Abstract
Objective:
To assess the patterns of angiotensin converting enzyme inhibitors and angiotensin receptor blockers (ACE-I/ARB) discontinuation in the setting of chronic kidney disease (CKD) progression in real-world clinical practice.
Patients and Methods:
We identified incident ACE-I/ARB users with a baseline estimated glomerular filtration rate (eGFR) ≥15mL/min/1.73m2 and without end-stage renal disease in the Geisinger Health System between January 1, 2004 and December 31, 2015. We investigated the associations of CKD stage, hospitalizations with and without acute kidney injury (AKI), serum potassium, bicarbonate level, thiazide, and loop diuretic use with ACE-I/ARB discontinuation.
Results:
Among the 53,912 ACE-I/ARB users, the mean age was 59.9 years and 50.6% were female. Over half discontinued ACE-I/ARB within 5 years of therapy initiation. The risk of ACE-I/ARB discontinuation increased with more advanced CKD stage. For example, patients who initiated ACE-I/ARB with CKD stage G4 (eGFR: 15-29ml/min/1.73m2) were 2.09-times [95% CI: 1.87-2.34] more likely to discontinue therapy than those with eGFR≥90ml/min/1.73m2. Potassium level>5.3mEq/L, systolic blood pressure≤90mmHg, bicarbonate level<22mmol/L, and intervening hospitalization – particularly AKI-related – were also strong risk factors for ACE-I/ARB discontinuation. Thiazide diuretic use was associated with lower risk, whereas loop diuretic use was associated with higher risk of discontinuation.
Conclusion:
In a real-world cohort, discontinuation of ACE-I/ARB was common, particularly in patients with lower eGFR. Hyperkalemia, hypotension, low bicarbonate level, and hospitalization (AKI-related, in particular) were associated with higher risk of ACE-I/ARB discontinuation. Additional studies are needed to evaluate the risk-benefit balance of discontinuing ACE-I/ARB in the setting of CKD progression.
Keywords: discontinuation, antihypertensive, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, chronic kidney disease, estimated glomerular filtration rate
Angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) are first-line antihypertensives for individuals with albuminuria, and are among the few clinically-proven therapies to delay chronic kidney disease (CKD) progression in patients with albuminuria.1–5 ACE-Is and ARBs may also provide benefits post myocardial infarction, with improvement in myocardial performance and survival compared with placebo.6–11 On the other hand, ACE-Is, ARBs, or their combination (ACE-I/ARB) may predispose to hyperkalemia and acute kidney injury (AKI), risks that are particularly high among patients with low estimated glomerular filtration rate (eGFR) or albuminuria.12,13
Currently, there is equipoise in the safety and efficacy of ACE-I/ARB use in advanced CKD, which motivated ongoing clinical trials such as the STOP-ACEi trial.14,15 The STOP-ACEi trial is a multi-center randomized controlled trial, which randomizes users of ACE-I/ARB with advanced progressive CKD to either discontinue or continue to receive ACE-I/ARB.14,15 Clinical guidelines reflect the uncertainty, and remain vague as to when ACE-I/ARB needs to be discontinued in patients with advanced CKD, leaving providers and patients to navigate these questions without clear scientific guidance. For example, the Kidney Disease Improving Global Outcomes (KDIGO) guideline recommends “temporary discontinuation” of ACE-I/ARB “in people with a GFR <60 ml/min/1.73 m2 (GFR categories G3a-G5) who have serious intercurrent illness that increases the risk of AKI”; yet also states “do not routinely discontinue [ACE-I/ARB therapy] in people with GFR <30 ml/min/1.73 m2 as they remain nephroprotective”.16 Although clinical trials suggest the rate of discontinuation of ACE-Is and ARBs is low,17 less is known about real-world practice in the setting of CKD progression.
The goal of this study was to describe ACE-I/ARB discontinuation patterns in a real-world setting using over ten years of data from a large, integrated healthcare delivery network, with a particular focus on patterns in the setting of CKD. As a secondary objective, we identified additional factors associated with ACE-I/ARB discontinuation, hypothesizing that hypotension, elevated potassium levels, low bicarbonate level, and recent AKI-related hospitalization would be among the strongest risk factors for discontinuing ACE-I/ARB therapy. Additionally, we assessed how ACE-I/ARB discontinuation was affected by concurrent use of loop and thiazide diuretics, two medication classes known to be associated with decreased risk of hyperkalemia.18 Finally, among discontinued users, we assessed antecedent events as well as the frequency of restarting ACE-I/ARB within six months of therapy discontinuation.
