Abstract
Background
Group B streptococcus (GBS) is a leading neonatal sepsis pathogen globally. Investment in GBS disease prevention, such as maternal vaccination, requires evidence of disease burden, particularly in high infant mortality regions like sub-Saharan Africa. We aimed to provide such evidence by conducting a systematic literature review and meta-analysis to estimate maternal colonization proportion, GBS disease incidence and GBS serotype distribution.
Methods
MEDLINE, MEDLINE in process and Cochrane Library were searched for studies published during 1990–2014, pertaining to sub-Saharan Africa. Eligible studies were used to estimate the proportion of pregnant women colonized with GBS, early-onset GBS disease incidence, late-onset GBS disease incidence and respective serotype distributions. Random effects meta-analysis was conducted to estimate weighted means and confidence intervals (CIs).
Results
We identified 17 studies of colonization, 9 of disease incidence, and 6 of serotype distribution meeting inclusion criteria. 21.8% (95% CI: 18.3, 25.5) of expectant women were colonized with GBS. The incidence of early-onset GBS disease was 1.3 per 1000 births (95% CI: 0.81, 1.9), that of late-onset GBS disease 0.73 per 1000 births (95% CI: 0.48, 1.0). The most common disease-causing serotype was 3, followed by 1a. Serotypes 1b, 2 and 5 were next most common in frequency.
Conclusion
Despite methodological factors leading to underestimation, GBS disease incidence appears high in sub-Saharan Africa. A small number of GBS serotypes cause almost all disease. GBS disease burden in sub-Saharan Africa suggests that safe, effective and affordable GBS disease prevention is needed.
Keywords: Africa, group B streptococcus, neonatal sepsis, neonatal meningitis, global health, meta-analysis
Streptococcus agalactiae, or group B streptococcus (GBS), is a leading neonatal sepsis pathogen globally and may have a particularly high burden of disease in Africa.1 Maternal rectovaginal GBS colonization is a risk factor for infant disease. In some high-income countries, intrapartum antibiotic prophylaxis (IAP) that targets pregnant women shown to be colonized with GBS on rectovaginal swabs collected several weeks before delivery has greatly reduced the burden of GBS disease by preventing early-onset cases (EOGBS disease, occurring days 0–6 of life).2 This strategy, however, is unlikely to be feasible in low- and some middle-income countries due to lack of resources. Risk factor-based IAP, which targets women with known risk factors such as intrapartum fever or preterm labor, is less costly than prenatal screening, but nonetheless challenging to implement in resource-poor settings. Moreover, IAP does not prevent late-onset GBS disease (LOGBS disease, occurring days 7–90 of life).
A serotype-specific maternal GBS polysaccharide-protein conjugate vaccine to prevent both EOGBS and LOGBS disease in newborns has completed phase II clinical trials in South Africa and Malawi,3,4 Although the trivalent vaccine candidate will not move forward to Phase III trials, planning for the development of a pentavalent vaccine has begun. International health organizations and funders need information on the potential impact of introducing a new vaccine, if encouraging results from clinical trials become available. Such information is also needed for health economic studies, where incidence of GBS disease has been shown to be a leading driver of cost–effectiveness.5 Thus, disease burden data are key to the evidence base that informs decision-making regarding new vaccine introduction. While global analysis has previously been conducted, relatively few studies from sub-Saharan Africa were included1 and detailed consideration of sub-Saharan Africa’s GBS disease burden is needed to guide policy decisions regarding prevention. We conducted a systematic literature review and evidence synthesis using meta-analysis, focusing on 3 metrics of GBS disease burden in sub-Saharan Africa: maternal colonization proportion, GBS disease incidence and GBS serotype distribution.
Methods
Eligibility Criteria
After standard guidelines,6 we conducted systematic literature reviews and meta-analyses for the following outcomes (1) proportion of pregnant women colonized with GBS; (2) incidence of EOGBS disease in infants; (3) incidence of LOGBS disease in infants and (4) proportions of GBS isolate serotypes (Ia, Ib, II, III and V) which would be covered by a potential pentavalent vaccine. Serotype studies were subcategorized by studies examining maternal colonizing serotypes and infant disease-causing serotypes. Because other candidate vaccines may be developed, we also collected information concerning the contributions of individual serotypes to overall serotype distribution, estimating cumulative serotype coverage sequentially by ordering GBS serotypes by frequency. Information regarding case fatality ratios (CFRs) was also abstracted from incidence studies.
The search strategy, inclusion and exclusion criteria are summarized in Table 1. The geographic scope of analysis was limited to sub-Saharan Africa.1 Only studies published from 1990 to 2014 were included. Published trials and observational studies, including prospective and retrospective cohort, case series, case–control, and cross-sectional studies evaluating maternal colonization, infant GBS disease incidence and GBS serotypes from expectant mothers or neonates were considered for eligibility. Only English language papers were considered for inclusion.
