Abstract
Biodiesel or renewable diesel fuels are alternative fuels produced from vegetable oil and animal tallow that are being considered to help reduce the use of petroleum-based fuels and emissions of air pollutants including greenhouse gases. Here, we analyzed the gene expression of inflammatory marker responses and the cytochrome P450 1A1 (CYP1A1) enzyme after exposure to diesel and biodiesel emission samples generated from an in-use heavy-duty diesel vehicle. Particulate emission samples from petroleum-based California Air Resource Board (CARB)-certified ultralow sulfur diesel (CARB ULSD), biodiesel, and renewable hydro-treated diesel all induced inflammatory markers such as cyclooxygenase-2 (COX)-2 and interleukin (IL)-8 in human U937-derived macrophages and the expression of the xenobiotic metabolizing enzyme CYP1A1. Furthermore, the results indicate that the particle emissions from CARB ULSD and the alternative diesel fuel blends activate the aryl hydrocarbon receptor (AhR) and induce CYP1A1 in a dose- and AhR-dependent manner which was supported by the AhR luciferase reporter assay and gel shift analysis. Based on a per mile emissions with the model year 2000 heavy duty vehicle tested, the effects of the alternative diesel fuel blends emissions on the expression on inflammatory markers like IL-8 and COX-2 tend to be lower than emission samples derived from CARB ULSD fuel. The results will help to assess the potential benefits and toxicity from biofuel use as alternative fuels in modern technology diesel engines.
Keywords: Aryl hydrocarbon receptor, Inflammation, Biodiesel, PM, Macrophages, Chassis dynamometer testing
1. Introduction
Airborne particulate matter (PM) and PM associated with diesel exhaust and diesel fuel combustion is known to have adverse health effects including cardiovascular disease (CVD) (Brook et al., 2010). As an approach to decrease ambient diesel PM exposure, control measures such as filters and alternative fuels have been introduced. It is also important to understand the potential health effects of the combustion of alternative fuels, such as biodiesel, which is becoming more widely incorporated as a supplement to petroleum-based diesel fuels. Further, the increased use of alternative fuels is being considered to reduce the carbon intensity of transportation fuels.
Long-term exposure to PM is associated with respiratory symptoms, effects on the immune system, and CVD (Craig et al., 2008). Patients with asthma, obstructive pulmonary disease, pneumonia, CVD, and diabetes are particularly vulnerable (Zanobetti et al., 2000). Exposure to fine PM is considered to be a risk factor for CVD mainly thought to be important as part of the pathway for systemic inflammation, atherosclerosis, and altered cardiac autonomic functions (Pope et al., 2004). These harmful effects on human health are strongly associated with exposure to traffic-derived fine PM (<2.5 μm, PM2.5) (Laden et al., 2000; Aguilera et al., 2016). Inhalation studies with diesel engine exhaust in men have demonstrated reductions in adverse vascular and prothrombotic effects associated with a reduction in PM when a particle trap was used, underlining the harmful effects of diesel engine exhaust inhalation (Lucking et al., 2011). In vitro studies have also indicated reduced cytotoxicity, oxidative, and pro-inflammatory effects through the application of a diesel particle filter, which has a beneficial effect on both PM mass emission and potential PM toxicity (Gerlofs-Nijland et al., 2013).
Inflammation, including oxidatively-induced inflammation, is considered to be an important pathway leading toward atherosclerosis and heart diseases (Brook et al., 2003), chronic lung diseases, immune suppression, and cancer (Marx, 2004). Several studies indicate that inflammation may provide a key to these pathological processes through inflammatory factors such as Cyclooxygenase 2 (COX-2), the chemokine Interleukin 8 (IL-8), or Tumor necrosis factor α (TNFα), which are mediators of the inflammatory response. Suwa et al. (2002) exposed a strain of hyperlipidemia-prone rabbits to ambient PM10 via inhalation and found progression of atherosclerotic lesions in the aorta, as well as a massive recruitment of macrophages to those lesions, which suggests an association of inflammatory reaction with exposure to PM10. Previously, we reported that the aryl hydrocarbon receptor (AhR) and the chemokine receptor CXCR2 activated by IL-8 are critical mediators of atherosclerosis induced by dioxin (Wu et al., 2011). Exhaust emissions from diesel engines are complex chemical mixtures containing compounds such as polycyclic aromatic hydrocarbons (PAHs), which can activate the AhR. The AhR is a ligand-activated transcription factor that mediates many of the responses to toxic environmental chemicals such as Benzo[a]pyrene (BaP), 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), or dioxin-like polychlorinated biphenyls (PCB). After ligand binding, the activated AhR translocates to the nucleus and associates with its partner protein AhR nuclear translocator (Arnt). The AhR-Arnt complex binds on so-called dioxin responsive elements (DRE) and induces the transcription of an array of target genes such as CYP1A1 (Denison and Nagy, 2003; Okey, 2007). Induction of target genes like COX-2 and IL-8 may involve alternative pathways of AhR signaling including NF-κB (Vogel, 2000; Vogel et al., 2007). Interestingly, lipophilic constituents of diesel exhaust particles (DEP) have been shown to induce pro-inflammatory responses and increase intracellular calcium concentration in endothelial cells via non-genomic but AhR-dependent signaling (Brinchmann et al., 2018a, 2018b).
Previously, we have shown that organic extracts from urban dust or diesel exhaust induce IL-8, COX-2, as well as CYP1A1, in an AhR-dependent manner (Vogel et al., 2005). In a recent study, we reported a correlation of increased IL-8 levels with elevated serum levels of PCBs in children (Tsuji et al., 2012). In vivo studies with mice (Finch et al., 2002) demonstrated that exposure to the exhaust of 100% soybean-derived fuel can generate a modest inflammatory effect in lungs of mice, which was also true for the fossil diesel fuels investigated in vivo by Reed et al. (2004, 2005). The direct comparison of biodiesel and fossil diesel in vivo showed that tissue damage, oxidative stress, inflammation, and cytokine response were more pronounced in mice exposed to biodiesel compared to fossil diesel exposed mice (Shvedova et al., 2013; Yanamala et al., 2013). On the other hand, exposure to petroleum diesel in healthy mice and a mouse model of allergic asthma induced inflammation and the production of TH2 cytokines as well as IL-17 which was not observed after exposure to the emission from soy-based biodiesel blends (Gavett et al., 2015). Furthermore, the exposure to emission from biodiesel blends but not petroleum diesel significantly increased the proliferation of resting peribronchiolar lymph node cells.
In vitro comparisons of biodiesel blend and fossil diesel fuel showed that the use of biodiesel blend resulted in an increased cytotoxicity and inflammatory response via IL-6 and IL-8 release compared to fossil diesel when the hazard was expressed on a per unit of mass basis (Gerlofs-Nijland et al., 2013). However, the PM oxidative potential was similar or reduced for the biodiesel blend compared to the fossil diesel. An increased expression of COX-2 and elevated levels of prostaglandin E2 (PGE2) were observed in alveolar macrophages after exposure to biodiesel blend and low sulfur petroleum diesel exhaust particles (Bhavaraju et al., 2014). In this study the exposure to a low concentration of biodiesel blend resulted in higher levels of PGE2 in vitro compared to petroleum diesel. A recent study found that biodiesel fuel blends showed a significant reduction of PM-related genotoxic effects compared to fossil diesel fuel (Yang et al., 2017). A study with human lung epithelial cells showed higher cytotoxicity and oxidative stress after exposure to biodiesel B100 relative to fossil diesel fuel (Betha et al., 2012) as well as increased expression of TNFα and IL-8 (Steiner et al., 2013) suggesting that biodiesel as well as fossil diesel fuel may induce cytotoxic and inflammatory effects on a cellular level.
