Abstract
Major advances have been made in the diagnosis, evaluation and management of children with epilepsy over the past 15 years. There has been a marked increase in genetic diagnoses of a number of key childhood-onset epilepsy syndromes, such as Dravet syndrome, which has been linked to mutations in the SCN1A gene. The reorganization and reclassification of epilepsies, devised by the International League Against Epilepsy, has stimulated specialists to reassess their diagnostic practices; however, many studies have not addressed the global issues in treating children with epilepsy—specifically, the challenges of diagnosis through to optimal, and appropriate, therapeutic management. Also, Class I evidence-based data that are needed as a foundation for the development of treatment guidelines worldwide are lacking. Epilepsy is common, and the impact of this disease crosses age ranges and should be managed at all levels of care from community to quaternary care. In this Review, existing data and new therapeutic management approaches are discussed with the aim of highlighting the incidence of standard practices that may not be based on clinical evidence.
Introduction
Childhood epilepsies present broad treatment challenges that are unique to this age group. These challenges include the possible diagnoses; the treatment options; the developmental, cognitive, and behavioural comorbidities that accompany epilepsies; and the likelihood that these different factors interact with developmental processes in the young brain.1
The effect of seizures on the developing brain are motivating factors to consider a therapeutic aim not only to achieve overt freedom from seizures, but also to actively abolish abnormal electrical activity (although evidence that this is definitively beneficial is lacking in the majority of patients).2–7 Achievement of these goals while avoiding unacceptable adverse effects from interventions remains an important challenge. In addition, there is evidence that comorbidities such as behavioural problems, learning difficulties and psychiatric manifestations have major effects on the cognitive development of children with epilepsy.2,8–10 For such children, the transition through to adolescence is fraught with its own unique challenges.11
Reorganization of the key concepts and terms in epilepsy diagnosis and treatment should enable consistency across studies and permit practical conclusions to be drawn from meta-analyses, which will lead to more evidence-based research and optimal therapeutic management. Revision of the epilepsy terminology is particularly pertinent to the infantile encephalopathy epilepsy syndromes, which are rare but have a tremendous effect on the individual owing to their associated life-long disability. An increasing number of children are being identified with an underlying genetic cause for their epilepsy; therefore, the potential to develop pharmacogenetic therapies is becoming more realistic.12–15 Nonpharmacological therapies are also becoming accepted and refined; these include interventions such as the ketogenic diet, resective surgical interventions or surgical implants and, most recently, state-of-the-art concepts such as closed-loop stimulation systems to control epileptic seizures.16–21 The selection of patients who might be suitable for epilepsy surgery is being addressed, and surgery is accepted worldwide as an appropriate intervention in carefully selected children across the full age range.22–24
Although advances in treatment have been made in so-called ‘developing nations’, the treatment gap between resource-equipped and resource-poor countries will continue to widen unless evidence-based therapeutic management strategies can be adapted and the capacity to deliver such therapy is addressed in all health-care settings.25 Developing and developed nations have different health-care priorities, which range from the need for primary prevention, to the recognition of seizures, and access to sustainable and appropriate therapy.
This Review aims to address key issues in the global therapeutic management of epilepsy in children, and to illustrate that childhood-onset epilepsy presents a unique set of challenges. The changes to seizure terminology recommended by the International League Against Epilepsy (ILAE) are particularly relevant to childhood epilepsies (Table 1). We also discuss the recommendations for treatment that are difficult to incorporate into clinical practice owing to a lack of evidence-based data—as is often the case in the paediatric age group—and provide a framework for implementation.
Table 1. New terms and concepts in the epilepsies.