Patients and Methods
Study design, population, and data source
We conducted a retrospective study using electronic health records (EHR) data from a community-based cohort receiving primary care in the Geisinger Health System. Geisinger Health System has 12 hospitals in central and northeastern Pennsylvania. This large, integrated EHR database combines patient-level information on demographic characteristics, outpatient prescriptions, problem lists, inpatient and outpatient encounters, and laboratory test results. Based on prescription records, we identified individuals who were first prescribed an ACE-I, an ARB, or the combination of both between January 1, 2004 and December 31, 2015 (Supplemental Table 1). Baseline for each patient was the date of the initial ACE-I/ARB prescription, and follow-up was available until January 2017. Further inclusion criteria were age 18 years or older; having an outpatient measurement of serum creatinine, potassium, bicarbonate level, and systolic blood pressure within one year before baseline; and having baseline eGFR ≥15 mL/min/1.73 m2 and no previous diagnosis of end-stage renal disease.
Outcome, exposure, and covariate definitions
The primary outcome was discontinuation after starting ACE-I/ARB therapy. Discontinuation was defined as a gap in therapy greater than 60 days. Switching to different medications within ACE-I, ARB, or between the two medication classes was not considered as discontinuation. Specifically, we classified discontinuation as the end date of a prescription if there was no subsequent prescription within 60 days and the individual continued to receive follow-up in the Geisinger system. We censored patients at the therapy end date if no subsequent outpatient encounter was observed more than 60 days after the end date.
CKD stage was defined based on the KDIGO guideline, with an eGFR ≥90, 60-89, 45-59, 30-44, 15-29, and <15 mL/min/1.73 m2 classified as G1, G2, G3a, G3b, G4, and G5, respectively.16 The eGFR was estimated from outpatient serum creatinine levels using the CKD-EPI equation.19 Baseline eGFR was the latest outpatient measure taken within one year prior to the initial ACE-I/ARB prescription. Time-dependent CKD stage was ascertained using time-updated outpatient measures of serum creatinine.
Other covariates included age at baseline, gender, race, initial class of therapy (i.e., ACE-I, ARB, or the combination of both), and calendar year of initial ACE-I/ARB prescription. Additionally, we defined both baseline and time-dependent variables for serum potassium, bicarbonate level, and systolic blood pressure using the latest outpatient measurement during the one-year period prior to initial ACE-I/ARB prescription and updated outpatient measures during the follow-up period, respectively. We also captured baseline and time-updated use of loop and thiazide diuretics using prescription records (Supplemental Table 1). Baseline comorbidities such as diabetes, congestive heart failure, and coronary artery disease were determined based on the presence of diagnostic codes prior to the initial prescription (Supplemental Table 2). A binary variable was created to indicate “albuminuria testing”, defined by whether a patient had an outpatient measure of albuminuria on or before the baseline date. We also defined a time-dependent “recent hospitalization” variable indicating hospitalization within the previous 30 days. For each hospitalization, we further classified it as AKI-related versus non-AKI-related based on diagnostic codes.
Statistical analysis
We described the baseline characteristics of the overall study cohort as well as stratified by baseline CKD stage. Cumulative incidence curves were used to depict time to discontinuation since initial ACE-I/ARB prescription by baseline CKD stage, accounting for the competing risk of death. Fine-Gray competing risk regression models were constructed to quantify the associations of ACE-I/ARB discontinuation with CKD stage as well as other factors that may affect discontinuation, with death as a competing event.20 We first ran a model (Model 1) that included the following baseline variables: CKD stage, drug class/classes of the initial prescription (ACE-I, ARB, the combination of ACE-I and ARB,), calendar year of initial prescription (2004-2007, 2008-2011, 2012-2015), age (18-44, 45-64, 65+), female sex, black race, diabetes, congestive heart failure, coronary artery disease, albuminuria testing, potassium level (≤3.5, 3.5-5, 5-5.3, >5.3 mEq/L), systolic blood pressure (≤90, 90-140, ≥140 mmHg), low bicarbonate level (<22 mmol/L), use of loop diuretics, and thiazide diuretics. Model 2 included the same variables except that CKD stage, potassium level, systolic blood pressure, low bicarbonate level, and use of loop and thiazide diuretics were captured as time-dependent variables to incorporate changes during the observation period. Model 2 also adjusted for the time-dependent variable of recent hospitalization status (no hospitalization, AKI-related hospitalization, non-AKI-related hospitalization within the previous 30 days).
Among discontinued users, we described the prevalence of the following risk factors preceding discontinuation: CKD progression (decline in eGFR ≥30% compared to the antecedent measure), hyperkalemia (serum potassium >5 mEq/L), recent hospitalizations with and without AKI, bicarbonate level <22 mmol/L, and systolic blood pressure ≤90 mmHg, stratified by CKD stage prior to discontinuation. We performed logistic regression to assess the associations of demographic and clinical characteristics with restarting therapy within six months of discontinuation.
Sensitivity analysis
We performed several sensitivity analyses. First, we defined discontinuation as a gap greater than 90 days without receiving ACE-I/ARB, and repeated the primary analyses. Second, we excluded individuals who were prescribed ACE-I/ARB at their first outpatient encounter in the Geisinger system. Finally, we evaluated the pattern of discontinuation across CKD stages of a comparison medication class, beta-blockers, to discern whether the observed associations were class-specific and not simply a marker of poor health.