The AhR is considered to be an important receptor protein mediating the toxic responses of dioxin-like PAHs which may occur as components of PM derived from complex chemical mixtures such as from urban PM, tobacco smoke, industrial exhaust as well as from petroleum and diesel engine exhaust (Esakky et al., 2015; Vogel et al., 2005; Jaguin et al., 2015; Brinchmann et al., 2018a,b; Weng et al., 2018). Here, we analyzed the expressions of inflammatory marker responses and CYP1A1 after exposure to diesel and biodiesel emission samples and test the hypothesis that the AhR plays a key role in this process. The samples were collected from an in-use heavy-duty truck operated on a chassis dynamometer with biodiesel and renewable diesel blends ranging from 20% to 100%. This study provides information on how the emissions and eventual exposure to toxic compounds present in the exhaust from biodiesel fuel can induce biomarkers of toxicity, which might be relevant for the development of chronic diseases like CVD.
2. Materials and methods
2.1. Reagents and PM collection
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, > 99% purity) was originally obtained from Dow Chemicals Co. (Midland, MI). Dimethylsulfoxide (DMSO) and Phorbol-12-myristate-13-acetate (TPA) were obtained from Sigma Chemical (St. Louis, MO). Other molecular biological reagents were purchased from Qiagen (Valencia, CA) and Roche (Indianapolis, IN). National Institute of Standards and Technology (NIST) Standard Reference Material diesel exhaust particulate sample, NIST SRM 2975, and SRM 1650a were purchased from NIST (Gaithersburg, MD). [y-32P] ATP (6000 Ci/mmol) was purchased from ICN (Costa Mesa, CA).
2.2. Test fuels
The fuels for the chassis dynamometer testing were a petroleum-based California Air Resource Board (CARB)-certified ultralow sulfur diesel (CARB ULSD) as the base fuel, and soy and animal-based biodiesels and a renewable hydro-treated diesel as blend stocks. The hydro-treated diesel originated from a tallow-based feedstock. These fuels were obtained from the same batches of primary fuels used for a larger engine and chassis dynamometer testing study (Hajbabaei et al., 2014; Na et al., 2015; Karavalakis et al., 2016). The fuel properties for the neat and blended fuels are provided in Table 1. Details of the blending procedures and detailed properties have been described previously (Hajbabaei et al., 2012, 2014; Karavalakis et al., 2016; Na et al., 2015). For the chassis testing, the fuels were tested at the 20% and 50%, blend levels, as well as the 100% fuel. These blends are designated with an “S” for soy-based biodiesel, an “A” for animal-based biodiesel, and a “R” for renewable diesel fuels. For example, the designations for 20%, 50%, and 100% soy are S20, S50, and S100, respectively.
Table 1.
Selected fuel properties.
| CARB ULSD | Soy-biodiesel | Animal-biodiesel | NExBTL Renewable HydroTreated Diesel | |
|---|---|---|---|---|
| API gravity (@ 60 °F) | 39.3 | 29 | 28.5 | 51.3 |
| Aromatics, vol. % | 18.7 | NA | NA | 0.4 |
| PNAs, wt. % | 1.5 | NA | NA | 0.1 |
| Cetane number, D613 | 55.8 | 47.7 | 57.9 | 72.3 |
| Cetane number IQT | 74.7 | |||
| Distillation, IBP | 337 | 350 °C | 347.5 °C | 326 |
| T10, °F | 408 | 426 | ||
| T50, °F | 519 | 521 | ||
| T90, °F | 612 | 547 | ||
| FBP | 659 | 568 | ||
| Free glycerin, mass % | NA | 0.001 | 0.008 | NA |
| Total glycerin, mass % | NA | 0.080 | 0.069 | NA |
| Sulfur, ppm | 4.7 | 0.7 | 2 | 0.3 |
NA = Not Available.
2.3. Heavy-duty vehicle and sampling
The test vehicle was a 2000 Freightliner Truck equipped with a 2000 Caterpillar C15 engine with the specifications summarized in Table 2. Samples were drawn from the dilution tunnel using stainless steel probes positioned in the same plane as the probes used for gravimetric PM measurements as described in Title 40 Code of Federal Regulations (40 CFR86) as previously described (Hajbabaei et al., 2014; Karavalakis et al., 2016; Na et al., 2015; Johnson et al., 2011). Briefly, a 1.5″ O.D. stainless steel probe was connected to an 8″ x 10” Hi Volume filter unit (Zefluor, Pall Life Sciences, Ann Arbor, MI). Flow rates were nominally 450 Lpm. Flows and sampling volumes used for calculations were adjusted to standard temperature and pressure (STP). Filter samples were stored at − 20 °C until shipment to the University of California, Davis investigators. For shipment these samples were placed in insulated containers packed with blue ice and shipped using overnight delivery. Filter samples were weighed, inventoried, and placed in a − 20 °C freezer upon receipt for storage. Filters were extracted using pressurized solvent extraction at 2000 psi, 100 °C using with Dichloromethane (Burdick and Jackson GC grade). Extracts were reduced under a stream of nitrogen and were transferred to a 2 ml amber vial., The extracts obtained were dried and then dissolved in dimethylsulfoxide (DMSO). We used the diesel extracts at concentrations corresponding to the total mass of particles at 5–50 μg/ml.
Table 2.
Test engine specifications.
| Engine Manufacturer | Caterpillar |
|---|---|
| Engine Model | C-15 |
| Model Year | 2000 |
| Engine Family Name | XH0893ERK |
| Engine Type | In-line 6 cylinder, 4 stroke |
| Displacement (liter) | 14.6 |
| Power Rating (hp) | 475 hp @ 2100 |
| Fuel Type | Diesel |
| Odometer reading at Initiation of testing | 34,000 |
| Induction | Turbocharger with after cooler |
2.4. Cell culture and transient transfection
Human U937 monocytic cells were obtained from the American Tissue Culture Collection (Manassas, VA) and maintained in RPMI 1640 medium containing 10% fetal bovine serum (Gemini, Woodland, CA). For differentiation into macrophages, U937 cells were treated with TPA and allowed to adhere for 48 h as described previously (Vogel et al., 2005). For transient transfection of U937 macrophages, luciferase reporter constructs were transfected via Nucleofector technology, as described previously (Vogel et al., 2007). The Nrf2 reporter construct was purchased from Promega (Madison, MI). The Nrf2 reporter plasmid expresses the firefly luciferase reporter gene under the control of Nrf2/ARE response element. The DRE reporter construct was a kind gift of Dr. Thomas Haarmann-Stemmann (Institute for Environmental Research, Duesseldorf, Germany). The DRE reporter plasmid expresses the firefly luciferase reporter gene under the control of the DRE1 sequence of the rat Cyp1a1 gene promoter region −1029 to −997 which binds the activated AhR complex as described previously (Berghard et al., 1993). Luciferase activities were measured with the Luciferase Reporter Assay System (Promega, Madison, MI) using a luminometer (Berthold Lumat LB 9501/16, Pittsburgh, PA).
2.5. Animals and bone-marrow derived cell culture
C57BL/6 mice aged 6–8 weeks were obtained from JAX West, Inc. (Davis, CA) and were euthanized in accordance with a protocol approved by the UC Davis Animal Resources Service. AhR-null (AhR−/−) mice were generated and kindly provided by Christopher Bradfield and coworkers from the McArdle laboratory for Cancer Research at the University of Wisconsin. Extracted bone marrow was depleted of red blood cells by ammonium chloride lysis. Cell culture medium used to culture bone marrow-derived macrophages (BMDM) was RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) as described earlier (Vogel et al., 2013). After 6 days of differentiation BMDM were treated for 24 h with S100, CARB ULSD or TCDD as indicated and prepared for RNA analysis.
