Abstract
The lethal consequences of prostate cancer are related to its metastasis to other organ sites. Epithelial-to-mesenchymal transition (EMT) has received considerable attention as a conceptual paradigm to explain invasive and metastatic behavior during cancer progression. EMT is a normal physiologic process by which cells of epithelial origin convert into cells bearing mesenchymal characteristics. It has been proposed that EMT is co-opted by cancer cells during their metastatic dissemination from a primary organ to secondary sites, but the extent to which this recapitulates physiologic EMT remains uncertain. However, there is ample evidence that EMT-like states occur in, and may contribute to, prostate cancer progression and metastasis, and so has become a very active area of research. Here we review this evidence and explore recent studies that have aimed to better define the role and mechanisms of EMT in prostate cancer. While definitive evidence of something akin to physiologic EMT is still lacking in human prostate cancer, this area of research has nonetheless provided new avenues of investigation into the longstanding puzzles of metastasis, therapeutic resistance, and prognostic biomarkers.
Introduction
EMT is a normal physiologic process in vertebrate development (for example, gastrulation) and tissue homeostasis (for example, wound healing), and is increasingly thought to be involved in disease states such as tissue fibrosis and cancer. As numerous excellent reviews have extensively covered these issues,1 here we will primarily focus on efforts to define the role of EMT in the specific pathophysiology of prostate cancer.
This is a very active area of prostate cancer research, with over 100 papers related to EMT and prostate cancer alone published in the past 3 years. Physiologic EMT triggered by local inductive cues is characterized by changes in gene expression, including decreases in epithelial genes, such as E-cadherin (also known as cadherin 1 [CDH1]), and increases in mesenchymal genes, such as vimentin, driven by transcription factors (such as those in the Snail, Twist and ZEB families), and is associated with altered cell morphology (loss of apical–basal polarity) and a concomitant change from a sessile to a migratory state. Two other important aspects of physiologic EMT are that, first, it is reversed in some cases via a mesenchymal-to-epithelial transition (MET); and, second, that these need not represent binary states, but rather a continuous spectrum of changes that occur to a greater or lesser degree depending on the biological context.
Dr Elizabeth Hay is widely credited with popularizing the notion that EMT may contribute to cancer meta-stasis in the early 1990s.2 This presents the very attractive hypothesis that EMT could enable epithelial-derived cancer cells to migrate to distant sites during metastasis.
In this way, a very intricate behavior of cancer cells could reflect a normal process occurring out of context, rather than a complex accumulation of genetic and epigenetic events. One could trace the roots of EMT research in prostate cancer to the same time period as the finding that E-cadherin expression is commonly lost or reduced in human prostate cancer specimens. Moreover, a preceding analysis had revealed that loss of E-cadherin expression within sublines of the Dunning rat model was correlated with metastatic colonization.3 Although these results were not interpreted in the context of EMT at the time—and loss of E-cadherin expression alone is not sufficient evidence for EMT—they presaged a number of the key issues discussed in the remainder of this Review regarding evidence for EMT in pathological specimens, and how studies involving cultured prostate cancer cell lines and animal models can be translated into understanding what happens in human disease. What has emerged is a more nuanced view of the possible role of EMT—or processes resembling EMT—in human prostate cancer.
Evidence for EMT in prostate cancer
Human cancer specimens
One of the most controversial aspects of EMT in cancer, including prostate cancer, is the degree to which one can find evidence for it in human pathological specimens.4,5 At one level, the Gleason grading system might be viewed as morphological evidence of EMT. Increasing Gleason grade is associated with a progressive loss of epithelial glandular architecture, including loss of defined basement membrane and cell polarity and increasingly invasive patterns characterized by cords, sheets, or individual cells invading the tumor stroma.6 Within the affected prostate, many of these patterns coexist amongst benign tissue, and different tumor foci may be present. This heterogeneity underlies the complexity of this disease and the difficulty in discriminating those patients with indolent disease from those with more aggressive prostate cancer. One interpretation of this pathology is that local tumor microenvironments might promote the high-grade patterns, which include changes that are consistent with EMT. However, this superficial resemblance does not constitute proof. Whether related to EMT or not, it is also worth noting that rare histological types of prostate cancer, such as phyllodes tumor and carcinosarcomas, clearly show elements of both transformed epithelial and mesenchymal cells.7,8
Further evidence is derived from analysis of molecular markers. The simplest expectation is that if EMT were facilitating metastatic dissemination, then changes in gene expression associated with EMT should be evident in primary tumors and enriched in metastases (Figure 1a). However, current evidence suggests that this is unlikely to be generally true. Numerous studies have evaluated E-cadherin expression in prostate cancer specimens (Table 1). While there is broad agreement that focal, aberrant E-cadherin protein expression is often observed in prostatectomy specimens (18–50%), the status of E-cadherin expression in metastases remains more questionable. While some studies report reduced E-cadherin expression in both bone and lymph node meta-stases,9,10 others dearly show that it is expressed at normal levels.11,12 There may be site-specific effects on E-cadherin expression, or the loss of E-cadherin expression may be transient, whereby expression is lost in primary tumors but re-expressed in metastatic sites.3,12 While inconsistent with a simple model of EMT, MET triggered by escape from EMT-inducing stimuli at the primary site and/or induction at the metastatic site could explain EMT reversal (Figure 1b). Consistent with this idea, TSU-Prl bladder cancer sublines with epithelial characteristics exhibited more-robust growth in bone following intratibial injection than those with mesenchymal teatures.13 However, convincing evidence for MET occurring in human prostate cancers has not yet emerged.
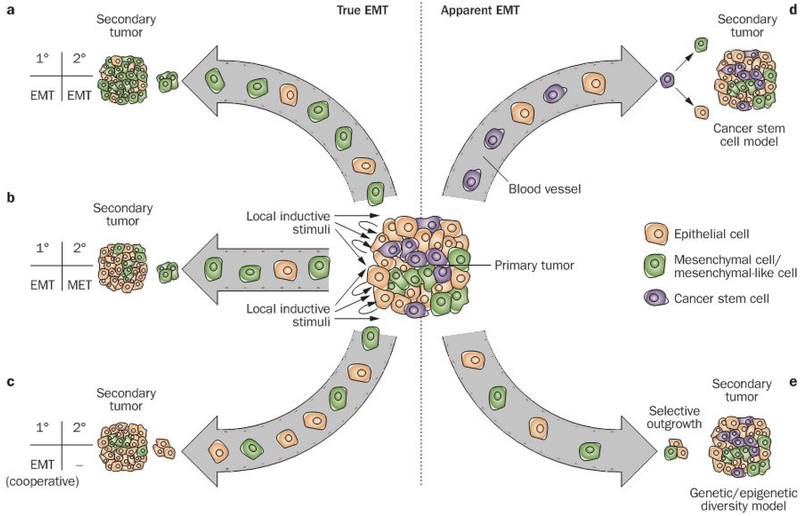
Figure 1 |.
