Abstract
Purpose
To determine whether early proteins from high-risk human papillomavirus (HPV) have the capacity to maintain cellular stemness.
Patients and methods
First, we isolated cancer stem cell like cells from two cervical cancer cell lines, SiHa and CaSki, using non-adhesive culture with serum-free medium. Second, we knocked down HPV16 E7 in SiHa sphere cells and overexpressed HPV16 E7 in U2OS sphere cells. Third, we used RNA-seq analysis and Western blotting to screen and identify the expression of differentially expressed genes in SiHa cells with HPV16 E7 knockdown.
Results
We found that both SiHa and CaSki cells grew as cell spheres (oncospheres) and shared the properties of cancer stem cells, including high expression of stem cell marker OCT4 and SOX2, self-renew, and resistance to chemotherapeutic drugs. The stem-like properties were deprived when HPV16 E7 was knocked down in SiHa sphere cells and maintained when HPV16 E7 was over-expressed in U2OS sphere cells. APH1B was up-regulated, among differential expression genes, in SiHa cells with HPV16 E7 knockdown and modulated cellular stemness and SiHa sphere cells with APH1B knockdown regained the stem-like properties deprived by E7 inhibition.
Conclusion
HPV16 E7 possesses the capacity to maintain cellular stemness and APH1B may participate in this process in cervical cancer sphere cells.
Keywords: cervical cancer, HPV, cancer stem cell, APH1B
Introduction
Cervical carcinoma still is the most common malignancy and identified as the leading cause of cancer death among females in the developing countries, with an estimated number of 998,000 new cases and 305,000 cancer deaths in China in 2015,1 among them, advanced disease accounting for 80% of deaths. The prognosis of cervical cancer patients with advanced-stage cervical cancer is poor due to insensitivity to chemotherapy and radiotherapy. Understanding the molecular mechanisms that drive cervical carcinoma progression may provide new therapeutic approaches for this disease.
Many studies have demonstrated that high-risk human papillomavirus (HR-HPV) infection is a causal factor in the initiation and development of cervical carcinoma, and the continuous overexpression of early viral protein E6 and E7 of HR-HPV is an essential event in the process of virus carcinogenesis and cancer progression. Evidence shows that cervical cancer develops from a specific anatomical site, called the transformation zone (TZ), where glandular epithelium transforms squamous epithelium. The basal cells located at the TZ have stem-like characteristics and are recognized as the headstream of cervical cancer development. Cancer stem cells (CSCs) are a small minority of cells that exist in cancer tissues.2 CSCs possess the capacity for tumor initiation, self-renewal, and chemo-radiotherapy resistance, implicating them as the root cause of tumor initiation, metastasis, and recurrence.3–5 Thus, the virus-infected basal cells at the TZ consistently maintain the characteristics of stemness, which may be a key mechanism in the persistent development and progression in cervical cancer. However, it is still unknown whether there is a link between HPV and the cellular stemness in cervical cancer up to date.
Here, we isolated and identified two populations of cancer stem cell-like cells, from cervical cancer cell lines SiHa and CaSki, which highly express stem cell marker OCT4 and SOX2 and exhibit sphere formation, self-renewal, and drug resistance. We then knocked down HPV16 E7 in SiHa and CaSki sphere cells and overexpressed HPV16 E7 in U2OS sphere cells, and found that viral gene E7 has the ability to maintain stem-like properties. Furthermore, we screened and identified the elevated expression of APH1B, among 320 differentially expressed genes, in SiHa sphere cells with HPV16 E7 knockdown, and found that APH1B also modulates the stemness and that APH1B knockdown makes SiHa sphere cells regain the stem-like properties deprived by E7 inhibition, suggesting that APH1B may participate in E7 maintaining the cellular stemness. Our findings may inform new approaches for advanced cervical cancer therapy.
Materials And Methods
Cell Culture And Treatment
Cervical cancer cell lines, SiHa and CaSki, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Media (DMEM) and Roswell Park Memorial Institute-1640 (RPMI-1640) with 10% fetal calf serum. U2OS cells were purchased from ATCC and cultured in McCoy’s 5A with 10% fetal bovine serum (FBS, Sijiqing, HZ, China). All the cells were cultured at 37°C with 5% CO2.
Oncosphere Formation Assay
Oncosphere formation assays were performed as described previously. One thousand cells per well were placed in an ultra-low attachment 6-well flask (Corning, NY, USA). Cells grew in serum-free Dulbecco’s Modified Eagle Media:Nutrient Mixture F-12 (DMEM-F12) (Hyclone, South Logan, UT, USA), supplemented with 2% B27 (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA), 2% N2 (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA), 20 ng/mL EGF (Peprotech, NJ, USA) and 20 ng/mL bFGF (Peprotech, NJ, USA). Bovine pituitary extract was excluded. Appropriate culture medium was added every 2 days. After 7–8 days, the oncospheres were photographed and counted using an Olympus microscope, and the data were outputted to DP2-BSW software (Olympus, Center Valley, PA, USA) every 3 days for analysis. Cells were harvested longitudinally and counted using a hemocytometer and a light microscope.
Reagents And Antibodies
Paclitaxel was purchased from Bristol-Myers Squibb Pharmaceutical Ltd. Cisplatin (#15663-27-1) was purchased from MedChem Express. Antibodies used for Western blotting included anti-SOX2 (#3579, CST), anti-OCT4 (#19857, Abcam), anti-β-actin (#1879-1, Epitomics), anti-mouse HRP (#7076, Cell Signaling Technology), and anti-rabbit HRP (#7074, Cell Signaling Technology).
