Abstract
Patient: Female, 49
Final Diagnosis: Metastatic metaplastic breast cancer
Symptoms: Progressive right breast mass
Medication: 5-fluorouracil • Cytoxan • Epirubicin (FEC 100) • Docetaxel • Capecitabine
Clinical Procedure: Mastectomy • Radiation Therapy
Specialty: Oncology
Objective:
Rare disease
Background:
Metaplastic breast cancer (MPBC) is a rare subtype of breast cancer that is difficult to manage and is resistant to therapy. Immunotherapy is becoming a promising option for care of several types of cancers including triple negative breast cancer.
Case Report:
We present a case of a patient with chemo-refractory metastatic metaplastic-type of breast cancer with failures to 3 active lines of chemotherapy. The patient achieved a complete and sustained response to chemo-immunotherapy combination with acceptable tolerability and minimal adverse events. The treatment was a combination of anti-PD-L1 antibody (durvalumab) immunotherapy in combination with the chemotherapeutic agent, paclitaxel.
Conclusions:
This is the first case reporting the successful use of durvalumab and paclitaxel combination for treatment of triple negative breast cancer in general, and in metastatic MPBC in specific.
MeSH Keywords: Antigens, CD274; Paclitaxel; Triple Negative Breast Neoplasms
Background
Metaplastic breast cancer (MPBC) has a poor response to conventional chemotherapy and carries an overall worse prognosis [1]. Although most MPBCs are estrogen receptor (ER), progesterone receptor (PR), and HER2-neu negative [2–4] (i.e., triple negative breast cancer [TNBC]), it is usually more aggressive than the other types of TNBC [5,6]. Optimal treatment of MPBC remains controversial and even though MPBC is usually treated in the same way as other TNBCs; it differs in several pathological and clinical aspects [5–7]. Response to systemic chemotherapy remains less than 20% [8,9].
Consistent with the recently reported efficacy of anti PD-L1 in combination with chemotherapy in the treatment of TNBC [10], we observed a remarkable response in a case of a chemo-refractory metastatic MPBC enrolled in an ongoing clinical trial of paclitaxel in combination with the anti PD-L1 durvalumab to treat TNBC patients.
Case Report
A 49-year-old premenopausal female was referred to our cancer center with diagnosis of right breast cancer based on core biopsy obtained on February 2016. Past medical history was only significant for hypothyroidism for which she was on replacement therapy. She had a workup in February 2016. Mammograpy revealed an abnormal mass in the right upper outer quadrant measuring 7.4×7×10 cm and extending into the central breast and attached to the skin with multiple ill-defined obscured masses in the lower inner quadrant. In addition, there was suspicious axillary lymph node as well as multiple masses in the left breast. Ultrasound described multiple bilateral cysts in both breasts, the largest within the left breast measuring 2.3×1.8×2.5 cm in addition to the index lesion.
Computed tomography (CT) scan of the chest, abdomen, and pelvis described right breast mass measuring 6.7×8.5×11 cm with central necrosis and this was associated with suspicious right axillary lymph node and there was no evidence of distant metastasis and the bone scan was negative. Pancreatic lesions were described. Magnetic resonance imaging (MRI) of the abdomen described multiple pancreatic cysts that were likely benign. Positron emission tomography (PET)/CT scan demonstrated uptake in the right breast lesion as well as suspicious right axillary lymph nodes but no evidence of distant metastasis. The final clinical stage was T4N1M0.
The biopsy showed tumor cells negative for estrogen and progesterone receptors as well as HER2 amplification (hence the cancer was designated TNBC) with squamous elements and histologically consistent with MPBC. Another nodule biopsy from the right breast at the 10 o’clock region showed fibroadenoma. Biopsy from the right axillary lymph node revealed no evidence of malignancy. Fine needle aspiration from the left breast cystic mass showed blood and macrophages, which is consistent with cyst content.