True and apparent EMT might both contribute to metastasis, yet be difficult to distinguish. Primary tumors contain a heterogeneous mixture of cells, including epithelial cells and cells that resemble mesenchymal cells. Whether this latter group of cells is the result of the transition of an individual cell from an epithelial to a mesenchymal phenotype, however, is unclear. The patterns observed in the primary and metastatic tumors can be reconciled by a variety of epistemological explanations. a | EMT occurs in the primary tumor, resulting in the entrance of both mesenchymal and epithelial cells into circulation. The mesenchymal cells are those that are chiefly capable of extravasation and colonization at a secondary site. b | Similarly, EMT occurs at the primary tumor, and mesenchymal cells exit the bloodstream at a new site to initiate a secondary tumor; however, in this model, the mesenchymal cells at the site of metastasis undergo MET back to an epithelial phenotype. c | in this instance, EMT again occurs at the primary site, but to a different end. Here, the mesenchymal cells facilitate the metastatic spread of epithelial cells. Because the mesenchymal cells serve a cooperative role at the point of departure, it is the epithelial cells that disseminate most effectively. Consequently, there is no need for MET at the secondary site in this model. d | The cancer stem cell population expands at the site of the primary tumor, perhaps under the influence of EMT, and make up the important subset of cells in circulatory transit. Upon arrival at the secondary site, these cells exercise stem-cell-like characteristics of asymmetrical division and pluripotency to generate both epithelial and mesenchymal cells. e | A subpopulation of epithelial cells begins to resemble mesenchymal cells due to genetic and/or epigenetic changes. conferring some of the invasive behaviors of mesenchymal cells and facilitating the steps of metastasis (Figure 3). The presence of this population reflects selective outgrowth, rather than transdifferentiation of individual cells. Abbreviations: EMT, epithelial-to-mesenchymal transition; MET, mesenchymal-to-epithelial transition.
Table.1 |.
Selective survey of EMT markers in clinical prostate cancer specimens
| Study | Number and type of specimens | Staining type | Results |
|---|---|---|---|
| E-cadherin | |||
| Sethi et al. (2010)18 | 10 primary PCa (all RP specimens), 10 bone metastases | Membranous | No statistically significant association between decreasing E-cadherin and bone metastasis (staining intensity, P=0.433; % of cells. P=0.122; low vs high expression, P=l) |
| Rubin et al. (2001)11 | Tissue microarray on 757 benign prostate, 41 high-grade PIN, 325 primary PCa, 97 hormone-refractory primary PCa | Membranous | Trend of increased E-cadherin expression in 128 clinically localized PCa samples; trends of aberrant expression associated with a high rate of PSA failure, positive surgical margins, and increasing Gleason score |
| Tomita et al. (2000)15 | 83 primary PCa (RP and TURP specimens) | Membranous | E-cadherin expression inversely correlated with N-cadherin (P=0.0008) |
| De Marzo et al. (1999)12 | 76 primary PCa (RP specimens) and 10 pelvic lymph node metastases | Membranous | Decreased E-cadherin expression correlated with advanced Gleason grade (P<0.003) and tumor stage (P<0.008); 9 of the 10 pelvic lymph node metastases displayed strong E-cadherin expression |
| Umbas et al. (1994)114 | 89 TURP specimens (33 primary PCa, 20 after recurrence) and 47 RP specimens (primary PCa) | Membranous | Decreased E-cadherin expression correlated with tumor grade and stage (P<0.005) and shorter overall survival (P<0.001); aberrant E-cadherin expression in primary tumor associated with the presence of metastases (P<0.001); post-RP progression associated with aberrant E-cadherin expression (P<0.005) |
| Umbas et al. (1992)9 | 84 PCa (37 TURP specimens with extensive local progression and 47 RP specimens of primary PCa). 8 metastases (7 lymph node, 1 testis) | Membranous | Decreasing E-cadherin expression correlated with increasing Gleason score (P<0.001); 6 of 8 metastases displayed either negative or intermediate heterogeneous E-cadherin staining |
| Vimentin | |||
| Sethi et al. (2010)18 | 10 primary PCa and 10 bone metastases | Cytoplasmic | No significant difference in vimentin expression between primary and metastatic lesions |
| Zhang et al. (2009)46 | 267 primary PCa (RP specimens) | Cytoplasmic | High vimentin expression correlated with shorter time to biochemical recurrence (P<0.004), independent of Gleason grade |
| Lang et al. (2002)115 | 54 primary PCa (TURP or needle biopsy specimens) and 8 bone metastases | Cytoplasmic | Positive vimentin staining was associated with bone metastases and poorly differentiated cancers—not correlated strongly enough to predict positive bone scans, yet positive bone scans were correlated with positive vimentin staining |
| ZEB1 | |||
| Sethi et al. (2010)18 | 10 primary PCa and 10 bone metastases | Nuclear | High expression of ZEB1 not found in either primary lesions or bone metastases |
| Graham et al. (2008)38 | Tissue microarrays of PCa and benign tissue | Nuclear | ZEB1 staining correlated with Gleason score (P<0.001) and was absent in normal tissue |
| TWIST | |||
| Kwok et al. (2005)74 | 46 PCa (needle biopsy or RP specimens) and 45 BPH | Cytoplasmic and nuclear | Expression higher in tumor tissue than in BPH (P<0.001), and correlated with Gleason grade >7 (P<0.05); TWIST expression was also increased in both bone and lymph node metastases |
| N-cadherin | |||
| Jaggi et al. (2006)116 | 44 RP specimens | Membranous | Increased N-cadherin expression associated with Gleason score >4 (P<0.05) and Gleason score 8–10 (P<0.001) |
| Kallakury et al. (2001)117 | 112 primary PCa (RP specimens) | Membranous | Only found to be present in 5% of samples, and demonstrated no correlation with any other evaluative metric |
| Tomita et al. (2000)15 | 83 primary PCa (RP and TURP specimens) and 12 benign acini | Dotted and membranous | No staining in benign samples, but expression increased with Gleason score in cancer specimens (P=0.001) |
Abbreviations: BPH, benign prostatic hyperplasia; PCa, prostate cancer; PIN. prostatic intraepithelial neoplasia; RP, radical prostatectomy; TURP, transurethral resection of the prostate.