Western Blotting
All cells were collected at the indicated time points. Western blotting was performed on whole-cell extracts that were separated by 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. Protein was transferred to PVDF membranes (Millipore, Bedford, MA, USA) and blocked with 5% BSA in TBST with primary antibodies at 4°C overnight, including anti-SOX2 (1:500), anti-OCT4 (1:500), and anti-β-actin (1:1000). The membranes were washed three times with PBST (PBS buffer containing 0.1% Tween-20) and then incubated with HRP-conjugated secondary antibodies (CST) for 1 hr. After the final wash with PBST, immunoblots were detected using ECL chemiluminescence reagents (Pierce, Thermo Fisher Scientific Inc., Waltham, MA, USA) with an Imagequant LAS 4000 mini (GE Healthcare) after incubation with secondary antibodies.
Cell Viability Assay
SiHa, CaSki, and U2OS cells were transfected with plasmid or siRNA for 48 hrs. Then, cells with an appropriate quantity were seeded (8000 cells per well) in 96-well plates. SiHa, CaSki, and U2OS cells were then exposed to paclitaxel at various concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, and 100 nM) and/or cisplatin various concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, and 100 μM) at for 48 hrs after adherence to the plates. The cell viability was determined by 3-(4,5-dimethylthiazole-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich) dye according to a standard protocol and measuring the absorbance at 490 nm was measured to calculate the number of viable cells.
Immunofluorescence
SiHa, CaSki, and U2OS cells and oncospheres were seeded and cultured on small confocal dishes. The cells were fixed with 4% paraformaldehyde for 0.5–1 hr at 4°C, permeabilized with 0.1% Triton X-100 (Solarbio, T8200-100) for 30 mins at room temperature, then blocked with 2% BSA for 1 hr at room temperature. The cells were then incubated with anti-SOX2 or anti-OCT4 antibodies overnight at 4°C, then washed by PBS, and then mounted in medium containing secondary antibodies (CST) and DAPI (Sigma-Aldrich, D9542). Images were obtained with an Olympus inverted fluorescence microscope (Olympus, Tokyo, Japan) and were outputted to the MetaMoph offline 7.7.8.0 software package (Olympus) for analysis.
Plasmids And siRNA Transfection
The construction of the pcDNA3.1(+)-E7 plasmid and pcDNA3.1(+)-APH1B plasmid was as follows. Fragments of HPV16 E7 mRNA (NM_001526.4) and APH1B mRNA (NM_000015.1) were synthesized by Sangon Biotech (Shanghai, China) and inserted into the EcoRI/BamHI sites of the pcDNA3.1(+) vector (Invitrogen, V79020). The pcDNA3.1(+) vector was designated as a mock control. SiHa and U2OS cells grew to 80% confluence before plasmid transfection. The ratio of X-tremeGENE HP DNA Transfection Reagent (Roche, 06366236001): DNA was 2:1, and 2 mg DNA was used for each well in 6-well plate. The transfection protocol followed the instructions of the manufacturer. The targeting sites for E7 and APH1B siRNAs were selected as follows: 5ʹ-GGACAGAGCCCAUUACAAU-3ʹ for E7 and 5ʹ-GCGUUUGUCUCUGUCUAUATT-3ʹ for APH1B. E7 and APH1B siRNAs were obtained from GenePharma (Shanghai, China) and delivered into SiHa and U2OS cells using Lipofectamine RNAiMAX Reagent (Invitrogen, 13778150) according to the instructions of the manufacturer.
Transcriptome Sequencing
Transcriptome sequencing and KEGG and GO analysis were performed by RiboBio Co., Ltd.
Quantitative Real-Time RT-PCR Validation
Validation of RNA-seq results was performed by a separate experimental group of SiHa oncospheres treated as described for the RNA-seq analysis experiment above. The RNA from cultured cells was extracted using RNA extraction kit (#9767, TaKaRa). The β-actin mRNA level was used for normalization. cDNAs were generated using the PrimeScript RT reagent Kit (#RR047, TaKaRa). Evaluation of target factor mRNA levels was performed using the SYBR Premix Ex Taq Kits (#RR042A TaKaRa) for qRT-PCR analysis. The primer sequences used were APC2 (forward: 5′-GCTGCACAACATCGTCTTCTC-3′) and (reverse: 5′-ACGGCGGTACTCCTCATCAA-3′), APH1B (forward: 5′-CGAGCCGTTGCGTATCATCTT-3′) and (reverse: 5′-CCAAACAAGGGACGAAATCAGT-3′), WNT9A (forward: 5′-AGCAGCAAGTTCGTCAAGGAA-3′) and (reverse: 5′-CCTTCACACCCACGAGGTTG-3′), and WNT5B (forward: 5′-GCTTCTGACAGACGCCAACT-3′) and (reverse: 5′-CACCGATGATAAACATCTCGGG-3′). The 2−ΔΔCt method was used to evaluate the gene expression fold changes among the groups. Three independent experiments were performed.
Statistical Analysis
Statistical analysis was performed using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). Student’s t-test or one-way ANOVA was used to determine statistical significance when two or more groups were compared. In all tests, p<0.05 was considered to be significant.