2.6. Cell viability assay
To assess the effect of PM on the viability of U937 macrophages, a trypan blue exclusion test was used (McAteer and Davis, 1994). A 10-μl portion of re-suspended cell pellet was placed in 190 μl PBS with 200 μl trypan blue (0.5% dilution in 0.85% NaCl). After 5 min, 10 μl of the cell suspension was loaded into a hemocytometer, and the proportion of nonviable to viable cells was determined.
2.7. Quantitative real-time reverse transcription–PCR
Total RNA was isolated from U937 cells using a high-pure RNA isolation kit (Roche), and cDNA synthesis following procedures, as previously described (Vogel et al., 2005). Quantitative detection of β-actin and differentially expressed genes was performed with a LightCycler Instrument (Roche Diagnostics, Mannheim, Germany) using the QuantiTect SYBR Green PCR Kit (Qiagen) according to the manufacturer’s instructions. The sequences of the primers used in this study are listed in Table 3. All PCR assays were performed in triplicate. The intra-assay variability was <7%. Data was analyzed with the LightCycler analysis software.
Table 3.
Sequences of primers for quantitative real time PCR analyses.
| Gene | Sequence |
|---|---|
| Mouse β-actin | FP: 5′-AGCCATGTACGTAGCCATCC-3′ RP: 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Mouse CYP1A1 | FP: 5′-GGCCACTTTGACCCTTACAA-3′ RP: 5′-CAGGTAACGGAGGACAGGAA-3′ |
| Mouse COX-2 | FP: 5′-TTTGTTGAGTCATTCACCAGACAGAT-3′ RP: 5′-CAGTATTGAGGAGAACAGATGGGATT-3′ |
| Human β-actin | FP: 5′-GGACTTCGAGCAAGAGATGG-3′ RP: 5′-AGCACTGTGTTGGCGTACAG-3′ |
| Human CYP1A1 | FP: 5′-GAGGCCAGAAGAAACTCCGT-3′ RP: 5′-CCCAGCTCAGCTCAGTACCT-3′ |
| Human COX-2 | FP: 5′-GGAACACAACAGAGTATGCG-3′ RP: 5′-AAGGGGATGCCAGTGATAGA-3′ |
| Human IL-8 | FP: 5′-TAGCAAAATTGAGGCCAAGG-3′ RP: 5′-AAACCAAGGCACAGTGGAAC-3′ |
2.8. Gel-mobility-shift assay (GMSA)
Nuclear extracts were isolated from U937 cells and GMSA was performed as described previously (Vogel et al., 2007). Briefly, 5 × 106 cells were treated with CARB ULSD, S100, or TCDD for 30 min, unless noted otherwise in the figure legends and harvested in Dulbecco’s PBS for preparation of nuclear extracts and subsequent GMSA.
2.9. Statistical analysis
All experiments were repeated a minimum of three separate times generally on separate days, and data are expressed as mean ± one standard deviation (SD). Differences were considered significant for P < 0.05. Comparison of two groups was made with an unpaired, two-tailed student’s t-test. Comparison of multiple groups was made with ANOVA followed by a Dunnett or Tukey test.
3. Results
3.1. Effect of emissions from biodiesel blends on CYP1A1 and inflammatory marker IL-8 and COX-2
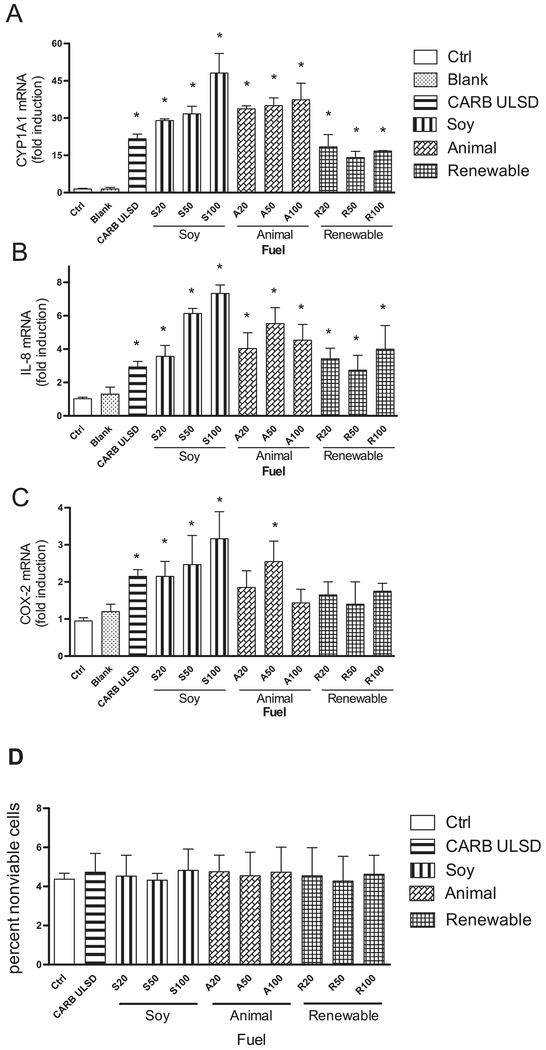
The results for the biodiesel emission samples generated from soy (S), animal (A) biodiesel, or renewable (R) diesel in blends of 20%, 50% or 100% with CARB ULSD are presented in Fig. 1. The results show that CARB diesel and all three biodiesel blends induced CYP1A1, IL-8, and COX-2 at statistically significant levels compared to control (Fig. 1A, B, and C) at a concentration of 10 μg/ml cell culture medium. The highest increase of CYP1A1 mRNA expression was found after treatment with 100% soy (S100) (50-fold) followed by 100%, 20% and 50% blends of animal biodiesel (A100, A20, A50) (35-fold). The extracts of the 20% and 50% soy blend (S20, S50) induced CYP1A1 about 30-fold above control level. The lowest increase of CYP1A1 mRNA expression (15-fold) was found after treatment with 20, 50, and 100% renewable diesel (R20, R50, R100). The treatment of macrophages with CARB fuel resulted in a 23-fold increase of CYP1A1 compared to control or blank treated cells (Fig. 1A).
Fig. 1.
Effect of various blends of Biodiesel and Diesel on CYP1A1, IL-8, and COX-2 mRNA expression in U937 macrophages. Increased mRNA levels of target genes A) CYP1A1, B) IL-8, and C) COX-2 are shown and corrected against the housekeeping gene β-actin. U937 macrophages were treated for 24 h with 10 μg/ml CARB Diesel (CARB ULSD), Soy-biodiesel (S20-S100), Animal-biodiesel (A20-A100), or Renewable (R20-R100) at 20% (20) and 50% (50) blended or not blended (100) with CARB ULSD. Values are given as mean ± SD of three independent experiments. An asterisk indicates significantly different from control cells (p < 0.05). D) Effect of biodiesel and diesel extracts on cell viability. U937 derived macrophages were treated with 50 μg/ml CARB ULSD and biodiesel blends for 24 h and cell viability was tested via trypan blue exclusion test.
Similar results were obtained analyzing the expression of the inflammatory marker IL-8 (Fig. 1B). A 7.5-fold induction of IL-8 was found by S100 followed by a 6.5-fold IL-8 increase by S50 compared to DMSO control. Treatment with A50 emission extracts led to a 5.8-fold induction of IL-8 followed by a 4.5-fold and 4.0-fold increase after treatment with A100 and A20 emission extracts, respectively. The treatment of U937 macrophages with CARB ULSD led to a 3-fold increase of IL-8 mRNA compared to the control. In general, the induction of COX-2 in macrophages was less significant than for IL-8 (Fig. 1C). The highest increase of COX-2 mRNA of about 3-fold above control level was found after treatment with S100 followed by S50 compared to a 2-fold elevated level of COX-2 by CARB ULSD. Only the A50 blend led to a significant increase of COX-2 whereas A20, A100, or the renewable (R) diesel blends did not significantly change the expression of COX-2 at a concentration of 10 μg/ml.