Table 1. Literature Review Methods and Data Abstracted: Maternal GBS Colonization, EOGBS Disease Incidence, LOGBS Disease Incidence and GBS Serotype Distribution.
| Databases searched | Medline, Medline In-Progress, Cochrane Library | ||||
| Unpublished data sources | Expert query, online abstracts for 2013 and 2014 international congresses, hand search of paper bibliographies | ||||
| Search terms | (“Streptococcus agalactiae” [Mesh] or “Streptococcus Group B” or “Group B Streptococcal”) and (“Africa” [Mesh] OR “developing countries” or “low-income countries”) | ||||
| Study time frame | 1990 to present | ||||
| Maternal GBS Colonization | Incidence |
GBS Serotype Distribution |
|||
| EOGBS | LOGBS | Maternal | Infant | ||
| Outcome of interest | Proportion rectovaginal swab (+) for GBS | Sterile site (blood, CSF) culture (+) for GBS | Sterile site (blood, CSF) culture (+) for GBS | Serotype results for rectovaginal swab (+) for GBS | Serotype results for sterile site culture (+) for GBS |
| Types of participants | Expectant women | Infants aged 0–6 d | Infants aged 7–89 d old | Expectant women in their last trimester | Infants 90 d old or less |
| Other inclusion/exclusion criteria | Time of screening: antepartum or intrapartum, otherwise exclude | Time of illness onset: approximately 0–6 d after birth, otherwise exclude* | Time of illness onset: approximately 7–90 d after birth, otherwise exclude* | Time of screening: antepartum or intrapartum Only studies in which serotyping was performed were included |
Sterile site cultures only (blood or CSF), otherwise exclude. Infant colonization studies excluded |
| Summary outcomes | Proportion of women colonized with GBS | Cases per 1000 live births of EOGBS disease | Cases per 1000 live births of LOGBS disease | Proportion covered by potential candidate vaccine | Proportion covered by potential candidate vaccine |
Studies that approximated these intervals were also included in the analysis.
Sources, Search and Study Selection
Studies were identified using the databases and search strategies, developed with input from a biomedical reference librarian, shown in Table 1. In addition, the bibliographies of papers selected for inclusion after full text review were searched for additional sources.
To mitigate publication bias, an extensive search for unpublished data was conducted. We identified unpublished sources of data in 2 steps1: we searched the online abstract books for 2013 and 2014 for 2 international congresses (IDWeek and European Society for Paediatric Infectious Diseases). The convening president of a third biannual international congress (World Society for Paediatric Infectious Diseases) held in 2013 was queried regarding relevant abstracts in their abstract book, not available online. The Working Group supporting this project—investigators working in neonatal sepsis and meningitis in sub-Saharan Africa—were asked to identify additional sources of abstract-level or unpublished data, either from their own groups or those of colleagues. The review of published literature was conducted in September 2014 and unpublished searches were finalized in January 2015.
Study selection was performed in a standardized manner, applying inclusion and exclusion criteria to titles and abstracts, and then to the full texts of studies. This assessment was performed by a research associate and then reviewed by an author (AS).
Data Collection
Data abstracted described the publication, characterized methods, characterized quality and summarized outcomes. Detailed data items abstracted from each study are summarized in Table 1.
Data were collected using a data extraction Excel spreadsheet. A research associate performed data extraction and an author (AS) reviewed the extracted data. Uncertainties were resolved by discussion between authors. Summary outcomes were as described under “Eligibility criteria.”
Meta-analysis
Random effects meta-analysis was performed for each outcome,7 using OpenMetaAnalyst,8 to estimate weighted means and associated 95% confidence intervals (CIs). Because some proportions were close to 1, we stabilized variances with a Freeman–Tukey arcsine transformation before pooling. The DerSimonian–Laird method for estimating random-effects, with a correction factor of 0.5 added to zero-valued cells, was implemented to obtain the estimates. Heterogeneity was examined using Q, a χ2 statistic, where a P < 0.05 suggests effects across all studies are not the same, and I2, a statistic used to quantify inconsistency across studies, with I2 values >50% representing substantial heterogeneity.9
Results
Study Selection and Study Characteristics
The results of the initial literature search and study selection are summarized in Table 2, which concisely summarizes the information that would be included in separate PRISMA charts for each of the outcomes. Two unpublished studies in progress were identified and included in the systematic literature review.
Table 2. Systematic Literature Review Results.
| Maternal GBS Colonization | Incidence |
GBS Serotype Distribution |
|||
|---|---|---|---|---|---|
| EOGBS | LOGBS | Maternal | Infant | ||
| Number of nonduplicate citations identified, published literature | 86 | 51 | 51 | 16 | 9 |
| Number of papers screened at abstract level | 86 | 51 | 51 | 16 | 9 |
| Exclusions after abstract review | 38 | 41 | 41 | 10 | 5 |
| Reasons for exclusion | No maternal colonization information (13) Year (13) Not human (5) Not GBS (3) Not sub-Saharan Africa (3) Not pregnant (1) |
Not EOGBS (24) No denominator (6) Not English (3) Review article (3) Not human (2) No blood or CSF test (1) Not sub-Saharan Africa (1) Secondary analysis of an included article (1) |
Not EOGBS (24) No denominator (6) Not English (3) Review article (3) Not human (2) No blood or CSF test (1) Not sub-Saharan Africa (1) Secondary analysis of an included article (1) |
No serotype information (10) | No serotype information (5) |
| Number of full text papers, retrieved and reviewed | 48 | 10 | 10 | 6 | 4 |
| Number of unpublished data sources identified | 0 | 0 | 0 | 0 | 0 |
| Exclusions after review | 32 | 1 | 2 | 1 | 1 |
| Reasons for exclusion | Review article (13) Second analysis of an included article (5) Not sub-Saharan Africa (3) Year (2) Not maternal colonization (2) Specimen collection method unspecified (1) Not English (1) Not GBS (1) Not pregnant (1) Not recto-vaginal swab (1) Post labor (1) Unavailable (1) |
Review article (1) | Review article (1) Not LOGBS (1) |
Insufficient information on serotype (1) | Method of sample collection unclear (1) |
| Number of unpublished sources included in meta-analysis | 0 | 0 | 0 | 0 | 0 |
| Number of studies included in quantitative synthesis* | 16 | 9 | 8 | 5 | 3 |
Tables 3 and 4 summarize study characteristics and provide a description of the studies. In total, 9 of 45 sub-Saharan African countries contributed data to at least one of the systematic literature reviews. Five of the 10 countries with the largest birth cohorts in sub-Saharan Africa contributed data to at least one of the systematic literature reviews. South Africa predominated as a contributor of data. 4094 (47.4%) observations included in the maternal colonization review were from South Africa; the next 2 leading contributors were Malawi and Zimbabwe, contributing 1954 (22.6%) and 1491 (17.3%) observations, respectively. A similar pattern was observed in the reviews for EOGBS disease incidence, LOGBS disease incidence, maternal serotype distribution and infant EOGBS/LOGBS disease serotype distribution. South Africa contributed the largest number of observations of any single country, but 54% of observations in the maternal colonization analysis, 26% of observations contributing to EOGBS disease incidence analysis, 42% contributing to the LOGBS disease incidence analysis and 43% of observations contributing to any serotype analysis were from low-income countries in sub-Saharan Africa. 21 of 25 studies were performed at urban sites only, while 4 studies described at least one rural site.