Results
Sphere Cells From Cervical Cancer Cell Lines Exhibit Stem-Like Properties
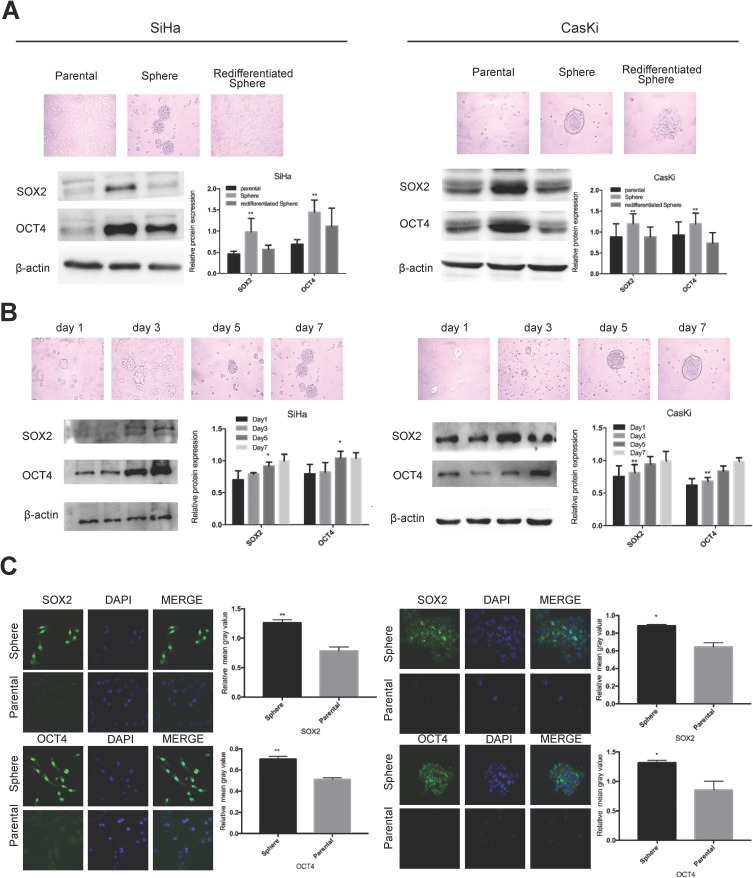
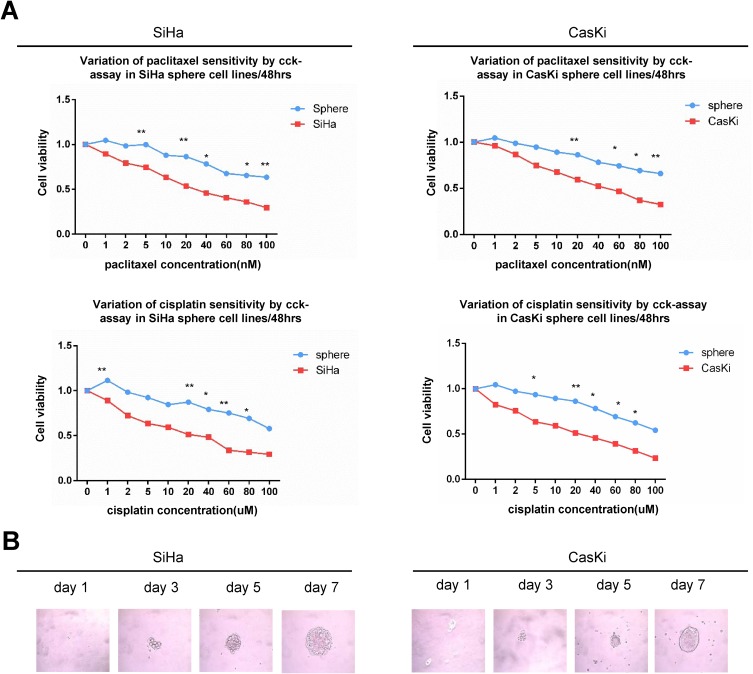
We previously successfully enriched stem-like cells from an ovarian cancer cell line in defined medium at low adherence.6 Here, we isolated and enriched stem-like cells from two human cervical cancer cell lines, SiHa and CaSki, in the same medium at low adherence and observed that cells grew as cell spheres (oncospheres). As expected, SiHa and CaSki oncospheres exhibited stem-like properties. By Western blotting and immunofluorescence imaging, we found that the expressions of SOX2 and OCT4 proteins, two stem cell markers, were higher in sphere cells than that in either parental cells or re-differentiated spheres that were derived from oncospheres cultured with 10% FBS medium (Figure 1A, C). Further, the expressions of SOX2 and OCT4 protein were gradually increased during the process of sphere formation when SiHa and CaSki cells were cultured in serum-free medium throughout days 1 to 7 (Figure 1B). Consistent with the expression of stem cell markers, SiHa and CaSki sphere cells exhibited resistance to chemotherapeutic drugs when they were exposed to paclitaxel or cisplatin at different concentrations for 48 hrs as compared to parental cells (Figure 2A), and single SiHa and CaSki oncospheres showed highly efficient clonal expansion with high efficiency (SiHa cells, 91/98 cells [93%]; and CaSki cells, 41/44 cells [93%]) (Figure 2B).
Figure 1.
Oncosphere from cervical cancer cells exhibits high expression of stem cell markers.
Notes: Phase contrast photomicrographs and Western blot detection of the expression of SOX2 and OCT4 proteins (A) in parental adherent SiHa and CaSki cells (left), oncospheres in low-adherence culture (middle), and re-differentiated oncospheres that returned to adherent culture with 10% FBS medium (right); and (B) at days 1, 3, 5, and 7 from parental adherent SiHa and CaSki cells through development of spheroid clusters to oncospheres. (C) Immunofluorescence images of SOX2 and OCT4 in parental adherent SiHa and CaSki cells and oncospheres using an anti-SOX2/OCT4 (green) antibody and DAPI staining (blue) to indicate the cell nucleus. Images on the left show cells stained with anti-SOX2/OCT4, images in the middle show cells stained with DAPI, and images on the right are merged anti-SOX2/OCT4 and DAPI. All of the contrast of images were taken under the same conditions. *P<0.05 and **P<0.01 when comparing experimental and control groups (two-tailed Student's t test).
Figure 2.
Oncosphere from cervical cancer cells exhibits self-renew and resistance to chemotherapeutic drugs.