Evaluation of cytotoxicity of CARB ULSD and biodiesel blends. A possible cytotoxic effect of CARB diesel and biodiesel blends was tested in U937 macrophages. Cells were exposed to CARB ULSD and biodiesel samples generated from soy (S), animal (A) biodiesel, or renewable (R) diesel in blends of 20%, 50% or 100% at 50 μg/ml which is the highest concentration used in the current study. The viability of control cells was tested with 0.1% DMSO added served as a solvent control. After incubating the cells for 24 h at 37°C, we determined cytotoxicity by trypan blue exclusion test. The percentage of nonviable control U937 macrophages was 4.2% (Fig. 1D). The treatment with 50 μg/ml of CARB ULSD or the biodiesel blends had no significant effect on cell viability compared to control cells.
3.2. Dose-dependent induction of CYP1A1, IL-8 and COX-2 by extracts of soy biodiesel and CARB diesel
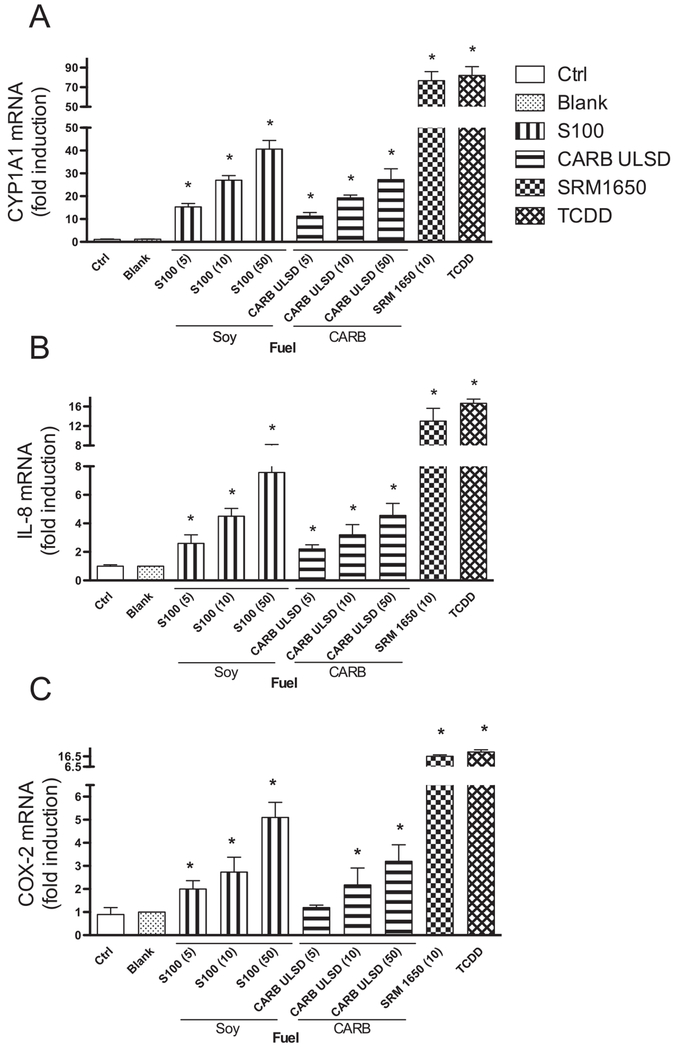
The increased mRNA level of the inflammatory markers and CYP1A1 after exposure to soy biodiesel and CARB ULSD showed a significant increase as early as 6 h after initial treatment, which peaked at 24 h (data not shown). Therefore, U937 macrophages were treated for 24 h with various concentrations (5–50 μg/ml) of soy biodiesel and compared to CARB ULSD to analyze dose-dependent effects on mRNA expression of the target genes. To address the dose-dependent effect of extracts from diesel and soy biodiesel exhaust, the mRNA expression of CYP1A1 and pro-inflammatory genes IL-8 and COX-2 with various concentrations of diesel extracts was studied. For dose-dependent and further mechanistic studies we selected the soy-based diesel since the S100 emission was the strongest inducer of CYP1A1 and IL-8 compared to the other alternative diesel fuels A100 or R100 tested in this study (Fig. 1). As shown in Fig. 2, treatment of U937 macrophages for 24 h with emission samples from soy-based biodiesel or CARB ULSD fuel in the range of 5–50 μg/ml cell culture medium led to a dose-dependent mRNA induction of CYP1A1, IL-8 and COX-2 (Fig. 2A–C). In addition, CYP1A1 mRNA and IL-8 mRNA expressions were significantly increased by both diesel samples compared to the control at the lowest concentration of 5 μg/ml used in this study. As in the experiments above the most conspicuous effect of the diesel extracts was found in the case of CYP1A1 (over 35-fold increase), followed by IL-8 and COX-2 mRNA expression in U937 macrophages.
Fig. 2.
Dose-dependent effect of Biodiesel and Diesel emission extracts on CYP1A1, IL-8, and COX-2 mRNA expression. Effect of organic extracts preparations of S100 and CARB Diesel on CYP1A1, IL-8, and COX-2 mRNA expression in U937 macrophages. U937 macrophages were treated for 24 h with 5, 10 and 50 μg/ml S100 or CARB ULSD. Increased mRNA levels of target genes are shown and corrected against the housekeeping gene β-actin. As a positive control, cells were treated with 10 μg/ml SRM 1650 or 1 nM TCDD. Values are given as mean ± SD of three independent experiments. An asterisk indicates significantly different from control cells (p < 0.05).
To estimate the toxic potency, the effects of soy-based biodiesel were compared with NIST SRM 1650 diesel particulate matter, which has been shown to activate AhR and induce CYP1A1 as well as inflammatory factors in U937 macrophages (Vogel et al., 2005). TCDD, a prototypical AhR ligand, was used as a pure AhR ligand and induced CYP1A1, IL-8 and COX-2 to a similar extent as NIST 1650. As shown in Fig. 2, treatment of macrophages with 10 μg/ml NIST 1650 led to a significantly higher increase of CYP1A1, IL-8 as well as COX-2 compared to the highest concentration of 50 μg/ml S100 or CARB ULSD.