Table 3. Characteristics of Studies Included for Meta-analysis.
| Maternal GBS Colonization10–24 | Incidence |
GBS Serotype Distribution |
|||
|---|---|---|---|---|---|
| EOGBS11,25–30 | LOGBS11,25,26,28–30 | Maternal10,16,19,31 | Infant10,25,31 | ||
| Countries | Ethiopia (2), Gambia, Kenya, Malawi (2), Mozambique (2), Nigeria, South Africa (2), Tanzania (1), Zimbabwe (4) | Kenya, Malawi, Mozambique Nigeria, South Africa (4), Zimbabwe | Kenya, Malawi, Mozambique Nigeria, South Africa (3), Zimbabwe | Ethiopia, Kenya, Malawi, South Africa, Zimbabwe | Malawi, South Africa (2) |
| Study design | Case–control (2), cross-sectional (13), longitudinal cohort (1) | Case–control (1), cross-sectional (5), retrospective (3) | Case–control (1), cross-sectional (5), retrospective (2) | Cross-sectional (5) | Cross-sectional (3) |
| Study population | Antepartum women (11), intrapartum women (5) | 0–2 d (2), 0–5 d (1), 0–6 d (4), 0–7 d (2) | <5 d (1), 2–28 d (2), 8–28 d (1), 7–90 d (4) | Antepartum women (3), intrapartum women (2) | 7–90 d (3) |
| Ascertainment of endpoints | Vaginal culture only (4), vaginal and rectal culture (12) | Blood culture only (3), blood and CSF culture (6) | Blood culture only (3), blood and CSF culture (5) | Vaginal culture only (3), vaginal and rectal culture (2) | Blood culture only (1), blood and CSF culture (2) |
| Notes | Timing of culture during antepartum period varied between 20 and 37+ weeks gestation | ||||
Table 4. Description of Studies Included in Meta-analyses.
| Country | First Author (Reference) | Year Published | N | Study Design | Case Ascertainment | Rural/Urban | Serotypes Reported? |
|---|---|---|---|---|---|---|---|
| Colonization | |||||||
| Nigeria | Uhiara21 | 1993 | 100 | Cross-sectional, hospital-based | In labor, high vaginal + rectal swabs | Urban | No |
| Gambia | Suara18 | 1994 | 136 | Cross-sectional, health center-based | In labor, vaginal + rectal swab | Rural | No |
| Mozambique | Osman13 | 1995 | 53 | Case–control, hospital-based | 25–36 weeks gestation, posterior vaginal fornix swab | Urban | No |
| Kenya | Mosabi19 | 1997 | 28 | Cross-sectional, hospital-based | Hospital-associated antenatal or post-natal clinic, high vaginal swab | Urban | Yes |
| Zimbabwe | Moyo16 | 2000 | 206 | Cross-sectional, hospital-based | All pregnant women, high vaginal + rectal swabs | Urban | Yes |
| Zimbabwe | Thinkhamrop17 | 2003 | 209 | Cross-sectional, hospital-based | 20–32 weeks gestation, cervix + lower vagina | Urban | No |
| Malawi | Dzowela22 | 2005 | 97 | Cross-sectional, hospital-based | >34 weeks gestation, lower vaginal and rectal swabs | Urban | No |
| Zimbabwe | Mavenyengwa24 | 2006 | 400 | Cross-sectional, clinic- and hospital-based | 20–30 weeks gestation, lower vaginal and rectal swabs | Both | No |
| Mozambique | de Steenwinkel12 | 2008 | 113 | Cross-sectional, health center-based | 35–37 weeks gestation, rectovaginal swabs | Urban | No |
| Tanzania | Joachim20 | 2009 | 300 | Cross-sectional, hospital-based | 37 weeks of gestation, high vaginal + rectal swabs | Urban | No |
| South Africa | Cutland11 | 2009 | 3964 | Prospective randomized clinical trial, hospital-based | In labor, lower vagina | Urban | No |
| Zimbabwe | Mavenyengwa15 | 2010 | 676 | prospective cohort, clinic- and hospital-based | ~26 weeks gestation and in labor, lower vaginal + rectal swab | Both | No |
| South Africa* | Madzivhandila31 | 2011 | 2561 | Prospective case series, hospital-based | In labor, vaginal swab | Urban | Yes |
| Malawi | Gray10 | 2011 | 1857 | Cross-sectional, hospital-based | In labor, low vagina + rectal swab | Urban | Yes |
| Ethiopia | Mohammed23 | 2012 | 139 | Cross-sectional, health center-based | 35–37 weeks gestation, lower 1/3 of vagina + anal region | Urban | No |
| South Africa | Kwatra32 | 2013 | 130 | Cross-sectional, community clinic-based | 20+ weeks gestation, lower vaginal and rectal swabs | Urban | No |
| Ethiopia | Mohammed* | In progress | 235 | Cross-sectional, hospital-based | Before delivery | Urban | Yes |
| EOGBS and LOGBS disease | |||||||
| Zimbabwe | Nathoo33 | 1990 | 12,917 | Prospective, hospital-based | Ages EOGBS < 48 h, 48 h–28 d; blood culture | Urban | No |
| South Africa | Haffejee26 | 1991 | 14,340 | Retrospective, hospital | Ages EOGBS 0–5 d, LOGBS >5 d; blood and/or CSF culture | Urban | No |
