Abstract
Objectives
To explore the in vitro and in vivo antibacterial activity of linezolid/fosfomycin combination against vancomycin-susceptible and -resistant enterococci (VSE and VRE), and provide a theoretical basis for the treatment of VRE.
Methods
The checkerboard method and time-kill curve study were used to evaluate the efficacy of linezolid combined with fosfomycin against VSE and VRE. The transmission electron microscopy (TEM) was employed to observe the cell morphology of bacteria treated with each drug alone or in combination, which further elucidate the mechanism of action of antibiotic combination therapy. The Galleria mellonella infection model was constructed to demonstrate the in vivo efficacy of linezolid plus fosfomycin for VSE and VRE infection.
Results
The fractional inhibitory concentration index (FICI) values of all strains suggested that linezolid showed synergy or additivity in combination with fosfomycin against five of the six strains. Time-kill experiments demonstrated that the combination of linezolid-fosfomycin at 1×MIC or 2×MIC led to higher degree of bacterial killing without regrowth for all isolates tested than each monotherapy. TEM images showed that the combination treatment damaged the bacterial cell morphology more obviously than each drug alone. In the Galleria mellonella infection model, the enhanced survival rate of the combination treatment compared with linezolid monotherapy (P<0.05) was revealed.
Conclusion
Our data manifested that the combination of linezolid and fosfomycin was a potential therapeutic regimen for VRE infection. The combination displayed excellent bacterial killing and inhibited amplification of fosfomycin-resistant subpopulations.
Keywords: linezolid, fosfomycin, vancomycin-resistant enterococci, Galleria mellonella
Introduction
Once considered a part of the normal gastrointestinal flora, enterococcus species have emerged as the second leading cause of healthcare-acquired infections in the United States, now associated with life-threatening infections such as pyelonephritis, intra-abdominal infections and bloodstream infections.1 They are intrinsically resistant to most commonly used antibiotics and readily acquire resistance.2 In 1988, the VRE clinical isolate was first reported in New York, from a wound secretion culture.3 Resistance to vancomycin is primarily mediated via acquisition of transferrable plasmids encoding modification of the primary binding site D-Ala-D-Ala. These peptidoglycan precursors are replaced with D-Ala-D-lactate or D-Ala-D-Serine, and vancomycin loses its affinity by approximately 1000-fold.4 VRE has been associated with 2.5 times higher mortality compared with VSE, which might be associated with postponing appropriate antibiotic therapy.5,6 Infections caused by VRE, incidences of which have been on the rise since 1988, posing significant challenges for antimicrobial therapy because there are fewer and fewer available antimicrobial agents.7 VRE has become problematic in the clinical setting due to the tendency for easy spreading and challenges in the antimicrobial management.8
Linezolid, which was approved by US Food and Drug Administration in 2000, is recommended as one of the first-line antimicrobial agents for the treatment of VRE infection.9 Linezolid is bacteriostatic, and adverse events attributed to the long-time use of linezolid such as neurotoxicity and bone marrow toxicity limit its use.10,11 In addition, Smith et al reported that prolonged exposure of linezolid increased the likelihood of the emergence of linezolid-resistant Enterococcus faecium.12 Fosfomycin is a bactericide against both Gram-positive and Gram-negative bacteria, including E. faecium.13 Fosfomycin has currently aroused renewed interest as a potential therapeutic choice for infections caused by VRE despite limited efficacy data.14,15 The rapid occurrence of fosfomycin resistance in vitro is the dominating limiting factor for its use as monotherapy in the clinical practice,16 which results in this old antibiotic often being specifically considered for use in combination with another agent.
In this paper, we studied the in vitro antimicrobial activity of linezolid in combination with fosfomycin against clinical VSE and VRE. The efficacy of this regimen in vivo was evaluated using the Galleria mellonella infection model. The experimental results highlighted the potential of this combination for treating infections caused by VRE.
Materials And Methods
Bacterial Isolates
Six strains were studied, including two vancomycin-susceptible strains (Enterococcus faecalis ATCC 29212 and E. faecium clinical isolate No.1) and four vancomycin-resistant strains (E. faecalis ATCC 51299, E. faecium clinical isolates No.2, No.3 and No.4). ATCC 29212, ATCC 51299 and No.1 were supplied by the First Affiliated Hospital of Anhui Medical University, China. No.2, No.3 and No.4 were obtained from Beijing Hospital, China. No.1, No.2, No.3 and No.4 were isolated from the urine of different patients. In addition, these clinical strains were not specifically isolated for this research, but they were part of the routine hospital microbiology laboratory procedure. This study was approved by the First Affiliated Hospital of Anhui Medical University institutional review board.