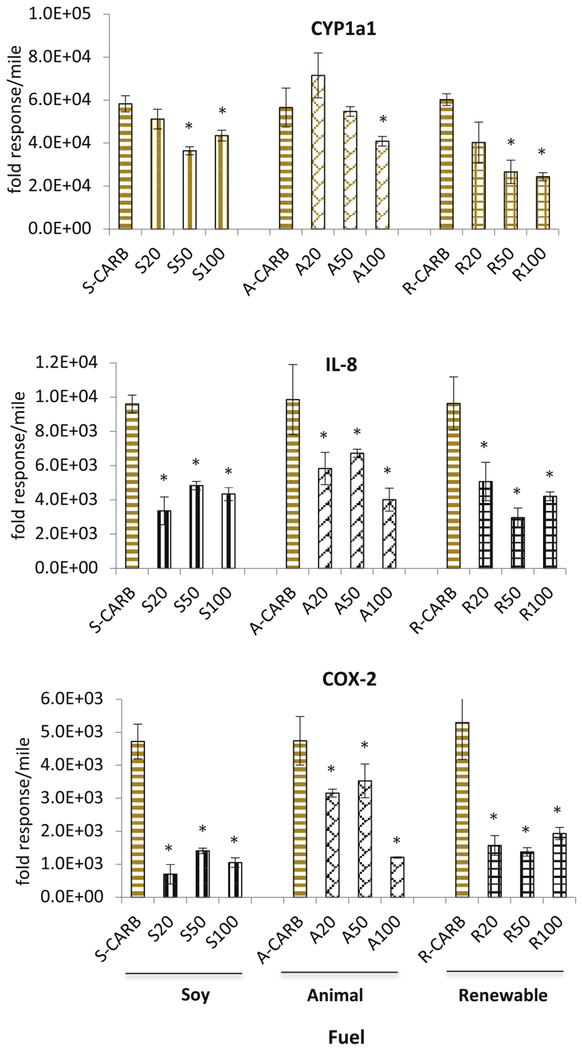
3.3. Emissions of CYP1A1 and IL-8 expression on a per mile basis
We calculated the CYP1A1, IL-8 and COX-2 mRNA level per mile of the various emission samples based on the amount of the particle mass equivalent added to the cells in vitro. The CARB diesel samples were collected matching the corresponding time period when the biodiesel and renewable diesel samples were taken. For example, S-CARB, A-CARB, and R-CARB represent the results for the CARB diesel fuel tested during the same time period as the soy, animal biodiesel, and renewable diesel, respectively.
The effects of the three different biodiesel blends on CYP1A1 and the inflammatory markers IL-8 and COX-2 tend to be lower than for the CARB diesel (Fig. 3). A lower response on a per mile basis by biodiesel blends is due to the lower amount of PM mass produced compared to CARB ULSD, as published elsewhere (Hajbabaei et al., 2014; Karavalakis et al., 2016; Na et al., 2015).
Fig. 3.
Effect of diesel and biodiesel fuels on CYP1a1, IL-8, and COX-2 expression in the macrophages on a per mile basis for the 2000 Caterpillar C-15 on the UDDS cycle. The error bars represent one standard deviation on the average value. An asterisk indicates significantly different from CARB ULSD treated cells (p < 0.05).
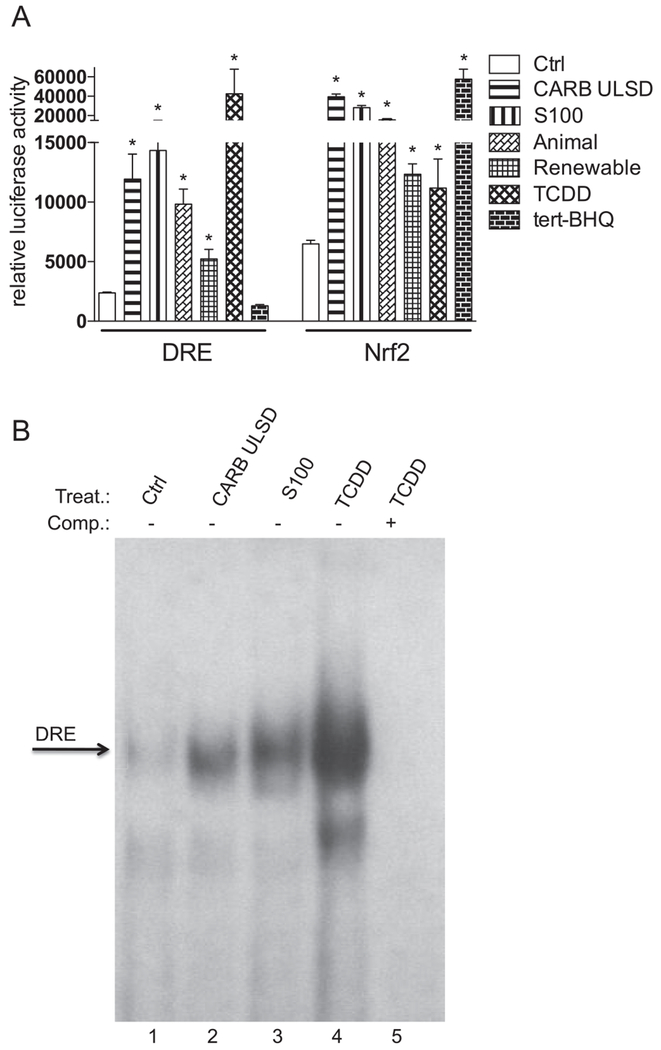
3.4. S-CARB activates DRE activity and AhR DNA binding
We have chemically characterized S-CARB for PAHs and substituted PAHs (Kobayashi et al., unpublished results) and for most of the PAHs, there are higher levels in the S-CARB fuel than the biodiesel and renewable diesel fuel emissions. Although some PAH compounds are known to be unreactive with AhR, we also detected PAHs present in nanogram quantities which are potential AhR ligands. To determine whether S-CARB is capable of activating AhR, we used an in vitro reporter assay to measure DRE luciferase activity in response to S-CARB. As shown in Fig. 4A, S100 increases DRE luciferase activity 6-fold. Animal biodiesel and renewable diesel also significantly increased DRE activity at the 10 μg/ml dose. In comparison, CARB ULSD induced DRE-reporter activity increased by about 5-fold compared to the control at the corresponding concentration. Comparatively, treatment with TCDD (1 nM), used as the prototypical ligand to activate AhR and DRE activity, lead to an 18-fold increase of DRE activity compared to untreated controls. Next, we measured Nrf2 reporter activity since in vitro treatment with diesel emission samples has been reported to generate ROS (Gerlofs-Nijland et al., 2013). Thus, we examined the effect of diesel on Nrf2 reporter activity. The results in Fig. 4A show that undiluted S100 and CARB ULSD at a concentration of 10 μg/ml led to a statistically significant 6-fold and 4.4-fold increase of Nrf2 luciferase activity, respectively, compared to the control cells. Animal biodiesel and renewable diesel also significantly increased Nrf2 activity by 3-fold and 2-fold, respectively, above control. The positive inducer tert-BHQ activated Nrf2 by about 9-fold.
Fig. 4.
Effect of diesel emission samples on AhR and Nrf2 activity. A) Activation of DRE and Nrf2 luciferase activity by diesel emission samples. U937 macrophages were transiently transfected with DRE- and Nrf2-luciferase reporter plasmids for 24 h and treated with S100 (10 μg/ml) and CARB ULSD (10 μg/ml) or TCDD (1nM), and tert-butylhydroquinone (tert-BHQ 10 μM) as positive controls. Values are given as mean ± SD of three independent experiments. An asterisk indicates significantly different from control cells (p < 0.05). B) Increased DNA binding of AhR by diesel emission samples. Nuclear protein extracts of non-stimulated (Ctrl), CARB ULSD (10 μg/ml), S100 (10 μg/ml), and 1 nM TCDD-stimulated U937 macrophages were incubated with a DRE consensus probe. Competition (Comp.) with a 100-fold excess of unlabeled DRE consensus (lane 5) oligonucleotide. One representative experiment of three independently performed experiments is shown.
GMSA studies (Fig. 4B) confirmed that AhR activation is associated with an increased AhR DNA binding activity on a DRE consensus element, which regulates the expression of CYP1A1. A slightly weaker AhR binding activity in CARB ULSD treated samples (lane 2) was observed compared to S100 (lane 3) or TCDD-treated samples (lane 4). The results show that CARB diesel and the soy-based biodiesel induce CYP1A1 production through compounds that bind to and activate the AhR.