| 1989 |
2010 |
||||
|---|---|---|---|---|---|
| Term | Definition | Comment | Term | Definition | Comment |
| Epilepsy or syndrome | |||||
| Idiopathic | No underlying cause, possible hereditary predisposition Defined by age of onset, and clinical and EEG characteristics Presumed genetic aetiology often interpreted to mean benign |
Genetic screening and whole-exome and genome sequencing enable diagnosis Most genetic causes of epilepsy not benign |
Genetic | Core genetic component produces the symptoms of epilepsy, particularly seizures independent of the structure of brain lesions | No implication that these disorders are benign |
| Symptomatic | Consequence of a known or suspected disorder of the CNS | All epilepsies are a symptom of underlying brain disorder 1989 definition is based on degree of disability as a marker of the type of underlying cause, with genetic aetiologies being benign and others less so |
Structural or metabolic | A distinct structural abnormality affects the brain and forms an epileptogenic lesion A chronic metabolic condition contributes to the propensity of patients to experience seizures |
Most of these epilepsies, especially in childhood, have a genetic basis Neuroinflammation is not explicitly addressed; should be considered |
| Cryptogenic | Cause is hidden or occult Presumed to be symptomatic but aetiology is unknown Characterized by age of onset but without well-defined EEG characteristics |
1989 definition is obfuscatory and confusing | Unknown cause | NA | No specific cause identified |
| Seizures | |||||
| Complex partial | Seizures begin in one cerebral hemisphere and involve impairment of consciousness, either from the outset or during the course of the seizure | Consciousness as the primary axis for classifying focal seizures does not have a clear rationale Depends on adequate testing of the patient and can be inconsistently interpreted |
NA | No further interpretation needed Descriptions based on the seizure manifestations;* for example, versive, hemiclonic or hypermotor |
No study to compare the 1989 definition and the proposed ictal semiology has been carried out Anecdotal reports suggest that the descriptive ictal semiology is easier than the old semiology to understand and apply, particularly among students |
| Simple partial | Seizures begin in one cerebral hemisphere and do not involve impairment of consciousness | Many seizures that do not truly impair consciousness may be dramatic and interfere with communication during the ictus | NA | Does not need further interpretation Descriptions based on seizure manifestations* |
No study to compare the 1989 definition and the proposed ictal semiology has been carried out |
The terminology from the 1989 report by the International League Against Epilepsy refers to epilepsy in the singular and makes an assumption that the cause is directly linked to the clinical presentation (that is, either idiopathic or cryptogenic).29
Clearly defined terminology of ictal events is described elsewhere.32 Abbreviation: NA, not applicable.
Classification and terminology
Clear communication is the cornerstone of scientific inquiry and competent clinical practice. The efforts of the ILAE to provide a common language with meaningful concepts were intended to facilitate these purposes. The aims of the ILAE in seizure reclassification and provision of new terminology have been described as follows: “to provide a common international terminology and classification—a precondition for comparability of results in research and therapy and for meaningful exchange of ideas.”26–28
The terms to describe the epilepsies proposed by the ILAE in 2010 led to major improvements in communication among practitioners; however, their value has been outstripped by our growing understanding of the causes and manifestations of epilepsy that is afforded by the tremendous advances in diagnostic technologies. These changes include—but are not limited to—developments in the fields of neuroimaging and genetics. Our understanding of molecular cell biology has enabled us to fill the chasm between the potential underlying structural and genetic mechanisms of many of these disorders. For these reasons, the old seizure terminology and concepts based on our knowledge of epilepsy, which were first developed in the late 1800s, have given way to more-descriptive, clearer terminology and concepts that are more aligned with our increased understanding of the epilepsies and the underlying pathophysiological mechanisms (Table 1). The 2010 report was not intended as a final report, and the terminology will not be finalized until we have learned all there is to know about how the brain works.
The goals of the current changes in classification and terminology are to emphasize the importance of the precise diagnosis of seizures, and the cause and type of epilepsy when it can be identified. For example, a child with epilepsy who exhibits severe developmental delay for which no cause has been found should not be diagnosed as having ‘symptomatic generalized epilepsy’. Instead, the child should be diagnosed with epilepsy of unknown cause (that is, a cause remains to be found) accompanied by severe developmental delay and generalized features. These changes, while not universally welcome, present an opportunity to integrate our increasing knowledge about the pathophysiology of the epilepsies learned in the laboratory with that obtained through research in the context of clinical care, with the overall aim of improving patient care through diagnosis and optimal treatment.