Antimicrobial Agents And Medium
Linezolid was obtained from Pfizer limited liability company (Shanghai, China). Vancomycin and fosfomycin were purchased from National Institutes for Food and Drug Control. Antibiotic stock solutions were freshly prepared in Milli-Q water (Labconco Corporation) which was sterilized by a 0.22-μm sterilizing filter (MET, the United States) each day (fosfomycin) or were reserved at −20°C and used within a month (linezolid 1280 μg/mL, vancomycin 1280 μg/mL).
Cation-adjusted Mueller–Hinton broth (CAMHB, Oxoid, England) containing Ca2+ of 25 mg/L and Mg2+ of 12.5 mg/L was used for all in vitro susceptibility analyses. Mueller-Hinton agar (MHA, Oxoid, England) was used for culturing bacteria, performing agar dilution method and quantifying colony counts.
Determination Of Antimicrobial Susceptibility Testing
Minimum inhibitory concentrations (MICs) of all antibiotics except fosfomycin were determined using broth microdilution methods. Bacteria that were cultured to the log-phase (approximately 1.5×108 CFU/mL) and then diluted 150-fold were seeded at 96-well plates which were added a series of 2-fold dilutions of antimicrobial agents. Plates were incubated in humidified 5% CO2 at 37°C for 24 hrs. After the incubation period, the lowest concentration of antibiotics at which no visible bacteria grew was determined as MIC. The MIC of fosfomycin was detected by agar dilution method, using MHA containing a series of two-fold diluted fosfomycin appended with glucose-6-phosphate of 25 μg/mL. The results were interpreted in the light of the MIC breakpoints of the Clinical and Laboratory Standards Institute (CLSI, 2018) antimicrobial susceptibility testing standards.17 ATCC 29212 was served as a quality control strain. All experiments were repeated three times.
Vancomycin Resistance Genotype
The genes encoding resistance to vancomycin such as VanA, Van B, Van C1 and VanC2/3 were detected with polymerase chain reaction (PCR) and sequenced by Sangon Biotech (Shanghai) Co., Ltd.
Multilocus Sequence Typing (MLST)
According to the established MLST scheme,18 seven housekeeping genes (adk, atpA, ddl, gdh, gyd, purK, and pstS) were amplified by PCR. The purified PCR products were sequenced by Sangon Biotech (Shanghai) Co., Ltd. The sequencing results were submitted to the MLST website (https://pubmlst.org) to determine the molecular typing.
Checkerboard Assays
The checkerboard broth microdilution assay was performed in 96-well microtitre plates with 2-fold dilutions of two antibiotics which were diluted in CAMHB. Linezolid ranging from 1/64×MIC to 2×MIC was dispensed in every row. Then, fosfomycin supplemented with 25 μg/mL of glucose-6-phosphate ranging between 1/64×MIC and 2×MIC was added in each column. An equal volume of standardized bacterial suspension of 1×106 CFU/mL was added and then all plates were incubated at 37°C in an aerobic atmosphere for 24 hrs. Fractional inhibitory concentrations (FICs) were calculated as the MIC of drug A or B in combination divided by the MIC of drug A or B alone, respectively, and the FIC index (FICI) was obtained by adding the two FIC values. To categorize the drug combination that consistently generated the lowest FICI after repeating the experiment in duplicate on two further occasions, the results can be grouped as follows: FICIs of ≤0.5 were interpreted as synergistic; FICIs of >0.5 but ≤1 were considered as additive; FICIs of >1 but ≤4 were considered as no interaction and FICIs >4 were interpreted as antagonistic. SBPI was calculated based on a previously described method.19
Time-Kill Studies
Time-kill studies were performed in triplicate on ATCC 29212, No.1, No.2 and No.4 based on a previously reported method.20 The concentration of linezolid and fosfomycin was selected on the basis of drug serum concentration in stable state that is achievable when administrated the optimal dosage. In brief, bacterial suspensions at the exponential-phase were diluted to the inoculum of approximately 1.0×106 CFU/mL. For the drug-containing tubes, bacterial suspensions were mixed with diverse final concentrations of linezolid (at 0.5×, 1× and 2×MIC) and fosfomycin (at 0.5×, 1× and 2× MIC) alone or in combination. The tubes were then incubated with shaking at 37°C. At 0, 2, 4, 6, 8, 12 and 24 hrs, the bacteria in each tube were diluted with 4°C 0.9% NaCl, and then seeded on MHA plates for viable colony counts. Synergy, additivity, indifference and antagonism were defined as ≥2 log10 CFU/mL kill, <2 but >1 log10 CFU/mL kill, ±1 log10 CFU/mL kill and >1 log10 CFU/mL growth, respectively.20 Bactericidal activity was defined as 99.9% reduction in cell numbers from the initial inoculum. Changes to fosfomycin MICs were measured for all strains that regrew after 24 hrs to detect whether these strains were resistant to fosfomycin.