The patient was treated with neo-adjuvant chemotherapy in the form of 3 cycles of 5-fluorouracil, Cytoxan, and epirubicin (FEC 100) followed by 3 cycles of docetaxel completed on June 2016 with minimal clinical response. Then the patient underwent right modified radical mastectomy on July 2016. Unfortunately, she had poor pathological response to chemo-therapy as there was a residual metaplastic carcinoma measuring 9.5×8×6 cm in size, squamous cell carcinoma subtype, unifocal and occupying the outer quadrant. The surgical margins were negative; however, the tumor was invading the skin with ulceration. One out of 2 sentinel lymph nodes were positive with no evidence of extra nodal extension. Axillary lymph node dissection revealed additional 8 lymph nodes negative for metastasis. Pathological stage was ypT4bypN1aMx. The patient had uneventful post-operative course and on September 2016, she completed 40.5 Gy in 15 fractions of adjuvant radiotherapy, to the right chest wall and to the right supraclavicular area.
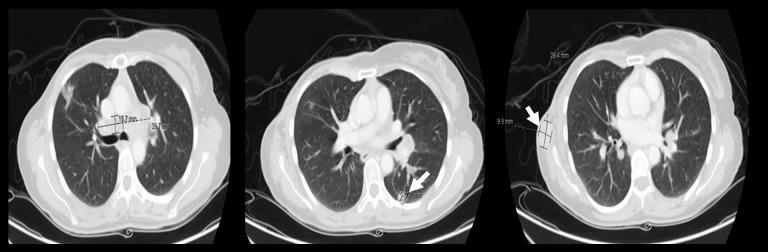
The patient was seen in the clinic in December 2016 with a 2-month history of a progressive right chest wall nodule. In addition, she had progressive shortness of breath for 1 month with dry cough. Clinical examination revealed a firm 4×3 cm mass on lateral edge of mastectomy incision, highly suspicious for local recurrence. Biopsy was consistent with recurrence of the triple negative MPBC, squamous cell subtype. Restaging CT chest, abdomen and pelvis on December 28, 2016 showed development of metastatic enhancing lesion at subcutaneous fat of the right chest wall inferior to right axilla and a new multiple necrotic metastatic lymph node in the mediastinum and hila with multiple metastatic bilateral pulmonary lesions (Figure 1). There were no other metastatic lesions in abdomen and pelvis. The bone scan was clear.
Figure 1.
Development of metastatic lesion months after neoadjuvant chemotherapy and mastectomy. Computed tomography chest, abdomen and pelvis on December 28, 2016 showing new development of metastatic enhancing lesion at subcutaneous fat of the right chest wall inferior to right axilla and new multiple necrotic metastatic lymph nodes in the mediastinum and hila with multiple pulmonary metastatic nodules. Lesions are indicated in white arrows