Notes: (A) Growth inhibition of parental SiHa and CaSki cells and oncospheres. Both parental cells and oncospheres were seeded in 96-well plates and treated with paclitaxel or cisplatin at different concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, 100 nM) for 48 hrs, and cell viability was determined by a modified MTT assay. OD values of each treated group were compared with controls at the same time point. (B) Representative photomicrographs of clonal expansion of SiHa and CaSki cells into single oncospheres in low-adherence cultures over a 7-day period. The cluster of the oncospheres after days 1, 3, 5, and 7 of the culture was measured. An oncosphere was defined as being composed of more than 15 cells. Error bars and mean with SD were from three independent experiments. *P<0.05 and **P<0.01 when comparing experimental and control groups (two-tailed Student’s t test).
HPV 16 E7 Maintains Stem-Like Properties In SiHa And U2OS Sphere Cells
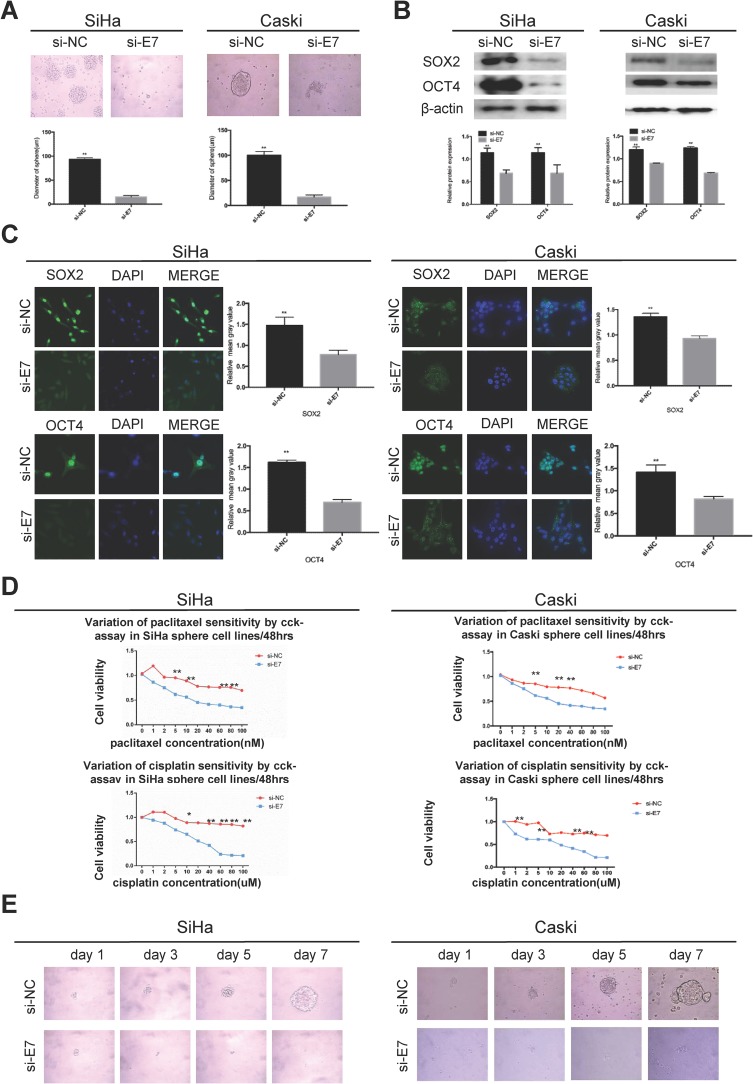
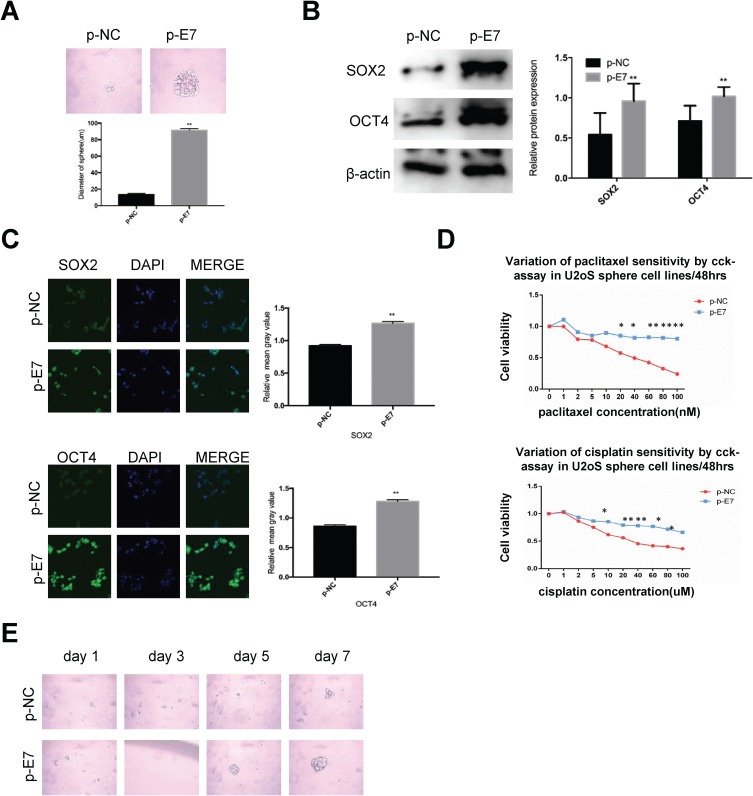
E7 is one of the oncogenes encoded by HR-HPV and plays a key role in cervical carcinogenesis.7 Here, we observed effect of E7 on the maintenance of cellular stemness. Western blotting and immunofluorescence imaging showed that SiHa and CaSki sphere cells with E7 knockdown failed to form spheres in serum-free medium (Figure 3A) and expressed the significantly decreased levels of SOX2 and OCT4 proteins compared to cells without E7 knockdown (Figure 3B and C). Consistently, SiHa and CaSki sphere cells with E7 knockdown exhibited higher sensitivity to chemotherapeutic drugs at different concentrations compared to controls (Figure 3D), and single SiHa and CaSki oncospheres with E7 knockdown failed to expand clonally compared to controls (Figure 3E). Contrarily, sphere cells of U2OS, a kind of osteosarcoma cell line, sphere cells with E7 overexpression presented stronger sphere-forming capacity in serum-free medium, higher expression of SOX2 and OCT4 proteins, resistance to chemotherapeutic drugs, and enhanced clonal capacity compared to those without E7 overexpression (Figure 4A–E).