| South Africa | Bomela27 | 2001 | 28,424 | Retrospective and prospective cohort, hospital-based | Ages EOGBS 0–6 d; blood and CSF culture | Urban | No |
| South Africa | Madhi25 | 2003 | 67,227 | Retrospective cohort, hospital-based | Ages EOGBS 0–6 d, LOGBS 7–90 d; blood and CSF culture | Urban | Yes |
| Kenya | Berkley28 | 2005 | 27,284 | Prospective cohort, hospital-based | Ages EOGBS 0–7 d, 8–90 d; blood culture | Rural | No |
| Nigeria | Ojukwu30 | 2006 | 4135 | Prospective cohort, hospital-based | Ages EOGBS 0–6 d, LOGBS 7–90 d; blood and CSF culture | Urban | No |
| Malawi | Gray29 | 2007 | 31,458 | Prospective cohort, hospital-based | Ages EOGBS 0–6 d, LOGBS 7–90 d; blood and CSF culture | Urban | Yes |
| South Africa | Cutland11 | 2009 | 8129 | Prospective randomized clinical trial, hospital-based | Ages EOGBS 0–2 d, LOGBS 3–28 d; blood and/or CSF culture | Urban | No |
| South Africa | Madzivhandila31† | 2011 | 2542 | Prospective case series, hospital-based | Ages EOGBS 0–6 d, LOGBS 7–90 d; blood and/or CSF culture | Urban | Yes |
| Mozambique | Sigauque* | In progress | 10,072 | cross-sectional, hospital-based | Ages EOGBS 0–7 d, LOGBS 8–28 d; blood and/or CSF culture | Urban | No |
Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Comoros, Dem. Rep. Congo, Rep. Congo, Côte d’Ivoire, Equatorial Guinea, Eritrea, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, Somalia, South Africa, South Sudan, Sudan, Swaziland, Tanzania, Togo, Uganda, Zambia, Zimbabwe. The island nations of Cabo Verde, São Tomé and Principe, and Seychelles were excluded.
Personal communications from first authors.
Contributed to serotype distribution analysis only.
Maternal GBS Colonization
Across the region, an average of 21.8% (95% CI: 18.3, 25.5) of women included in the studies identified were colonized with GBS either antenatally or during labor. There was heterogeneity across studies in this proportion (Fig. 1A), as indicated by a χ2 P value of <0.0001 and I2 value of 94%. In addition to possible true population differences, other sources of heterogeneity may have included methodological differences in the collection of specimens and microbiological processing. Four published studies collected only vaginal specimens for culture; 11 studies collected rectovaginal specimens but only 7 sampled both lower vagina and rectum, as is recommended to maximize yield.2 Three published studies did not describe the use of selective enrichment broths, such as Todd-Hewitt or Lim broth, also recommended to maximize yield.2
Figure 1.
Studies of maternal GBS colonization in sub-Saharan Africa. A: Forest plot from random-effects meta-analysis of proportion of pregnant women colonized with GBS in sub-Saharan Africa. Studies in which a swab for culture was collected from women antepartum or in labor were included10–24 (Musa Mohammed, personal communication, January 27, 2015). B: Proportion (%) of women colonized by the number of women cultured in each study. Larger studies consistently showed a colonization rate of ~21%–22% and smaller studies are arrayed symmetrically around this average.
Figure 1B suggests that variability among study results may be related to small study size, with larger studies consistently showing a colonization rate of ~21%–22% and smaller studies arrayed symmetrically around this average. In sensitivity analysis, when data from South Africa were removed and data from the remaining countries reanalyzed, the pooled average proportion was 20.7% (15.4, 26.7). When studies were restricted to the 7 that sampled both body sites with the highest yield (lower vagina and rectum), the proportion of women colonized with GBS rose to 26.0% (95% CI: 17.2, 35.9).
Incidence of EOGBS and LOGBS Disease
The estimated average incidence of EOGBS disease was 1.3 cases per 1000 births (Fig. 2A). The estimated incidence of LOGBS disease was 0.73 per 1000 births (Fig. 2B), roughly 1/2 the EOGBS disease incidence. As with maternal colonization, there was substantial heterogeneity across studies reporting EOGBS (LOGBS) incidence with χ2 P values <0.0001 for both outcomes and I2 values of 90% (59%). Of note, 2 of 8 published studies reported intrapartum antibiotic use in the study population; the remainder did not provide this information.
Figure 2.