Characterization Of Cell Morphology
TEM was used to explore the influence of the linezolid-plus-fosfomycin on the cellular structure and morphology of vancomycin-susceptible Enterococcus faecium No.1 and vancomycin-resistant Enterococcus faecium No.2. Bacteria that were cultured to the logarithmic phase were transferred and then diluted 100-fold into the tube, treated with 2 mg/L linezolid, 128 mg/L fosfomycin, or both antibiotics, continuing culturing for 4 hrs in the light of the time-kill experiments. Samples were centrifuged for 10 mins at 3300 rpm and 4°C three times. Supernatants were discarded and bacteria in the bottom of the tube were washed with 1 mL phosphate-buffered saline (PBS) during centrifugation procedures. After the final centrifugation procedure, the supernatants were abandoned, and then bacterial pellets were resuspended and fixed in 1 mL PBS with 2.5% glutaraldehyde at 4°C overnight. After fixed, tubes were centrifuged at 3300 rpm for 10 mins, the fixed agent was removed, bacterial pellets were washed three times in 1 mL PBS as described earlier, and then dehydrated gradiently with 30%, 50%, 70%, 80%, 90% and 100% ethanol. Each time, it was placed for 15 mins and centrifuged for 10 mins at 3300rpm. At last, bacterial pellets were washed with 100% ethanol twice as described earlier and then resuspended in 1 mL 100% ethanol. The prepared samples were observed under TEM at Southeast University, China.
Galleria mellonella Infection Model
The Galleria mellonella infection model was constructed according to a previously reported method with slight variations.21 G. mellonella larvae were stored in the darkness at 2–10°C and were used within 7 days of receipt. Larvae weighing 250–350 mg, milky white and active, without grey marks were selected for all experiments. The bacterial suspensions at log-growth phase were centrifuged, washed and resuspended in 0.9% NaCl three times. All inocula were determined by bacterial colony counts on MHA. In order to determine 80% lethal dose of No.1 and No.2, eight G. mellonella larvae of each group were injected with 10 μL bacterial suspensions of three different concentrations of 10-fold dilution using a 25 μL Hamilton microliter syringe via the last left proleg. Larvae in Petri dishes were reared at 37°C in an aerobic and humid atmosphere and were observed every 24 hrs until 96 hrs. Larva whose body was blackening and showed no movement in response to touch was considered dead. The doses of linezolid and fosfomycin were calculated according to the doses administered in the human body. Ninety-six larvae were randomly selected and equally assigned to each of the following six groups: (i) linezolid alone (10 mg/kg), (ii) fosfomycin alone (200 mg/kg), (iii) linezolid (10 mg/kg) and fosfomycin (200 mg/kg) in combination, (iv) linezolid (5 mg/kg) and fosfomycin (100 mg/kg) in combination, (v) linezolid (2.5 mg/kg) and fosfomycin (50 mg/kg) in combination or (vi) no treatment. Larvae were inoculated with 80% lethal dose of either No.1 or No.2 as previously described, following by 10 μL injections of the tested drug or 0.9% NaCl as a control within 2 hrs after injection, and then observed as performed earlier. Treatment was given only once. Blank and 0.9% NaCl controls were set for each experiment. The results of any experiment with more than one dead larva in either control group were abandoned. All experiments were performed twice on different occasions.
Statistical Analysis
All statistical analyses were performed with Graphpad Prism, version 5.01 (GraphPad Software Inc., San Diego, CA, USA). Survival curves were constructed using the Kaplan–Meier method and analyzed using the log-rank test. P-values <0.05 were considered statistically significant.
Results
Antimicrobial Susceptibility Testing
The results of in vitro susceptibility testing are listed in Table 1. The MICs of linezolid against all six tested strains ranged from 1 to 4 μg/mL. The MICs of fosfomycin against all organisms were 128 μg/mL. In short, no bacteria were resistant to linezolid and fosfomycin.
Table 1.