Loss of E-cadherin expression alone is not sufficient evidence for EMT, as this molecule is known to be regulated in cancer at many levels that might be unrelated to EMT.14 Loss of E-cadherin immunostaining in prostatectomy specimens and lymph node metastases is accompanied by increased expression of N-cadherin (also known as cadherin 2 [CDH2)) and cadherin 11, both of which are mesenchymal cadherins. This led to the development of the concept of ‘cadherin switching’, which is consistent with an EMT-like state.15 In prostate cancer cell lines, E-cadherin can be co-expressed with N-cadherin, consistent with the idea of partial EMT.16,17 Other markers have been evaluated, with vimentin and transcriptional regulators of EMT having drawn the most attention (Table 1). The latter are of particular importance, as they are arguably the best diagnostic markers of EMT to date.18
However, concluding whether or not EMT occurs in human prostate cancer on the basis of static analysis of pathologic specimens is difficult for several reasons: first, the lack of unambiguous markers, or even a coherent definition of EMT in prostate or other cancers; second, the typical concerns related to the specificity of immunostaining or possible misinterpretation due to stromal staining; and third, the lack of longitudinal evaluation. The purportedly dynamic and, perhaps, transient nature of EMT in the tumor microenvironment necessitates some effort to show that such a process occurs over time in a particular lineage of cancer cells. Snapshot views of the pathology, as we currently have, do not exclude other models to explain differences in marker expression. Such evidence may be difficult to obtain from human cancer specimens.
Prostate cancer cell lines
Much of what is reported about EMT in prostate and other cancers is based on studies involving cultured cells. An oft-repeated finding is that prostate cancer cell lines display a high degree of heterogeneity, manifest in the coexistence of distinct subpopulations of cells representing a spectrum of changes associated with EMT. This includes cell lines derived from the Dunning rat model,3 the prostate-specific Pten-knockout mouse,19 and has also been observed in the PC-3,17,20 DU145,21 and ARCaP22 human prostate cancer cell lines, each of which were originally isolated from metastatic lesions. In each case, subpopulations with either epithelial or mesenchymal features have been isolated by various means. Many of the details of these investigations are described in the following sections. The common findings are that cells with more mesenchymal features exhibit a more-invasive phenotype in vitro and display more-aggressive behavior in metastatic colonization models, although these trends are not without exception.23 Three-dimensional culture systems might also prove useful for analysis of EMT, as more features of polarized epithelial cells can be evaluated. MET has also been reported to occur in these systems.24–26
If EMT does occur in primary prostate cancers, how, if at all, does the mesenchymal state relate to the normal development and homeostasis of the prostate? Work from the Weinberg laboratory raised the possibility that SNAI1-driven EMT in breast cancer produces cells with stem-cell-like and enhanced tumor-initiating properties.27,28 Alternatively, work from the Chinnaiyan group shows that, via the concerted action of TMPRSS2-ERG gene fusions and the EZH2 histone methyltransferase, androgen receptor (AR)-driven differentiation is blocked, producing cells with stem-cell-like characteristics.29 The existence of prostate cancer stem cells has not yet been definitively established;30,31 therefore, it is difficult to say whether EMT and cancer stem cell behavior are indeed linked in prostate cancer. Nevertheless, experiments with prostate cancer cell lines have suggested this possibility (Figure 1d).32,33
While it is tempting to speculate that the heterogeneity observed in prostate cancer cell lines is reflective of EMT in tumors, these findings must be interpreted cautiously, as cell culture might select for outgrowth of variants arising ex vivo. This also applies to experimental manipulations that purport to induce or reverse EMT phenotypes in vitro. Indeed, it is critically important to distinguish between true EWT, which involves a phenotypic conversion of a lineage of cells in response to some inductive stimulus, and apparent EMT, in which cells bearing the hallmarks of EMT arise due to selective outgrowth of cells from pre-existing variants within a diverse tumor cell population, whether in vitro or in vivo.34 The latter may resemble EMT, but arise through a quite different mechanism (Figure 1e). For these reasons, and those discussed in the previous section, we favor the term “EMT-like”, as proposed by others in reference to EMT in cancer, as this reflects uncertainty in both its molecular definition and mechanistic origins.35 Considering this, Figure 1 summarizes several possible models for the involvement of EMT-like states in prostate cancer meta-stasis, whereby similar pathological presentations can be accomplished by different avenues of procession.
Mechanisms of EMT-like states in cancer
EMT-like states in prostate cancer are likely to become manifest by the concerted actions of a host of mechanisms, which we have organized into inducers (cell-extrinsic inductive stimuli), controllers (cell-intrinsic means of interpreting such stimuli) and effectors (proteins that mediate EMT-like behaviors) (Figure 2). The diverse array of mechanisms reported to induce EMT-like states in cultured cancer cells alone suggests that the definition of what EMT is in cancer should be re-evaluated.
Figure 2 |.
Many roads lead to EMT: the molecular basis for the mechanisms of EMT and EMT-like states. Inductive stimuli in the tumor microenvironment, including hypoxia and growth factors (e.g. TGF-β, FGF and IGF-1), trigger downstream signaling. These pathways, via MAPK, Smad, GSK3β, and NFκB, result in increased activity of transcriptional repressors in the ZEB, Twist, and Snail families, which repress E-cadherin and other epithelial cell adhesion proteins and induce other mesenchymal proteins. Epigenetic mechanisms involving EZH2 can promote EMT-like states, whereas miRNAs (such as miR-101 or those in the miR-200 family) might act to maintain epithelial status. Importantly, this picture presents numerous mutually reinforcing mechanisms that might promote the EMT-like state. Abbreviations: CDH1, cadherin-1 (E-cadherin); ERβ, estrogen receptor β; FGF, fibroblast growth factor; GSK3β, glycogen synthase kinase 3β; HIF-1α, hypoxia inducible factor 1α; IGF, insulin-like growth factor; ITGβ4; integrin β4; LAM332, laminin-332; MAPK, mitogen-activated protein kinase; miRNA, micro RNA; NFκB, nuclear factor κB; PKD1, protein kinase D1; RKIP, Raf kinase inhibitor protein; sFRP, secreted frizzled-related protein; TGF-β, transforming growth factor β; VEGF-A, vascular endothelial growth factor A; WIF, Wnt inhibitory factor.
Inducers
A key tenet of the hypothesis that EMT contributes to prostate cancer progression is that the tumor micro-environment provides local cues to induce EMT. Numerous growth factors are potential mediators of paracrine (or autocrine) induction of EMT-like states in prostate cancer, including endothelial growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1) and platelet-derived growth factor (PDGF).36–39 Two growth factors deserving of particular consideration are fibroblast growth factor (FGF) and transforming growth factor β (TGF β). both of which are known to have roles in physiologic EMT.