Incidence of GBS disease in sub-Saharan infants. A: Forest plot of the incidence of EOGBS disease for sub-Saharan infants. Studies with infants <7 days old with GBS-positive blood or CSF test were included11,25–30 (Betuel Sigauque, personal communication). B: Forest plot of the incidence of LOGBS disease for sub-Saharan infants. Studies with infants 7–90 days old with GBS-positive blood or CSF culture were included.11,25,26,28–30
In addition to any true population differences, methodological differences may have contributed to heterogeneity. Studies differed in the method of case ascertainment: Five of the published studies were prospective; 3 of the studies were retrospective; 2 collected blood only for culture, as opposed to 5 that collected both blood and/ or cerebrospinal fluid (CSF) for culture. It is likely that clinician thresholds for drawing sterile site cultures and the ability to capture infants on the day of birth varied across studies, although this information was difficult to abstract from published reports. Studies also varied in setting, with the majority of studies conducted in urban settings. All studies appeared facility-based but varied in whether facilities were outpatient (health centers and clinics) or inpatient.
All studies undergoing abstract review for EOGBS or LOGBS incidence were also screened for information concerning CFRs. All incidence studies also presented information on CFRs. However, they reported CFRs after subcategorizing EOGBS and LOGBS disease in different ways and using different patient age cut-offs, making meta-analysis impractical because CFRs were defined using different numerators and denominators. For example, a study from Malawi34 defined EOGBS and LOGBS disease as GBS isolated from blood or CSF sample from patients from birth to 6 days of age and from 7 to 90 days of age, respectively. It reported a CFR of 38% (95% CI: 20, 56) for EOGBS disease and a CFR of 29% (95% CI: 12, 45) for LOGBS disease. A large study from South Africa25 also defined EOGBS and LOGBS disease as positive blood or CSF sample from patients from birth to 6 days old and from 7 to 90 days, respectively; it reported a CFR of 20% (95% CI: 13, 27) for EOGBS disease and 14% (95% CI: 5, 22) for LOGBS disease. A second study from Malawi35 and a study from Mozambique36 reported CFRs for all GBS septicemia and meningitis occurring between the ages of 0–30 days of 21% and 26%, respectively (insufficient information to calculate 95% CI). By comparison, a much lower CFR for all GBS disease occurring between 0 and 30 days (12%) was reported in a study in progress in Mozambique (95% CI: 1, 23) (Betuel Sigauque, personal communication, February 15, 2015).
GBS Serotype Distribution
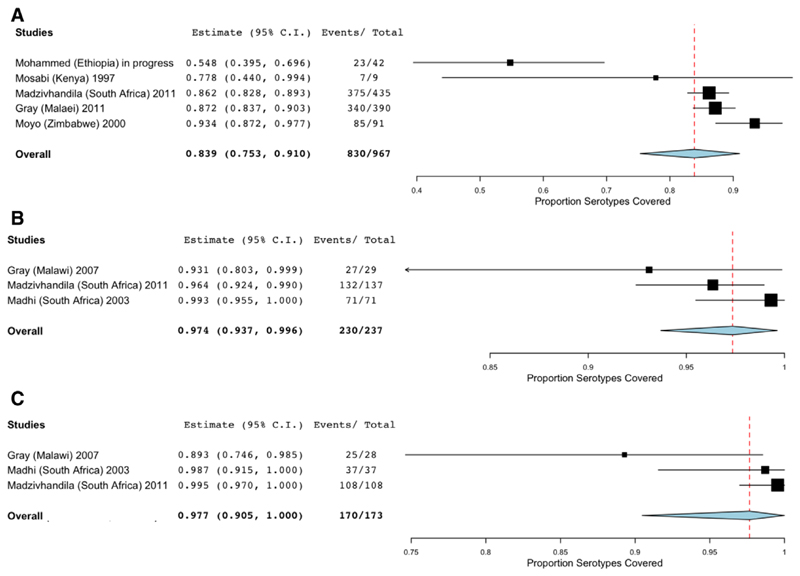
A total of 967 maternal colonizing samples from 5 studies were included in the meta-analysis of the proportion of maternal colonizing serotypes that would be covered by a pentavalent vaccine (Ia, Ib, II, III, and V). The average proportion of vaccine-covered maternal colonizing serotypes was 83.9% (95% CI: 75.3, 91.0; Fig. 3A). These studies comprise a subset of those included in the maternal colonization analysis and as a result, significant heterogeneity was present with a P value <0.001 and I2 value of 84%.
Figure 3.
Proportion of GBS serotypes in a pentavalent vaccine (Ia, Ib, II, III and V). A, Forest plot of GBS serotype proportion covered for maternal colonization. Studies in which women received swab for culture antepartum or in labor were included10,16,19,31 (Musa Mohammed, personal communication). B, Forest plot of GBS serotype proportion covered for EOGBS disease. Studies with infants <7 days old with GBS-positive blood or CSF culture were included.10,25,31 C, Forest plot of GBS serotype proportion covered for LOGBS disease. Studies with infants 8–90 days old with GBS-positive blood or CSF culture were included.10,25,31
A total of 237 serotyped infant samples from 3 studies conducted in Malawi and South Africa were included in the meta-analysis of the proportion EOGBS disease-causing serotypes covered by a pentavalent vaccine; for LOGBS disease-causing isolates, 173 samples and 3 studies were analyzed. The average proportion of EOGBS isolates that were vaccine-covered serotypes was 97.4% (95% CI: 93.7, 99.6; Fig. 3B), while for LOGBS the proportion was 97.7 (95% CI: 90.5, 100; Fig. 3C). Heterogeneity was not found in the analysis of EOGBS disease-causing isolates but was found in the analysis of LOGBS disease-causing isolates (P = 0.045, I2 = 68%).
