Abstract
Background
Heart failure (HF) is a common cardiovascular complication of type 2 diabetes (T2D). This secondary analysis investigated baseline factors and treatment differences associated with risk of hospitalization for HF (hHF), and the possible association between severe hypoglycemia and hHF.
Methods
DEVOTE was a treat-to-target, double-blind cardiovascular outcomes trial in patients (n = 7637) with T2D and high cardiovascular risk randomized to insulin degludec (degludec) or insulin glargine 100 units/mL (glargine U100). The main endpoint of this secondary analysis was time to first hHF (standardized MedDRA Query definition). Severe hypoglycemia was adjudicated (American Diabetes Association definition). The main endpoint and the temporal association between severe hypoglycemia and hHF were analyzed with a Cox proportional hazards regression model. Predictors of time to first hHF were identified using baseline variables.
Results
Overall, 372 (4.9%) patients experienced hHF (550 events). There was no significant difference in the risk of hHF between treatments (hazard ratio [HR] 0.88 [0.72;1.08]95% CI, p = 0.227). Prior HF (HR 4.89 [3.90;6.14]95% CI, p ≤ 0.0001) was the strongest predictor of future hHF events. The risk of hHF significantly increased after (HR 2.2), and within a week after (HR 11.1), experiencing a severe hypoglycemic episode compared with before an episode.
Conclusions
In patients with T2D and high cardiovascular risk there were no treatment differences in terms of hHF. Prior HF was the strongest predictor of future hHF events, and there was an association between severe hypoglycemia and subsequent hHF. Further research should evaluate whether the risk of hHF can be modified by treatments aimed at reducing hypoglycemia.
Trial Registration NCT01959529
Keywords: Clinical trial, Hospitalization for heart failure, Severe hypoglycemia, Insulin degludec, Type 2 diabetes
Background
Type 2 diabetes (T2D) is becoming increasingly prevalent worldwide and is one of the leading causes of death in the United States [1, 2]. Patients with diabetes are two to three times more likely to have cardiovascular disease (CVD) compared with those without diabetes [3], and CVD is the leading cause of death among these patients [4]. The increased risk of CVD in patients with T2D may be mediated in part through sub-optimal glycemic control, especially chronic hyperglycemia [5, 6], but also hypoglycemia. With respect to the latter, a clear association has been established, although a direct causal relationship remains unclear [7–12]. As T2D progresses, maintaining glycemic control becomes more challenging, and many patients require treatment intensification using insulin. Insulin-treated T2D increases the risk of severe hypoglycemia [13], which is associated with an increased rate of cardiovascular (CV) events [14, 15] and the possible risk of hospitalization for heart failure (HF) [16–22].
T2D is an independent risk factor for the development of HF [16]. Indeed, HF has emerged as one of the most common CV complications of T2D [23, 24]. It is estimated that 6.5 million Americans have HF [25], and of these, approximately 40% are reported to have T2D [26]. HF is associated with a poor prognosis; 30–40% of those diagnosed with HF die within 1 year and from then on, mortality rates remain high at 9% per year [27]. Not surprisingly, patients with HF and diabetes have an increased risk of mortality compared with patients with diabetes and no HF [28]. With the prevalence of T2D and HF predicted to increase in the foreseeable future, the coexistence of HF and T2D will become even more pronounced [25].
Recognizing the importance of HF as a complication of T2D, it has been suggested to include HF in a broader 5-point major adverse CV event (MACE) definition when performing T2D CV outcomes trials (CVOTs) [29–31]. A recent feasibility study using the Medical Dictionary for Regulatory Activities (MedDRA)-based endpoints suggested that these MedDRA query-derived endpoints may have utility as they closely approximate the adjudicated estimates reported in CVOTs [32].
Insulin degludec (degludec) is a basal insulin with an ultra-long duration of action, which has been shown to lead to lower rates of overall confirmed, nocturnal confirmed and severe hypoglycemia compared with insulin glargine 100 units/mL (glargine U100) across different patient populations, including the general diabetes population, elderly patients and patients with type 1 diabetes [33–40]. In the CVOT Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE), the CV safety of degludec versus glargine U100 was assessed in patients with T2D at high risk of CV events [38]. The primary analysis of DEVOTE showed that degludec was non-inferior to glargine U100 in terms of a 3-point MACE composite endpoint (CV death, non-fatal myocardial infarction [MI] and non-fatal stroke) (hazard ratio [HR] 0.91 [0.78; 1.06]95% CI) [38]. A 4-point MACE composite endpoint that included hospitalization for unstable angina demonstrated similar results. In addition, treatment with degludec, compared with glargine U100, resulted in a lower rate of both severe and nocturnal severe hypoglycemia (HR 0.60 [0.48; 0.76]95% CI and 0.47 [0.31; 0.73]95% CI, respectively; both p < 0.001). In a pre-specified secondary analysis of DEVOTE, a temporal association between severe hypoglycemia and all-cause mortality was identified [11].
DEVOTE presents an opportunity to investigate the risk of HF in a large patient cohort (n = 7637) with T2D at high risk of CV events and treated with insulin. The aims of this pre-specified secondary analysis were to investigate baseline factors and treatment differences that are associated with an increased risk of hospitalization for HF (hHF), and to gain a better understanding of the possible association between severe hypoglycemia and the subsequent increased risk of hHF.
Methods
Trial design
The present pre-specified secondary analysis included patients from DEVOTE, a prospective, treat-to-target, randomized, double-blind, active-comparator CVOT. The trial was event driven, and designed to continue until at least 633 episodes of first MACE had occurred. The events (MACE and severe hypoglycemia, but not hHF) were confirmed by a central, blinded, independent Event Adjudication Committee (EAC). The median observation time was 2.0 years in both treatment arms. A more detailed description of the trial protocol, methods and the primary results has been published previously [38, 41].
