear that it may promote sensitization and pain through neuro-immune crosstalk, although specific pathways and interactions need to be elucidated (Fig. 2). Thus, sensitization could occur rapidly when DAMPs directly bind TLRs expressed by sensory neurons, and can be perpetuated indirectly via innate immune cells (mainly macrophages, and also mast cells) present in the synovium, but also in the DRGs.
Intriguing links have been proposed between TLR signaling and NGF, a neurotrophic factor secreted by many cells including innate immune cells, fibroblasts, and chondrocytes, that is essential for nociceptor sensitization and pain [14]. In culture models of human intervertebral discs, it has been shown that TLR2 (but not TLR4) agonists trigger NGF production [75]. It has also been described that TLR4 activation of human monocytes increases their expression of the high-affinity receptor for NGF, TrkA. When NGF binds TrkA, this results in modulated TLR4 signaling and an attenuated pro-inflammatory response [76]. While there are currently only few such observations describing a bidirectional relationship between TLRs and NGF, they do invite a deeper dive into these mechanisms in the context of OA pain, especially in light of the imminent approval of tanezumab, a neutralising anti-NGF antibody for OA pain [77]. Similarly, there have been reports on communication between sensory neurons and macrophages, indicating that the neuropeptide, calcitonin gene‐related peptide (CGRP, released from sensory nerves) can modulate TLR4‐activated murine macrophages toward an anti-inflammatory phenotype [78]. These observations of neuro-immune modulation of inflammatory responses, along with the new insights that the DAMP biglycan can signal through TLR4/CD44 to ultimately increase M2 polarized macrophages in a renal injury model, suggest that the ultimate in vivo effect of TLR signaling in multiple cell-types after tissue injury is clearly more complex than previously thought. Therefore, it can be expected that detailed research in animal models of OA will shed light on these complex interactions. Attention to roles of TLR signaling in specific cells and joint tissues, and at specific stages of disease may enhance our understanding of which TLRs could be targeted therapeutically to manage joint damage and pain in OA. Inhibition of TLR pathways can be achieved by (1) blocking binding of ligands/DAMPs to the TLR, (2) disrupting intracellular signaling pathways, or (3) inhibiting TLR co-factors/enhancers (i.e., MD2, CD14…). In the first group, neutralizing antibodies have been developed that preclude binding of alarmins to TLRs (reviewed in [79]); specifically, there are antibodies available against TLR2, against TLR4, and one against TLR4/MD2. Based on the widespread expression of TLRs in the joint, it is likely that targeting multiple TLRs may be necessary, perhaps through targeting of adapter or co-receptor molecules. Alternatively, targeting high abundance DAMPs such as the 32-mer may be a feasible strategy. Subsets of patients with high levels of synovitis may also benefit from targeting S100A8/A9. In all these cases, the timing of the therapy must be considered since generation of DAMPs from the extracellular matrix, influx of TLR-expressing leukocytes, and expression patterns of TLRs by participating cells (including nociceptors) may occur in a temporal fashion in this slowly progressive disease.
Clinical grade reagents already exist to modulate TLR pathways through these different approaches. As examples, antibodies targeting TLR4 (NI-0101) have already been in phase 1 clinical trials for rheumatoid arthritis (clinicaltrials.gov identifier ) [80], and anti-CD14 antibodies are currently being tested in neurodegenerative disease and lung injury (IC14) (clinicaltrials.gov identifiers , ). To our knowledge, none have been tested in OA. Given the mounting evidence for TLR pathways both at the pre-clinical and clinical level, and availability of therapeutics targeting these pathways, the likelihood of future work in this area leading to clinically effective new therapies is high.
Acknowledgements
REM is supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (K01AR070328). AMM (R01AR064251 and R01AR060364) is supported by NIAMS. CRS is supported by the VA Rehabilitation Research and Development Service (RX001757), and funding from the University of Pennsylvania Perelman School of Medicine.