Figure 3.
HPV 16 E7 maintains stemness in SiHa and Caski oncospheres.
Notes: (A) Phase-contrast photomicrographs of SiHa and Caski cells with HPV16 E7 knockdown in low-adherence culture for 7 days. (B) Western blot detection of the expression of SOX2 and OCT4 proteins in SiHa and Caski oncospheres with HPV16 E7 knockdown. (C) Immunofluorescence images of SOX2 and OCT4 in SiHa and Caski oncospheres with HPV16 E7 knockdown using an anti-SOX2/OCT4 (green) antibody. DAPI staining (blue) indicates cell nuclei. Images on the left show cells stained with anti-SOX2/OCT4, images in the middle show cells stained with DAPI, and images on the right are merged anti-SOX2/OCT4 and DAPI. All of the contrast images were taken under the same conditions. (D) Growth inhibition of in SiHa and Caski oncospheres with HPV16 E7 knockdown. Both were seeded in 96-well plates and treated with paclitaxel or cisplatin at different concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, 100 nM) for 48 hrs, and cell viability was determined by a modified MTT assay. OD values of each treated group were compared with controls at the same time point. (E) Representative photomicrographs of clonal expansion of single oncospheres from SiHa and Caski with HPV16 E7 knockdown in low-adherence cultures over a 7-day period. The cluster of the oncospheres after days 1, 3, 5, 7 of culture was measured. Western blot expression levels were normalized to those of β-actin. Error bars and mean with SD were from three independent experiments. *P<0.05 and **P<0.01 when comparing experimental and control groups (two-tailed Student’s t test).
Figure 4.
HPV 16 E7 maintains stemness in U2OS oncospheres.
Notes: (A) Phase-contrast photomicrographs of U2OS cells with HPV16 E7 overexpression in low-adherence culture for 7 days. (B) Western blot detection of the expression of SOX2 and OCT4 proteins in U2OS oncospheres with HPV16 E7 overexpression. (C) Immunofluorescence images of SOX2 and OCT4 in U2OS oncospheres with HPV16 E7 overexpression using an anti-SOX2/OCT4 (green) antibody. DAPI staining (blue) indicates cell nuclei. Images on the left show cells stained with anti-SOX2/OCT4, images in the middle show cells stained with DAPI, and images on the right are merged anti-SOX2/OCT4 and DAPI. All of the contrast images were taken under the same conditions. (D) Growth inhibition of U2OS oncospheres with HPV16 E7 overexpression. Both were seeded in 96-well plates and treated with paclitaxel or cisplatin at different concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, 100 nM) for 48 hrs, and cell viability was determined by a modified MTT assay. OD values of each treated group were compared with controls at the same time point. (E) Representative photomicrographs of clonal expansion of single oncospheres from U2OS with HPV16 E7 overexpression in low-adherence cultures over a 7-day period. The cluster of the oncospheres after days 1, 3, 5, 7 of culture was measured. Western blot expression levels were normalized to those of β-actin. Error bars and mean with SD were from three independent experiments. *P<0.05 and **P<0.01 when comparing experimental and control groups (two-tailed Student’s t test).
APH1B Is Involved In Modulating Stemness In SiHa Sphere Cells
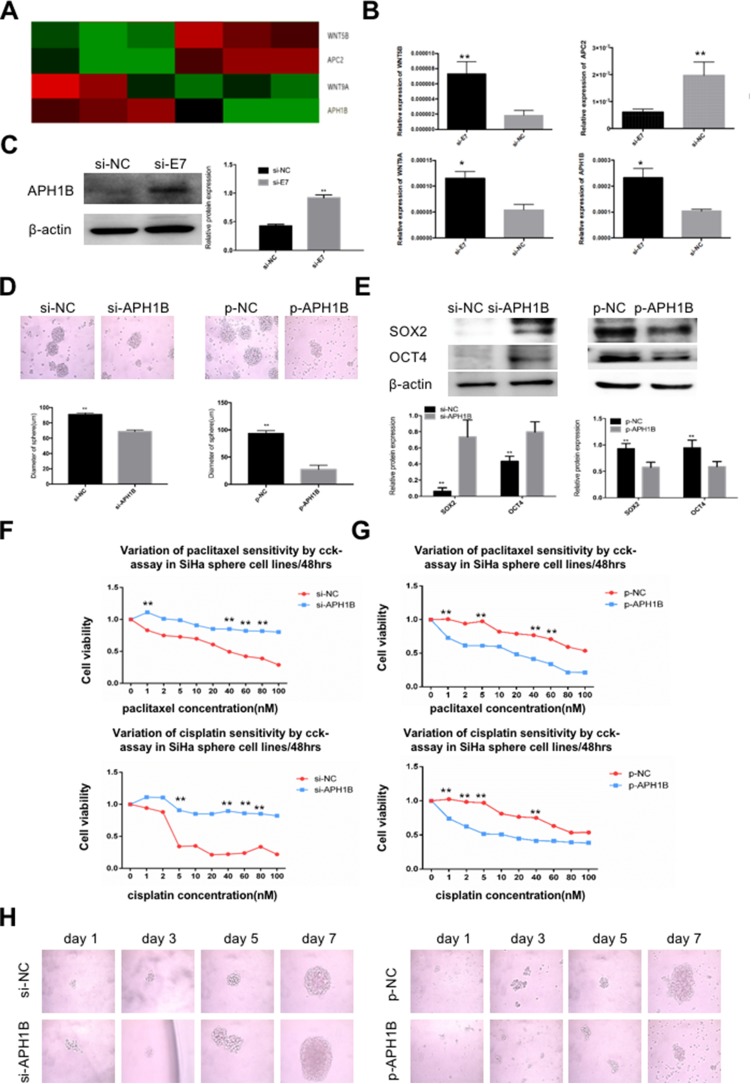