Aetiology-based terminology
The terms ‘idiopathic’ (meaning epilepsy of no known cause but presumed to be genetic), ‘symptomatic’ (meaning epilepsy secondary to a condition affecting the brain), and ‘cryptogenic’ (meaning epilepsy of unknown cause but presumed to be symptomatic) have been abandoned.29 In place of these terms, temporary substitutions have been proposed, including ‘genetic’, ‘structural–metabolic’, and ‘unknown’. Of these, ‘unknown’ is perhaps the most clear and useful term; the other two are a work in progress.30,31
Seizure classification
The concepts of generalized or focal seizures were redefined with respect to the engagement of local unilateral versus bilateral distributed networks. Crucial to this reconceptualization is the recognition that generalized seizures can sometimes be the result of very focal pathology and that focal manifestations can occur in the setting of a diffuse brain disorder. ‘Generalized’ seizures were originally named and represented by their ictal semiology, as recorded on EEG; for example myoclonic or tonic seizures. No major changes have been made to this terminology since its introduction
For focal seizure manifestations, a parallel approach was suggested in which seizures are simply named according to their primary ictal semiology. Thus, the old terms ‘simple’ and ‘complex partial’ seizure have been abandoned, and the use of common and well-defined terms to describe ictal semiology is recommended.32 According to the ILAE recommendations, a clonic seizure restricted to one side of the body should be called a hemiclonic seizure, and a seizure involving tonic eye and head deviation to one side should be referred to as a tonic versive seizure. Previously, either or both might have been called a simple partial seizure, resulting in a loss of diagnostic specificity. The potential improvements in communication and diagnosis are considerable; for example, for determining whether or not an event is an epileptic seizure, as well as the type of seizure and which brain regions are involved. In many ways, descriptive terms, as promoted by the new recommendations, might be much easier to use than the older terms, especially in resource-poor settings where records of seizures may be purely descriptive, and any inferences about impairment of consciousness could be of dubious validity or value.
Generalized and focal epilepsy
The epilepsy itself is a product of the underlying cause. In children, the manifestations of epilepsy, especially the seizures, are expressed in the context of a developing brain and can change over time. In addition, these manifestations (that is, the seizure type) and the underlying causes are not always concordant. For example, Dravet syndrome (previously known as severe myoclonic epilepsy of infancy) is caused by a mutation in the sodium channel gene SCN1A, and the majority of patients present with focal-onset hemiclonic seizures. Dravet syndrome is not a ‘focal epilepsy’ although some of its manifestations are focal in the early course of the disease, and it is not an appropriate target for epilepsy surgery or for common drugs used for treating focal seizures in adults. Similarly, patients with West syndrome (also known as infantile spasms) present with very diffuse, bilateral manifestations on EEG. Individuals with West syndrome should always be evaluated for the possibility of a surgically resectable lesion despite the apparently ‘generalized’ disease manifestations. In either circumstance, it is not helpful to call the epilepsy focal or generalized on the basis of the manifestations, as this approach can lead to therapeutic errors.
Epidemiology and aetiology
In North America and Europe, the highest incidence of epilepsy is in the first year of life, reported at 90–212 per 100,000 people.33–41 This number drops steeply there-after and reaches a low of 20–70 per 100,000 people per year34–37 in later childhood and mid-adolescence, only rising again in the sixth to seventh decade of life. These variations are attributable to age-specific shifts in the underlying causes of epilepsy; similarly, the incidence of epilepsy varies worldwide, owing to differing causes of neurological morbidity.
In 2010, epilepsy was reported to contribute to 0.7% of the global burden of disease,42 with epilepsy in Africa contributing to 0.261% of this burden (or 37% of the total burden of epilepsy).43 These models underestimate the burden of epilepsy in the resource-poor areas of the world, since epilepsies secondary to CNS infections or stroke are not included.42 Furthermore, these data are extrapolated from high-income countries, such as North America, due to the lack of data in the low-income and-middle income countries, such as Malawi. Mortality is high in Africa,44 and data from China (standardized mortality 36.6)45 and Kenya (mortality rate ratio 4.4–22.5)46 indicate that premature mortality is very high, particularly affecting older children and adults.