Figure 4 shows individual serotype contributions. Serotypes Ia, Ib, II, III and V were dominant strains, with serotype III alone contributing 54% to the overall serotype distribution for EOGBS disease and 79% to the overall distribution for LOGBS disease. The same serotypes were the most common maternal colonizing strains, although serotype III was a less dominant contributor.
Figure 4.
Distribution of GBS serotypes in sub-Saharan mothers and infants. A, Maternal colonization (n =1073 isolates), (B) EOGBS disease (n = 237 isolates) and (C) LOGBS disease (n = 173 isolates). Error bars indicate the 95% CIs. The line plot indicates the cumulative proportion of GBS serotypes10,16,19,25,31 (Musa Mohammed, personal communication).
Discussion
The estimated incidence in sub-Saharan Africa of EOGBS disease, 1.3 cases per 1000 live births, and LOGBS disease, 0.7 cases per 1000 live births, suggest a burden of disease at least comparable to that found in high income settings before the use of intrapartum antibiotics.37 For example, in early 1990s in the US EOGBS incidence was 1.3–1.7 cases per 1000 live births. However, these estimated incidences for sub-Saharan Africa are likely to be underestimates of the true rates due to factors typical of health systems in low-income countries that make it difficult to identify GBS disease accurately. Such factors include access to care, availability of microbiologic laboratories, many febrile infants at risk for sepsis and/or meningitis not receiving blood and CSF sampling, antibiotic use before blood and CSF sampling, early mortality before clinical care and home births. In addition, the limited sensitivity of blood and CSF culture for disease even when properly performed38 contributes to underestimating disease incidence.
Maternal rectovaginal colonization with GBS is an important risk factor for EOGBS disease, with a relative risk of approximately 30.37 Similar to the US, the proportion of expectant mothers colonized with GBS was 21%–22% and this was true when studies from South Africa, a large contributor to the data, were excluded. Also similar to the US, serotypes Ia and Ib, II, III and V were predominant in both colonizing and disease-causing GBS isolates. Of note, when considering a potential pentavalent vaccine covering these 5 serotypes, the serotype coverages for both EOGBS and LOGBS disease were higher than serotype coverage for maternal colonization; the most important contributor to the difference between colonizing and disease-causing serotypes was serotype III, which has been associated with higher invasiveness potential in other studies.39
As have other investigators,32 we found substantial heterogeneity across studies concerning maternal GBS colonization and EOGBS/LOGBS disease incidence. For maternal colonization, the number of studies was large, and we could best examine patterns between study characteristics and study outcome. Our results suggested that small sample sizes and unstable estimates for some smaller studies might have contributed to variability in results. In addition, only 7 studies collected specimens from both rectal and lower vaginal swabs, as is recommended to maximize yield during antepartum screening. Finally, some studies did not use selective enrichment broths and the failure to do so can result in a 50% false-negative rate.2 For EOGBS and LOGBS incidence, as well as serotype distribution, the number of studies was smaller but reasons for heterogeneity could include different approaches to case ascertainment, denominators used and sites of microbiological culture taken.
The EOGBS incidence for sub-Saharan Africa estimated in this analysis was roughly double that found by other investigators for the sub-Saharan Africa region in a global systematic literature review by Edmond et al.1 This may be due to our inclusion of 4 studies, published in 1990,33 1991,26 200127 and 2003,25 which did not meet the date range criteria of the global review, in addition to including a study from Mozambique currently in progress. Our choice to use a longer time window, 1990 to 2014, was informed by the knowledge that data from sub-Saharan Africa are relatively sparse, and the longer time window enabled the analysis to capture 4 large studies (cumulative subjects = 122,908) that would not otherwise have been included. This difference between the 2 systematic reviews had less impact on estimation of LOGBS, where our finding (0.7 per 1000 births) was similar to that of the global review by Edmond et al.1 Serotype distribution estimates were similar with both studies finding upward of 85% of disease-causing isolates to be covered by the trivalent candidate vaccine that is no longer in trials. A potential pentavalent vaccine was not considered by the earlier analysis. We find that a pentavalent vaccine including serotypes Ia, Ib, II, III and V would cover over 95% of isolates.
Limitations to this study include lack of consideration of maternal risk factors, such as maternal fever, preterm versus term delivery and prolonged rupture of membranes. We were unable to include consideration of these risk factors due to variable reporting practice. Different distributions of these risk factors for GBS disease across studies could also have contributed to the heterogeneity observed. In addition, consideration of HIV exposure could also have been important. For example, a recent study from South Africa, published after completion of these analyses, found that HIV-exposed infants may have a 64% higher risk of disease, compared with non-HIV-exposed infants (4.46 GBS cases vs. 2.72 cases per 1000 live births).40 Of note, with an incidence of 2.72 per 1000 live births, this study also documented a significant disease burden in non-HIV-exposed infants. Because the literature review was confined to English, publications from French-speaking West Africa may have been excluded.