Results
Study population
A total of 53,912 individuals from the Geisinger Health System met the inclusion criteria (Supplemental Figure 1). The study population was 50.6% female and had a mean (SD) age of 59.9 (14.9) years. The majority (88.0%) of the initial prescriptions were for ACE-I, with 11.3% for ARB, and 0.7% for both (Table 1). At baseline, 23,069 (42.8%), 23,158 (43.0%), 5,123 (9.5%), 2,029 (3.8%), and 533 (1.0%) patients were classified as G1, G2, G3a, G3b, and G4 CKD stage, respectively. The proportion of patients initially prescribed ARBs was higher among those with more advanced baseline CKD stages.
Table 1.
Baseline characteristics of incident users of ACE-I/ARB by baseline CKD stage in the Geisinger health system, N (%)a
| CKD Stage at treatment initiation | Overall | G1 | G2 | G3a | G3b | G4 |
|---|---|---|---|---|---|---|
| N=53912 | N=23069 (42.8%) |
N=23158 (43.0%) |
N=5123 (9.5%) |
N=2029 (3.8%) |
N=533 (1.0%) |
|
| Drug class | ||||||
| ACE-I | 47472 (88.0) | 20906 (90.6) | 20314 (87.7) | 4296 (83.9) | 1594 (78.6) | 362 (67.9) |
| ARB | 6068 (11.3) | 2021 (8.8) | 2703 (11.7) | 784 (15.3) | 408 (20.1) | 152 (28.5) |
| ACE-I and ARB | 372 (0.7) | 142 (0.6) | 141 (0.6) | 43 (0.8) | 27 (1.3) | 19 (3.6) |
| Calendar year | ||||||
| 2004-2007 | 18103 (33.6) | 6834 (29.6) | 8094 (35.0) | 2078 (40.6) | 867 (42.7) | 230 (43.2) |
| 2008-2011 | 18504 (34.3) | 8448 (36.6) | 7681 (33.2) | 1552 (30.3) | 638 (31.4) | 185 (34.7) |
| 2012-2015 | 17305 (32.1) | 7787 (33.8) | 7383 (31.9) | 1493 (29.1) | 524 (25.8) | 118 (22.1) |
| Age group | ||||||
| 18-44 | 8347 (15.5) | 6939 (30.1) | 1232 (5.3) | 102 (2.0) | 44 (2.2) | 30 (5.6) |
| 45-64 | 24580 (45.6) | 13437 (58.3) | 9819 (42.4) | 937 (18.3) | 284 (14.0) | 103 (19.3) |
| 65+ | 20985 (38.9) | 2693 (11.7) | 12107 (52.3) | 4084 (79.7) | 1701 (83.8) | 400 (75.1) |
| Female | 27256 (50.6) | 10846 (47.0) | 11822 (51.1) | 3031 (59.2) | 1268 (62.5) | 289 (54.2) |
| Black race | 1319 (2.5) | 893 (3.9) | 343 (1.5) | 57 (1.1) | 15 (0.7) | 11 (2.1) |
| Diabetes | 16641 (30.9) | 7798 (33.8) | 6245 (27.0) | 1594 (31.1) | 776 (38.3) | 228 (42.8) |
| Congestive heart failure | 3991 (7.4) | 791 (3.4) | 1768 (7.6) | 794 (15.5) | 488 (24.1) | 150 (28.1) |
| Coronary artery disease | 9823 (18.2) | 2530 (11.0) | 4795 (20.7) | 1533 (29.9) | 772 (38.1) | 193 (36.2) |