The prevalence of epilepsy is higher in rural areas than in urban areas.47 Population measures of epilepsy are often inaccurate due to both underdiagnosis and overdiagnosis. On the basis of the substantial gap in knowledge about diagnosis and treatment between many regions, we suspect that the published epidemiological data provide only a rough sense of the frequency of epilepsies worldwide.25 A study in rural Kenya revealed that 89% of children with epilepsy were either undiagnosed or were managed with antiepileptic drugs (AEDs).48 Health-care facilities, and workers, in many resource-poor settings are not equipped to diagnose children with epilepsy.49
The misdiagnosis of epilepsy is of equal importance, with huge negative implications for treatment. Paroxysmal events are often confused with epilepsy in children.50 In a large cohort of infants, 255 of 2,860 (8.9%) had paroxysmal events, whereas only 17 had febrile seizures and two had epilepsy.51 Of 223 children referred to a tertiary centre for investigation of paroxysmal events, 192 (86%) were already receiving AEDs, 87 (39%) of whom were found not to have epilepsy.52
Witness accounts of seizures are essential, and video EEG is the ‘gold standard’ that should, ideally, be implemented in situations where the diagnosis is unclear. One of the almost unnoticed diagnostic revolutions was the introduction of video-EEG monitoring in daily clinical practice, and another key development has been the facility to film seizures with smartphones in everyday nonclinical situations. Given that video EEG is a scarce resource in low-income countries, carers can instead be encouraged to use their cell phones to record events.53 These new approaches further illustrate the diversity of seizure types in childhood epilepsies, enabling better descriptions, classification and reorganization of seizure types and epilepsies.
Aetiological diagnoses
The greatest advance in the management of the childhood epilepsies has been in determination of the underlying cause, aided by imaging and genetics. Advances in MRI have enabled the detection of structural brain malformations in many children with drug-resistant epilepsy. Optimized imaging protocols are now recognized as essential to gain maximal information, particularly in the very young where myelination is incomplete and repeat imaging may be required.54 In older children, magnets of higher strength than standard 1.5 T might enhance the quality of MRI.55 Regardless of the imaging protocol, the acquired images must be reviewed by an experienced neuroradiologist who is aware of the developmental stages of the brain.56 This requirement is particularly relevant in the focal epilepsies, in which subtle focal brain malformations can be amenable to curative surgical resection. More-generalized brain malformations, as well as focal malformations, could contribute to epilepsy syndromes and indicate a subsequent genetic diagnosis.57 Other advanced technologies, such as PET, single-photon emission CT, functional MRI of language and motor activity, and magnetoencephalography, have a specific role in presurgical evaluation rather than any diagnostic function in childhood epilepsies.56
Detailed genetic analysis of pedigrees is revealing an increasing number of genetic causes of epilepsy, specifically in early-onset epileptic encephalopathies58,59 as well as late-onset epilepsies.60 Although few specific treatments are currently available even for epilepsies with a known genetic cause, this situation may change with further advances in basic research. Furthermore, discovery of a genetic cause has substantial benefits, in that it enables genetic counselling for parents who are planning future pregnancies. Dravet syndrome is an excellent example of these advances in mutational analysis. Dravet syndrome is an electroclinical syndrome (a group of clinical entities that are reliably identified by a cluster of electroclinical and developmental characteristics), for which appropriate care pathways are delineated. Up to 80% of patients with this condition have an SCN1A mutation, but in up to 95% of patients this mutation has arisen de novo in the child, with no consequent implications for other family members.61
The potential benefits of mutation analysis to the therapeutic management of patients have been assessed, and the results support early screening in patients with a suspected underlying genetic cause of epilepsy.62 Although specific genotype–phenotype associations are not always possible,63 a positive diagnosis enables acceptance on the part of the families, and helps avoid unnecessary investigations by providing accurate genetic information to the rest of the family (Table 2).62 Examples of epilepsy syndromes for which a genetic basis has been identified are provided in Table 2. A correct genetic diagnosis may also aid selection of the best treatments; for example, a ketogenic diet is the treatment of choice in GLUT1 deficiency syndrome (caused by mutations in SLC2A1),64 and classic sodium channel blockers should be avoided in Dravet syndrome. The role of whole-exome sequencing has yet to be fully realized. The focus of attention in epilepsy, however, is likely to shift from the technical molecular challenges of genetic diagnosis to intelligent interpretation of the genetic or exome analysis results.