To explore the mechanism of E7-maintained stemness, we added E7 RNAi into SiHa sphere cells and performed total RNA sequencing (RNA-seq) on nontarget (NT) RNAs. RNA-seq analysis showed a total of 320 significantly differentially expressed genes between sphere cells with and without E7 knockdown. Of those, 38 genes showed higher expression and 282 had lower expression (Table S1). KEGG analysis identified four stem cell pathway-related genes, up-regulated WNT5B, APH1B, and WNT9A, and down-regulated APC2, in SiHa oncospheres with E7 knockdown (Figure 5A), and qPCR analysis verified these four differentially expressed genes (Figure 5B), and Western blotting analysis further verified the increased expression of APH1B in cells with E7 RNAi (Figure 5C). Thus, APH1B was considered as a candidate downstream gene of viral E7. We down- and up-regulated APH1B by RNA interference (RNAi) and an overexpression plasmid, respectively, in SiHa sphere cells and found that sphere cells with APH1B knockdown still formed spheres in serum-free medium (Figure 5D), and they more highly expressed SOX2 and OCT4 proteins (Figure 5E), exhibited resistance to chemotherapeutic drugs at different concentrations than controls (Figure 5F), and presented similar clonal expansion (Figure 5H) as controls. In contrast, SiHa sphere cells with APH1B overexpression formed fewer spheres in serum-free medium, had lower expression of SOX2 and OCT4 proteins, were more sensitive to chemotherapeutic drugs, and had decreased clonal expansion, compared to those without APH1B overexpression (Figure 5D–H).
Figure 5.
APH1B is involved in modulating stemness in SiHa oncospheres.
Notes: (A) Heat-map of four stem cell pathway-related genes, WNT5B, APC2, WNT9A, and APH1B. (B) Real-time RT-PCR detection of the expression of WNT5B, APC2, WNT9A, and APH1B mRNAs in si-E7 and si-NC SiHa oncospheres. (C) Western blot detection of APH1B protein in si-E7 and si-NC SiHa oncospheres. (D) Phase-contrast photomicrographs of SiHa oncospheres with APH1B inhibition or overexpression in low-adherence cultures for 7 days. (E) Western blot detection of SOX2 and OCT4 proteins in SiHa oncospheres with APH1B inhibition or overexpression. (F and G) Growth inhibition of SiHa oncospheres with APH1B inhibition or overexpression. Both were seeded in 96-well plates and treated with paclitaxel or cisplatin at different concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, 100 nM) for 48 hrs, and cell viability was determined by a modified MTT assay. OD values of each treated group were compared with controls at the same time point. (H) Representative photomicrographs of clonal expansion of single oncospheres from SiHa with APH1B inhibition or overexpression in low-adherence cultures over a 7-day period. The cluster of oncospheres after days 1, 3, 5, 7 of culture was measured. Western blot expression levels were normalized to those of β-actin. Error bars and mean with SD were from three independent experiments. *P<0.05 and **P<0.01 when comparing experimental and control groups (two-tailed Student’s t test).
APH1B May Participate In E7 Maintenance Of Stemness In SiHa Sphere Cells
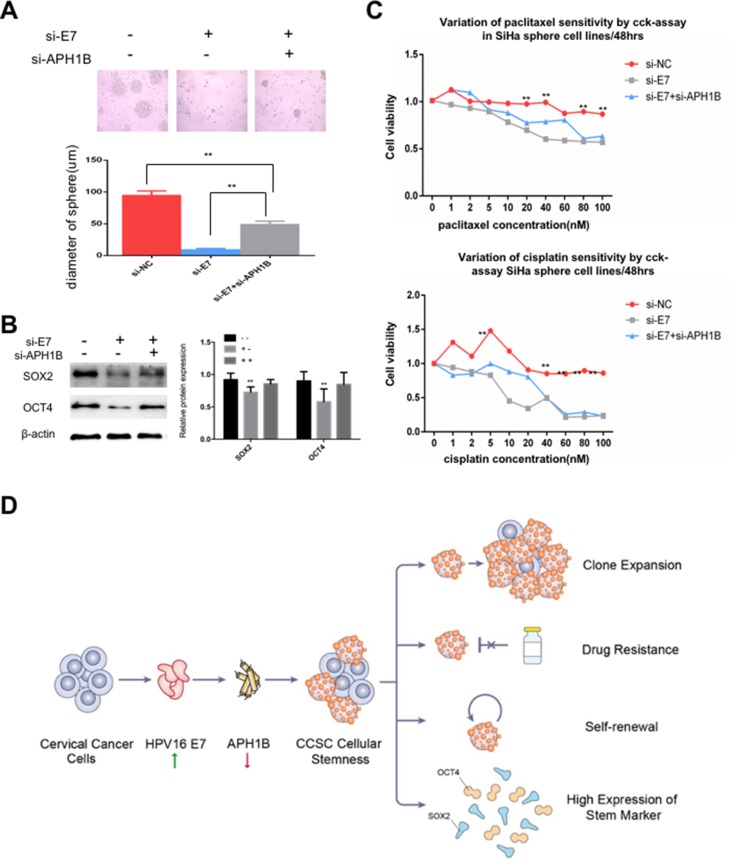
We downregulated HPV16 E7 and APH1B by E7 RNA interference (RNAi) and APH1B RNA interference (RNAi) in SiHa sphere cells, and found that cells with E7 knockdown alone failed to form spheres, expressed lower SOX2 and OCT4, and higher sensitivity to chemotherapeutic drugs compared to controls, but cells with both E7 and APH1B knockdown re-formed spheres in serum-free medium, showed upregulated SOX2 and OCT4 expression, and reduced sensitivity to chemotherapeutic drugs (Figure 6A–C). These results suggest that APH1B downregulation makes SiHa sphere cells regain, at least partially, the stem-like properties that have been deprived by E7 knockdown, and that APH1B may participate in E7 maintenance of stemness in SiHa sphere cells.