FGF signaling
Altered FGF signaling has been implicated in prostate cancer progression.40 In a very compelling set of experiments, Acevedo et al.41 used a novel mouse model to show that inducible activation of this pathway via FGF receptor 1 (FGFR1) led to an EMT-like state in the mouse prostate that was associated with increased lymph node and liver metastasis, and the metastatic foci were found to retain mesenchymal features. Gene expression studies in tumors derived from these mice implicated upregulation of Wnt signaling and the transcription factor SOX9—the latter of which is known to synergize with Snail family members during neural crest developmenr42—as mechanisms mediating this effect. While this constitutes strong evidence for the induction of EMT via this pathway, the authors provide caution that the promoter driving the expression of the FGFR1 construct could be expressed at low levels in the stroma, possibly contributing to the observed changes. Other studies have shown that FGFR4 and matrix metalloproteinase (MMP)-14 co-localize at the invasive edges of prostate cancers and are co-expressed in certain prostate cancer cell lines. Both a particular allelic variant of FGFR4 associated with aggressive disease outcomes and MMP-14 are required for invasive behavior in vitro and aggressive tumor phenotypes. Conversely, over-expression of MMP-14 induced an EMT-like state, including shedding of E-cadherin (which is noted in clinical cases) and upregulation of Wnt5A.43,44 Although the mechanism(s) by which FGFR4 facilitates MMP-14 activity and how this contributes to EMT are yet to be clarified, this pathway might particularly contribute to invasive behavior in the collagen-rich interstitial matrix.
TGF-β signaling
TGF-β signaling has been the most intensively studied pathway with regard to its role in EMT and cancer progression, and remains a very complex and active area of research.45 TGF-β can induce EMT-like states in various prostate cancer cell lines, as shown by a number of groups. Zhang et al.46 found that TGF-β induced nuclear accumulation of nuclear factor κB (NFκB), and that pharmacologic inhibition of NFκB blocked development of EMT characteristics, consistent with other studies demonstrating a role for NFκB signaling in EMT.47 Importantly, a signature of TGF-β, NFκB, and vimentin expression in a tissue microarray could predict biochemical recurrence independently of Gleason grade. On the other hand, and seemingly contrary to the idea that TGF-β signaling promotes EMT-like states, expression of dominant-negative TGF-β receptor type 2 (TGF-βR2) from the metallothionein promoter led to reduced E-cadherin in the TRAMP mouse model of prostate cancer, consistent with previous studies that showed, using the same approach, that dominant-negative TGF-βR2 expression led to increased metastasis.48,49 In either case, the effects on EMT-like states and/or metastasis that result from blocking TGF-β signaling in this manner could have been due to actions outside of the prostatic epithelium. Dialog between the cancer-associated stroma and epithelium, mediated by TGF-β. interleukin 6, and MMPs, is a likely candidate for supplying microenvironmental cues that could trigger EMT-like states in primary prostate tumors.50–52 Furthermore, other TGF-β superfamily members might also have a role in EMT or MET.53,54
Estrogen receptor β
Hypoxia is another tumor microenvironmental influence that has been suggested to be a cue for EMT, although this topic has received less attention in prostate cancer compared to other tumor types.55 An interesting 2010 study by Mak et al.56 showed that the effects of both hypoxia and TGF-β on an EMT-like state might be mediated by estrogen receptor β (ERβ). Expression of ERβ in the prostate epithelium is downregulated in high Gleason grade patterns. In cultured prostate cells, both hypoxia and TGF-β lead to concomitant downregulation of ERβ expression and changes in EMT markers, and ERβ knockdown alone is sufficient to induce an EMT-like state. Further studies support the idea that ERβ, in response to its endogenous ligand 5α-androstane-3β, 17β-diol, normally enforces an epithelial phenotype via multiple repressive effects on hypoxia inducible factor 1α (HIF-1α) and its target gene, vascular endothelial growth factor A (VEGF-A). The latter has been implicated as an autocrine regulator of an EMT-like state.57 Though a number of the mechanistic details for this model are still unclear, substantiation of these findings could lead to new prognostic and therapeutic approaches.
Controllers
Environmental stimuli are ultimately converted into intracellular signals that drive the changes associated with EMT. Three general mechanisms work in concert to regulate EMT-like states: signal transduction cascades, transcription factors, and epigenetic mechanisms, including chromatin modification, DNA methylation, and RNA interference, each of which has been found to have a role in prostate cancer progression. Prominent signal transduction pathways (such as Ras-MAPK, PI3K-AKT and NFκB) have been implicated in EMT-like states in addition to their numerous other roles in cancer.
DAB2IP
Recent studies suggest an interesting role for DAB2IP (disabled homolog 2-interacting protein) in this context. DAB2IP is a multifunctional scaffold protein with RAS GTPase activating activity,58,59 and loss of DAB2IP expression via an epigenetic mechanism is commonly observed in prostate cancer specimens.60,61 Xie et al.62 found that knockdown of DAB2IP resulted in an EMT-like state in prostate cancer cell lines, and implicated DAB2IP in the modulation of Wnt signaling. DAB2IP knockdown promoted metastasis in an orthotopic xenograft model, and Dab2ip-knockout mice developed prostatic hyperplasia that exhibited mesenchymal characteristics. Concordantly, Min et al.63 independently found that DAB2IP knockdown promoted an EMT-like state and metastasis in orthotopic models. In this case, the selective outgrowth model was excluded. These authors went on to show that DAB2IP coordinately activates both Ras and NFκB signaling and is a target for EZH2-mediated gene silencing, which has also been implicated in prostate cancer metastasis.61,64 Together, these studies point to a critical role for DAB2IP in mediating an EMT-like state and context-dependent metastatic behavior via its effects on multiple signaling pathways. Moreover, they raise the possibility that intrinsic epigenetic changes could trigger EMT-like states.
Wnt signaling pathway
The Wnt signaling pathway has been implicated in both physiologic EMT and EMT-like states in numerous cancers, including those of the prostate. Stable overexpression of the secreted Wnt antagonists sFRPs (secreted frizzled-related proteins) or WIF1 (Wnt inhibitory factor 1) results in increased expression of epithelial markers, decreased invasiveness, and corresponding downregulation of SNAI2 and TWIST1.65,66 This is consistent with the idea that canonical Wnt signaling activates glycogen synthase kinase 3β (GSK3β), resulting both in the stabilization and subsequent nuclear translocation of β-catenin as well as phosphorylation of SNAI1, which promotes its nuclear localization and, therefore, activity as a transcription factor. Recent results make an interesting connection between TMPRSS2-ERG gene fusions and Wnt pathway activation, demonstrating that ERG can drive the expression of the Wnt receptor Frizzled-4, which, in turn, is required for expression of a number of EMT markers.67 Again, like with DAB2IP, this indicates that EMT-like states may arise through primary genetic or epigenetic events. Opposing the activation of Wnt signaling is protein kinase D1, which has been shown to phosphorylate SNAI1 at different sites from GSK3β, promoting its nuclear export and thereby extinguishing its transcriptional activity.68 G-protein-coupled receptor signaling might also influence an EMT-like state via the Wnt pathway.69
Androgen receptor signaling
Given its central role in disease progression, it is not surprising that AR signaling has also been implicated in driving EMT-like states. Using a mouse tissue recombination model, Cai et al.70 found that wild-type cellular Src, when co-expressed with AR, led to an EMT-like state and invasive tumor behavior. Activation of MAPK signaling was also implicated in these phenotypes. Interestingly, in prostate cancer cell lines derived from Pten-deficient mice, those from androgen-replete animals exhibited EMT-like changes compared to those from castrated animals.19 In contrast, reduced AR expression in human prostate cancer cell lines was associated with an EMT-like state in response to androgens.71 Importantly, TMPRSS2-ERG gene fusions collaborate with AR and EZH2 to establish a dedifferentiated, EMT-like state, furthering the notion that this can arise through mutational events as opposed to local inductive cues.29 Further clarification of the role of AR signaling in EMT-like states is awaited.