| Measured albuminuria | 14222 (26.4) | 6692 (29.0) | 5420 (23.4) | 1384 (27.0) | 577 (28.4) | 149 (28.0) |
| Potassium level | ||||||
| 3.5 mEq/L or lower | 2039 (3.8) | 918 (4.0) | 844 (3.6) | 193 (3.8) | 68 (3.4) | 16 (3.0) |
| 3.5-5 mEq/L | 49764 (92.3) | 21573 (93.5) | 21449 (92.6) | 4608 (90.0) | 1726 (85.1) | 408 (76.6) |
| 5-5.3 mEq/L | 1573 (2.9) | 460 (2.0) | 674 (2.9) | 219 (4.3) | 158 (7.8) | 62 (11.6) |
| Above 5.3 mEq/L | 536 (1.0) | 118 (0.5) | 191 (0.8) | 103 (2.0) | 77 (3.8) | 47 (8.8) |
| Systolic blood pressure | ||||||
| 90 mmHg or lower | 221 (0.4) | 67 (0.3) | 76 (0.3) | 42 (0.8) | 26 (1.3) | 10 (1.9) |
| 90-140 mmHg | 24370 (45.2) | 10388 (45.0) | 10015 (43.3) | 2562 (50.0) | 1126 (55.5) | 279 (52.4) |
| 140 mmHg or above | 29321 (54.4) | 12614 (54.7) | 13067 (56.4) | 2519 (49.2) | 877 (43.2) | 244 (45.8) |
| Bicarbonate lower than 22mmol/L | 1022 (1.9) | 456 (2.0) | 290 (1.3) | 101 (2.0) | 89 (4.4) | 86 (16.1) |
| Loop diuretic use | 6016 (11.2) | 1509 (6.5) | 2570 (11.1) | 1083 (21.1) | 641 (31.6) | 213 (40.0) |
| Thiazide diuretic use | 14343 (26.6) | 6059 (26.3) | 6383 (27.6) | 1311 (25.6) | 457 (22.5) | 133 (25.0) |
ACE-I = angiotensin converting enzyme inhibitors; ACE-I/ARB = angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or their combination; ARB = angiotensin receptor blockers; CKD = chronic kidney disease
Association between discontinuation and the severity of CKD
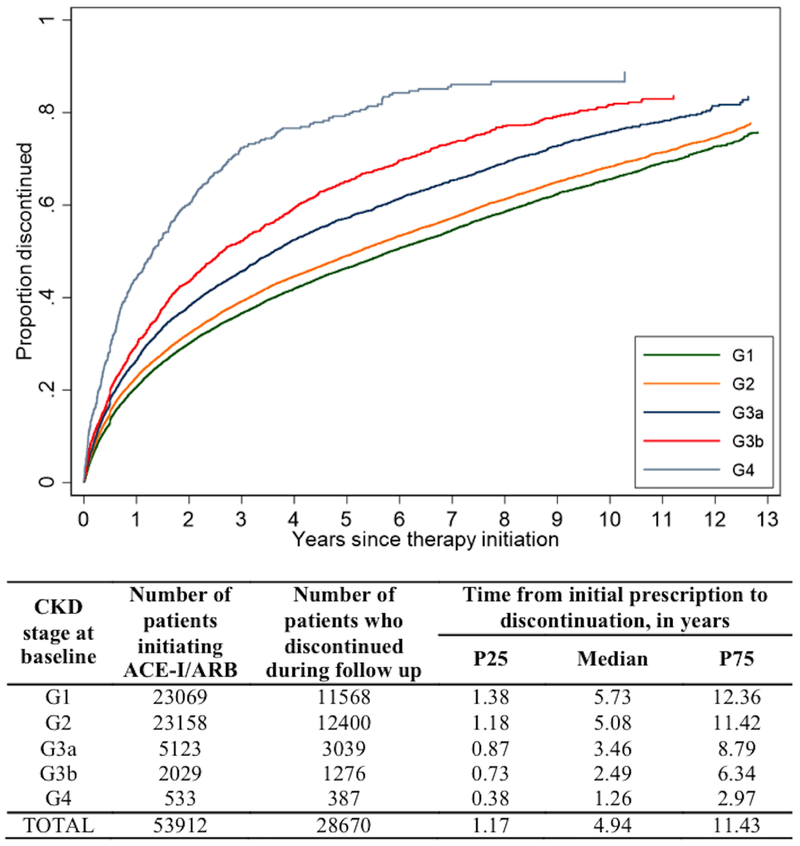
In total, 28,670 patients discontinued ACE-I/ARB therapy during follow-up. More advanced CKD stage at baseline was associated with greater risk of discontinuation (Figure 1; P<.001 for all comparisons between G2, G3a, G3b, G4, and G1). Based on the cumulative incidence of ACE-I/ARB discontinuation accounting for the competing risk of death, we estimated that 20.7% [95% confidence interval (CI): 20.2-21.3%], 22.9% [95% CI: 22.3-23.4%], 26.7% [95% CI: 25.4-27.9%], 30.1% [95% CI: 28.1-32.3%], and 45.3% [95% CI: 41.0-49.8%] had discontinued ACE-I/ARB use by one year of therapy initiation among patients with G1, G2, G3a, G3b, and G4 CKD stage at baseline, respectively. These proportions rose to 46.8% [95% CI: 46.1-47.5%], 49.7% [95% CI: 49.0-50.4%], 58.8% [95% CI: 57.2-60.3%], 68.4% [95% CI: 65.9-70.8%], and 83.7% [95% CI: 79.5-87.4%] by five years after treatment initiation.
Figure 1. Discontinuation of ACE-I/ARB among incident users, by CKD stage at baseline.