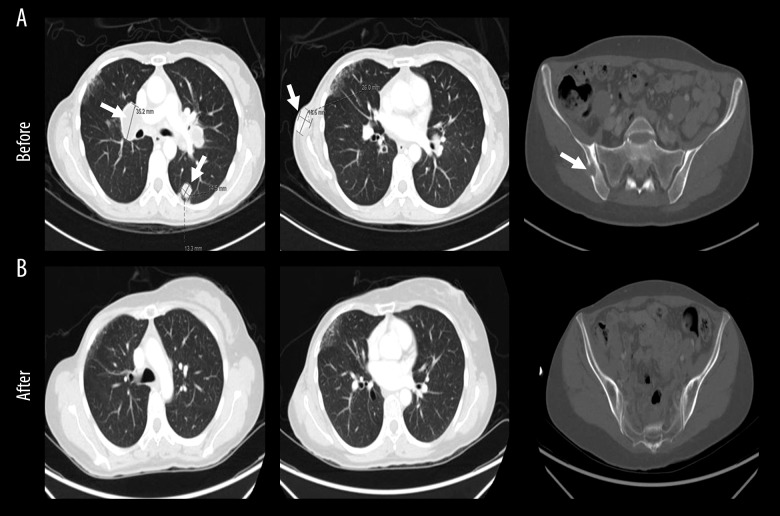
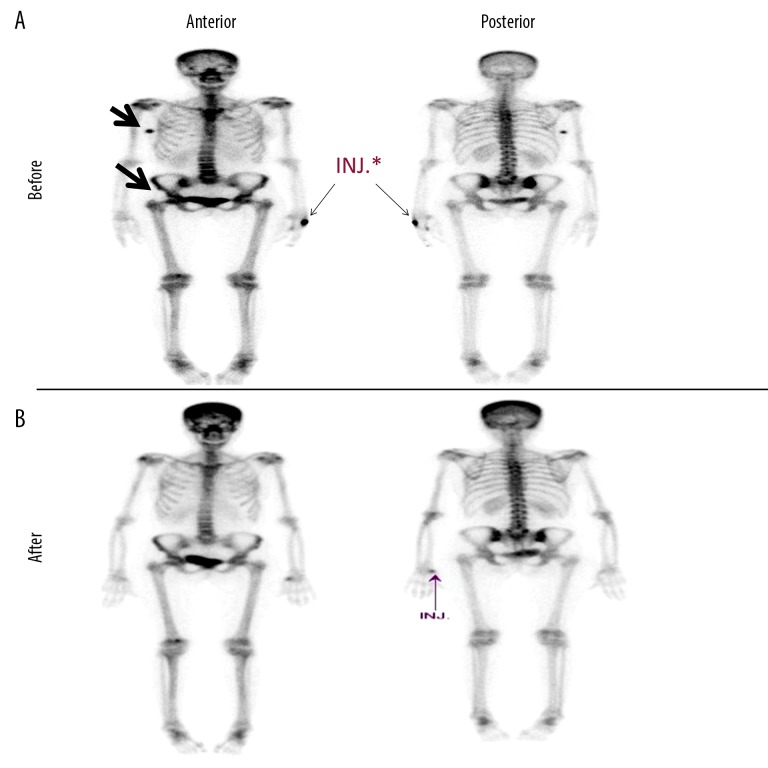
The patient received 4 cycles of capecitabine [11]. CT chest, abdomen and pelvis done on April 17, 2017 showed progressive disease with an increase in size and number of pulmonary nodules and mediastinal lymph nodes. In addition, there was a newly developed lytic lesion with soft tissue component in the right iliac bone (Figure 2A). Bone scan confirmed pelvic bony metastasis in the right iliac bone and in the right femoral head (Figure 3A).
Figure 2.
Dramatic radiological response after chemo-immunotherapy. Computed tomography chest, abdomen and pelvis showing: (A) pretreatment; an increase in size of metastatic lymph nodes in the mediastinum and hila, multiple pulmonary metastatic lesions (left and middle) and a newly developed lytic lesion with soft tissue component in the right iliac bone (right), (B) post treatment; an excellent response with no suspicious pulmonary nodules (left and middle) and disappearance of right iliac bone soft tissue mass (right). Lesions are indicated in white arrows.
Figure 3.
Resolution of bone lesions after chemo-immunotherapy. Bone scan showing: (A) pretreatment; interval development of a focus of diffuse radiotracer uptake in the medial aspect of the right iliac and right femoral head, (B) post treatment; interval improvement in the overall scan appearance. Site lesions are indicated in black arrows. INJ – radiotracer injection.
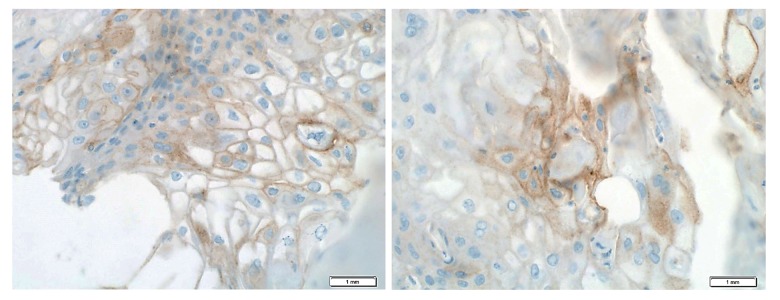
Due to lack of an effective standard therapy to manage metastatic MPBC, the patient was offered enrollment in a clinical chemo-immune therapy trial designed for TNBC patients using the anti-PD-L1 durvalumab in combination with paclitaxel. PD-L1 expression was checked by immunohistochemistry and was positive in 20% of tumor cells with medium intensity while there was no tumor infiltrating lymphocytes in the examined section (Figure 4). The patient was enrolled in a phase I/II institutional clinical trial testing the anti PD-L1 durvalumab in combination with paclitaxel (NCT02628132).
Figure 4.