Transcription factors
In physiologic EMT, a cadre of transcription factors mediates the changes in gene expression underlying the dramatic alterations in cell identity and behavior. Prominent examples are members of the Snail, Twist, and ZEB families, but also included are ETS-family members such as ERG and PDEF (Table 1).72 These and other transcription factors affecting EMT are often co-expressed; how their overlapping activities are coordinated to produce EMT-like states in prostate or other cancers is still unclear. SNAI1 directly represses E-cadherin and, interestingly, RKIP (Raf kinase inhibitor protein), which has been identified as a metastasis suppressor in prostate cancer via its inhibition of signaling through the Ras-MAPK and NFκB pathways.73 As mentioned above, these pathways act upstream of SNAI1, suggesting the possibility of a positive feedback loop that maintains an EMT-like state. TWIST1 induces EMT-like states, including upregulation of N-cadherin.74 Nuclear localization of TWIST1 in PC-3 cells depends on interaction between β1 - integrin and fibronectin, indicating that EMT-like states might be modulated by the matrix microenvironment.75 A gain-of-function p53 allele was shown to regulate TW1ST1 expression, suggesting yet another mechanism by which a genetic change may influence EMT-like states.76 ZEB1 is also a direct repressor of E-cadherin in prostate cancer cell lines, and its level of expression correlates with Gleason score.17,38 IGF-1, via the Ras-MAPK pathway, increases ZEB1 expression and induces an EMT-like state in ARCaPE cells.38
Epigenetics
Epigenetic mechanisms are also involved in controlling EMT-like states, although it is still largely unclear whether these are a cause or an effect of this state. An attractive feature of these mechanisms is that, unlike mutations, they can be reversed, which is consistent with the reversible nature of EMT. The E-cadherin promoter is methylated in a substantial proportion of prostate cancer cases, but is often found unmethylated in bone metastases, consistent with immunohistochemistry findings.77,78 As mentioned above, the EZH2 histone methyltransferase, a component of the polycomb repressive complex 2 (PRC2), has been implicated in prostate cancer progression, and acts to maintain an undifferentiated state in embryonic stem cells.64 In addition to its role in repressing DAB2IP expression, overexpression of EZH2 has also been shown to repress E-cadherin expression.79 Interestingly, this was not associated with subsequent DNA methylation, indicating that these mechanisms are not linked at this locus. Precisely how PRC2 acts on specific targets is unclear, but, in other contexts, SNAI1 can direct PRC2 to targets such as CDH1 and PTEN.80 Moreover, the TMPRSS2-ERG gene fusions commonly found in prostate cancer activate EZH2 expression and direct EZH2 complexes to target loci.29 In a related vein, the polycomb repressive complex 1 (PRC1) component BMI-1 might also regulate epigenetic status, leading to EMT-like states. BMI-1 is necessary for self-renewal of prostate stem cells, and its expression is elevated in prostate cancer.81,82
Micro RNAs
Micro RNAs (miRNAs) have also been implicated in the control of the epithelial–mesenchymal axis; this is particularly true for the maintenance of the epithelial phenotype. Moreover, they are inter-related with each of the mechanisms discussed above that control EMT-like states. One interesting example involves a reciprocal negative feedback loop between miR-200 family members (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) and ZEB proteins.83–85 Exposure to EMT inducers such as TGF-β can break this mutually inhibitory loop and facilitate ZEB protein expression with miR-200 repression via reversible DNA methylation.86 Similarly, Kong et al.87 found that PDGF-D reduces miR-200 expression, allowing upregulation of epithelial-mesenchymal axis controllers (including ZEB1, ZEB2, and SNAI2). miRNA profiling efforts in prostate cancer cell lines and clinical specimens have identified downregulation of miR-203 and miR-205.88,89 In both of these cases, enforced expression of these miRNAs in prostate cancer cells restores epithelial characteristics, including E-cadherin expression. Both of these miRNAs repress ZEB2, but they are likely to regulate other targets that contribute to an EMT-like state. A compelling finding that links several mechanisms discussed here involves miR-101. EZH2 is a predicted (and subsequently confirmed) target of miR-101 that frequently exhibits genomic deletions in prostate cancer.90,97 Re-expression of miR-101 reduced EZH2 expression and activity, and produced MET-like changes.
Thus, taken together with the findings mentioned above, here is another example of how an underlying genetic mutation can result in a series of events that produce an EMT-like state. While there is much more to learn about the role of epigenetic mechanisms in prostate cancer progression, their connection to producing EMT-like states in association with aggressive disease is likely to be a fruitful area of research.
Effectors
The combined actions of the inducers and controllers described above lead to changes in the expression or activity of a variety of genes that carry out EMT-like behaviors. Although these effectors are known to mediate epithelial polarity, cell adhesion, and the development of invasive phenotypes, as well as alter cell survival, the protein players and the mechanisms underlying precisely how this occurs are not clearly characterized.
Cell migration and invasion
As presented previously, cadherin switching is a typical EMT-like change that is thought to facilitate homotypic cell–cell adhesion, which may promote metastatic dissemination. Tran et al.20 showed that N-cadherin-positive PC-3 cells were able to invade muscle more readily than those expressing E-cadherin. More recently, our lab showed that an N-cadherin-positive PC-3 subpopulation migrated more efficiently across an endothelial monolayer than those expressing both E-cadherin and N-cadherin.17 Other roles for N-cadherin in cancer progression include mediating FGF signaling.92 Importantly, recent studies have shown that N-cadherin is upregulated in castrate-resistant disease, both in animal models and in human specimens, and that forced N-cadherin expression alone, quite surprisingly, can drive castrate-resistant disease, EMT-like states, invasion and meta-stasis.93,94 The mechanism by which N-cadherin supports these phenotypes is not yet elucidated, but promises to be an exciting area of future research.