Abbreviations: ACE-I/ARB, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or their combination; CKD, chronic kidney disease
In adjusted analysis, more advanced CKD stage at baseline continued to be significantly associated with higher risk of discontinuation (hazard ratio (HR): 1.06 [95% CI: 1.03-1.09], 1.22 [95% CI: 1.17-1.28], 1.40 [95% CI: 1.32-1.49], and 2.09 [95% CI: 1.87-2.34] respectively for G2, G3a, G3b, and G4, compared with G1) (Model 1, Table 2). When CKD stage was treated as time-dependent, a similar pattern persisted but the effects were more pronounced, i.e. G2, G3a, G3b, and G4 yielded HRs of 1.08 [95% CI: 1.04, 1.11], 1.25 [95% CI: 1.19, 1.30], 1.45 [95% CI: 1.36, 1.55], and 2.43 [95% CI: 2.17, 2.72], respectively, compared with G1 (Model 2). Although we excluded patients with G5 CKD stage at baseline, some patients progressed to G5 during follow up, which was associated with a substantially elevated risk of discontinuation (HR: 4.62 [95% CI: 3.27, 6.52], compared with G1).
Table 2.
Association of demographic and clinical factors with discontinuation of ACE-I/ARB, subhazard ratio and 95% confidence intervala,b
| Demographic and Clinical Factors | Model 1 | Model 2 |
|---|---|---|
| CKD stage (referent: G1) | ||
| G2 | 1.06 (1.03, 1.09) | 1.08 (1.04, 1.11) |
| G3a | 1.22 (1.17, 1.28) | 1.25 (1.19, 1.30) |
| G3b | 1.40 (1.32, 1.49) | 1.45 (1.36, 1.55) |
| G4 | 2.09 (1.87, 2.34) | 2.43 (2.17, 2.72) |
| G5c | 4.62 (3.27, 6.52) | |
| Drug class (referent: ACE-I alone) | ||
| ACE-I and ARB | 0.95 (0.84, 1.08) | 0.93 (0.80, 1.07) |
| ARB alone | 0.99 (0.95, 1.02) | 0.99 (0.95, 1.03) |
| Calendar year (referent: 2004-2007) | ||
| 2008-2011 | 1.14 (1.10, 1.17) | 1.13 (1.09, 1.16) |
| 2012-2015 | 1.24 (1.20, 1.28) | 1.20 (1.16, 1.24) |
| Age group (referent: 18-44) | ||
| 45-64 | 0.82 (0.79, 0.85) | 0.80 (0.77, 0.83) |
| 65+ | 0.94 (0.90, 0.98) | 0.82 (0.78, 0.85) |
| Female | 1.15 (1.13, 1.18) | 1.15 (1.12, 1.18) |
| Black race | 1.07 (0.99, 1.16) | 1.07 (0.99, 1.16) |
| Diabetes | 0.99 (0.96, 1.03) | 0.94 (0.91, 0.98) |
| Congestive heart failure | 1.00 (0.95, 1.05) | 0.79 (0.74, 0.84) |
| Coronary artery disease | 1.15 (1.11, 1.18) | 1.07 (1.03, 1.10) |
| Measured albuminuria | 0.93 (0.89, 0.96) | 0.96 (0.92, 0.99) |
| Potassium level (referent: 3.5-5 mEq/L) | ||
| 3.5 mEq/L or lower | 1.04 (0.98, 1.11) | 1.12 (1.03, 1.21) |
| 5-5.3 mEq/L | 1.09 (1.02, 1.16) | 1.06 (0.99, 1.14) |
| Above 5.3 mEq/L | 1.24 (1.11, 1.38) | 1.94 (1.74, 2.17) |
| Systolic blood pressure (referent: 90-140 mmHg) | ||
| 90 mmHg or lower | 1.23 (1.01, 1.49) | 1.84 (1.57, 2.15) |
| 140 mmHg or above | 0.93 (0.90, 0.95) | 1.07 (1.04, 1.10) |
| Bicarbonate lower than 22mmol/L | 1.14 (1.05, 1.24) | 1.11 (1.01, 1.22) |
| Loop diuretic use | 1.14 (1.09, 1.18) | 1.34 (1.29, 1.39) |
| Thiazide diuretic use | 0.90 (0.88, 0.93) | 0.85 (0.82, 0.87) |
| Hospitalization status (referent: no hospitalization) | ||
| Non-AKI-related | - | 4.54 (4.22, 4.89) |
| AKI related | - | 7.10 (6.11, 8.25) |
ACE-I = angiotensin converting enzyme inhibitors; ACE-I/ARB = angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or their combination; AKI = acute kidney injury; ARB = angiotensin receptor blockers; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Shaded fields indicate time-dependent form of the corresponding variables.
Patients with CKD G5 stage at baseline were excluded but some patients progressed to G5 after therapy initiation.