MICs Of Vancomycin, Linezolid, Fosfomycin And Linezolid–Fosfomycin Combination Against Six Enterococci Strains
| Strains | MIC (μg/mL) | MIC Combination | ||||
|---|---|---|---|---|---|---|
| VAN | LIN | FOS | LIN+FOS | FICI | SBPI | |
| ATCC 29212 | 2 | 2 | 128 | 1+64 | 1.0 | 3 |
| ATCC 51299 | 256 | 2 | 128 | 0.03125+64 | 0.52 | 65 |
| No.1 | 1 | 2 | 128 | 0.5+32 | 0.5 | 6 |
| No.2 | 512 | 2 | 128 | 0.5+32 | 0.5 | 6 |
| No.3 | 256 | 1 | 128 | 0.25+128 | 1. 25 | 8.5 |
| No.4 | 512 | 4 | 128 | 1+64 | 0.75 | 3 |
Notes: VAN: ≤4 μg/mL, susceptible (S); 8–16 μg/mL, intermediate (I); ≥32 μg/mL, resistant (R). LIN: ≤2 μg/mL, susceptible (S); 4 μg/mL, intermediate (I); ≥8 μg/mL, resistant (R). FOS: ≤64 μg/mL, susceptible (S); 128 μg/mL, intermediate (I); ≥256 μg/mL, resistant (R).
Abbreviations: MIC, minimum inhibitory concentration; VAN, vancomycin; LIN, linezolid; FOS, fosfomycin; LIN+FOS, linezolid–fosfomycin combination; FICI, fractional inhibitory concentration index; SBPI, susceptible breakpoint index.
Vancomycin Resistance Genotype And Molecular Typing
The results of genotype test showed that VanA genotype was detected for 3 VRE, and no VanB genotype was detected. One MLST type (ST78) was identified in No.2, No.3 and No.4.
In Vitro Synergy Testing With The Checkerboard Method
The FICI values of all strains suggested that linezolid showed synergy or additivity in combination with fosfomycin against five of the six strains (Table 1). No antagonistic effect was observed against all isolates evaluated. For No.1 and No.2, the existence of fosfomycin at 0.25×MIC reduced the MIC of linezolid from 2 μg/mL to 0.5 μg/mL; A FICI ≤0.5 was seen for both strains, demonstrating a synergistic interaction. No significant synergism was observed in ATCC 29212, ATCC 51299, No.3 and No.4. However, an SBPI >2 was discovered in all six isolates tested, which manifested potential synergism (Table 1).
Time-Kill Studies
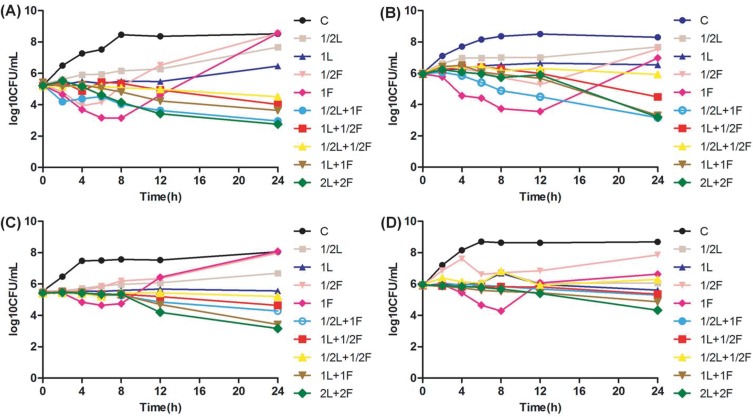
Linezolid at 1×MIC showed bacteriostatic activity to all four strains (Figure 1). For VSE ATCC 29212 and No.1, fosfomycin at 1×MIC resulted in 2.1 and 2.4 log10 CFU/mL colony decrease at 8 hrs and 12 hrs, respectively (Figure 1A and B). Another, fosfomycin at 1×MIC generated 0.8 and 1.6 log10 CFU/mL reduction in bacterial growth after 8 hrs incubation against No.2 and No.4, respectively (Figure 1C and D). However, regrowth appeared after 24 hrs in all four isolates and paralleled the growth of the controls in two strains (Figure 1). Fosfomycin resistance was noted after 24 hrs for monotherapy. MICs of fosfomycin for all isolates obtained from the final timepoint of the time-kill assay were >1024 μg/mL, representing at least an eight-fold MIC elevation.
Figure 1.
Time-kill study performed on (A) vancomycin-susceptible Enterococcus faecalis (ATCC 29212), (B) vancomycin-susceptible Enterococcus faecium (No.1), (C) vancomycin-resistant Enterococcus faecium (No.2), (D) vancomycin-resistant Enterococcus faecium (No.4) using linezolid and fosfomycin alone or in combination.
Abbreviations: CFU, colony-forming units; L, linezolid; F, fosfomycin; C, control group; 1/2, 1/2×MIC, and so forth.
