Abstract
NAD+ plays a pivotal role in regulating many biological processes. A recent study by Palzer et al. demonstrated that ACMSD is a key regulator of NAD+ metabolism and overexpression of human ACMSD leads to niacin-dependency for NAD+ biosynthesis in mice, providing important insights into human diseases associated with niacin/NAD+ deficiency.
Keywords: NAD+, ACMSD, tryptophan, niacin, metabolism
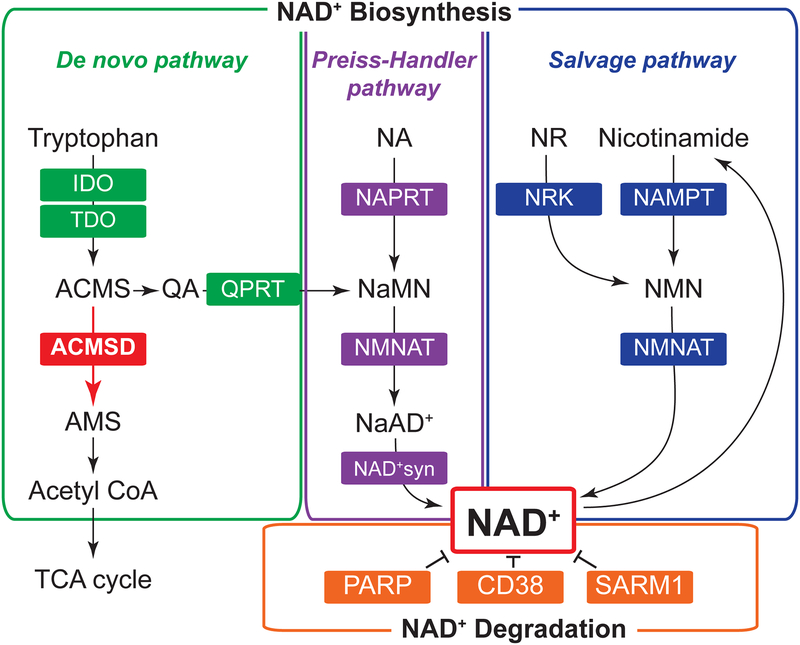
Nicotinamide adenine dinucleotide (NAD+) is an important redox coenzyme found in all species. Recent studies have identified new functions for this old molecule in many biological processes, such as metabolism, circadian rhythms, aging, and inflammation. Importantly, defects in NAD+ metabolism play a causative role in various diseases, such as type 2 diabetes, cardiovascular disease, cancer, and Alzheimer’s disease [1–3]. In mammals, NAD+ is dynamically regulated by multiple enzymatic reactions in de novo biosynthetic pathway starting from tryptophan, Preiss-Handler and salvage biosynthetic pathways starting from niacin (nicotinic acid, nicotinamide, nicotinamide riboside [NR]), and degradative pathway (Figure 1). Over the past decade, many studies have reported the importance of NAD+ biosynthetic and degradative enzymes under physiological and pathophysiological conditions. For example, genetic deletion of nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme in the salvage NAD+ biosynthetic pathway, causes severe metabolic dysfunction in a tissue-specific manner [2]. Increased activity of NMN adenylyltransferase (NMNAT), another salvage enzyme, prevents axon degeneration, whereas SARM1 activation promotes axon degeneration through NAD+ decomposition [3]. Genetic ablation of quinolinate phosphoribosyltransferase (QPRT), a key de novo NAD+ biosynthetic enzyme, results in increased susceptibility to ischemic renal injury [4]. In addition, genetic and pharmacological inhibition of the major NAD+-degradative enzyme, such as CD38 and poly(ADP-ribose) polymerase (PARP), protects mice against metabolic stresses [1, 3]. These discoveries shed light on the pathophysiological significance and therapeutic potential of NAD+ biosynthetic and degradative enzymes.
Figure 1. NAD+ biosynthetic and degradative enzymes.