Other factors associated with discontinuation
AKI-related hospitalization was among the strongest risk factors of ACE-I/ARB discontinuation (HR: 7.10 [95% CI: 6.11-8.25]); non-AKI-related hospitalization also elevated the risk of discontinuation (HR: 4.54 [95% CI: 4.22-4.89]). High potassium levels (5-5.3, and >5.3 mEq/L) were associated with higher risk of discontinuation compared with potassium levels in the normal range (3.5-5 mEq/L). Similarly, low systolic blood pressure (≤90 mmHg) and low bicarbonate level (<22 mmol/L) were risk factors for discontinuation. Concurrent use of thiazide diuretics was associated with decreased risk of ACE-I/ARB discontinuation whereas loop diuretics were associated with increased risk of discontinuation. There was no difference in risk of discontinuation between patients with an initial prescription of ACE-I and those initially prescribed ARB.
Events preceding discontinuation and restarting therapy within six months of discontinuation
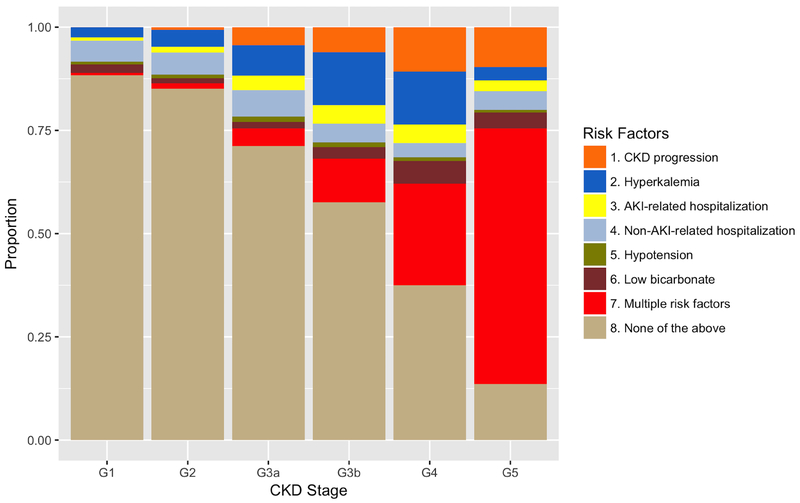
Among the 28,670 patients who discontinued ACE-I/ARB use, the proportion with antecedent CKD progression, hyperkalemia, recent hospitalizations with and without AKI, bicarbonate level <22 mmol/L, and systolic blood pressure ≤90 mmHg increased from G1 to G5 (Figure 2). Most patients who discontinued ACE-I/ARB at G1 or G2 stage did not manifest any of these risk factors before discontinuation; whereas the majority who discontinued at G4 or G5 stage experienced at least one of these risk factors prior to discontinuation.
Figure 2. Events preceding discontinuation of ACE-I/ARB, by CKD stage.
Abbreviations: ACE-I/ARB, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or their combination; CKD, chronic kidney disease; AKI, acute kidney injury
There were 6,135 (21.4%) patients who restarted ACE-I/ARB within six months of discontinuation, and 632 (2.2%) patients who died within six months of discontinuation without restarting therapy. Patients who discontinued at G3a were more likely to restart ACE-I/ARB within 6 months of discontinuation (odds ratio (OR): 1.18 [95% CI: 1.07, 1.31]) whereas those who discontinued at G4 or G5 were less likely to restart (OR: 0.83 [95% CI: 0.69, 0.99]), compared with G1 (Supplemental Table 3). No significant differences were detected between G1 and other G-stages. Patients who discontinued after a recent non-AKI-related hospitalization were more likely to restart therapy. Additionally, women were less likely to restart than men.
Sensitivity analysis
The sensitivity analyses using a 90-day gap as the threshold to define discontinuation and excluding individuals prescribed ACE-I/ARB at the first outpatient encounter yielded substantively similar findings.
Among 44,634 incident users of beta-blockers, there was a completely different pattern of time to discontinuation across CKD stages in comparison to ACE-I/ARB, with G1 stage showing the highest rate of discontinuation and other CKD stages showing fairly similar rates of discontinuation (Supplemental Figure 2).
This study was approved by the Johns Hopkins University Institutional Review Board and the Geisinger Medical Center Institutional Review Board.
Discussion
Main findings
In this study of 53,912 incident users of ACE-I/ARB observed over a decade in a community-based health system, we estimated that the majority had a discontinuation in treatment by 5 years. There was no difference in risk of discontinuation between people initially prescribed an ACE-I compared to those prescribed an ARB, but there were strong associations of advanced CKD stages with therapy discontinuation, with patients with G4 disease (eGFR: 15-29 ml/min/1.73 m2) more than twice as likely to discontinue therapy compared to people with eGFR ≥90 mL/min/1.73m2. This pattern was specific to ACE-I and ARB therapy and not observed in incident beta-blocker users. We also observed that recent hospitalization, particularly hospitalization with AKI, was a strong risk factor for discontinuation. Our findings highlight how commonly ACE-Is and ARBs are discontinued among individuals with CKD, and suggest that post-hospitalization periods are critical junctures to re-evaluate appropriate ACE-I and ARB use. Given the associations with acidosis and thiazide diuretics, we propose that correcting acidosis and treating with thiazide diuretics may provide an opportunity to extend the duration of ACE-I/ARB therapy, although this requires formal testing.