Conflict Statement
R. E. Miller and C.R. Scanzello have no conflicts to declare. A.M. Malfait has received research funding from Galapagos N.V. and from GSK, and consulting fees from Eli-Lilly/Pfizer, EMD Serono, and Vizuri.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Collaborators GDaIIaP: Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390(10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puig-Junoy J, Ruiz Zamora A: Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin Arthritis Rheum 2015, 44(5):531–541. [DOI] [PubMed] [Google Scholar]
- 3.Hawker GA, Croxford R, Bierman AS, Harvey PJ, Ravi B, Stanaitis I, Lipscombe LL: All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One 2014, 9(3):e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA: The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol 2012, 26(5):637–647. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman IN, Kemp JL, Crossley KM, Culvenor AG, Hinman RS: Hip and Knee Osteoarthritis Affects Younger People, Too. J Orthop Sports Phys Ther 2017, 47(2):67–79. [DOI] [PubMed] [Google Scholar]
- 6.Castagnini F, Sudanese A, Bordini B, Tassinari E, Stea S, Toni A: Total Knee Replacement in Young Patients: Survival and Causes of Revision in a Registry Population. J Arthroplasty 2017, 32(11):3368–3372. [DOI] [PubMed] [Google Scholar]
- 7.Orlowsky EW, Kraus VB: The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol 2015, 42(3):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanzello CR: Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 2017, 29(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syx D, Tran PB, Miller RE, Malfait AM: Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr Rheumatol Rep 2018, 20(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neogi T: Structural correlates of pain in osteoarthritis. Clin Exp Rheumatol 2017, 35 Suppl 107(5):75–78. [PubMed] [Google Scholar]
- 11.Basbaum AI, Bautista DM, Scherrer G, Julius D: Cellular and molecular mechanisms of pain. Cell 2009, 139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woller SA, Eddinger KA, Corr M, Yaksh TL: An overview of pathways encoding nociception. Clin Exp Rheumatol 2018, 36(1):172. [PubMed] [Google Scholar]
- 13.Matsuda M, Huh Y, Ji RR: Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019, 33(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denk F, Bennett DL, McMahon SB: Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci 2017, 40:307–325. [DOI] [PubMed] [Google Scholar]
- 15.Pinho-Ribeiro FA, Verri WA Jr., Chiu IM: Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol 2017, 38(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM: CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 2012, 109(50):20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nefla M, Holzinger D, Berenbaum F, Jacques C: The danger from within: alarmins in arthritis. Nat Rev Rheumatol 2016, 12(11):669–683. [DOI] [PubMed] [Google Scholar]
- 18.Lacagnina MJ, Watkins LR, Grace PM: Toll-like receptors and their role in persistent pain. Pharmacol Ther 2018, 184:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki T, Kawai T: Toll-like receptor signaling pathways. Front Immunol 2014, 5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokolove J, Lepus CM: Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013, 5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Bosch MHJ: Inflammation in osteoarthritis: is it time to dampen the alarm(in) in this debilitating disease? Clin Exp Immunol 2019, 195(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G: TLR4 signalling in osteoarthritis--finding targets for candidate DMOADs. Nat Rev Rheumatol 2015, 11(3):159–170. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal AK: Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol 2011, 23(2):170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RE, Belmadani A, Ishihara S, Tran PB, Ren D, Miller RJ, Malfait AM: Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol 2015, 67(11):2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadachi R, Hargreaves KM: Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 2006, 85(1):49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero AT, Pinto LG, Cunha FQ, Ferreira SH, Alves-Filho JC, Verri WA Jr., Cunha TM: Mechanisms underlying the hyperalgesic responses triggered by joint activation of TLR4. Pharmacol Rep 2016, 68(6):1293–1300. [DOI] [PubMed] [Google Scholar]
- 27.Tortorella MD, Malfait AM: Will the real aggrecanase(s) step up: evaluating the criteria that define aggrecanase activity in osteoarthritis. Curr Pharm Biotechnol 2008, 9(1):16–23. [DOI] [PubMed] [Google Scholar]
- 28.Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA: Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem 1991, 266(24):15579–15582. [PubMed] [Google Scholar]
- 29.Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, Fosang AJ: Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol 2015, 67(5):1240–1249. [DOI] [PubMed] [Google Scholar]