Regional data were dominated by the contribution of South Africa, highlighting the need for research investment and information on disease burden from low-income countries within the region. As an example, information on disease-causing serotypes was only available from one low-income country, Malawi. Serotype data will be important to understanding the effectiveness of prevention through maternal vaccination. Information on death due to EOGBS and LOGBS disease was reported in a variable fashion, making estimation of regional GBS mortality burden difficult. Importantly, our analysis did not include the pathogen’s contributions to maternal puerperal sepsis, stillbirth and potentially preterm delivery, all of which may be part of its disease burden.41 Additional investments to estimate disease burden should include the contributions of these syndromes, as the full estimation of GBS disease burden will be a key driver in understanding the value of GBS-targeted prevention efforts.5
Despite these gaps, the data reviewed in this analysis suggest a substantial disease burden, a finding that is supported by broader evidence syntheses documenting the important contribution of sepsis to neonatal mortality.42 An existing set of antepartum, intrapartum and postpartum strategies to prevent neonatal sepsis and meningitis and its consequences are available.43 Other strategies, including the development of effective maternal GBS vaccines, are in development. If supported by further disease burden data and cost–effectiveness analyses, investments in these strategies may have the potential to avert cases and save newborn lives in the short and long term.
Acknowledgments
The authors would like to thank Kim Klingler for expert technical assistance. The GBS Vaccine Cost-Effectiveness Analysis in sub-Saharan Africa Working Group authors are Rudi Eggers, Immunization Vaccines and Biologicals Department (IVB), Expanded Program on Immunization (EPI), World Health Organization; Neil French, Institute of Infection and Global Health, University of Liverpool, Liverpool, UK; Robert Heyderman, Malawi-Liverpool Wellcome Trust; Liverpool School of Tropical Medicine, Liverpool, UK; Beate Kampmann, Medical Research Unit—The Gambia and Imperial College, London Medical Research Center—The Gambia; Shabir A. Madhi, National Institute for Communicable Diseases; University of Witwatersrand; Medical Research Council: Respiratory and Meningeal Pathogens Research Unit; Richard Mihigo, Routine Immunization and New Vaccines Programme, World Health Organization/Regional Office for Africa; Haroon Saloojee, Division of Community Paediatrics, Department of Paediatrics and Child Health, University of Witwatersrand, Johannesburg South Africa; and Anna C. Seale, KEMRI-Wellcome Trust Research Programme; Centre for Tropical Medicine and Global Health, University of Oxford, Oxford, UK.
The GBS Vaccine Cost-Effectiveness Analysis in sub-Saharan Africa Working Group authors are listed in the Acknowledgments.
N.H., R.S.H. and S.A.M. have received funding for a GBS vaccine trial from Novartis Vaccines and Diagnostics, Inc. S.A.M. has received honoraria for advisory board participation from Pfizer, Inc. This study was supported by award OPP1105076 from the Bill and Melinda Gates Foundation.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Edmond KM, Kortsalioudaki C, Scott S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- 2.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR–10):1–36. [PubMed] [Google Scholar]
- 3.Novartis Vaccines (Novartis) Magnitude of the Antibody Response to and Safety of a GBS Trivalent Vaccine in HIV Positive and HIV Negative Pregnant Women and Their Offsprings. [Accessed September 9, 2015]; Available at: https://clinicaltrials.gov/show/NCT01412801.
- 4.Novartis Vaccines (Novartis) A Phase Ib/II Randomized, Observer-blind, Controlled, Single Centre Study of a Trivalent Group B Streptococcus Vaccine in Healthy Non-pregnant Women Leading Into a Dose-ranging Study in Pregnant Women in South Africa. [Accessed September 9, 2015]; Available at: https://clinicaltrials.gov/show/NCT01193920.
- 5.Kim SY, Russell LB, Park J, et al. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine. 2014;32:1954–1963. doi: 10.1016/j.vaccine.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 9.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: Wiley-Blackwell; 2008. [Google Scholar]
- 10.Gray KJ, Kafulafula G, Matemba M, et al. Group B streptococcus and HIV infection in pregnant women, Malawi, 2008-2010. Emerg Infect Dis. 2011;17:1932–1935. doi: 10.3201/eid1710.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutland CL, Madhi SA, Zell ER, et al. PoPS Trial Team Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet. 2009;374:1909–1916. doi: 10.1016/S0140-6736(09)61339-8. [DOI] [PubMed] [Google Scholar]
- 12.de Steenwinkel FD, Tak HV, Muller AE, et al. Low carriage rate of group B streptococcus in pregnant women in Maputo, Mozambique. Trop Med Int Health. 2008;13:427–429. doi: 10.1111/j.1365-3156.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- 13.Osman NB, Folgosa E, Bergström S. An incident case-referent study of threatening preterm birth and genital infection. J Trop Pediatr. 1995;41:267–272. doi: 10.1093/tropej/41.5.267. [DOI] [PubMed] [Google Scholar]
- 14.Kwatra G, Madhi SA, Cutland CL, et al. Evaluation of Trans-Vag broth, colistin-nalidixic agar, and CHROMagar StrepB for detection of group B streptococcus in vaginal and rectal swabs from pregnant women in South Africa. J Clin Microbiol. 2013;51:2515–2519. doi: 10.1128/JCM.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavenyengwa RT, Afset JE, Schei B, et al. Group B streptococcus colonization during pregnancy and maternal-fetal transmission in Zimbabwe. Acta Obstet Gynecol Scand. 2010;89:250–255. doi: 10.3109/00016340903398029. [DOI] [PubMed] [Google Scholar]