Figure 6.
APH1B may participate in E7 maintenance of stemness in SiHa oncospheres.
Notes: (A) Phase-contrast photomicrographs of SiHa oncospheres with or without HPV16 E7 inhibition and APH1B inhibition in low-adherence cultures for 7 days. (B) Western blot detection of SOX2 and OCT4 proteins in SiHa oncospheres with or without HPV16 E7 inhibition and APH1B inhibition in low-adherence cultures for 7 days. (C) Growth inhibition of SiHa oncospheres with or without HPV16 E7 inhibition and APH1B inhibition. All sphere cells were seeded in 96-well plates and treated with paclitaxel or cisplatin at different concentrations (0, 1, 2, 5, 10, 20, 40, 60, 80, 100 nM) for 48 hrs, and cell viability was determined by a modified MTT assay. OD values of each treated group were compared with controls at the same time point. (D) Proposed model: APH1B may participate in the process of E7 maintaining cellular stemness. Western blot expression levels were normalized to those of β-actin. Measurements of the diameters of colonies are shown in the bar graph. Error bars and mean with SD were from three independent experiments. **P<0.01 when comparing experimental and control groups (two-tailed Student’s t-test).
Discussion
Accumulated evidence suggests that CSCs, as a small population in cancer tissues,8–13 possess the capacity of self-renewal, differentiation, and can drive tumor initiation, progression, and metastasis. CSCs can be isolated from solid tumor tissues14,15 or cancer cell lines,16,17using available experimental methods, such as sorting subpopulations identified on the basis of the surface markers or by non-adhesive culture with serum-free medium, and others. Among these, culturing in serum-free medium is thought to be a more effective and less time-consuming method.18–20 In previous studies, CSCs have been successfully isolated and enriched by this method from established human squamous cell carcinoma cell lines.21,22 In the present study, we also successfully isolated and enriched stem-like cells from SiHa and CaSki cell lines using similar method, and observed that cells grew as cell spheres (oncospheres). We further found that those sphere cells had high expression of stem cell markers, self-renewal, and resistance to chemotherapeutic drugs, suggesting that a non-adhesive culture system with serum-free medium can obtain cancer stem cell like cells from human cervical cancer cell lines.
Chemotherapy is still one of the main treatments for advanced cervical cancer, but its efficacy is poor and the exact mechanism of resistance remains unclear. It has been verified that nearly all of cervical cancers are produced by HR-HPV, and over 60% of them are associated with HPV16.23 However, how HR-HPV regulates and maintains stemness of cervical cancer cells are largely unknown. Accumulated evidence implies that there is a potential link between the virus and CSCs in virus-associated cancers.24,25 Previous studies have revealed that some cancer-associated viruses possess the capacity to modulate cellular stemness. For instance, Epstein-Barr virus (EBV)-encoded protein can induce stem cell-like properties in epithelial cells.26–28 Hepatitis C(HCV)-infected hepatocytes display sphere formation on ultralow binding plates with characteristic activation of CSC signaling and tumorigenicity in NOD-SCID IL2Rgammanull (NSG) mice.29 In this study, we forcibly modulated E7 expression in SiHa, CaSki and U2OS sphere cells, respectively, and found that both SiHa and CaSki cells with E7 knockdown had weakened sphere-forming capacity in serum-free medium, decreased expression of stem cell markers, increased sensitivity to chemotherapeutic drugs, and decreased clonal expansion, and we found the opposite effect when E7 was overexpressed, suggesting that HPV16 E7 possesses the capacity to maintain the stemness of cervical cancer stem cell like cells.
To explore the mechanism by which E7 maintains cellular stemness, we screened differentially expressed genes between SiHa sphere cells with and without HPV16 E7 knockdown, using RNA-seq and KEGG analysis, and identified that four stem cell pathway-related genes, upregulated WNT5B, APH1B and WNT9A, and downregulated APC2 in HPV16 E7-knockdown cells. Of those, the expression of APH1B was most significantly upregulated. APH1B, together with APH1a, PEN2, and Nicastrin proteins, is one of the elements of the γ-secretase complex.30 The γ-secretase is located at the cell membrane and releases the intracellular domain (ICD) of Notch (NICD), and NICD then translocates to the nucleus and binds to transcription factors to modify the expression of certain genes.31,32 The function of Notch signaling in modulating the properties of stem cells implies that APH1B may participate in E7 maintenance of cellular stemness. As expected, we found that SiHa sphere cells with APH1B knockdown showed enhanced sphere formation in serum-free medium, elevated expression of stem cell markers, and resistance to chemotherapeutic drugs, and we found the opposite with overexpression, suggesting that APH1B acts as a modulator in the maintenance of the cellular stemness. Further, we knocked down of HPV16 E7 and APH1B simultaneously in SiHa sphere cells and found that APH1B down-regulation made SiHa sphere cells regain, at least partially, the properties of stemness deprived by E7 inhibition, suggesting that APH1B may participate in E7 maintenance of stemness in SiHa sphere cells.