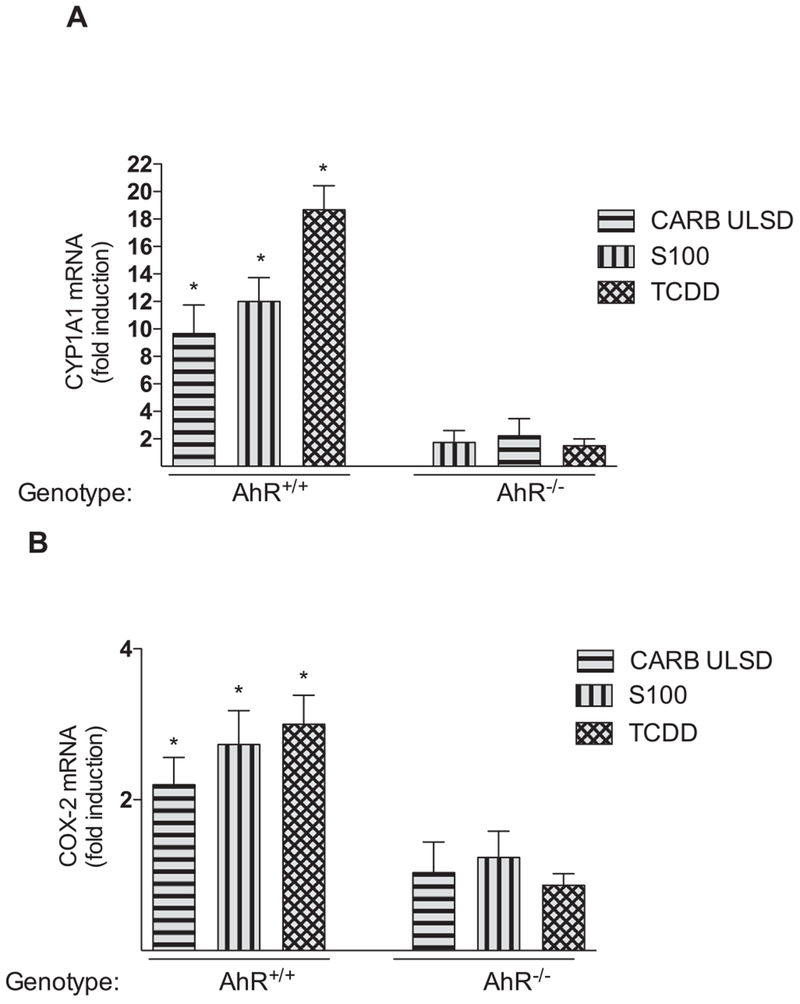
To further investigate the involvement of AhR in soy-based biodiesel-mediated effects we used BMDM from B6 wild type (wt) and B6 AhR null mice (mice deficient in AhR; AhR−/−). S100 and CARB ULSD induced CYP1A1 mRNA expression in BMDM from wt mice by 12-fold and 10-fold, respectively (Fig. 5A). The mRNA expression of COX-2 was increased about 2-fold by S100 and 2.6-fold after treatment with CARB ULSD (Fig. 5B). The results show that treatment with TCDD, S100 or CARB ULSD did not significantly increase the expression of CYP1A1 or COX-2 in BMDM derived from AhR−/− mice (Fig. 5A and B).
Fig. 5.
mRNA expression analyses of target genes in BMDM derived from wt (AhR+/+) or AhR null mice (AhR−/−). BMDM were treated with CARB ULSD (10 μg/ml), S100 (10 μg/ml), and TCDD (1 nM) used as positive control. Increased mRNA levels of A) CYP1A1 and B) COX-2 are shown and corrected against the housekeeping gene β-actin. Total RNA was prepared 24 h after treatment of BMDM derived from wt (AhR+/+) or AhR null mice ( AhR−/−). *, Values are the mean ± SD of three independent experiments and are significantly different from control (P < 0.005). Ctrl, Control.
4. Discussion
The current study shows that the emissions from alternative fuels derived from vegetable oils or animal fats induce molecular markers of inflammation IL-8 and COX-2. The fuel emissions are also effective activators of the AhR and induce down-stream AhR-regulated genes, such as CYP1A1, as well as IL-8 and COX-2 in human U937-derived macrophages. Previous studies have shown that U937-derived human macrophages are a suitable cell model to detect potential AhR activity and inflammatory responses after exposure to ambient PM from urban dust or diesel exhaust PM (Vogel et al., 2005). The current study shows that the effect of soy-based biodiesel emissions on the above target genes is more pronounced compared to animal-based biodiesel, renewable or CARB ULSD emissions expressed on a per unit of mass basis. The combustion of biodiesel fuels has been shown to generate less mass emission of PM compared to petroleum-based diesel (Garshick et al., 2004). As previously reported, the PM emissions decreased as the levels of biodiesel or renewable diesel increased in the blend with ULSD (Hajbabaei et al., 2014; Karavalakis et al., 2016; Na et al., 2015). The current data in Fig. 3 calculated on a per mile basis indicated that S100 or A100 biodiesel or renewable diesel R100 generate a lower response than CARB ULSD for the induction of CYP1A1 and IL-8 in macrophages. The Luciferase reporter assay and GMSA showed that treatment of U937-derived macrophages with S100 as well CARB ULSD induce AhR-activity and increase AhR-DNA binding activity similar to the prototypical AhR ligand TCDD or NIST1650. However, the activation of AhR was less pronounced in CARB ULSD treated samples compared to S100- or TCDD-treated cells. The results indicate that S100 may contain a higher amount or more potent AhR ligands than CARB ULSD. Using BMDM derived from AhR−/− mice showed that CARB ULSD- and S100-mediated induction of CYP1A1 and COX-2 depends on a functional expression of the AhR. The role of AhR in mediating the effects of diesel exhaust particles on the differentiation of thymocytes has been emphasized earlier in vitro (Ito et al., 2006; van Voorhis et al., 2013) as well as in vivo (O’Driscoll et al., 2018). The results suggest that S100 and CARB ULSD contain components capable of activating CYP1A1 in an AhR-dependent manner. Treatment with S100 and CARB ULSD led to a significant activation of Nrf2. The results indicate that S100 contain specific components, such as fatty acid esters, aldehydes, nitrogen oxides or transition metals, which are capable of generating reactive oxygen species (ROS) and mediating the activation of Nrf2. It is also possible that Nrf2 activation contributes to AhR-mediated effects. Recent studies show that AhR and Nrf2 may interact to induce the expression NAD(P)H:quinoneoxidoreductase 1 (NQO1) for instance (Wang et al., 2013). Other possible cross-talks between the AhR and Nrf2 signaling pathway have been hypothesized (Köhle and Bock, 2007). Miao et al. (2005) identified Nrf2 as a downstream target of the AhR, which could lead to synergistic effects through activation of both AhR and Nrf2. Interestingly, from in vivo studies it has been reported that a low dose fossil diesel emission exposure induces oxidant stress in mice, and that Nrf2 is required for a functional host anti-oxidant responses to prevent diesel-induced allergic airway inflammation (Li et al., 2010).
5. Conclusions
We have chemically characterized S100 and found that they contain PAHs, alkyl-PAHs and nitro-PAHs. (Kobayashi et al., unpublished results). In summary, the results from the current study suggest that S100, despite its lower effect on a per mile basis due to decreased levels of PM emissions, may on an equal mass basis produce compounds that induce markers of inflammation compared to petroleum-based diesel exposure. Furthermore, increased organic matter including unsaturated aldehyde components from S100 combustion may result in enhanced oxidative stress as indicated by Nrf2 activity. Further studies are needed to identify and characterize biodiesel and renewable diesel emissions and chemically characterize the different emissions and their effects on oxidative stress and inflammation. The data from this study will be relevant for toxicity and safety assessments of diesel, biodiesel and renewable diesel blends.