On the contrary, linezolid in combination with fosfomycin showed better bacterial killing activity and no regrowth was observed for all the isolates in comparison with any agent alone. The combination treatment at 1×MIC demonstrated synergistic bacterial killing against No.1 and No.2, and also produced an additive effect against ATCC 29212 and No.4. The combination of 2×MIC was more effective than the combination of 1×MIC for all four strains evaluated.
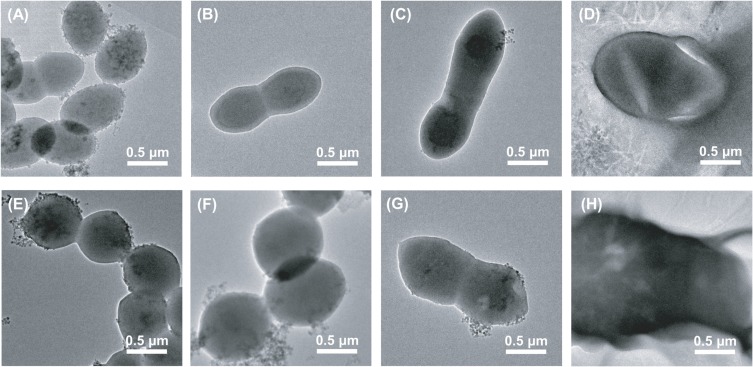
Influence Of Linezolid And Fosfomycin Alone And In Combination On The Cell Morphology Of Vancomycin-Susceptible And -Resistant Enterococcus faecium
Figure 2 shows TEM results of vancomycin-susceptible Enterococcus faecium No.1 and vancomycin-resistant Enterococcus faecium No.2 following therapy with linezolid (1×MIC), fosfomycin (1×MIC), or both. For No.1, the cells without treatment were observed with elliptical shapes and integrated cell membranes (Figure 2A). Both linezolid monotherapy and fosfomycin monotherapy resulted in significantly longer cell compared with the untreated group and the cell appeared to be undergoing cell division (Figure 2B and C). In the combination treatment group, the bacterial cell surface became cracked (Figure 2D). For No.2, the cell morphology of the untreated bacteria was round shape and unbroken cell membrane (Figure 2E). Linezolid monotherapy had minimal impact on the morphology of the bacterial cells compared with the control group (Figure 2F). Compared to the untreated group, the bacterial cell treated with fosfomycin monotherapy displayed uneven and rough, and the cell length increased to approximately double (Figure 2G). Linezolid/fosfomycin combination led to obvious cell membrane damage, with leakage of cell cytoplasm (Figure 2H).
Figure 2.
The TEM images of No.1 (A–D) and No.2 (E–H). (A) and (E) represent the control group without treatment. (B) and (F) were treated with 2 mg/L linezolid. (C) and (G) were treated with 128 mg/L fosfomycin. (D) and (H) were treated with the combination of linezolid and fosfomycin.
Activities Of Linezolid And Fosfomycin In Infected Galleria mellonella Larvae
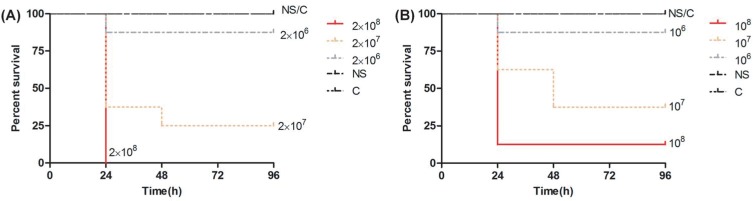
As the bacterial concentration increased, the mortality of the Galleria mellonella larvae also increased, and most of the deaths of the infected larvae occurred within the first 24 hrs. The 80% lethal dose of No.1 and No.2 was approximately 2×107 CFU/mL and 108 CFU/mL, respectively (Figure 3).
Figure 3.
Survival curves of Galleria mellonella larvae infected with (A) No.1 and (B) No.2 at three different concentrations.
Abbreviations: NS, 0.9% NaCl; C, control group.
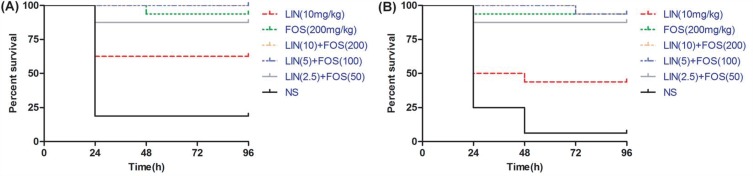
The linezolid/fosfomycin combination of high doses was superior to the combination of low doses but no significance was observed. A statistically significant higher survival rate was observed for No.1 and No.2 in the combination of linezolid and fosfomycin compared with linezolid monotherapy (P <0.05). Interestingly, fosfomycin showed excellent antibacterial efficacy against both No.1 and No.2, which was approximately equivalent to the efficacy of the combination treatment group (P >0.05) (Figure 4).