While EMT-like states are generally associated with increased invasive behavior, our mechanistic understanding of the effectors involved in this complex phenotype is incomplete. HGF is well known to induce cell scattering—an EMT-like behavior—due in part to its effects on relocalizing lysosomes to the cell periphery and increased cathepsin B secretion.95 Vimentin was shown to be necessary for the invasive phenotype in an aggressive LNCaP-derivative cell line, but was not sufficient to confer this phenotype to parental LNCaP cells.96 Indeed, EMT-like states can produce counterintuitive findings with regard to cell invasion. We found that while a PC-3 subpopulation that endogenously expresses ZEB1 is proficient at transendothelial migration, it is poor at migration and invasion in other contexts.17 Underlying this behavior, ZEB1 directly represses laminin-332 (a promigratory molecule) and its receptor, integrin β4. These and other results suggest that different populations of cancer cells—some exhibiting EMT-like states and others not—may cooperate to achieve an overall invasive tumor phenotype (Figure 1c).23,97
Cell survival
Although far less explored than its impact on invasive behavior, EMT-like states are also known to be associated with increased cell survival under various conditions, including those involved with therapeutic resistance.1 As mentioned above, N-cadherin upregulation is associated with castrate-resistant disease and has been shown to regulate the antiapoptotic protein BcI-2.98 In addition to its role in driving an EMT-like state in prostate cancer, TMPRSS2-ERG fusions repress AR expression, perhaps providing a selective force for AR amplification leading to castrate-resistant prostate cancer.29 SNAI1 has been shown to transcriptionally repress PTEN, contributing to upregulation of PI3K–AKT signaling and improved radioresistance. This is interesting in the context of prostate cancer (where monoallelic PTEN deletions are common), and suggests that SNAI1 may further abrogate PTEN function.99 BMI-1 has been shown to promote resistance to docetaxel, which is currently the only chemotherapy option available for castrate-resistant, metastatic prostate cancer.100 Both SNAI1 and TWIST1 have been implicated in the survival and proliferation of cancer cells in the bone microenvironment, in part by contributing to bone remodeling.101,102 However, this must be reconciled with the observation that bone metastases typically retain E-cadherin expression. Although not yet substantiated in prostate cancer, an intriguing study indicated that both TWIST1 and TWIST2 might provide a route for cancer cells to bypass oncogene-induced senescence.103 This suggests the novel idea that oncogene activation provides a selective pressure for an EMT-like state early in tumorigenesis, with the invasive phenotype being an unfortunate byproduct. Taken together, these effectors of EMT-like states might contribute at multiple steps of the metastatic cascade to promote an aggressive phenotype (Figure 3).
Figure 3 |.
The possible roles of EMT-like states in the metastatic cascade. The loss of cell–cell adhesion that accompanies the loss of E-cadherin-mediated adherens junctions is a characteristic feature of EMT induced in vitro. This decreased cell–cell adhesion is accompanied by changes in the expression of proteases and matrix proteins that allow motile cells to invade through the basement membrane, resulting in invasive carcinoma. Similarly, these events can facilitate entrance into and escape from the bloodstream. Once at a secondary tissue, metastatic colonization requires survival, proliferation, and, possibly, MET. Abbreviations: EMT, epithelial-to-mesenchymal transition; MET, mesenchymal-to-epithelial transition.
Clinical implications of EMT-like states
Diagnosis and prognosis
The pressing need to develop better strategies for early identification of patients with high-risk disease and effective treatments for advanced disease provides the motivation for investigating EMT-like states in prostate cancer. E-cadherin has not yet emerged as a standard prognostic marker in prostate cancer, but combinations of markers related to EMT do show promise.46 PRC1 and PRC2 components and their targets are also strong candidates.82,104 Indeed, if the model is correct that EMT-like states are driven by localized microenvironments, it may be difficult to capture with standard needle biopsy approaches.105
Circulating tumor cells (CTCs) and disseminated tumor cells at sites such as bone have provided a new window to the metastatic process.106 To date, CTCs have demonstrated limited prognostic value in prostate cancer;107 however, current methods for CTC capture rely on epithelial markers, such as EpCAM (epithelial cell adhesion molecule).106 Potentially more aggressive cells in an EMT-like state (that is, those displaying mesenchymal surface markers) may go undetected with this approach. The use of other surface markers reflective of EMT, such as N-cadherin, may improve the prognostic value of CTCs. However, isolation of what might be a minor subpopulation from an already difficult-to-recover cell population could prove a daunting task. The utility of EMT-like states as prognostic biomarkers may, therefore, remain confined to the evaluation of prostatectomy specimens.
Future therapeutic potential
Is there any potential therapeutic utility for our knowledge of EMT-like states? A number of potential anti-EMT compounds do exist. The natural products genistein and silibinin have been reported to reverse EMT-like states in prostate cancer cell lines and/or inhibit progression and an EMT-like state in TRAMP mice, although the mechanisms that mediate these effects are unclear.108–110 Moreover, the proteasome inhibitor salinosporamide A (also known as NPI-0052) and the nitric oxide donor DETANONOate have been reported to inhibit Snail expression and increase RKIP expression.111,112 Finally, antibodies against the secreted form of clusterin can block TGF-β-induced EMT in breast and prostate cancer cell lines.113
Whether reversal of EMT is a desirable therapeutic goal is debatable, as metastases often exhibit epithelial features. If metastatic dissemination driven by an EMT-like state is an early event, one might not have an opportunity to intervene. However, given the relatively slow course of disease progression, EMT-inhibiting drugs could conceivably forestall the extent of metastasis, perhaps by blunting the expansive growth of micro-metastases. Moreover, if EMT-like states promote survival and therapeutic resistance, EMT-inhibitors, such as those that might inhibit the activities of PRC1 or PRC2, could augment other therapies, including chemotherapy and hormonal therapy. Thus, it is difficult to dismiss the possibility that such an approach could provide therapeutic benefit. An alternative approach to reversing or inhibiting an EMT-like state is to selectively target features of an EMT-like state. Toward this end, a very promising development has been reported by Tanaka and colleagues,94 who have developed monoclonal antibodies against N-cadherin, which are active in preclinical models of castrate-resistant prostate cancer.
Conclusions
After nearly two decades of research, there is not yet compelling evidence of a process identical to physiologic EMT in human prostate cancer (that is, a reversible, lineage-specific transdifferentiation in response to local inductive stimuli) that could account for the pathological findings. Yet, as described in this Review, there is an abundance of evidence that EMT-like states exist and are important for various aspects of prostate cancer progression and metastasis. This raises questions as to how these changes originate and what they really reveal about tumor biology. Extrinsic cues may trigger changes, but there are also examples in prostate cancer of intrinsic genetic and epigenetic events, such as TMPRSS2-ERG gene fusions, deletion of miR-101 and activation of EZH2, that drive EMT-like states. It is not entirely clear what selective pressures exist for the emergence of a motile and invasive phenotype in normally sessile epithelial cells during tumor evolution; however, if an EMT-like state reflects one means by which cancer cells can survive the stresses of aberrant oncogenic signaling, hypoxia, and inappropriate growth factor stimulation, then these forces may provide the missing selective pressure, with invasive and metastatic behavior being an unfortunate consequence. These unanswered questions will continue to provide an impetus for research in this area, with the ultimate aim of using this knowledge to improve the lives of patients.
Review criteria.