DEVOTE is registered with ClinicalTrials.gov number NCT01959529 and was conducted in accordance with the Declaration of Helsinki and ICH Good Clinical Practice Guideline [42, 43]. The protocol was approved by independent ethics committees or institutional review boards for each center; written informed consent was obtained from each patient before any trial-related activities.
Patients and treatments
Eligible patients included those with T2D treated with at least one oral or injectable antihyperglycemic agent with A1C ≥ 7.0% (53 mmol/mol), or with ≥ 20 units/day of basal insulin, and were either aged ≥ 50 years with at least one co-existing CV or renal condition, or aged ≥ 60 years and had at least one pre-specified CV risk factor.
Patients were randomized 1:1 to receive either degludec or glargine U100 administered once daily between dinner and bedtime, in addition to standard of care. As the study was double-blinded, both treatments were provided in identical 100 U/mL, 10 mL vials. All patients were allowed to continue their pre-trial antihyperglycemic therapy with the exception of basal and premix insulin, which were discontinued.
Endpoints
The primary endpoint in DEVOTE was the time from randomization to first occurrence of MACE, a composite of death from CV causes, non-fatal MI or non-fatal stroke. The main endpoint of this secondary analysis was time to first hHF, an endpoint not adjudicated by the EAC. This endpoint was defined using the standardized MedDRA Query (SMQ; version 19.0) definition of cardiac failure which is restricted to specific terminology and symptoms, signs and investigational findings that are pathognomonic for cardiac failure (see Additional file 1: Additional Methods) along with a requirement for hospitalization, defined as an admission to an inpatient unit or a visit to an emergency department requiring at least a 12-h stay. The number of EAC-confirmed severe hypoglycemic episodes was a confirmatory endpoint. Severe hypoglycemia was defined in accordance with the American Diabetes Association criteria as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon or to take other corrective actions [44].
Statistical analysis
The main endpoint and associated sensitivity analyses were analyzed with a Cox proportional hazards regression model, with treatment group as a fixed factor. To identify significant predictors of time to first hHF, available baseline and medical history variables were considered in a stepwise model selection procedure in SAS PHREG [45] with p-value thresholds of 0.1 and 0.05 determining whether a single predictor should be added or removed from the model, respectively. Based on a Cox regression model that included all the significant baseline predictors simultaneously, the relative importance of a baseline predictor of hHF was calculated based on the Chi square contribution of each variable relative to the total Chi square. This indicated the relative degree to which baseline variables could predict hHF.
Several different sensitivity analyses were conducted. The time to first hHF was also defined by a broad MedDRA search that included patients with signs, symptoms or investigational findings highly suggestive of cardiac failure; this search also included the requirement for hospitalization. In another, a pre-specified set of baseline variables (sex, region, age, estimated glomerular filtration rate [eGFR], smoking status, diabetes duration, CV risk, and whether or not the patients were insulin naïve) were included as explanatory effects in the model. Similar sensitivity analyses were carried out for the primary endpoint in DEVOTE, as reported previously [38]. An additional sensitivity analysis was conducted that analyzed time to first hHF (SMQ definition) or HF leading to death (SMQ definition).
As hHF was not adjudicated in DEVOTE, two additional sensitivity analyses were conducted using information from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial adjudication of hHF (that utilized the broad MedDRA search), which included a patient population at high risk of CV events similar to DEVOTE [46]. Based on LEADER data, the proportion of positively adjudicated hHF relative to all adjudicated hHF events was calculated. These LEADER positive adjudication probabilities were used to resample the data from DEVOTE for a weighted analysis (see Additional file 1: Table S1). Serious adverse events captured by the broad MedDRA search were picked at random with a probability equal to the LEADER adjudication probabilities. This random resampling was repeated 100 times and mean values were reported. For the few preferred terms available in DEVOTE which were not available in LEADER, probabilities of 100% and 0%, respectively, were used in two separate sensitivity analyses. It should be noted that the preferred terms used in the adjudication of hHF were more extensive than those used in the DEVOTE SMQ definition.
In the main analysis, missing data were assumed to be missing at random. In order to assess the robustness of this assumption with respect to the conclusions, a tipping point analysis was conducted. In this case, non-informative censored patients randomized to degludec were assumed to have hHF the day after being censored, starting with the earliest non-informative censored patient relative to the individual randomization date and then moving forward until the upper-bound of the 95% confidence interval (CI) was above the pre-specified non-inferiority limit of 1.3 or until end.
Severe hypoglycemia and subsequent risk of experiencing hHF were analyzed with a Cox proportional hazards regression model with treatment and previous experience of severe hypoglycemia (Yes/No) as a time-varying covariate. Similar analyses to adjust for general frailty were carried out using the following baseline variables: sex, age, smoker status, geographic region from (US/Non-US), diabetes duration, insulin naïve, CV risk as fixed factors as well as eGFR and A1C as fixed covariates.
Results
hHF and baseline characteristics
Using the SMQ definition of hHF, 372 (4.9%) patients experienced hHF, reporting a total of 550 events during the 2 years of observation. Of these events, 499 were classed as cardiac disorders (see Additional file 1: Table S2). Of those patients who experienced hHF during the trial, 58.9% had a diagnosis of HF before the trial. Using the broad MedDRA search with the LEADER match, there were 618 (8.1%) patients who experienced hHF, with 948 reported events, the majority (784 events) of which were classed as cardiac disorders (see Additional file 1: Table S2).