Conclusion
In summary, we observed that sphere cells, isolated from cervical cancer cell lines using non-adhesive culture with serum-free medium have the properties of CSCs, including high expression of stem cell markers, self-renewal, resistance to chemotherapeutic drugs, and clonal expansion. We found for the first time to our knowledge, that HPV16 E7 possesses the capacity to maintain the stemness of cervical cancer sphere cells, and that APH1B may participate in the process of E7 maintenance of cellular stemness (Figure 6D). Our findings may inform a new approaches for advanced cervical cancer therapy.
Acknowledgments
We gratefully acknowledge the kind cooperation of Profs. Yan Lu, Linyu Lu, and Xiaohang Yang, as well as all the members of our Lab. This study was funded by the National Science Foundation of China (No. 81572549); the Medical and Health Science and Technology Project of Zhejiang Province (No. 2017KY009); the National Key Research and Development Program: research on prevention and control of major chronic non-infective diseases, China (No. 2016YFC1302900); the Molecular Typing and Precision Control of Cervical Cancer Based on Proteomics Feature (No. 2016YFC0902900); and the Gynecological Oncology Research Collaborative Network Construction (No. 2015BA113B05).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854 [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12(11):767–775. doi: 10.1038/nrc3368 [DOI] [PubMed] [Google Scholar]
- 4.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8(7):545–554. doi: 10.1038/nrc2419 [DOI] [PubMed] [Google Scholar]
- 5.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499 [DOI] [PubMed] [Google Scholar]
- 6.Shi MF, Jiao J, Lu WG, et al. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol Life Sci. 2010;67(22):3915–3925. doi: 10.1007/s00018-010-0420-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley RR, Duensing S, Brake T, et al. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63(16):4862–4871. [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke RB, Anderson E, Howell A, et al. Regulation of human breast epithelial stem cells. Cell Prolif. 2003;36(Suppl 1):45–58. doi: 10.1046/j.1365-2184.36.s.1.5.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taipale J, Beachy PA. The hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219 [DOI] [PubMed] [Google Scholar]
- 11.Gournay J, Auvigne I, Pichard V, et al. In vivo cell lineage analysis during chemical hepatocarcinogenesis in rats using retroviral-mediated gene transfer: evidence for dedifferentiation of mature hepatocytes. Lab Invest. 2002;82(6):781–788. doi: 10.1097/01.LAB.0000017363.11489.AD [DOI] [PubMed] [Google Scholar]
- 12.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 13.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343 [DOI] [PubMed] [Google Scholar]
- 14.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 17.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 19.Zhong Y, Guan K, Guo S, et al. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010;299(2):150–160. doi: 10.1016/j.canlet.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 20.Hueng DY, Sytwu HK, Huang SM, et al. Isolation and characterization of tumor stem-like cells from human meningiomas. J Neurooncol. 2011;104(1):45–53. doi: 10.1007/s11060-010-0469-1 [DOI] [PubMed] [Google Scholar]
- 21.Chen SF, Chang YC, Nieh S, et al. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One. 2012;7(2):e31864. doi: 10.1371/journal.pone.0031864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Guo H, Lin C, et al. Enrichment and characterization of cancer stemlike cells from a cervical cancer cell line. Mol Med Rep. 2014;9(6):2117–2123. doi: 10.3892/mmr.2014.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S, Jaffer NN, Khan MN, et al. Human papillomavirus subtype 16 is common in Pakistani women with cervical carcinoma. Int J Infect Dis. 2007;11(4):313–317. doi: 10.1016/j.ijid.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Mao Z, Huang J, et al. Blocking the NOTCH pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy. Biochem Biophys Res Commun. 2014;444(4):670–675. doi: 10.1016/j.bbrc.2014.01.164 [DOI] [PubMed] [Google Scholar]
- 25.Modur V, Thomas-Robbins K, Rao K. HPV and CSC in HNSCC cisplatin resistance. Front Biosci (Elite Ed). 2015;7:58–66. [DOI] [PubMed] [Google Scholar]
- 26.Kong QL, Hu LJ, Cao JY, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6(6):e1000940. doi: 10.1371/journal.ppat.1000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo S, Wakisaka N, Muramatsu M, et al. Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol. 2011;85(21):11255–11264. doi: 10.1128/JVI.00188-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Port RJ, Pinheiro-Maia S, Hu C, et al. Epstein-Barr virus induction of the hedgehog signalling pathway imposes a stem cell phenotype on human epithelial cells. J Pathol. 2013;231(3):367–377. doi: 10.1002/path.4245 [DOI] [PubMed] [Google Scholar]
- 29.Kwon YC, Bose SK, Steele R, et al. Promotion of cancer stem-like cell properties in hepatitis C virus-infected hepatocytes. J Virol. 2015;89(22):11549–11556. doi: 10.1128/JVI.01946-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marlow L, Canet RM, Haugabook SJ, et al. APH1, PEN2, and nicastrin increase abeta levels and gamma-secretase activity. Biochem Biophys Res Commun. 2003;305(3):502–509. doi: 10.1016/S0006-291X(03)00797-6 [DOI] [PubMed] [Google Scholar]
- 31.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93(4):649–660. doi: 10.1016/S0092-8674(00)81193-9 [DOI] [PubMed] [Google Scholar]
- 32.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083 [DOI] [PubMed] [Google Scholar]