- 30.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ: Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest 2007, 117(6):1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, Fosang AJ, Malfait AM: An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 2018, 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M et al. : The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 2005, 115(8):2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L: De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol 2014, 35:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreto G, Soininen A, Ylinen P, Sandelin J, Konttinen YT, Nordstrom DC, Eklund KK: Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther 2015, 17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avenoso A, D’Ascola A, Scuruchi M, Mandraffino G, Calatroni A, Saitta A, Campo S, Campo GM: The proteoglycan biglycan mediates inflammatory response by activating TLR-4 in human chondrocytes: Inhibition by specific siRNA and high polymerized Hyaluronan. Arch Biochem Biophys 2018, 640:75–82. [DOI] [PubMed] [Google Scholar]
- 36.Poluzzi C, Nastase MV, Zeng-Brouwers J, Roedig H, Hsieh LT, Michaelis JB, Buhl EM, Rezende F, Manavski Y, Bleich A et al. : Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int 2019, 95(3):540–562. [DOI] [PubMed] [Google Scholar]
- 37.Roedig H, Nastase MV, Wygrecka M, Schaefer L: Breaking down chronic inflammatory diseases: the role of biglycan in promoting a switch between inflammation and autophagy. FEBS J 2019. [DOI] [PubMed] [Google Scholar]
- 38.Sica A, Mantovani A: Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012, 122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chockalingam PS, Glasson SS, Lohmander LS: Tenascin-C levels in synovial fluid are elevated after injury to the human and canine joint and correlate with markers of inflammation and matrix degradation. Osteoarthritis Cartilage 2013, 21(2):339–345. [DOI] [PubMed] [Google Scholar]
- 40.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G et al. : Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009, 15(7):774–780. [DOI] [PubMed] [Google Scholar]
- 41.Zuliani-Alvarez L, Marzeda AM, Deligne C, Schwenzer A, McCann FE, Marsden BD, Piccinini AM, Midwood KS: Mapping tenascin-C interaction with toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat Commun 2017, 8(1):1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamura N, Hasegawa M, Nakoshi Y, Iino T, Sudo A, Imanaka-Yoshida K, Yoshida T, Uchida A: Deficiency of tenascin-C delays articular cartilage repair in mice. Osteoarthritis Cartilage 2010, 18(6):839–848. [DOI] [PubMed] [Google Scholar]
- 43.Matsui Y, Hasegawa M, Iino T, Imanaka-Yoshida K, Yoshida T, Sudo A: Tenascin-C Prevents Articular Cartilage Degeneration in Murine Osteoarthritis Models. Cartilage 2018, 9(1):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato J, Agalave NM, Svensson CI: Pattern recognition receptors in chronic pain: Mechanisms and therapeutic implications. Eur J Pharmacol 2016, 788:261–273. [DOI] [PubMed] [Google Scholar]
- 45.Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, Ji RR: TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep 2016, 6:28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasi S, Ea HK, Chobaz V, van Lent P, Liote F, So A, Busso N: Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent toll-like receptors (TLRs) in a murine model of osteoarthritis. Joint Bone Spine 2014, 81(4):320–324. [DOI] [PubMed] [Google Scholar]
- 47.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL: Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation 2013, 10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL: Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011, 152(12):2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambamurthy N, Zhou C, Nguyen V, Smalley R, Hankenson KD, Dodge GR, Scanzello CR: Deficiency of the pattern-recognition receptor CD14 protects against joint pathology and functional decline in a murine model of osteoarthritis. PLoS One 2018, 13(11):e0206217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inglis JJ, McNamee KE, Chia SL, Essex D, Feldmann M, Williams RO, Hunt SP, Vincent T: Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum 2008, 58(10):3110–3119. [DOI] [PubMed] [Google Scholar]
- 51.Blom AB, van Lent PL, Abdollahi-Roodsaz S, van der Kraan PM, van den Berg WB: Toll Like Receptor-2 Prevents Cartilage Damage in Osteoarthritis Models that Display Synovial Activation In: Orthopaedic Research Society Annual Meeting. San Francisco; 2012. [Google Scholar]
- 52.Schelbergen RF, de Munter W, van den Bosch MH, Lafeber FP, Sloetjes A, Vogl T, Roth J, van den Berg WB, van der Kraan PM, Blom AB et al. : Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis 2016, 75(1):218–225. [DOI] [PubMed] [Google Scholar]
- 53.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, Cats H, Vogl T, Roth J, van den Berg WB: Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 2012, 64(5):1466–1476. [DOI] [PubMed] [Google Scholar]
- 54.Schelbergen RF, Geven EJ, van den Bosch MH, Eriksson H, Leanderson T, Vogl T, Roth J, van de Loo FA, Koenders MI, van der Kraan PM et al. : Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann Rheum Dis 2015, 74(12):2254–2258. [DOI] [PubMed] [Google Scholar]
- 55.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R: TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol 2005, 174(8):5016–5023. [DOI] [PubMed] [Google Scholar]