Several baseline characteristics were significantly different for patients who experienced hHF during the trial compared with those who did not (Table 1). Overall, 93.8% of patients who experienced hHF during the trial had established CVD/chronic kidney disease (CKD) at baseline, compared with 84.8% of patients not experiencing hHF (p < 0.0001). Patients who experienced hHF during the trial had a higher body mass index versus those who did not (35.5 kg/m2 vs. 33.5 kg/m2, p < 0.001) and a lower eGFR (59.0 mL/min/1.73 m2 vs. 68.4 mL/min/1.73 m2, p < 0.001). Additionally, a higher proportion of patients who experienced hHF during the trial had hepatic impairment (9.9%) compared with those who did not have hHF (2.2%) (p < 0.0001).
Table 1.
Baseline characteristics and medical history by hHF during the trial
| hHF during trial, n = 372 | No hHF during trial, n = 7265 | p-value | |
|---|---|---|---|
| Age, years | 65.8 (± 8.1) | 64.9 (± 7.3) | 0.022 |
| Male, n (%) | 222 (59.7) | 4556 (62.7) | NS |
| Region, n (% from North America) | 299 (80.4) | 4972 (68.4) | 0.0001 |
| Established CVD/CKD ≥ 50 years, n (%) | 349 (93.8) | 6160 (84.8) | < 0.0001 |
| Hepatic impairment, n (%) | 37 (9.9) | 159 (2.2) | < 0.0001 |
| Current smoker, n (%) | 40 (10.8) | 812 (11.2) | NS |
| Insulin naïve, n (%) | 36 (9.7) | 1192 (16.4) | 0.0006 |
| A1C, % | 8.6 (± 1.8) | 8.4 (± 1.6) | NS |
| FPG, mmol/L | 9.8 (± 4.5) | 9.5 (± 3.9) | NS |
| Duration of diabetes, years | 17.6 (± 9.1) | 16.4 (± 8.9) | 0.006 |
| BMI, kg/m2 | 35.5 (± 7.3) | 33.5 (± 6.8) | < 0.001 |
| Body weight, kg | 101.2 (± 23.8) | 95.8 (± 22.8) | < 0.001 |
| eGFR, mL/min/1.73 m2 | 59.0 (± 22.6) | 68.4 (± 21.4) | < 0.001 |
| Systolic blood pressure (mmHg) | 137.1 (± 21.2) | 135.5 (± 17.9) | NS |
| Diastolic blood pressure (mmHg) | 75.1 (± 12.4) | 76.2 (± 10.3) | 0.039 |
| Pulse, beats/min | 73.2 (11.6) | 73.1 (11.3) | – |
| Prior heart failure, n (%) | 219 (58.9) | 1115 (15.3) | – |
| Prior myocardial infarction, n (%) | 182 (48.9) | 2424 (33.4) | – |
| Atrial fibrillation, n (%) | 97 (26.1) | 627 (8.6) | – |
| Macular edema, n (%) | 5 (1.3) | 24 (0.3) | – |
| Proteinuria (microalbuminuria and gross proteinuria), n (%) | 107 (28.8) | 1710 (23.5) | – |
| Insulin, n (%) | |||
| Long acting | 241 (64.8) | 4356 (60.0)b | – |
| Intermediate actinga | 59 (15.9) | 1015 (14.0)b | – |
| Bolus | 186 (50.0) | 2645 (36.4)b | – |
| Premix | 46 (12.4) | 736 (10.1)b | – |
| Antihypertensive therapy, n (%) | |||
| Beta-blockers | 279 (75.0) | 4121 (56.7)c | – |
| Calcium channel blockers | 136 (36.6) | 2322 (32.0)c | – |
| Angiotensin-converting enzyme inhibitors | 163 (43.8) | 3464 (47.7)c | – |
| Angiotensin receptor blockers | 139 (37.4) | 2416 (33.3)c | – |
| Others | 62 (16.7) | 715 (9.8)c | – |
| Diuretics, n (%) | |||
| Loop diuretics | 226 (60.8) | 1512 (20.8)c | – |
| Thiazides | 68 (18.3) | 1674 (23.0)c | – |
| Others | 95 (25.5) | 976 (13.4)c | – |
| Lipid-modifying medications, n (%) | |||
| Statins | 297 (79.8) | 5705 (78.5)c | – |
| Fibrates | 44 (11.8) | 807 (11.1)c | – |
| Ezetimibe | 18 (4.8) | 328 (4.5)c | – |
| Others | 11 (3.0) | 257 (3.5)c | – |
| Platelet aggregation inhibitors, n (%) | |||
| Acetylsalicylic acid | 253 (68.0) | 4739 (65.2)c | – |
| Others | 108 (29.0) | 1689 (23.2)c | – |
| Anti-thrombotic medication, n (%) | 78 (21.0) | 519 (7.1)c | – |
Values are mean (± SD), unless otherwise stated
Hepatic impairment defined as having a score of > 2 on a modified Child–Pugh criteria scale using only bilirubin and albumin values
A1C glycosylated hemoglobin, BMI body mass index, CKD chronic kidney disease, CVD cardiovascular disease, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, hHF hospitalization for heart failure, NS not statistically significant, SD standard deviation
aIntermediate acting insulin cover human insulin, neutral protamine Hagedorn and unknown types of insulin
bSix patients have missing initiation drug date; they are assumed to be on treatment at baseline
cNine patients have missing initiation drug date; they are assumed to be on treatment at baseline
In patients who experienced hHF during the trial versus those who did not experience hHF during the trial, a greater proportion at baseline used bolus insulin (50.0 vs. 36.4%), β-blockers (75.0 vs. 56.7%), diuretics (77.7 vs. 48.5%) and anti-thrombotics (21.0 vs. 7.1%), respectively.