Figure 4.
Effect of linezolid alone, fosfomycin alone and the combination of different doses on survival rate of Galleria mellonella larvae infected with (A) No.1 and (B) No.2.
Abbreviations: LIN, linezolid; FOS, fosfomycin; LIN+FOS, linezolid–fosfomycin combination; NS, 0.9% NaCl.
Discussion
Infections caused by VRE have been on the rise worldwide in recent years, complicating the therapeutic options.1,22 The increasing use of linezolid, one of the last-resort antibiotics, enhances the selective pressure for developing resistance to it in VRE strains.23 Currently, the speed of research and development of novel antimicrobial agents cannot keep pace with the increasing antibiotic resistance rates, so more and more unconventional combinations for the infection of VRE seem to be attractive options.24 The study conducted by Luther et al showed that the combination of linezolid and gentamicin enhanced antimicrobial activity against VRE.21 Tang et al reported that teicoplanin combined with fosfomycin revealed excellent synergistic activity against VRE.25 Previous studies have confirmed the potent synergism of the combination of linezolid and fosfomycin against another common multidrug-resistant gram-positive pathogen, methicillin-resistant Staphylococcus aureus (MRSA).26 However, reports about this combination therapy against VRE are scarce.
In the current study, the FICI values of all strains suggested that linezolid showed synergy or additivity in combination with fosfomycin against five of the six strains. No antagonistic effect was observed against all isolates evaluated. However, an SBPI >2 was discovered in all strains. SBPI is a parameter predicting the efficacy of antimicrobial combination therapies. Additionally, due to the in-depth analysis of the pharmacokinetic/pharmacodynamic indicator, SBPI is more related to clinical outcome than FICI. An SBPI >2 indicates potential synergy.19 In the time-kill curves, linezolid only displayed bacteriostatic liveness, in agreement with the results demonstrated by Oliva et al.27 For this reason, linezolid monotherapy is associated with high failure rates for severe VRE infections, especially VRE bloodstream infections.28 Fosfomycin initially exhibited excellent bacterial killing, following by regrowth after 8 hrs or 12 hrs, which is consistent with the result reported in a recent study conducted in an experimental foreign-body infection model.27 This phenomenon may be interpreted by “fosfomycin heteroresistance”. Heteroresistance to fosfomycin has been exhibited in Streptococcus pneumoniae, and MurA (UDP-N-acetylglucosamine enolpyruvyl transferase) is responsible for the heteroresistance.29 There is a growing body of evidence suggesting that resistance to fosfomycin can emerge with monotherapy.16,27 The high-level resistance of enterococcus to fosfomycin may result from mutations of the target enzyme MurA, accompanied by a slight decrease in catalytic activity.30 Therefore, fosfomycin monotherapy is problematic for treating infections caused by VRE. In contrast, the combination of linezolid and fosfomycin at 1×MIC or 2×MIC resulted in a higher degree of bacterial kill without regrowth for each of the isolates than either monotherapy regimen. The combination treatment at 1×MIC showed a synergistic effect against No.1 and No.2, and also displayed an additive activity against ATCC 29212 and No.4. The synergism of the linezolid plus fosfomycin was described in several studies, especially against MRSA.26,31 To our knowledge, only one study was found that explored the effectiveness of linezolid combined with fosfomycin against VRE in vitro via time-kill curve experimentation and similar results were detected in that study.32 Fosfomycin inactivates MurA via covalently combining to the thiol group of a cysteine located in the active site of MurA, causing the early synthesis of the peptidoglycan precursor of bacterial cell wall to be suppressed and therefore causing bacterial death.15 According to the above analysis, it is speculated that the mechanism for the augmented bacterial killing revealed in the combination is inhibition of bacterial cell wall biosynthesis by fosfomycin, which results in easier entry of linezolid into bacterial cells.
TEM image results showed the damage of the bacteria was more obvious in the combination treatment group, which further confirmed the synergistic effect of linezolid combined with fosfomycin and tentatively clarified the mechanism of action of the combination. After retrieving similar investigations, it appears the study was the first to observe the impact of linezolid combined with fosfomycin on VRE’s cell morphology using TEM, and the results are mostly in line with the above in vitro results.