The PubMed database was searched using the terms: “epithelial mesenchymal transition prostate cancer”, “EMT prostate cancer”. “E-cadherin prostate cancer”, and “vimentin prostate cancer”, Based on a review of abstracts identified from this search, selected original, full-text, articles published in English were retrieved. The search was not limited by date of publication; however, preference was given to papers published since 2007. The reference lists of retrieved articles were searched for additional relevant articles.
Key points.
Epithelial-to-mesenchymal transition (EMT) is a normal physiologic process that involves lineage-specific, reversible transdifferentiation of epithelial cells in response to local inductive stimuli
Prostate cancers exhibit EMT-like states, characterized by changes in the expression of various markers, such as E-cadherin and vimentin, which are associated with invasive behavior
Many mechanisms have been reported to produce EMT-like states in prostate cancer
The degree to which EMT-like states in prostate cancer result from processes similar to those that produce physiologic EMT is unclear
EMT-like states are associated with metastatic behavior and therapeutic resistance, so are potential targets for biomarker development or novel therapeutics
Acknowledgments
We would like to thank members of the Henry laboratory and Dr Christopher Stipp for critical reading of the manuscript, and Dr Beatrice Knudsen for sharing unpublished results. Work related to this topic in the Henry laboratory has been supported by NIH grant R01 CA130916 and DOD grant W81XWH-10-1-0313. We apologize to colleagues whose work we were unable to cite due to space constraints.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Thiery JP, Acloque H, Huang RY & Nieto MA Epithelial-mesenchymal transitions in development and disease. Cell 1139 871–890 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Hay ED Role of cell-matrix contacts in cell migration and epithelial-mesenchymal transformation. Cell Differ. Dev 32, 367–375 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Bussemakers MJ et al. Decreased expression of E-cadherin in the progression of rat prostatic cancer. cancer Res 52, 2916–2922 (1992). [PubMed] [Google Scholar]
- 4.Thompson EW, Newgreen DF & Tarin D Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res 65, 5991–5995 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Tarin D, Thompson EW & Newgreen DF fallacy of epithelial mesenchymal transition in neoplasia. cancer Res 65, 599–000 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Gleason DF Classification of prostatic carcinomas. Cancer Chemother. Rep 50, 125–128 (1966). [PubMed] [Google Scholar]
- 7.Randolph TL, Amin MB, Ro JY & Ayala AG Histologic variants of adenocarcinoma and other carcinomas of prostate: pathologic criteria and clinical significance. Mod. Pathol 10, 612–629 (1997). [PubMed] [Google Scholar]
- 8.McCarthy RP et al. Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am. J. Pathol 165 1395–1400 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umbas R et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res 52, 5104–5109 (1992). [PubMed] [Google Scholar]
- 10.Cheng L, Nagabhushan M, Pretlow TP, Amini SB & Pretlow TG Expression of E-cadherin in primary and metastatic prostate cancer. Am. J. Pathol 148, 1375–1380 (1996). [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin MA et al. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum. Pathol 32 690–697 (2001). [DOI] [PubMed] [Google Scholar]
- 12.De Marzo AM, Knudsen B, Chan-Tack K & Epstein JI E-cadherin expression as a marker of tumor aggressiveness in routinely processe radical prostatectomy specimens. Urology 53, 707–713 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Chaffer c. L. et al. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. cancer Res 66, 11271–11278 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Berx G & van Roy F Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect Biol 1, a003129(2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita K et al. Cadherin switching in human prostate cancer progression. Cancer Res 60, 3650–3654 (2000). [PubMed] [Google Scholar]
- 16.Rokhlin OW & Cohen MB Expression of cellular adhesion molecules on human prostate tumor cell lines. Prostate 26, 205–212 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Drake JM, Strohbehn G, Bair TB, Moreland JG & Henry MD ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol. Biol. Cell 20, 2207–2217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S, Macoska J, Chen W & Sarkar FH Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer metastasis. Am. J. Transl. Res 3 90–99 (2010). [PMC free article] [PubMed] [Google Scholar]
- 19.Liao CP et al. Mouse prostate cancer cell lines established from primary and post-castration recurrent tumors. Horm. Cancer 1, 44–54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran NL, Nagle RB, Cress AE & Heimark RL N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with Stromal cells. Am. J. Pathol 155, 787–798 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chunthapong J et al. Dual roles of E-cadherin in prostate cancer invasion. J. Cell. Biochem 91, 649–661 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Zhau HE et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin. Exp. Metastasis 25 601–610 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake JM et al. ZEB1 coordinately regulates laminin-332 and β4 integrin expression altering the invasive phenotype of prostate cancer cells. J. Biol. Chem 285, 33940–33948 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harma V et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 5, e10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu JH, Yu S, Hayward SW & Chan FL Development of a three-dimensional culture model of prostatic epithelial cells and its use for the study of epithelial-mesenchymal transition and inhibition of PI3K pathway in prostate cancer. Prostate 69 428–442 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Yates CC, Shepard CR, Stolz DB & Wells A Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 96, 1246–1252 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani SA et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyak K & Weinberg RA Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9 265–273 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Yu J et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. cancer Cell 17 443–454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ZA & Shen MM Revisiting the concept of cancer stem cells in prostate cancer. Oncogene 30, 1261–1271 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Goldstein AS, Stoyanova T & Witte ON Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol. Oneal 4, 385–396 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong D et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE 5, e12445(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klarmann GJ et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 26, 433–446 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke XS et al. Epithelial to mesenchymal transition of a primary prostate cell line with switches of cell adhesion modules but without malignant transformation. PLoS ONE 3 e3368(2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klymkowsky MW & Savagner P Epithelial–mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am. J. Pathol 174, 1588–1593 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan Y et al. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Wells CM, Ahmed T, Masters JR & Jones. G. E. Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil. Cytoskeleton 62, 180–194 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Graham TR et al. Insulin-like growth factor-l-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. cancer Res 68 2479–2488 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Kong D et al. Platelet-derived growth factor-D overexpression contributes to epithelial–mesenchymal transition of PC3 prostate cancer cells. Stem Cells 26,1425–1435 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giri D, Ropiquet F & lttmann M Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin. cancer Res 5, 1063–1071 (1999). [PubMed] [Google Scholar]
- 41.Acevedo VD et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. cancer Cell 12, 559–571 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Sakai D, Suzuki T, Osumi N & Wakamatsu Y Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development 133, 1323–1333 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Cao J et al. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J. Biol. Chem 283,6232–6240 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Rashid MG et al. Posttranslational truncation and inactivation of human E-cadherin distinguishes prostate cancer from matched normal prostate. cancer Res. 61, 489–492 (2001). [PubMed] [Google Scholar]
- 45.Massagué J TGFβ in cancer. Cell 134, 215–230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q et al. Nuclear factor-κB-mediated transforming growth factor-β-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin. cancer Res 15,3557–3567 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Huber MA et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest 114, 569–581 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pu H et al. Dysfunctional transforming growth factor-β receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. cancer Res 69, 7366–7374 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu WH et al. The loss of TGF-β signaling promotes prostate cancer metastasis. Neoplasia 5 267–277 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giannoni E et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer sternness. Cancer Res 70, 6945–6956 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Ao M, Williams K, Bhowmick NA & Hayward SW Transforming growth factor-β promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res 66, 8007–8016 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuxhorn JA et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res 8, 2912–2923 (2002). [PubMed] [Google Scholar]