Prior HF (HR 4.89), prior hepatic impairment (HR 3.08), eGFR (log regression) (HR 0.44), prior atrial fibrillation (HR 1.95), total insulin dose at week 1 (HR 1.53) and prior MI (HR 1.54) were all associated with a significantly higher risk of experiencing hHF during the trial (all p ≤ 0.0001), with a relative importance (i.e. the relative degree to which baseline variables could predict hHF) of 54.6%, 11.0%, 10.0%, 7.2%, 5.9% and 4.3%, respectively. Other significant baseline predictors included macular edema, A1C, proteinuria and systolic blood pressure (Table 2). Baseline variables considered, but not having a significant effect on time to first hHF are listed in Additional file 1: Additional Methods.
Table 2.
Predictors of time to first hHF (SMQ definition)
| Baseline predictor | Hazard ratio [95% CI] | Relative importance | P-value |
|---|---|---|---|
| Prior heart failure (Y vs. N) | 4.89 [3.90; 6.14] | 54.6 | < 0.0001 |
| Hepatic impairment (Y vs. N) | 3.08 [2.15; 4.41] | 11.0 | < 0.0001 |
| eGFR (log regression) | 0.44 [0.34; 0.58] | 10.0 | < 0.0001 |
| Atrial fibrillation (Y vs. N) | 1.95 [1.50; 2.55] | 7.2 | < 0.0001 |
| Total insulin dose (U/kg) at week 1 | 1.53 [1.27; 1.84] | 5.9 | < 0.0001 |
| Prior myocardial infarction (Y vs. N) | 1.54 [1.23; 1.91] | 4.3 | 0.0001 |
| Macular edema (Y vs. N) | 3.77 [1.40; 10.2] | 2.0 | 0.0087 |
| A1C (squared regression) | 1.00 [1.00; 1.01] | 1.8 | 0.0137 |
| Proteinuria (microalbuminuria and gross proteinuria) | 1.36 [1.06; 1.73] | 1.8 | 0.0140 |
| Systolic blood pressure at baseline | 1.01 [1.00; 1.01] | 1.5 | 0.0251 |
Variables identified by stepwise selection − FAS. Relative importance is calculated as 100 × Chi square/Total Chi square, where the Chi squares are from a model simultaneously considering all effects mentioned in the table
A1C glycosylated hemoglobin, CI confidence interval, eGFR estimated glomerular filtration rate, FAS full analysis set, hHF hospitalization for heart failure, N no, SMQ standardized Medical Dictionary for Regulatory Activities Query, U units, Y yes
Treatment differences in time to first hHF
Using the SMQ definition, the main endpoint (hHF) occurred in 4.6% of patients with a rate of 3.42 events/100 patient-years of observation (PYO) in the degludec group and in 5.2% of patients with a rate of 3.85 events/100 PYO in the glargine U100 group (HR 0.88 [0.72; 1.08]95% CI, p = 0.227). A 5-point MACE composite endpoint (CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina and hHF) demonstrated similar results to the primary 3-point MACE composite endpoint (HR 0.92 [0.82; 1.04]95% CI).
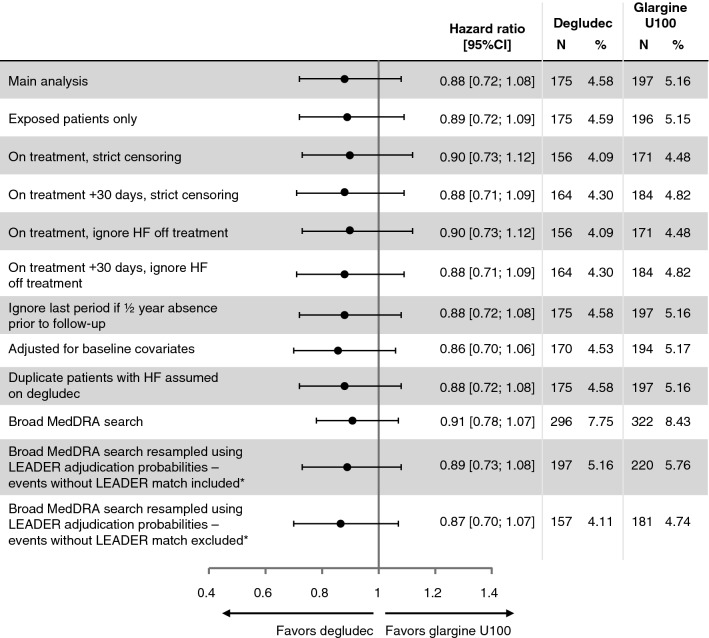
All sensitivity analyses (e.g. using the broad MedDRA search, exposed patients only, various censoring definitions, disregarding hHF off-treatment, adjusting for baseline covariates, and broad MedDRA resampled according to LEADER adjudication probabilities) were consistent with the findings of the main analysis, showing that the risk of hHF was not substantially different between degludec and glargine U100 (HR from 0.87 to 0.91, with all 95% CIs including 1.0) (Fig. 1). Using the broad MedDRA search, hHF occurred in 7.8% of patients with a rate of 5.80 events/100 PYO in the degludec group and in 8.4% of patients with a rate of 6.73 events/PYO in the glargine U100 group (HR 0.91 [0.78; 1.07]95% CI, p = 0.251). There were 12 patients (0.16%; degludec: n = 7; glargine U100: n = 5) who had HF leading to death but no hHF. When these events were included in the SMQ analysis a similar result to the main result was observed (HR 0.90 [0.73; 1.09]95% CI, p = 0.276).
Fig. 1.