Discontinuation of treatment has been relatively understudied compared to the initiation and escalation of a given therapy. Among patients with CKD, the risk of treatment discontinuation or treatment withholding may be particularly high. For example, the utilization of coronary angiography is lower in patients with CKD compared to those without, which may be due to perceived uncertainties of the procedure’s risks and benefits in the CKD population.21,22 A previous study showed that patients who initiated ACE-I/ARB therapy for renal indications were more likely to discontinue therapy than those who initiated ACE-I/ARB therapy for other indications.23 Although our study cannot tease out the reason for ACE-I/ARB prescription, it does suggest that patients with lower GFR take therapy for shorter amounts of time, which may diminish the beneficial effects of ACE-I/ARB use in CKD. Indeed, the KDIGO guideline does not recommend routine discontinuation of ACE-I and ARB in patients with GFR < 30 mL/min/1.73 m2.16 It is also worth noting that our study only revealed the pattern among individuals who initiated ACE-I/ARB; there are many patients with CKD who would benefit from ACE-I/ARB use but never initiated the therapy.
Our results expand upon existing data on ACE-I and ARB discontinuation rates, which largely originated from clinical trials.24 A meta-analysis of 22,542 patients without heart failure across eight randomized controlled trials showed a pooled discontinuation rate of 6.5% and 4.9% for ACE-I and ARB users, respectively, over an average of 3.4 years of follow-up.17 In comparison, our study reflected real-world ACE-I/ARB use, had a greater representation of patients with advanced CKD, and observed a much higher rate of discontinuation, with over 50% ACE-I/ARB users discontinuing therapy by 5 years. Our discontinuation rate was consistent with a population-based study in the UK, which showed that 56.8% discontinued ACE-I use at 5 years post-initiation.23
An interesting finding in our study was that thiazide diuretics were associated with lower risk of ACE-I/ARB discontinuation. In contrast, loop diuretics were associated with higher risk of discontinuation. This may be due to the fact that thiazide diuretics have greater effect on lowering serum potassium than loop diuretics, thus preventing hyperkalemia;25–30 whereas loop diuretics may result in greater water diuresis, predisposing to AKI. We also found that lower bicarbonate level was a risk factor for ACE-I/ARB discontinuation. These results provide important insights for clinical practice, suggesting that thiazide diuretics and/or correction of metabolic acidosis may prolong the safe use of ACE-I/ARB. On the other hand, the associations may simply reflect the sicker patient population that develops metabolic acidosis and requires loop diuretics.
There are several limitations to our study. First, our study population was primarily white, limiting generalizability to other races/ethnicities. Second, ACE-I/ARB use was ascertained using prescription data and we did not validate whether these medications were actually dispensed or taken. Thus, our study demonstrates prescription patterns in clinical practice rather than patient adherence to medication. Finally, we do not know the reason a medication was discontinued, and patterns cannot be interpreted as causal.
Strengths of our study included the large community-based cohort with over a decade of follow-up, which provided sufficient power and a high level of precision in study results. Clinical measures were ascertained not only at baseline, but also throughout the follow-up period. We demonstrated robustness of our findings through multiple sensitivity analyses including using a more conservative threshold of medication discontinuation, and comparing patterns to a negative control class of medications.
Conclusion
In conclusion, among 53,912 incident users of ACE-I/ARB in a large community-based cohort, over half discontinued therapy within 5 years, with higher risks of discontinuation among those with more advanced CKD. Risk of discontinuation was particularly high subsequent to AKI-related hospitalization. Use of thiazide diuretics was associated with a lower risk of ACE-I/ARB discontinuation whereas loop diuretics were associated with a higher risk of discontinuation. Our findings suggest a need for more precise assessment of risk-benefit balance of ACE-I/ARB discontinuation in patients with advanced CKD, as both overuse and underuse can be harmful to health outcomes.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by R01 DK115534 (PI: Dr. Grams and Inker) and R01 DK100446 (PI: Dr. Grams) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH).
Financial support and conflict of interest disclosure:
Research reported in this publication was supported by R01 DK115534 (PI: Dr. Grams and Inker) and R01 DK100446 (PI: Dr. Grams) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). The funding sources had no role in the design and conduct of the study, analysis, or interpretation of the data; and preparation or final approval of the manuscript prior to publication.