- 16.Moyo SR, Mudzori J, Tswana SA, et al. Prevalence, capsular type distribution, anthropometric and obstetric factors of group B streptococcus (Streptococcus agalactiae) colonization in pregnancy. Cent Afr J Med. 2000;46:115–120. doi: 10.4314/cajm.v46i5.8533. [DOI] [PubMed] [Google Scholar]
- 17.Thinkhamrop J, Limpongsanurak S, Festin MR, et al. Infections in international pregnancy study: Performance of the optical immunoassay test for detection of group B streptococcus. J Clin Microbiol. 2003;41:5288–5290. doi: 10.1128/JCM.41.11.5288-5290.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suara RO, Adegbola RA, Baker CJ, et al. Carriage of group B Streptococci in pregnant Gambian mothers and their infants. J Infect Dis. 1994;170:1316–1319. doi: 10.1093/infdis/170.5.1316. [DOI] [PubMed] [Google Scholar]
- 19.Mosabi JM, Arimi SM, Kang’ethe EK. Isolation and characterization of group B streptococci from human and bovine sources within and around Nairobi. Epidemiol Infect. 1997;118:215–220. doi: 10.1017/s0950268897007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joachim A, Matee MI, Massawe FA, et al. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. 2009;9:437. doi: 10.1186/1471-2458-9-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhiara JE. Group B streptococcal carriage among parturients and their neonates in Zaria, Nigeria. Afr J Med Med Sci. 1993;22:79–83. [PubMed] [Google Scholar]
- 22.Dzowela T, Komolafe OO, Igbigbi A. Prevalence of group B streptococcus colonization in antenatal women at the Queen Elizabeth Central Hospital, Blantyre—a preliminary study. Malawi Med J. 2005;17:97–99. [Google Scholar]
- 23.Mohammed M, Asrat D, Woldeamanuel Y, et al. Prevalence of group B streptococcus colonization among pregnant women attending antenatal clinic of Hawassa Health Center, Hawassa, Ethiopia. Ethiop J Health Dev. 2012;26:36–42. [Google Scholar]
- 24.Mavenyengwa RT, Masunga P, Meque E, et al. Streptococcus agalactiae (group B streptococcus (GBS)) colonisation and persistence, in pregnancy; a comparison of two diverse communities (rural and urban) Cent Afr J Med. 2006;52:38–43. [PubMed] [Google Scholar]
- 25.Madhi SA, Radebe K, Crewe-Brown H, et al. High burden of invasive Streptococcus agalactiae disease in South African infants. Ann Trop Paediatr. 2003;23:15–23. doi: 10.1179/000349803125002814. [DOI] [PubMed] [Google Scholar]
- 26.Haffejee IE, Bhana RH, Coovadia YM, et al. Neonatal group B streptococcal infections in Indian (Asian) babies in South Africa. J Infect. 1991;22:225–231. doi: 10.1016/s0163-4453(05)80003-9. [DOI] [PubMed] [Google Scholar]
- 27.Bomela HN, Ballot DE, Cooper PA. Is prophylaxis of early-onset group B streptococcal disease appropriate for South Africa? S Afr Med J. 2001;91:858–860. [PubMed] [Google Scholar]
- 28.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 29.Gray KJ, Bennett SL, French N, et al. Invasive group B streptococcal infection in infants, Malawi. Emerg Infect Dis. 2007;13:223–229. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ojukwu JU, Abonyi LE, Ugwu J, et al. Neonatal septicemia in high risk babies in South-Eastern Nigeria. J Perinat Med. 2006;34:166–172. doi: 10.1515/JPM.2006.030. [DOI] [PubMed] [Google Scholar]
- 31.Madzivhandila M, Adrian PV, Cutland CL, et al. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One. 2011;6:e17861. doi: 10.1371/journal.pone.0017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwatra G, Cunnington MC, Valencia C, et al. Maternal colonization with group B streptococcus: Do rates vary across regions?. 8th World Congress of the World Society for Pediatric Infectious Diseases; November 19–22; Cape Town, South Africa: 2013. [Google Scholar]
- 33.Nathoo KJ, Mason PR, Chimbira TH. Neonatal septicaemia in Harare Hospital: aetiology and risk factors. The Puerperal Sepsis Study Group. Cent Afr J Med. 1990;36:150–156. [PubMed] [Google Scholar]
- 34.Katherine JG, Sally LB, Neil F, et al. Invasive group B streptococcal infection in infants, Malawi. Emerg Infect Dis. 2007;13:223. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milledge J, Calis JC, Graham SM, et al. Aetiology of neonatal sepsis in Blantyre, Malawi: 1996-2001. Ann Trop Paediatr. 2005;25:101–110. doi: 10.1179/146532805X45692. [DOI] [PubMed] [Google Scholar]
- 36.Sigaúque B, Roca A, Mandomando I, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 37.Edwards MS, Nizet V. Group B streptococcal infections. In: Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado Y, editors. Infectious Diseases of the Fetus and Newborn. 7th ed. Philadelphia, PA: Elsevier Saunders; 2011. pp. 419–469. [Google Scholar]
- 38.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103:e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 39.Davies HD, Adair C, McGeer A, et al. Antibodies to capsular polysaccharides of group B streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis. 2001;184:285–291. doi: 10.1086/322029. [DOI] [PubMed] [Google Scholar]
- 40.Cutland CL, Schrag SJ, Thigpen MC, et al. Increased risk for group B streptococcus sepsis in young infants exposed to HIV, Soweto, South Africa, 2004–2008. Emerg Infect Dis. 2015;21 doi: 10.3201/eid2104.141562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31(suppl 4):D7–12. doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Lawn JE, Blencowe H, Oza S, et al. Lancet Every Newborn Study Group Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 43.Bhutta ZA, Das JK, Bahl R, et al. Lancet Newborn Interventions Review Group Lancet Every Newborn Study Group. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]