Main and sensitivity analyses of treatment differences in time to first hHF (SMQ definition). *Broad MedDRA search weighted by proportion confirmed in LEADER by the Event Adjudication Committee. There were only 100 events using the broad MedDRA search without the LEADER match, 31 of which were classed as cardiac disorders. CI confidence interval, glargine U100 insulin glargine 100 units/mL, hHF hospitalization for heart failure, HF heart failure, N number of patients, % proportion of patients, SMQ standardized Medical Dictionary for Regulatory Activities Query
There were 64 patients in the degludec group and 67 patients in the glargine U100 group with non-informative censoring. In the tipping point analysis, having the most conservative assumption about these patients prematurely discontinuing the trial, it was assumed that patients in the degludec group did have hHF the day after the end of trial and those randomized to glargine U100 did not have hHF. To exceed the non-inferiority limit of 1.3 for the upper-bound of the 95% CI, it was estimated that it would be necessary to impute 38 (59%) additional first events for patients with incomplete information (non-informative censoring) treated with degludec in order to overturn the conclusion from the main endpoint. That is, with 38 additional first events added to the degludec arm the HR (degludec versus glargine U100) was 1.07 [0.88; 1.30]95% CI.
In the degludec treatment group, four patients were lost to follow up, compared with one patient in the glargine U100 treatment group. Four additional first events were imputed in the degludec group and after that, there was still a similar risk of time to first hHF with degludec versus glargine U100 (HR 0.90 [0.74; 1.11]95% CI).
Temporal association between severe hypoglycemia and the subsequent risk of hHF
The risk of hHF (at any time until the end of the trial) more than doubled (HR 2.2, p = 0.0002) after experiencing an episode of severe hypoglycemia compared with before an episode (Table 3). In addition, the risk of hHF increased more than tenfold (HR 11.1, p < 0.0001) within 7 days of experiencing an episode of severe hypoglycemia compared with before and more than 7 days after the episode. When adjusting for different sets of baseline variables the strength of this temporal association weakened, but there was still a significantly higher risk of hHF after experiencing severe hypoglycemia.
Table 3.
Temporal association between severe hypoglycemia and subsequent risk of experiencing hHF in DEVOTE and LEADER
| Time window (days after each severe hypoglycemic event) | DEVOTE (SMQ definition) | LEADER (SMQ definition) | LEADER (EAC confirmed) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Degludec | Glargine U100 | Main analysis | Sensitivity analysisa | Liraglutide | Placebo | Main analysis | Sensitivity analysisa | Liraglutide | Placebo | Main analysis | Sensitivity analysisa | |||||||||||||
| N | E | N | E | HRb | p-value | HRb | p-value | N | E | N | E | HRb | p-value | HRb | p-value | N | E | N | E | HRb | p-value | HRb | p-value | |
| 0–end trial | 173 | 10 | 238 | 18 | 2.2 | 0.0002 | 1.7 | 0.0119 | 106 | 8 | 136 | 17 | 3.0 | < 0.0001 | 2.3 | < 0.0001 | 108 | 5 | 141 | 17 | 3.2 | < 0.0001 | 2.4 | 0.0001 |
| 0–7 | 173 | 1 | 238 | 2 | 11.1 | < 0.0001 | 9.8 | < 0.0001 | 106 | 1 | 136 | 3 | 32.7 | < 0.0001 | 23.1 | < 0.0001 | 108 | 0 | 141 | 2 | N/A | – | N/A | – |
| 8–end trial | 173 | 10 | 238 | 18 | 1.6 | 0.0358 | 1.3 | 0.2507 | 106 | 8 | 136 | 17 | 2.3 | <0.0001 | 1.7 | 0.0109 | 108 | 5 | 141 | 17 | 3.3 | < 0.0001 | 2.4 | < 0.0001 |
Total number of patients experiencing severe hypoglycaemia: DEVOTE, degludec n = 187, glargine U100 n = 252 [38]; LEADER, liraglutide n = 114, placebo n = 153 [46]
For events occurring on the same day as a hypoglycemic event, 0.5 days were added to the day of the event
CV cardiovascular, E number of events, EAC Event Adjudication Committee; eGFR estimated glomerular filtration rate, glargine U100 insulin glargine 100 units/mL, hHF hospitalization for heart failure, HR hazard ratio, N number of patients, N/A analysis did not converge, SMQ standardized Medical Dictionary for Regulatory Activities Query
aAdjusted for sex, region, age, eGFR, smoking status, diabetes duration, CV risk, and whether or not the patients were insulin naïve
bhHF with prior severe hypoglycemia in window versus hHF without severe hypoglycemia in window
When the SMQ definition of hHF was applied to LEADER, similar results were observed compared with the DEVOTE SMQ hHF analysis and the LEADER EAC-confirmed hHF analysis, even after adjusting for baseline variables (Table 3). Similarly in the LEADER trial, patients experiencing severe hypoglycemia were at significantly higher risk of subsequent EAC-confirmed hHF before the end of the trial than those who did not experience severe hypoglycemia (Table 3).
Discussion
The results from this pre-specified secondary analysis of DEVOTE demonstrated that there was no significant difference in the risk of experiencing hHF with degludec versus glargine U100.
Prior HF at baseline was the strongest predictive factor for experiencing hHF during the trial. Other baseline factors associated with hHF during the trial included hepatic impairment, lower eGFR, atrial fibrillation, higher total insulin dose at week 1, prior MI, macular edema, higher A1C, proteinuria and higher systolic blood pressure. Furthermore, severe hypoglycemia during the trial increased the risk of subsequent hHF. These baseline characteristics are all hallmark characteristics of long-standing diabetes as well as both hepatic impairment and atrial fibrillation potentially being signs of established HF. In particular, a recent survey highlighted that patients with T2D with high blood glucose levels (> 11.1 mmol/L) admitted to hospital with HF had an increased mortality risk [47]. Furthermore, glycemic variability has also been shown to be independently related to an increased risk of all-cause mortality in patients with T2D and HF [48]. When simultaneously considering other baseline variables, diabetes duration was not a strong predictor of hHF. Likewise, age was significantly associated with hHF during the trial in a single-factor analysis, but when considering other predictors of hHF simultaneously this association was no longer significant. Therefore, suggesting that it is the complications associated with age and diabetes duration, and not the age or diabetes duration in itself, leading to an increased risk of hHF.