PD-L1 is expressed on tumor cells. Representative immunohistochemical images of formalin-fixed paraffin-embedded tumor tissue sections stained for PD-L1 (brown) at 40× using fully automated Benchmark platform (Ventana/Roche, USA). Nuclei were counterstained using hematoxylin (blue).
As per the trial protocol, the patient received 1 cycle of paclitaxel alone followed by a combination of paclitaxel and durvalumab on May 2017. Paclitaxel was given as 80 mg/m2 weekly on days 1, 8, and 15 of every 28 days. There was subjective and objective clinical response to single agent weekly paclitaxel.
Upon clinical examination, there was a decrease in locally recurrent mass from 4×3 cm to 2×2 cm. Durvalumab was given in addition to paclitaxel starting from cycle 2 and it was given at a fixed dose of 750 mg on days 1 and 15 of every 28 days. After the 4th cycle the patient had clinically complete response in chest wall nodules and her symptoms of cough and shortness of breath improved. Bone scan on December 17, 2017 revealed interval improvement in the overall scan appearance (Figure 3B).
On March 13, 2018, a CT scan indicates a complete clearance of pulmonary nodules, nodal disease, as well as chest wall lesion, mediastinal nodes, and pulmonary metastasis. There was resolution of the associated soft tissue component of lytic right iliac bone (Figure 2B).
The patient had multiple CT scans from May 2018 until August 4, 2019 and all were consistent with complete response with no evidence of local disease or lung nodules. The last bone scan was in October 2018 and showed normalization of uptake in the right iliac bone (Figure 3).
The patient was last seen in the clinic in August 2019 which was more than 2 years since the patient received the first dose of the durvalumab-paclitaxel combination. The patient is clinically doing very well with no evidence of relapse and she is tolerating the biweekly durvalumab well. The only reported side effect was an increase in serum cholesterol and she was given a statin with favorable response. Her thyroid function remained stable.
Discussion
Metaplastic breast cancer (MPBC) is a rare and histologically diverse subtype of breast carcinoma. It accounts for less than 1% of all breast cancers. Histologically, these tumors encompass adenocarcinoma with an admixture of spindle cell, squamous, chondroid or bone-forming neoplastic cells [12].
Most MPBCs are triple negative (i.e., TNBC) [2–4] and tend to have a worse prognosis than other TNBC [5,6]. Optimal treatment of MPBC remains controversial and even though MPBC is usually treated in the same way as other TNBC; it differs in several pathological and clinical aspects [5–7]. Due to its rarity and resistance to conventional chemotherapy, there are no specific guidelines for the management of advanced MPBC. Literature indicates that MPBC is associated with a poor response to standard chemotherapeutic agents, thus generally considered to be chemo-resistant with poorer prognosis than typical infiltrating ductal carcinomas (IDC) of the breast including TNBC [5,7].
The positive PD-L1 expression in our case was in concordance with the results of comprehensive profiling of a large cohort of MPBC published previously [13]. In the previous report, Joneja et al. reported on PD-L1 expression in a significantly higher proportion of MPBCs (46%) than in other subtypes (6% each in hormone-positive and HER2-positive breast cancers, and 9% in TNBC, not otherwise specified, P<0.001) [13]. The overexpression of PD-L1 in primary MPBC, suggests that PD-1/PD-L1 inhibitors might be used in the treatment of aggressive, resistant MPBCs. The PDL-1 expression seems to be an important predictive marker for the response to immuno-therapy in advanced TNBC [14,15].
MPBC subtype of breast cancer has pathological features characterized by transformation of neoplastic epithelium into mesenchymal-like elements in a process known biologically as epithelial-to mesenchymal-transition (EMT) [16]. Typically, in EMT, epithelial-like cells lose some of their epithelial traits and acquire instead mesenchymal features. Interestingly, we and others have previously shown that this transition upregulates PD-L1 in breast cancer and other types of cancers [17,18], therefore promoting the immune evasion of cancer cells. In addition, EMT is known to induce cancer stem cells (or cancer initiating cells) that are known to be chemo-resistant [19]. Very recently, we have shown that indeed PD-L1 promotes cancer stem-like cells by maintaining the PI3K/AKT pathway and their downstream stem-related embryonic antigens OCT4 and Nanog [20]. Indeed, MPBCs were found to be enriched with cells expressing makers for cancer initiating cells [21] and to respond to the PI3K/AKT/mTOR pathway inhibitors more than non MPBCs [22,23].