HIGHLIGHTS.
Biodiesel or renewable diesel fuels induce inflammatory markers in macrophages.
Diesel and biodiesel emission samples induce cytochrome P450 1A1.
Emissions samples from diesel and the alternative diesel fuel blends activate the aryl hydrocarbon receptor.
Effects of the alternative diesel fuel blends emissions on the expression on inflammatory markers tend to be lower than emission samples derived from California ultra-low sulphur diesel fuel.
Acknowledgements
The authors are grateful to Christina La, Uka Enkhayar, and Brian Do for their critical support, Tullie Flower, Don Chernich, Mark Burnitzki, Lex Mitchell, William Robertson, Thomas Ladzinki, Ralph Rodas, George Gatt, and Keshav Sahay who worked closely with us during this project. We thank Alvaro Alvarado for helpful comments and the National Biodiesel Board and Neste Oil for donating the fuels for this project. We are grateful to the California Air Resources Board and the South Coast Air Quality Management District for their support. This work was supported in part by funding through the National Institute of Environmental Health Sciences, United States grants R01ES019898 and R01ES029126 (CV). The statements and opinions expressed in this paper are solely the authors’ and do not represent the official position of the University of California or the California Air Resources Board. The mention of trade names, products, and organizations does not constitute endorsement or recommendation for use.
Funding
We are grateful to the California Air Resources Board and the South Coast Air Quality Management District for their support. This work was supported in part by funding through the National Institute of Environmental Health Sciences, United States grants R01ES019898 and R01ES029126 (CV).
Abbreviations:
- AhR
Aryl hydrocarbon receptor
- CARB ULSD
California Air Resource Board-certified ultralow sulfur diesel
- CVD
cardiovascular disease
- COX-2
cyclooxygenase 2
- CYP1A1
cytochrome P450 1A1
- DEP
diesel exhaust particulate
- DRE
dioxin responsive element
- IL
Interleukin
- NIST
National Institute of Standards & Technology
- SRM
Standard Reference Material
- PM
particulate matter
- PAH
polycyclic aromatic hydrocarbon
- PCB
polychlorinated biphenyls
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TNFα
Tumor necrosis factor α
- S
Soy Biodiesel
- A
Animal Biodiesel, Renewable Biodiesel
- ROS
Reactive Oxygen Species
Footnotes
Experimental animals
Bone marrow-derived macrophages were generated from C57BL/6 mice obtained from JAX West, Inc. (Davis, CA). Mice were euthanized in accordance with a protocol approved by the UC Davis Animal Resources Service.
References
- Aguilera I, Dratva J, Caviezel S, Burdet L, de Groot E, Ducret-Stich RE, Eeftens M, Keidel D, Meier R, Perez L, Rothe T, Schaffner E, Schmit-Trucksäss A, Tsai M-Y, Schindler C, Künzli N, Probst-Hensch N, 2016. Particulate matter and subclinical atherosclerosis: associations between different particle sizes and sources with carotid intima-media thickness in the SAPALDIA study. Environ. Health Perspect. 124 (11), 1700–1706. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghard A, Gradin K, Pongratz I, Whitelaw M, Poellinger L, 1993. Cross-coupling of signal transduction pathways: the dioxin receptor mediates induction of cytochrome P-450IA1 expression via a protein kinase C-dependent mechanism. Mol. Cell Biol 13, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betha R, Pavagadhi S, Sethu S, Hande M, Balasubramanian R, 2012. Comparative in vitro cytotoxicity assessment of airborne particulate matter emitted from stationary engine fuelled with diesel and waste cooking oil-derived biodiesel. Atmos. Environ 61, 23–29. [Google Scholar]
- Bhavaraju L, Shannahan J, William A, McCormick R, McGee J, Kodavanti U, Madden M, 2014. Diesel and biodiesel exhaust particle effects on rat alveolar macrophages with in vitro exposure. Chemosphere 104,126–133. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann BC, Skuland T, Rambøl MH, Szoke K, Brinchmann JE, Gutleb AC, Moschini E, Kubátová A, Kukowski K, Le Ferrec E, Lagadic-Gossmann D, Schwarze PE, Låg M, Refsnes M, Øvrevik J, Holme JA, 2018a. Lipophilic components of diesel exhaust particles induce pro-inflammatory responses in human endothelial cells through AhR dependent pathway(s). Part. Fibre Toxicol 15 (1), 21 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann BC, Le Ferrec E, Podechard N, Lagadic-Gossmann D, Shoji KF, Penna A, Kukowski K, Kubátová A, Holme JA, Øvrevik J, 2018b. Lipophilic chemicals from diesel exhaust particles trigger calcium response in human endothelial cells via aryl hydrocarbon receptor non-genomic signalling. Int. J. Mol. Sci 19 (5). May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Rajagopalan S, 2003. Air pollution: the “Heart” of the problem. Feb Curr. Hypertens. Rep 5 (1), 32–39 (submitted for publication). [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, 2010. American heart association council on epidemiology and prevention, council on the kidney in cardiovascular disease, and council on nutrition, physical activity and metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American heart association. Circulation 121 (21), 2331–2378. June 1. [DOI] [PubMed] [Google Scholar]
- Craig L, Brook JR, Chiotti Q, Croes B, Gower S, Hedley A, Krewski D, Krupnick A, Krzyzanowski M, Moran MD, Pennell W, Samet JM, Schneider J, Shortreed J, Williams M, 2008. Air pollution and public health: a guidance document for risk managers. J. Toxicol. Environ. Health 71 (9–10), 588–698. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR, 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol 43, 309–334. [DOI] [PubMed] [Google Scholar]
- Esakky P, Hansen DA, Drury AM, Moley KH, 2015. Cigarette smoke-induced cell cycle arrest in spermatocytes [GC-2spd(ts)] is mediated through crosstalk between Ahr-Nrf2 pathway and MAPK signaling. J. Mol. Cell Biol 7 (1), 73–87. February. [DOI] [PubMed] [Google Scholar]
- Finch GL, Hobbs CH, Blair LF, Barr EB, Hahn FF, Jaramillo RJ, Kubatko JE, March TH, White RK, Krone JR, Ménache MG, Nikula KJ, Mauderly JL, Van Gerpen J, Merceica MD, Zielinska B, Stankowski L, Burling K, Howell S, 2002. Effects of subchronic inhalation exposure of rats to emissions from a diesel engine burning soybean oil-derived biodiesel fuel. Inhal. Toxicol 14 (10), 1017–1048. October. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE, 2004. Lung cancer in railroad workers exposed to diesel exhaust. Environ. Health Perspect 112 (15), 1539–1543. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett SH, Wood CE, Williams MA, Cyphert JM, Boykin EH, Daniels MJ, Copeland LB, King C, Krantz TQ, Richards JH, Andrews DL, Jaskot RH, Gilmour MI, 2015. Soy biodiesel emissions have reduced inflammatory effects compared to diesel emissions in healthy and allergic mice. Inhal. Toxicol 27 (11), 533–544. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Totlandsdal AI, Tzamkiozis T, Leseman DL, Samaras Z, Låg M, Schwarze P, Ntziachristos L, Cassee FR, 2013. Cell toxicity and oxidative potential of engine exhaust particles: impact of using particulate filter or biodiesel fuel blend. Environ. Sci. Technol 47 (11), 5931–5938. June 4. [DOI] [PubMed] [Google Scholar]
- Hajbabaei M, Johnson KC, Okamoto RA, Mitchell A, Pullman M, Durbin TD, 2012. Evaluation of the impacts of biodiesel and second generation biofuels on NO(x) emissions for CARB diesel fuels. Environ. Sci. Technol 46 (16), 9163–9173. August 21. [DOI] [PubMed] [Google Scholar]
- Hajbabaei M, Karavalakis G, Johnson KC, Guthrie J, Mitchell A, Durbin TD, 2014. Impacts of biodiesel feedstock and additives on criteria emissions from a heavy-duty engine. Fuel Process. Technol 126, 402–414. October. [Google Scholar]
- Ito T, Nagai H, Lin TM, Peterson RE, Tohyama C, Kobayashi T, Nohara K, 2006. Organic chemicals adsorbed onto diesel exhaust particles directly alter the differentiation of fetal thymocytes through arylhydrocarbon receptor but not oxidative stress responses. J. Immunot 3 (1), 21–30. January 1. [DOI] [PubMed] [Google Scholar]
- Jaguin M, Fardel O, Lecureur V, 2015. AhR-dependent secretion of PDGF-BB by human classically activated macrophages exposed to DEP extracts stimulates lung fibroblast proliferation. Toxicol. Appl. Pharmacol 285 (3), 170–178. June 15. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Durbin TD, Jung H, Cocker DR 3rd, Bishnu D, Giannelli R, 2011. Quantifying in-use PM measurements for heavy duty diesel vehicles. Environ. Sci. Technol 45 (14), 6073–6079. July 15. [DOI] [PubMed] [Google Scholar]
- Karavalakis G, Johnson KC, Hajbabaei M, Durbin TD, 2016. Application of low-level biodiesel blends on heavy-duty (diesel) engines: feedstock implications on NOx and particulate emissions. Fuel 181 (1), 259–268. October. [Google Scholar]
- Kobayashi R, Liu X, Na K, Durbin TD, Robertson W, Vogel CF, Okamoto RA, Kado NY, Polycyclic aromatic hydrocarbon emissions from a 2000 heavy-duty vehicle tested with biodiesel fuels. Unpublished results.