In this study, 58.9% of patients who experienced hHF had a diagnosis of HF before the trial, compared with 15.3% of patients who did not experience hHF. A greater proportion of patients who experienced hHF during the trial were using β-blockers and diuretics at baseline compared with those who did not experience hHF during the trial. This was expected, as these treatments are standard of care for patients with a prior diagnosis of HF.
Treatment with insulin increases the risk of severe hypoglycemia [13], which is associated with an increased risk of all-cause mortality and CV events, including stroke, coronary heart disease, CV disease and all-cause hospitalization (including heart failure) [16–22]. In a previous secondary analysis of DEVOTE, it was demonstrated that there was a significant association as well as a temporal association between severe hypoglycemia and all-cause mortality [11]. These results are similar to those observed in the LEADER trial, where it was demonstrated that patients experiencing severe hypoglycemia were more likely than those without severe hypoglycemia to experience MACE, CV death and all-cause mortality [8]. Analyses from the Veterans Affairs Diabetes Trial (VADT) also demonstrated that a severe hypoglycemic event was an independent predictor of death at 90 days [44, 49]. Furthermore, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, patients who had one or more severe hypoglycemic episodes had higher rates of death than those who did not experience such episodes [50]. In the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, severe hypoglycemia increased the risk of arrhythmic death, all-cause death and CV death [10]. In addition, in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), severe hypoglycemia was associated with an increased risk of CV events, all-cause death and CV death [12]. In the current analysis, patients who experienced severe hypoglycemia were at a significantly higher risk of subsequently experiencing hHF than those who did not experience severe hypoglycemia. A strong temporal relationship (with the highest risk observed within a week of the event) further supports this hypothesis. Furthermore, such an association was also demonstrated in LEADER. The relatively weaker strength of an association when adjusting for baseline covariates suggests that the association may partly be explained by more frail patients suffering from comorbidities and complications, which in itself are risk factors of both severe hypoglycemia and hHF [12, 51]. Furthermore, although the hazard ratio for hHF did not reach statistical significance, the point estimate was in favor of degludec. This is consistent with the significantly lower rates of severe hypoglycemia with degludec versus glargine U100 observed in the primary DEVOTE trial [38, 41], and the significantly higher risk of hHF following an occurrence of severe hypoglycemia observed in this secondary analysis. The reason for the hazard ratio for hHF not reaching statistical significance, despite the other two strong significant associations, may be because factors other than treatment may affect the risk of severe hypoglycemia. Furthermore, the trial was not powered to detect a significant treatment effect for hHF. It is also important to note that our investigation into the possible association between severe hypoglycemia and subsequent hHF is relatively novel in that, aside from the TECOS and EMPA-REG OUTCOME trials, this association has not been explored by other trials [12, 52]. In TECOS, a significant association between severe hypoglycemia and subsequent hHF was not identified [12]. However, a post hoc exploratory analysis of the EMPA-REG OUTCOME trial demonstrated that hypoglycaemia was associated with an increased risk of subsequent hHF [52]. Our results are in line with those from EMPA-REG OUTCOME trial as well as LEADER, which had a similar patient population to DEVOTE. However, it is currently unclear whether there is a direct pathophysiological link between severe hypoglycemia and hHF, or whether severe hypoglycemia is primarily a marker of vulnerability for patients at risk of hHF. Overall, our results support an association between severe hypoglycemia and subsequent hHF even when adjusting for potential confounders and it is most likely that hypoglycemia is just a single contributory factor to hHF events in a much larger multifactorial landscape.
This study has a number of limitations. This was a pre-specified secondary analysis from a trial that was not powered to compare differences in the risk of hHF when treated with different basal insulin. It was also not designed or powered to compare the difference in risk of experiencing hHF based on HF prior to the trial or indeed the relationship between severe hypoglycemia and risk of hHF. However, the findings support those from other trials and therefore, this analysis supports and adds to the existing body of evidence.
Furthermore, the inclusion criteria of this trial were designed to recruit a cohort who were at a high CV risk, so the different outcomes observed may not be generalizable to the wider T2D population or to those not fulfilling the inclusion criteria. A further limitation is that hHF events were not adjudicated by an EAC in this trial. However, the sensitivity analysis based on LEADER adjudicated probabilities demonstrated similar results to analyses without adjudication.
Strengths of this study include the large sample size, the double-blind active-control design and the independent adjudication of severe hypoglycemic events. The prospective design and international multicenter nature of this trial, as well as the high levels of patient follow-up are additional strengths. Robustness was also increased through the use of a MedDRA search matched with LEADER EAC criteria, as well as use of the SMQ definition.
Conclusions
This secondary analysis from DEVOTE of patients with T2D at high risk of CV events, treated with basal insulin (degludec or glargine U100) demonstrated no treatment differences with degludec versus glargine U100 in terms of hHF, that prior HF was the strongest predictor of future hHF events, and that there was an association between severe hypoglycemia and subsequent hHF, which was further supported by the similar results from LEADER.
Supplementary information
Additional file 1: Additional Methods. Description of the SMQ definition of cardiac failure and the hHF definition used by the EAC in LEADER. Table S1. LEADER positive adjudication probabilities. Table S2. hHF by system organ class: hHF events by SMQ and broad MedDRA definitions.