Dr. Alexander is Chair of FDA’s Peripheral and Central Nervous System Advisory Committee; serves as a paid advisor to IQVIA; serves on the advisory board of MesaRx Innovations; holds equity in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Abbreviations:
- ACE-I
angiotensin converting enzyme inhibitors
- ACE-I/ARB
angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or their combination
- AKI
acute kidney injury
- ARB
angiotensin receptor blockers
- CI
confidence interval
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- KDIGO guideline
Kidney Disease Improving Global Outcomes guideline
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 2.Marin R, Ruilope LM, Aljama P, et al. A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19(10):1871–1876. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 4.Qin Y, Chen T, Chen Q, et al. The effect of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on mortality in patients with chronic kidney disease: a meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2016;25(5):503–511. doi: 10.1002/pds.3941 [DOI] [PubMed] [Google Scholar]
- 5.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. [DOI] [PubMed] [Google Scholar]
- 6.Borghi C, Omboni S, Novo S, Vinereanu D, Ambrosio G, Ambrosioni E. Efficacy and Safety of Zofenopril Versus Ramipril in the Treatment of Myocardial Infarction and Heart Failure: A Review of the Published and Unpublished Data of the Randomized Double-Blind SMILE-4 Study. Adv Ther. 2018;35(5):604–618. doi: 10.1007/s12325-018-0697-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332(2):80–85. doi: 10.1056/NEJM199501123320203 [DOI] [PubMed] [Google Scholar]
- 8.Borghi C, Ambrosioni E, Survival of Myocardial Infarction Long-term Evaluation Study Group. Effects of zofenopril on myocardial ischemia in post-myocardial infarction patients with preserved left ventricular function: the Survival of Myocardial Infarction Long-term Evaluation (SMILE)-ISCHEMIA study. Am Heart J. 2007;153(3):445.e7–14. doi: 10.1016/j.ahj.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1999;33(3):598–604. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001 [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292 [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson LA, Abel GA, Chaudhry AN, et al. ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PIoS One. 2013;8(11):e78465. doi: 10.1371/journal.pone.0078465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahuja TS, Freeman D, Mahnken JD, Agraharkar M, Siddiqui M, Memon A. Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. Am J Nephrol. 2000;20(4):268–272. doi: 10.1159/000013599 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Jorna T, Bhandari S. Should We STOP Angiotensin Converting Enzyme Inhibitors/Angiotensin Receptor Blockers in Advanced Kidney Disease? Nephron. 2016;133(3):147–158. doi: 10.1159/000447068 [DOI] [PubMed] [Google Scholar]
- 15.Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31(2):255–261. doi: 10.1093/ndt/gfv346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf. [DOI] [PubMed]
- 17.Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers in Patients Without Heart Failure? Insights From 254,301 Patients From Randomized Trials. Mayo Clin Proc. 2016;91(1):51–60. doi: 10.1016/j.mayocp.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 18.Chang AR, Sang Y, Leddy J, et al. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertens Dallas Tex 1979. 2016;67(6):1181–1188. doi: 10.1161/HYPERTENSIONAHA.116.07363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 21.Chertow GM, Normand S-LT, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol JASN. 2004;15(9):2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B [DOI] [PubMed] [Google Scholar]
- 22.Weisbord SD. AKI and medical care after coronary angiography: renalism revisited. Clin J Am Soc Nephrol CJASN. 2014;9(11):1823–1825. doi: 10.2215/cjN.09430914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoudpour SH, Asselbergs FW, Souverein PC, de Boer A, Maitland-van der Zee AH. Prescription patterns of angiotensin-converting enzyme inhibitors for various indications: A UK population-based study. Br J Clin Pharmacol. June 2018. doi: 10.1111/bcp.13692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J Am Coll Cardiol. 2018;71(13):1474–1482. doi: 10.1016/j.jacc.2018.01.058 [DOI] [PubMed] [Google Scholar]
- 25.Moser M The diuretic dilemma and the management of mild hypertension. J Clin Hypertens. 1986;2(2):195–202. [PubMed] [Google Scholar]
- 26.Mukete BN, Rosendorff C. Effects of low-dose thiazide diuretics on fasting plasma glucose and serum potassium-a meta-analysis. J Am Soc Hypertens JASH. 2013;7(6):454–466. doi: 10.1016/j.jash.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 27.Roush GC, Sica DA. Diuretics for Hypertension: A Review and Update. Am J Hypertens. 2016;29(10): 1130–1137. doi: 10.1093/ajh/hpw030 [DOI] [PubMed] [Google Scholar]
- 28.Savage PJ, Pressel SL, Curb JD, et al. Influence of long-term, low-dose, diuretic-based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: The Systolic Hypertension in the Elderly Program. SHEP Cooperative Research Group. Arch Intern Med. 1998; 158(7): 741–751. [DOI] [PubMed] [Google Scholar]
- 29.Roush GC, Ernst ME, Kostis JB, Tandon S, Sica DA. Head-to-head comparisons of hydrochlorothiazide with indapamide and chlorthalidone: antihypertensive and metabolic effects. Hypertens Dallas Tex 1979. 2015;65(5):1041–1046. doi: 10.1161/HYPERTENSI0NAHA.114.05021 [DOI] [PubMed] [Google Scholar]
- 30.Tannen RL. Diuretic-induced hypokalemia. Kidney Int. 1985;28(6):988–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.