- 53.Bokobza SM, Ye L, Kynaston H & Jiang WG Growth and differentiation factor 9 (GDF-9) induces epithelial-mesenchymal transition in prostate cancer cells. Mol. Cell. Biochem 349, 33–40 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Buijs JT et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am. J. Pathol 171, 1047–1057 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imai T et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am. J. Pathol 163, 1437–1447 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mak P et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated Snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Moreno O et al. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp. Cell Res 316, 554–567 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Xie D et al. DAB2IP coordinates both PI3K–Akt and ASK1 pathways for cell survival and apoptosis. Proc. Natl Acad. Sci. USA 106, 19878–19883 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z et al. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC·2/DAB2. J. Biol. Chem 277, 12622–12631 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Chen H, Toyooka S, Gazdar AF & Hsieh JT Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J. Biol. Chem 278,3121–3130 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Tu SW & Hsieh JT Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J. Biol. Chem 280, 22437–22444 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Xie D et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc. Natl Acad. Sci. USA 107, 2485–2490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min J et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κB. Nat. Med 16, 286–294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varambally S et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Zi X et al. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. cancer Res 65, 9762–9770 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Yee DS et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer 9, 162(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta S et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. cancer Res 70, 6735–6745 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Du C, Zhang C, Hassan S, Biswas MH & Balaji KC Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 70, 7810–7819 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Shah GV, Muralidharan A, Gokulgandhi M, Soan K & Thomas S Cadherin switching and activation of β-catenin signaling underlie proinvasive actions of calcitonin-calcitonin receptor axis in prostate cancer. J. Biol. Chem 284, 1018–1030 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai H, Babic I, Wei X, Huang J & Witte ON Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. cancer Res. 71, 862–872 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu ML & Kyprianou N Role of androgens and the androgen receptor in epithelial mesenchymal transition and invasion of prostate cancer cells. FASEB J. 24, 769–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu X et al. Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. cancer Res 67, 4219–4226 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Beach S et al. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene 27, 2243–2248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwok WK et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. cancer Res 65, 5153–5162 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Alexander NR et al. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. cancer Res 66,3365–3369 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Kogan-Sakin I et al. Mutant p53R175H upregulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ. 18, 271–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li LC et al. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J. Urol 166 705–709 (2001). [PubMed] [Google Scholar]
- 78.Saha B et al. Unmethylated E-cadherin gene expression is significantly associated with metastatic human prostate cancer cells in bone. Prostate 68, 1681–1688 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Cao Q et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27,7274–7284 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herranz N et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell. Biol 28, 4772–4781 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lukacs RU, Memarzadeh S, Wu H & Witte ON Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7, 682–693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Leenders GJ et al. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur. Urol 52, 455–463 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Gregory PA et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol 10, 593–601 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Korpal M, Lee ES, Hu G & Kang Y The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem 283, 14910–14914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SM, Gaur AB, Lengyel E & Peter ME The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22, 894–907 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gregory PA et al. An autocrine TGF-β/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mot. Biol. Cell doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kong D et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 27, 1712–1721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saini S et al. Regulatory role of miR-203 in prostate cancer progression and metastasis. Clin. cancer Res. doi: 10.1158/1078-0432.CCR-10-2619. [DOI] [PubMed] [Google Scholar]
- 89.Gandellini P et al. miR-205 exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cε. Cancer Res. 69, 2287–2295 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Varambally S et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao P et al. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and Hlf-1α/HIF-1β. Mol. cancer 9 108(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suyama K, Shapiro I, Guttman M & Hazan RB A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. cancer Cell 2, 301–314 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Jennbacken K et al. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr. Relat. cancer 17, 469–479 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Tanaka H et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat. Med 16, 1414–1420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steffan JJ, Williams BC, Welbourne. T. & Cardelli, J. A. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+−H+ exchangers. J. Cell Sci 123, 1151–1159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh S et al. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. cancer Res 63, 2306–2311 (2003). [PubMed] [Google Scholar]
- 97.Tsuji T et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. cancer Res 68, 10377–10386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tran NL, Adams DG, Vaillancourt RR & Heimark RL Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J. Biol. Chem 277 32905–32914 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Escriva M et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol. Cell. Biol 28, 1528–1540 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crea F et al. BMI1 silencing enhances docetaxel activity and impairs antioxidant response in prostate cancer. int. J. cancer 128, 1946–1954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Odero-Marah VA et al. Receptor activator of NF-κB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res 18, 858–870 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Yuen HF et al. TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis 29, 1509–1518 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Ansieau S et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. cancer Cell 14, 79–89 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Yu J et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. cancer Res 67, 10657–10663 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Ruijter E et al. Heterogeneous expression of E-cadherin and p53 in prostate cancer: clinical implications. BIOMED-II Markers for Prostate Cancer Study Group. Mod. Pathol 11, 276–281 (1998). [PubMed] [Google Scholar]
- 106.Pantel K & Alix-Panabières C Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol. Med 16, 398–406 (2010). [DOI] [PubMed] [Google Scholar]
- 107.de Bono JS et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. cancer Res. 14, 6302–6309 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Singh RP, Raina K, Sharma G & Agarwal R Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin. Cancer Res. 14, 7773–7780 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang LL et al. A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol. Sin 29, 1060–1068 (2008). [DOI] [PubMed] [Google Scholar]
- 110.Wu K et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol. Rep 23,1545–1552 (2010). [PubMed] [Google Scholar]
- 111.Baritaki S et al. Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: inhibition of the metastasis-inducer Snail and induction of the metastasis-suppressor RKIP. Cell Cycle 9, 4931–4940 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baritaki S et al. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NP1–0052: pivotal roles of Snail repression and RKIP induction. Oncogene 28,3573–3585 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Lenferink AE et al. Transcriptome profiling of a TGF-β-induced epithelia-to-mesenchymal transition reveals extracellular clusterin as a target for therapeutic antibodies. Oncogene 29, 831–844 (2010). [DOI] [PubMed] [Google Scholar]
- 114.Umbas R et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. cancer Res 54, 392–3933 (1994). [PubMed] [Google Scholar]
- 115.Lang SH et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 52, 253–263 (2002). [DOI] [PubMed] [Google Scholar]
- 116.Jaggi M et al. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate 66, 193–199 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Kallakury BV, Sheehan CE & Ross JS Co-downregulation of cell adhesion proteins α- and β-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum. Pathol 32 849–855 (2001). [DOI] [PubMed] [Google Scholar]