Acknowledgements
We thank the trial investigators, staff and patients for their participation; and Francesca Hemingway and Beverly La Ferla of Watermeadow Medical for providing medical writing and editorial support (sponsored by Novo Nordisk). DEVOTE research activities were supported at numerous US centers by Clinical and Translational Science Awards from the National Institutes of Health’s National Center for Advancing Translational Science.
Abbreviations
- A1C
glycated hemoglobin
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- CI
confidence interval
- CKD
chronic kidney disease
- CV
cardiovascular
- CVD
cardiovascular disease
- CVOTs
cardiovascular outcomes trials
- Degludec
insulin degludec
- DEVOTE
Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events
- EAC
Event Adjudication Committee
- eGFR
estimated glomerular filtration rate
- Glargine U100
insulin glargine 100 units/mL
- hHF
hospitalization for HF
- HF
heart failure
- HR
hazard ratio
- LEADER
Liraglutide Effect and Action in DiabetesEvaluation of Cardiovascular Outcome Results
- MACE
major adverse cardiovascular events
- MedDRA
Medical Dictionary for Regulatory Activities
- MI
myocardial infarction
- ORIGIN
Outcome Reduction with Initial Glargine Intervention
- PYO
patient-years of observation
- SMQ
standardized MedDRA Query
- T2D
type 2 diabetes
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- VADT
Veterans Affairs Diabetes Trial
Authors’ contributions
All authors (REP, MH, IL, TRP, TM, HAS, DVM, BZ) made substantial contributions to the interpretation of data for the manuscript, drafted and critically revised the manuscript. TM carried out the statistical analyses and validation of the data. All authors are responsible for the integrity of the work as a whole. All authors read and approved the final manuscript.
Funding
DEVOTE and this secondary analysis were sponsored and funded by Novo Nordisk (Bagsvaerd, Denmark). The trial sponsor was involved in the design of the trial, the collection and analysis of data, and writing the clinical report.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The protocol was approved by independent ethics committees or institutional review boards for each center; written informed consent was obtained from each patient before any trial-related activities.
Consent for publication
Not applicable.
Competing interests
REP’s fees for services were paid directly to AdventHealth (formerly Florida Hospital), a non-profit organization and therefore he has no financial relationship with the following companies, except for Sanofi. Consultancy and speaker fees from AstraZeneca, Takeda and Novo Nordisk; consultancy fees from Boehringer Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical Co. Ltd., Janssen Scientific Affairs LLC, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Pfizer and Eisai, Inc.; research grant from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Sanofi US LLC and Takeda. MH has received consulting fees or honoraria from AstraZeneca, Boehringer-Ingelheim, Janssen, Merck and Novo Nordisk. IL received funds for research, consulting, editorial support and/or travel expenses from: Novo Nordisk, Eli Lilly, Sanofi, Astra Zeneca, Boehringer-Ingelheim, Merck, Novartis, Intarcia, MannKind, TARGETPharma, Pfizer, Valeritas and Mylan. TRP has received research support from Novo Nordisk and AstraZeneca (paid directly to the Medical University of Graz); personal fees as a consultant from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk and Roche Diabetes Care. TRP is also the Chief Scientific Officer of CBmed (Center for Biomarker Research in Medicine), a public-funded biomarker research company. HAS and DVM are full-time employees of Novo Nordisk A/S. TM is a full-time employee of, and holds stock in, Novo Nordisk A/S. BZ has received grant support from Boehringer-Ingelheim, AstraZeneca and Novo Nordisk; and consulting fees from AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-019-0960-8.
References
- 1.Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS ONE. 2017;12(1):e0170219. doi: 10.1371/journal.pone.0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaboration NRF Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . IDF Diabetes Atlas. 8. Brussels: International Diabetes Federation; 2017. [PubMed] [Google Scholar]
- 4.International Diabetes Federation . IDF Diabetes Atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 5.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 6.Pistrosch F, Natali A, Hanefeld M. Is hyperglycemia a cardiovascular risk factor? Diabetes Care. 2011;34(Suppl 2):S128–S131. doi: 10.2337/dc11-s207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, Chen CH, Chou P, Chuang SY. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care. 2013;36(4):894–900. doi: 10.2337/dc12-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB. Hypoglycemia, cardiovascular outcomes and death: the LEADER experience. Diabetes Care. 2018;41(8):1783–1791. doi: 10.2337/dc17-2677. [DOI] [PubMed] [Google Scholar]
- 9.Heller SR, Bergenstal RM, White WB, Kupfer S, Bakris GL, Cushman WC, Mehta CR, Nissen SE, Wilson CA, Zannad F, et al. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: The EXAMINE trial. Diabetes Obes Metab. 2017;19(5):664–671. doi: 10.1111/dom.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellbin LG, Ryden L, Riddle MC, Probstfield J, Rosenstock J, Diaz R, Yusuf S, Gerstein HC. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013;34(40):3137–3144. doi: 10.1093/eurheartj/eht332. [DOI] [PubMed] [Google Scholar]
- 11.Pieber TR, Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pratley RE, Woo V, Heller S, Lange M, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61(1):58–65. doi: 10.1007/s00125-017-4422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standl E, Stevens SR, Armstrong PW, Buse JB, Chan JCN, Green JB, Lachin JM, Scheen A, Travert F, Van de Werf F, et al. Increased risk of severe hypoglycemic events before and after cardiovascular outcomes in TECOS suggests an at-risk type 2 diabetes frail patient phenotype. Diabetes Care. 2018;41(3):596–603. doi: 10.2337/dc17-1778. [DOI] [PubMed] [Google Scholar]
- 13.Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev. 2008;24(2):87–92. doi: 10.1002/dmrr.796. [DOI] [PubMed] [Google Scholar]