In mammals, nicotinamide adenine dinucleotide (NAD+) homeostasis is regulated by multiple enzymes in de novo biosynthetic pathway starting from tryptophan, Preiss-Handler and salvage biosynthetic pathways starting from niacin (nicotinamide, nicotinic acid [NA], nicotinamide riboside [NR]), and degradative pathway. A new study by Palzer [5] identified alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase (ACMSD) as an important regulator of NAD+ metabolism. ACMSD coverts aminocarboxymuconic semialdehyde (ACMS) into aminomuconic semialdehyde (AMS) in the de novo NAD+ biosynthetic pathway. Increasing ACMSD expression shifts the balance from de novo NAD+ biosynthesis toward acetyl-CoA production, leading to the development of niacin-dependency.
Aberration: IDO, indoleamine-pyrrole 2,3-dioxygenase; TDO, tryptophan 2,3-dioxygenase; QPRT, quinolinate phosphoribosyltransferase; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyltransferase; NRK, nicotinamide riboside kinase; NAPRT, nicotinic acid phosphoribosyltransferase; NAD+syn, NAD+ synthase; QA, quinolinic acid; NMN, nicotinamide mononucleotide; NaMN, nicotinic acid mononucleotide; NaAD+, nicotinic acid adenine dinucleotide; PARP, poly(ADP-ribose) polymerase; SARM1, sterile alpha and TIR motif containing 1; TCA cycle, tricarboxylic acid cycle.
A recent study conducted by Palzer et al. [5] revealed a novel function of alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase (ACMSD) in NAD+ metabolism and mouse physiology. ACMSD is an important enzyme involved in regulating tryptophan degradation along the kynurenine pathway and converts aminocarboxymuconic semialdehyde (ACMS) into aminomuconic semialdehyde (AMS), which is utilized for generating acetyl-CoA into the tricarboxylic acid (TCA) cycle (Figure 1). Palzer et al. hypothesized that increasing ACMSD activity would inhibit conversion of ACMS into quinolinic acid (QA), a key intermediate in de novo NAD+ biosynthetic pathway, and lead to niacin-dependency. To test this hypothesis, the authors generated a novel mouse model overexpressing human ACMSD (hACMSD) gene under doxycycline (DOX) control, namely “acquired niacin dependency (ANDY)” mouse. Control (water)- and DOX-treated ANDY mice were studied under three different dietary conditions; niacin-free diet (ND1), ND1 diet containing a moderate amount (30 mg/kg) of niacin (CD1), and regular chow diet containing 63 mg/kg of niacin. They found that blood NAD+ levels were decreased in DOX-treated ANDY mice on niacin-free diet (ANDY/DOX/ND1), compared to DOX-treated or control ANDY mice on niacin-replete diet (ANDY/DOX/CD1 or ANDY/water/CD1) or on regular chow diet. ANDY/DOX/ND1 mice also showed marked decreases in NAD+ levels in liver, kidney, spleen, and brain, compared to ANDY/DOX/CD1 mice. In addition, NAD+ phosphate (NADP+) levels were reduced in ANDY/DOX/ND1 mice. Intriguingly, blood NAD+ and NADP+ levels were fully restored in ANDY/DOX/ND1 mice after switching the diet from niacin-free ND1 to niacin-replete CD1. As expected, overexpression of hACMSD enhanced hepatic acetyl-CoA production regardless of niacin intake. Taken together, these results are consistent with the hypothesis and demonstrate that ACMSD critically regulates tryptophan catabolism by shifting the balance from de novo NAD+ biosynthesis toward acetyl-CoA production and thus ANDY mice overexpressing hACMSD become dependent on dietary niacin intake for NAD+ biosynthesis.