In this case report the patient had a rapid response after 2 doses of durvalumab given in combination with paclitaxel despite being initially taxane-refractory when given alone in the neoadjuvant setting as recurrence happened within 6 months of completing docetaxel. This patient kept responding to treatment until she achieved a complete response in the lung and lymph nodes, chest wall, and with dramatic response in the bone after around 10 cycles of treatment with durvalumab. Our case is consistent with recently reported efficacy of the anti PD-L1 atezolizumab in combination with chemotherapy [10].
However, our case is the first to report on durvalumab and paclitaxel combination to treat metastatic MPBC.
Conclusions
This is the first report on a case of durvalumab and paclitaxel combination for the treatment of metastatic TNBC in general, and MPBC in specific. The limited therapeutic options available for MPBC support further clinical trials with chemo-immunotherapy for this type of highly refractory breast cancer.
Footnotes
Conflict of interests
None.
References:
- 1.Salemis NS. Metaplastic carcinoma of the breast with mesenchymal differentiation (carcinosarcoma). A unique presentation of an aggressive malignancy and literature review. Breast Dis. 2018;37(3):169–75. doi: 10.3233/BD-170313. [DOI] [PubMed] [Google Scholar]
- 2.Al Sayed AD, El Weshi AN, Tulbah AM, et al. Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncologica. 2006;45(2):188–95. doi: 10.1080/02841860500513235. [DOI] [PubMed] [Google Scholar]
- 3.Tse GM, Tan PH, Putti TC, et al. Metaplastic carcinoma of the breast: A clinicopathological review. J Clin Pathol. 2006;59(10):1079–83. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat. 2009;117(2):273–80. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 5.Bae SY, Lee SK, Koo MY, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126(2):471–78. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- 6.Jung SY, Kim HY, Nam BH, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120(3):627–37. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 7.Rayson D, Adjei AA, Suman VJ, et al. Metaplastic breast cancer: Prognosis and response to systemic therapy. Ann Oncol. 1999;10(4):413–19. doi: 10.1023/a:1008329910362. [DOI] [PubMed] [Google Scholar]
- 8.Chen IC, Lin CH, Huang CS, et al. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130(1):345–51. doi: 10.1007/s10549-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 9.El Zein D, Hughes M, Kumar S, et al. Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: A study from a single institution and review of literature. Clin Breast Cancer. 2017;17(5):382–91. doi: 10.1016/j.clbc.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Z, Yang Y, Li L, et al. Capecitabine monotherapy in advanced breast cancer resistant to anthracycline and taxane: A meta-analysis. J Cancer Res Ther. 2018;14(Suppl.):S957–63. doi: 10.4103/0973-1482.187384. [DOI] [PubMed] [Google Scholar]
- 12.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101(3):349–53. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 13.Joneja U, Vranic S, Swensen J, et al. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J Clin Pathol. 2017;70(3):255–59. doi: 10.1136/jclinpath-2016-203874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21):2460–67. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 16.Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107(35):15449–54. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsuliman A, Colak D, Al-Harazi O, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: Significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dongre A, Rashidian M, Reinhardt F, et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77(15):3982–89. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28(25):4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almozyan S, Colak D, Mansour F, et al. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer. 2017;141(7):1402–12. doi: 10.1002/ijc.30834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Toy KA, Kleer CG. Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol. 2012;25(2):178–84. doi: 10.1038/modpathol.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basho RK, Yam C, Gilcrease M, et al. Comparative effectiveness of an mTOR-based systemic therapy regimen in advanced, metaplastic and nonmeta-plastic triple-negative breast cancer. Oncologist. 2018;23(11):1300–9. doi: 10.1634/theoncologist.2017-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang MH, Chen IC, Lu YS. PI3K inhibitor provides durable response in metastatic metaplastic carcinoma of the breast: A hidden gem in the BELLE-4 study. J Formos Med Assoc. 2019;118(9):1333–38. doi: 10.1016/j.jfma.2018.12.004. [DOI] [PubMed] [Google Scholar]