- Köhle C, Bock KW, 2007. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. June 15 Biochem. Pharmacol. 73 (12), 1853–1862. submitted for publication). [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, 2000. Schwartz J Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Perspect 108, 941–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I, 2010. Nrf2 is closely related to allergic airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin. Immunol 137 (2), 234–241. November. [DOI] [PubMed] [Google Scholar]
- Lucking AJ, Lundbäck M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, Blomberg A, 2011. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 123 (16), 1721–1728. April 26. [DOI] [PubMed] [Google Scholar]
- McAteer, Davis JM, 1994. Basic cell culture: a practical approach, basic cell culture and the maintenance of cell lines In: Davis JM (Ed.), Basic Cell Culture: a Practical Approach. Oxford University Press, New York. [Google Scholar]
- Marx J, 2004. Cancer research. Inflammation and cancer: the link grows stronger. Science 306 (5698), 966–968. November 5. [DOI] [PubMed] [Google Scholar]
- Miao W, Hu L, Scrivens PJ, Batist G, 2005. Transcriptional regulation of NF-E2 p45-regulated factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway. J. Biol. Chem 280, 20340–20348. [DOI] [PubMed] [Google Scholar]
- Na K, Sahay K, Okamoto B, Mitchell AS, 2015. Impact of biodiesel and renewable diesel on emissions of regulated pollutants and greenhouse gases on a 2000 heavy duty diesel truck. Atmos. Environ 107, 307–314. April. [Google Scholar]
- O’Driscoll CA, Owens LA, Gallo ME, Hoffmann EJ, Afrazi A, Han M, Fechner JH, Schauer JJ, Bradfield CA, Mezrich JD, 2018. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part. Fibre Toxicol 15 (1), 35 August 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB, 2007. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol. Sci 98 (1), 5–38. July. [DOI] [PubMed] [Google Scholar]
- Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ, 2004. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109 (1), 71–77. January 6. [DOI] [PubMed] [Google Scholar]
- Reed MD, Blair LF, Burling K, Daly I, Gigliotti AP, Gudi R, Mercieca MD, McDonald JD, Naas DJ, O’Callaghan JP, Seilkop SK, Ronskoh NL, Wagner VO, Kraska RC, 2005. Health effects of subchronic exposure to diesel-water emulsion emission. Inhal. Toxicol 17, 851–870. [DOI] [PubMed] [Google Scholar]
- Reed MD, Gigliotti AP, McDonald JD, Seagrave JC, Seilkop SK, Mauderly JL, 2004. Health effects of subchronic exposure to environmental levels of diesel exhaust. Inhal. Toxicol 16, 177–193. [DOI] [PubMed] [Google Scholar]
- Steiner S, Czerwinski J, Comte P, Popovicheva O, Kireeva E, Müller L, Heeb N, Mayer A, Fink A, Rothen-Rutishauser B, 2013. Comparison of the toxicity of diesel exhaust produced by bio- and fossil diesel combustion in human lung cells in vitro. Atmos. Environ 81, 380–388. [Google Scholar]
- Shvedova AA, Yanamala N, Murray AR, Kisin ER, Khaliullin T, Hatfield MK, Tkach AV, Krantz QT, Nash D, King C, Ian Gilmour M, Gavett SH, 2013. Oxidative stress, inflammatory biomarkers, and toxicity in mouse lung and liver after inhalation exposure to 100% biodiesel or petroleum diesel emissions. J. Toxicol. Environ. Health 76 (15), 907–92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF, 2002. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol 39 (6), 935–942. March 20. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Vogel CF, Koriyama C, Akiba S, Katoh T, Kawamoto T, Matsumura F, 2012. Association of serum levels of polychlorinated biphenyls with IL-8 mRNA expression in blood samples from asthmatic and non-asthmatic Japanese children. Chemosphere 87 (11), 1228–1234. June. [DOI] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD, 2013. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One 8 (12), e82545 December 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, 2000. Prostaglandin H synthases and their importance in chemical toxicity. Curr. Drug Metabol. 1 (4), 391–404. December. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F, 2005. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect 113 (11), 1536–1541. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F, 2007. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol 21 (12), 2941–2955. December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CC, Wu D, Goth SR, Baek J, Lollies A, Domhardt R, Grindel A, Pessah IN, 2013. Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol. Cell Biol 91 (9), 568–575. October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, He X, Szklarz GD, Bi Y, Rojanasakul Y, Ma Q, 2013. The aryl hydrocarbon receptor interacts with nuclear factor erythroid 2-related factor 2 to mediate induction of NAD(P)H:quinoneoxidoreductase 1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch. Biochem. Biophys 537 (1), 31–38. June 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng CM, Wang CH, Lee MJ, He JR, Huang HY, Chao MW, Chung KF, Kuo HP, 2018. Aryl Hydrocarbon Receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy. April 19. [DOI] [PubMed] [Google Scholar]
- Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, Matsumura F, Vogel CF, 2011. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E−/− mice. Arterioscler. Thromb. Vasc. Biol 31 (6), 1260–1267. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamala N, Hatfield MK, Farcas MT, Schwegler-Berry D, Hummer JA, Shurin MR, Birch ME, Gutkin DW, Kisin E, Kagan VE, Bugarski AD, Shvedova AA, 2013. Biodiesel versus diesel exposure: enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol. Appl. Pharmacol 272 (2), 373–383. October 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PM, Wang CC, Lin YC, Jhang SR, Lin LJ, Lin YC, 2017. Development of novel alternative biodiesel fuels for reducing PM emissions and PM-related genotoxicity. Environ. Res 156, 512–518. July. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J, Gold D, 2000. Are there sensitive subgroups for the effects of airborne particles? Environ. Health Perspect. 108 (9), 841–845. September. [DOI] [PMC free article] [PubMed] [Google Scholar]