The Galleria mellonella larva infection model has been previously used for the research of the virulence of numerous human pathogens and the efficacy of the antimicrobial agents.33–35 G. mellonella has also been effectively employed to test the effects of rifampicin combination therapy against enterococcal infections in the past.36 When compared with mammalian models, G. mellonella is cheaper to obtain and free of ethical constraints.37 Additionally, G. mellonella possesses both cellular and humoral immune responses, which function analogously to vertebrate immune systems.38 In the study, the combination of linezolid and fosfomycin improved survival rate significantly over linezolid alone. Interestingly, no significant difference was detected between the combination treatment group and fosfomycin group. This efficacy observed in vivo is better than the one in vitro, it may result from the immunomodulatory activity of fosfomycin.39 These results were partly in accord with the results of in vitro and might preliminarily predict clinical outcomes, and also indicated that combination therapy with linezolid and fosfomycin might be a good therapeutic option for serious VRE infections.
In conclusion, linezolid combined with fosfomycin has excellent in vitro and in vivo activity against VSE and VRE in contrast to linezolid or fosfomycin monotherapy. Importantly, the combination also inhibits amplification of fosfomycin-resistant subpopulations. Even so, further mammal experiments and clinical studies are needed to confirm the activity of this combination on VRE and the exact mechanism of the combination.
Acknowledgments
This study was supported by the National Natural Science Fund of China (0601021203); the Fund of Excellent Talents in Colleges and Universities of Anhui Province, China (gxbjZD06); and the Fund of Academic Leaders of Anhui Province, China (2015D068).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2015;34(01):1–14. doi: 10.1086/668770 [DOI] [PubMed] [Google Scholar]
- 2.Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2014;3(5):421–569. doi: 10.4161/viru.21282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frieden TR, Munsiff SS, Williams G, et al. Emergence of vancomycin-resistant enterococci in New York City. Lancet. 1993;342(8863):76–79. doi: 10.1016/0140-6736(93)91285-t [DOI] [PubMed] [Google Scholar]
- 4.Munita JM, Bayer AS, Arias CA. Evolving resistance among gram-positive pathogens. Clin Infect Dis. 2015;61(suppl 2):S48–S57. doi: 10.1093/cid/civ523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diazgranados CA, Zimmer SM, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–333. doi: 10.1086/430909 [DOI] [PubMed] [Google Scholar]
- 6.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time is of the essence: the impact of delayed antibiotic therapy on patient outcomes in hospital-onset enterococcal bloodstream infections. Clin Infect Dis. 2016;62(10):1242–1250. doi: 10.1093/cid/ciw110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuro NJ, Davis SL, Zervos MJ, Herc ES. Combatting resistant enterococcal infections: a pharmacotherapy review. Expert Opin Pharmacother. 2018;19(9):979–992. doi: 10.1080/14656566.2018.1479397 [DOI] [PubMed] [Google Scholar]
- 8.Reyes K, Bardossy AC, Zervos M. Vancomycin-resistant enterococci. Infect Dis Clin North Am. 2016;30(4):953–965. doi: 10.1016/j.idc.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis. 2003;36(2):159–168. doi: 10.1086/345744 [DOI] [PubMed] [Google Scholar]
- 10.Frippiat F, Derue G. Causal relationship between neuropathy and prolonged linezolid use. Clin Infect Dis. 2004;39(3):439. doi: 10.1086/422147 [DOI] [PubMed] [Google Scholar]
- 11.Bernstein WB, Trotta RF, Rector JT, Tjaden JA, Barile AJ. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann Pharmacother. 2003;37(4):517. doi: 10.1345/aph.1C361 [DOI] [PubMed] [Google Scholar]
- 12.Smith TT, Tamma PD, Do TB, et al. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 2018;91(2):161–163. doi: 10.1016/j.diagmicrobio.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 13.Sastry S, Doi Y. Fosfomycin: resurgence of an old companion. J Infect Chemother. 2016;22(5):273–280. doi: 10.1016/j.jiac.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56(11):5744–5748. doi: 10.1128/AAC.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev. 2016;29(2):321–347. doi: 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15(11):e732–e739. doi: 10.1016/j.ijid.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 29th ed. CLSI Supplement M100. Wayne(PA): CLSI; 2019. [Google Scholar]
- 18.Homan WL, Tribe D, Poznanski S, et al. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol. 2002;40(6):1963–1971. doi: 10.1128/jcm.40.6.1963-1971.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne KE, Gould IM. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J Antimicrob Chemother. 2010;65(1):82–90. doi: 10.1093/jac/dkp384 [DOI] [PubMed] [Google Scholar]