Finally, the authors investigated in vivo metabolic phenotypes in ANDY mice. Interestingly, niacin-deficient ANDY/DOX/ND1 mice displayed significant decreases in body weight and adipose tissue mass independently of food intake, compared to niacin-replete ANDY/DOX/CD1 mice. Consistent with these results, ANDY/DOX/ND1 mice also had pronounced decreases in hepatic lipid accumulation, pyruvate content and NAD+/NADH ratios, indicating impaired energy and redox metabolism. In addition, ANDY/DOX/ND1 mice showed lethargy-like behavior by progressively reducing voluntary ambulatory physical activity. Remarkably, similar phenotypes are often observed in people with niacin deficiency. Given that people use niacin much more efficiently than tryptophan to synthesize NAD+ [6], many aspects in ANDY mice could closely mimic NAD+ metabolism in people. Therefore, the study by Palzer et al. provides important groundwork for future studies that explore the molecular mechanisms of human diseases associated with niacin/NAD+ deficiency.
The findings by Palzer et al. [5] have important implications for NAD+ biology research and raise new exciting questions. For example, what are molecular links between NAD+ deficiency and metabolic and neurological disorders? Interestingly, recent studies have shown that NAD+-dependent protein deacetylase SIRT1 regulates adipogenesis, energy metabolism, and physical activity [7], suggesting reduced SIRT1 activity is likely involved in functional defects in niacin-deficient ANDY mice. It is also possible that other NAD+-dependent enzymes, such as CD38 and PARP, other sirtuin(s) or redox species are downstream mediators. Another important question concerns the complex role of ACMSD in the brain. Data obtained from studies conducted in ANDY mice suggest increased ACMSD activity contributes to the development of neurological disorders [5]. However, previous studies have found that ACMSD deficiency or mutation is also linked to neurological diseases, such as epilepsy and Parkinson’s disease [8]. These apparent conflicting results could emphasize the importance of QA (Figure 1), a key NAD+ intermediate which is known to cause neurotoxicity [9]. Future studies are warranted to investigate the functions of ACMSD and QPRT simultaneously and dissect the mechanisms regulating the balance between QA accumulation and NAD+ biosynthesis from tryptophan. Lastly, therapeutic potential of ACMSD remains to be explored. Strikingly, in line with the recent work by Palzer et al. [5], Auwerx’s group recently reported that pharmacological inhibition of ACMSD enhances NAD+ levels in liver and kidney and protects mice from nonalcoholic fatty liver disease (NAFLD) and acute kidney injury (AKI) [10]. There is also evidence that nicotinamide mononucleotide (NMN), a product of NAMPT reaction (Figure 1), and chemical inhibitor of CD38 treat NAFLD and AKI [1–3]. Therefore, it will be of great importance to determine whether ACMSD inhibition and other NAD+ boosters can synergistically increase NAD+ levels and achieve therapeutic effects.
In conclusion, the findings by Palzer and colleagues [5] greatly increase our understanding of complex and sophisticated regulatory mechanisms of NAD+ metabolism. In addition, a novel transgenic mouse model, namely ANDY mouse, provides important insights into human diseases associated with niacin/NAD+ deficiency. These findings will undoubtedly accelerate translation of basic NAD+ biology research into clinical application.
Acknowledgments
J.Y. is supported by grants from the National Institutes of Health: DK104995 and DK56341 (Nutrition Obesity Research Center).
References
- 1.Rajman L et al. (2018) Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab 27 (3), 529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshino J et al. (2018) NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab 27 (3), 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chini EN et al. (2018) The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci 39 (4), 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyan Mehr A et al. (2018) De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 24 (9), 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palzer L et al. (2018) Alpha-Amino-Beta-Carboxy-Muconate-Semialdehyde Decarboxylase Controls Dietary Niacin Requirements for NAD(+) Synthesis. Cell Rep 25 (5), 1359–1370 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitt MK et al. (1981) Niacin-tryptophan relationships for evaluating niacin equivalents. Am J Clin Nutr 34 (3), 423–7. [DOI] [PubMed] [Google Scholar]
- 7.Satoh A et al. (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18 (3), 416–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marti-Masso JF et al. (2013) The ACMSD gene, involved in tryptophan metabolism, is mutated in a family with cortical myoclonus, epilepsy, and parkinsonism. J Mol Med (Berl) 91 (12), 1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarcz R et al. (1983) Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219 (4582), 316–8. [DOI] [PubMed] [Google Scholar]
- 10.Katsyuba E et al. (2018) De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 563 (7731), 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]