- 56.Su SL, Tsai CD, Lee CH, Salter DM, Lee HS: Expression and regulation of Toll-like receptor 2 by IL-1beta and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage 2005, 13(10):879–886. [DOI] [PubMed] [Google Scholar]
- 57.Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, Kim HY: The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum 2006, 54(7):2152–2163. [DOI] [PubMed] [Google Scholar]
- 58.Hwang HS, Park SJ, Cheon EJ, Lee MH, Kim HA: Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res Ther 2015, 17:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Hui W, Litherland GJ, Barter MJ, Davidson R, Darrah C, Donell ST, Clark IM, Cawston TE, Robinson JH et al. : Differential Toll-like receptor-dependent collagenase expression in chondrocytes. Ann Rheum Dis 2008, 67(11):1633–1641. [DOI] [PubMed] [Google Scholar]
- 60.Sillat T, Barreto G, Clarijs P, Soininen A, Ainola M, Pajarinen J, Korhonen M, Konttinen YT, Sakalyte R, Hukkanen M et al. : Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop 2013, 84(6):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barreto G, Sandelin J, Salem A, Nordstrom DC, Waris E: Toll-like receptors and their soluble forms differ in the knee and thumb basal osteoarthritic joints. Acta Orthop 2017, 88(3):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su SL, Yang HY, Lee CH, Huang GS, Salter DM, Lee HS: The (−1486T/C) promoter polymorphism of the TLR-9 gene is associated with end-stage knee osteoarthritis in a Chinese population. J Orthop Res 2012, 30(1):9–14. [DOI] [PubMed] [Google Scholar]
- 63.Balbaloglu O, Sabah Ozcan S, Korkmaz M, Yilmaz N: Promoter polymorphism (T-1486C) of TLR-9 gene is associated with knee osteoarthritis in a Turkish population. J Orthop Res 2017, 35(11):2484–2489. [DOI] [PubMed] [Google Scholar]
- 64.Yang HY, Lee HS, Lee CH, Fang WH, Chen HC, Salter DM, Su SL: Association of a functional polymorphism in the promoter region of TLR-3 with osteoarthritis: a two-stage case-control study. J Orthop Res 2013, 31(5):680–685. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Chen K, Kang H, Yan Y, Liu K, Guo C, Qi J, Yang K, Wang F, Guo L et al. : Double-stranded RNA released from damaged articular chondrocytes promotes cartilage degeneration via Toll-like receptor 3-interleukin-33 pathway. Cell Death Dis 2017, 8(11):e3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang ZY, Stabler T, Pei FX, Kraus VB: Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 2016, 24(10):1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Z, Kraus VB: Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol 2016, 12(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalaitzoglou E, Lopes EBP, Fu Y, Herron JC, Flaming JM, Donovan EL, Hu Y, Filiberti A, Griffin TM, Humphrey MB: TLR4 Promotes and DAP12 Limits Obesity-Induced Osteoarthritis in Aged Female Mice. JBMR Plus 2019, 3(4):e10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guss JD, Ziemian SN, Luna M, Sandoval TN, Holyoak DT, Guisado GG, Roubert S, Callahan RL, Brito IL, van der Meulen MCH et al. : The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthritis Cartilage 2019, 27(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lloyd-Jones KL, Kelly MM, Kubes P: Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J Immunol 2008, 181(2):1446–1453. [DOI] [PubMed] [Google Scholar]
- 71.Nair A, Kanda V, Bush-Joseph C, Verma N, Chubinskaya S, Mikecz K, Glant TT, Malfait AM, Crow MK, Spear GT et al. : Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum 2012, 64(7):2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daghestani HN, Pieper CF, Kraus VB: Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 2015, 67(4):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang ZY, Perry E, Huebner JL, Katz B, Li YJ, Kraus VB: Biomarkers of inflammation - LBP and TLR- predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthritis Cartilage 2018, 26(12):1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, Wolfe F, Schnitzer TJ, Moreland LW, Manzi S et al. : Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum 2005, 52(7):2015–2025. [DOI] [PubMed] [Google Scholar]
- 75.Krock E, Currie JB, Weber MH, Ouellet JA, Stone LS, Rosenzweig DH, Haglund L: Nerve Growth Factor Is Regulated by Toll-Like Receptor 2 in Human Intervertebral Discs. J Biol Chem 2016, 291(7):3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minnone G, De Benedetti F, Bracci-Laudiero L: NGF and Its Receptors in the Regulation of Inflammatory Response. Int J Mol Sci 2017, 18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller RE, Block JA, Malfait AM: What is new in pain modification in osteoarthritis? Rheumatology (Oxford) 2018, 57(suppl_4):iv99–iv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baliu-Pique M, Jusek G, Holzmann B: Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur J Immunol 2014, 44(12):3708–3716. [DOI] [PubMed] [Google Scholar]
- 79.Gao W, Xiong Y, Li Q, Yang H: Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front Physiol 2017, 8:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monnet E, Lapeyre G, Poelgeest EV, Jacqmin P, Graaf K, Reijers J, Moerland M, Burggraaf J, Min C: Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin Pharmacol Ther 2017, 101(2):200–208. [DOI] [PubMed] [Google Scholar]