- 14.Munnee K, Bundhun PK, Quan H, Tang Z. Comparing the clinical outcomes between insulin-treated and non-insulin-treated patients with type 2 diabetes mellitus after coronary artery bypass surgery: a systematic review and meta-analysis. Medicine. 2016;95(10):e3006. doi: 10.1097/MD.0000000000003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther. 2011;13(Suppl 1):S65–S74. doi: 10.1089/dia.2011.0019. [DOI] [PubMed] [Google Scholar]
- 16.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 17.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ. 2016;354:i3477. doi: 10.1136/bmj.i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–1169. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossello X, Ferreira JP, McMurray JJ, Aguilar D, Pfeffer MA, Pitt B, Dickstein K, Girerd N, Rossignol P, Zannad F. Impact of insulin-treated diabetes on cardiovascular outcomes following high-risk myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2019;8(3):231–241. doi: 10.1177/2048872618803701. [DOI] [PubMed] [Google Scholar]
- 20.Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853–872. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, Petrie MC, McMurray JJ, Petrie JR, McAlister FA. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care. 2010;33(6):1213–1218. doi: 10.2337/dc09-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, Cohn JN, Cosmi D, De Berardis G, Dickstein K, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20(5):888–895. doi: 10.1002/ejhf.1146. [DOI] [PubMed] [Google Scholar]
- 23.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 24.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A. Hemingway H. type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHugh KR, DeVore AD, Mentz RJ, Edmonston D, Green JB, Hernandez AF. The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: a focus on cardiorenal outcomes. Clin Cardiol. 2018;41(9):1259–1267. doi: 10.1002/clc.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R, Midlands Research Practices C Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES) Eur Heart J. 2007;28(9):1128–1134. doi: 10.1093/eurheartj/ehm102. [DOI] [PubMed] [Google Scholar]
- 28.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf Accessed 07 Aug 2019.
- 30.European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf. Accessed 07 Aug 2019.
- 31.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2(10):843–851. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 32.Patel T, Tesfaldet B, Chowdhury I, Kettermann A, Smith JP, Pucino F, Almario EEN. Endpoints in diabetes cardiovascular outcome trials. Lancet. 2018;391(10138):2412. doi: 10.1016/S0140-6736(18)31184-X. [DOI] [PubMed] [Google Scholar]
- 33.Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, Troelsen L, Kvist K, Norwood P. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: The SWITCH 2 randomized clinical trial. JAMA. 2017;318(1):45–56. doi: 10.1001/jama.2017.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53(9):787–800. doi: 10.1007/s40262-014-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novo Nordisk. Tresiba insulin degludec injection 100 U/mL, 200 U/mL. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203314s009lbl.pdf. Accessed 07 Aug 2019.
- 37.Schnell O, Standl E, Catrinoiu D, Genovese S, Lalic N, Lalic K, Skrha J, Valensi P, Ceriello A. Report from the 3rd Cardiovascular Outcome Trial (CVOT) Summit of the Diabetes & Cardiovascular Disease (D&CVD) EASD Study Group. Cardiovasc Diabetol. 2018;17(1):30. doi: 10.1186/s12933-018-0667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Haahr PM, Lange M, Brown-Frandsen K, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–732. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heller SR, Hans Devries J, Wysham CH, Hansen CT, Hansen MV, Frier BM. Insulin degludec has lower hypoglycemia risk than insulin glargine U100 in older people with type 2 diabetes (T2D) Diabetes. 2018;67(Supplement 1):107-OR. doi: 10.2337/db18-107-OR. [DOI] [Google Scholar]
- 40.Tentolouris A, Eleftheriadou I, Tentolouris N. Insulin degludec U100 is associated with lower risk for severe and symptomatic hypoglycemia as compared with insulin glargine U100 in subjects with type 1 diabetes. Ann Transl Med. 2018;6(3):63. doi: 10.21037/atm.2017.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Haahr PM, Lange M, Frandsen KB, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events)—DEVOTE 1. Am Heart J. 2016;179:175–183. doi: 10.1016/j.ahj.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 42.International Conference on Harmonisation ICH harmonised tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47(3):199–203. [PubMed] [Google Scholar]
- 43.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 44.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the american diabetes association and the endocrine society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SAS/STAT® 15.1 User’s Guide The PHREG Procedure. In: SAS Institute Inc 2018 SAS/STAT® 151 User’s Guide. Cary, NC: SAS Institute Inc.; 2018.
- 46.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itzhaki Ben Zadok O, Kornowski R, Goldenberg I, Klempfner R, Toledano Y, Biton Y, Fisman EZ, Tenenbaum A, Golovchiner G, Kadmon E, et al. Admission blood glucose and 10-year mortality among patients with or without pre-existing diabetes mellitus hospitalized with heart failure. Cardiovasc Diabetol. 2017;16(1):102. doi: 10.1186/s12933-017-0582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):96. doi: 10.1186/s12933-018-0739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 50.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee AK, Warren B, Lee CJ, McEvoy JW, Matsushita K, Huang ES, Sharrett AR, Coresh J, Selvin E. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018;41(1):104–111. doi: 10.2337/dc17-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitchett D, Inzucchi SE, Wanner C, Mattheus M, George JT, Vedin O, Zinman B, Johansen OE. Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA-REG OUTCOME® trial. Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Additional Methods. Description of the SMQ definition of cardiac failure and the hHF definition used by the EAC in LEADER. Table S1. LEADER positive adjudication probabilities. Table S2. hHF by system organ class: hHF events by SMQ and broad MedDRA definitions.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.