- 20.Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother. 2008;61(2):365–370. doi: 10.1093/jac/dkm457 [DOI] [PubMed] [Google Scholar]
- 21.Luther MK, Arvanitis M, Mylonakis E, LaPlante KL. Activity of daptomycin or linezolid in combination with rifampin or gentamicin against biofilm-forming Enterococcus faecalis or E. faecium in an in vitro pharmacodynamic model using simulated endocardial vegetations and an in vivo survival assay using Galleria mellonella larvae. Antimicrob Agents Chemother. 2014;58(8):4612–4620. doi: 10.1128/AAC.02790-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simner PJ, Adam H, Baxter M, et al. Epidemiology of vancomycin-resistant enterococci in Canadian hospitals (CANWARD study, 2007 to 2013). Antimicrob Agents Chemother. 2015;59(7):4315–4317. doi: 10.1128/AAC.00384-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheetz MH, Knechtel SA, Malczynski M, Postelnick MJ, Qi C. Increasing incidence of linezolid-intermediate or -resistant, vancomycin-resistant Enterococcus faecium strains parallels increasing linezolid consumption. Antimicrob Agents Chemother. 2008;52(6):2256–2259. doi: 10.1128/AAC.00070-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim J, Smith JR, Rybak MJ. Role of combination antimicrobial therapy for vancomycin-resistant enterococcus faecium infections: review of the current evidence. Pharmacotherapy. 2017;37(5):579–592. doi: 10.1002/phar.1922 [DOI] [PubMed] [Google Scholar]
- 25.Tang HJ, Chen CC, Zhang CC, et al. In vitro efficacy of fosfomycin-based combinations against clinical vancomycin-resistant Enterococcus isolates. Diagn Microbiol Infect Dis. 2013;77(3):254–257. doi: 10.1016/j.diagmicrobio.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Chai D, Liu X, Wang R, Bai Y, Cai Y. Efficacy of linezolid and fosfomycin in catheter-related biofilm infection caused by methicillin-resistant Staphylococcus aureus. Biomed Res Int. 2016;2016:1–7. doi: 10.1155/2016/6413982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva A, Furustrand Tafin U, Maiolo EM, Jeddari S, Betrisey B, Trampuz A. Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58(3):1284–1293. doi: 10.1128/AAC.02583-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant Enterococcal bloodstream infection: a national cohort study of veterans affairs patients. Clin Infect Dis. 2015;61(6):civ444. doi: 10.1093/cid/civ444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansjürg E, Javier G-F, Christine F, et al. Heteroresistance to fosfomycin is predominant in Streptococcus pneumoniae and depends on the murA1 gene. Antimicrob Agents Chemother. 2013;57(6):2801–2808. doi: 10.1128/AAC.00223-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Tomich AD, McElheny CL, et al. High-level fosfomycin resistance in vancomycin-resistant Enterococcus faecium. Emerg Infect Dis. 2017;23(11):1902–1904. doi: 10.3201/eid2311.171130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang HJ, Chen CC, Cheng KC, et al. In vitro efficacy of fosfomycin-containing regimens against methicillin-resistant Staphylococcus aureus in biofilms. J Antimicrob Chemother. 2012;67(4):944–950. doi: 10.1093/jac/dkr535 [DOI] [PubMed] [Google Scholar]
- 32.Descourouez JL, Jorgenson MR, Wergin JE, Rose WE. Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob Agents Chemother. 2013;57(3):1518–1520. doi: 10.1128/AAC.02099-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colomer-Winter C, Flores-Mireles AL, Baker SP, et al. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog. 2018;14(9):e1007102. doi: 10.1371/journal.ppat.1007102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook SM, Mcarthur JD. Developing Galleria mellonella as a model host for human pathogens. Virulence. 2013;4(5):350–353. doi: 10.4161/viru.25240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai CJ, Loh JM, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7(3):214–229. doi: 10.1080/21505594.2015.1135289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner K, Sandoe JAT, Rajendran R, Ramage G, Lang S. Efficacy of rifampicin combination therapy for the treatment of enterococcal infections assessed in vivo using a Galleria mellonella infection model. Int J Antimicrob Agents. 2017;49(4):507–511. doi: 10.1016/j.ijantimicag.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Glavis-Bloom J, Muhammed M, Mylonakis E. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol. 2012;710:11–17. doi: 10.1007/978-1-4419-5638-5_2 [DOI] [PubMed] [Google Scholar]
- 38.Wojda I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017;24(3):342–357. doi: 10.1111/1744-7917.12325 [DOI] [PubMed] [Google Scholar]
- 39.Sauermann R, Marsik C, Steiner I, et al. Immunomodulatory effects of fosfomycin in experimental human endotoxemia. Antimicrob Agents Chemother. 2007;51(5):1879–1881. doi: 10.1128/AAC.00914-06 [DOI] [PMC free article] [PubMed] [Google Scholar]