Table 2. Typical childhood epilepsy syndromes of genetic origin.
| Syndrome | Gene | Key diagnostic marker or outcome | Comment |
|---|---|---|---|
| Epilepsy and mental retardation157 | PCDH19 | Female epilepsy syndrome—transmitted by unaffected fathers to daughters Onset in infancy, often provoked by fever Seizures occur in clusters Rigid personalities (possible autistic spectrum disorder) Seizures remit in adolescence Intellectual disability and behavioural disturbances are common |
Previously, patients with this mutation were diagnosed with Dravet syndrome—now regarded as a separate entity |
| Infantile spasms (X-linked) | CDKL5158,159 | Female epilepsy syndrome—transmitted by unaffected fathers to daughters Early-onset epileptic encephalopathy before 5 months of age (10 days to 3 weeks of age) Infantile spasms, including multiple seizure types Rett syndrome-like features include hand stereotypies* and deceleration in head growth during early childhood Severe mental retardation (absence of speech) |
Severely affected males reported in some cohorts Phenotype with dysmorphology Mutation type relates to severity of disease58,158 Patients have residual hand function, poor eye fixation with avoidance of eye contact, and feeding difficulties159 |
| STXBP1160 | Short periods of control with AEDs but frequent relapses EESB Rare 10% of patients of patients with EESB Infantile spasms described without preceding EESB Seizure types include tonic seizures, focal and generalized seizures Most patients are refractory to treatment Age of onset 1 day to 6 months of age Severe developmental delay |
Clinical spectrum can be diverse; overlap with Ohtahara syndrome Few cases reported with frontal hypoplasia and thin and dysmorphic corpus callosum Some patients become seizure-free in the first year of life160 |
|
| ARX161,162 | Occurs in boys Early infantile epileptic encephalopathy Ohtahara syndrome Seizure onset in the neonatal period Predominantly tonic spasms Patients eventually develop West syndrome EEG—burst suppression Severe mental retardation and dystonia AED-resistant |
ARX mutations with polyalanine expansion associated with risk of mental retardation, dystonia and epilepsy Severity depends on length of polyalanine tract Brain malformations not detectable on neuroimaging Some males have a micropenis with evidence of delayed puberty |
|
| Malignant migrating partial seizures of infancy163 |
KCNT1 (in up to 50% of patients);164 SCN1A, PLCB1, TBC1D24, SLC25A22163 |
Infantile epileptic encephalopathy syndrome Treatment-resistant Developmental delay Polymorphous epilepsy with symptom onset before 6 months of age Seizure migrates across from different regions of the brain Estimated prevalence 0.11 per 100,000 children |
Expanded criteria includes gut dysmotility Seizure phenotype can include hypsarryhthmia and burst suppression165 KCNT1 genotyping first is recommended; the other mutations should be considered if this result is negative |
Awareness of the typical phenotypes could support targeted genetic analyses in patients with atypical presentation.
Symmetrical movements at the midline.
Abbreviations: AED, antiepileptic drug; EESB, epileptic encephalopathy with suppression bursts.
The possibility that some of the acute-onset encephalopathies associated with epilepsy may have an autoimmune aetiology is increasingly recognized. In the context of acute onset of seizures with encephalopathy, or with a possible movement disorder and/or acute psychiatric disorder, consideration should be given to screening for autoantibodies. An assessment of the clinical phenotype in addition to analysis of serum and cerebrospinal fluid is vital to identify known or novel autoantibodies;65 for example, autoantibodies against the NMDA receptor, or the epilepsy-related proteins LGI1 and Caspr2.66,67 In many patients with a clinical picture indicative of such a disorder, however, no autoantibodies can be detected.66 Other conditions, including fever-induced refractory epileptic encephalopathy and Rasmussen encephalitis, are suspected to have an autoimmune component that is not well-defined.68–70 Children who present with acute-onset encephalopathy and found to have high titres of autoantibodies might respond well to immunosuppressant therapy; however, difficulties emerge in monitoring the relationships between antibody titre and clinical course, and in determining whether or not a clinical response occurs after a reduction in antibody titre.
Interventions
Antiepileptic drugs
In the past 15 years, new AEDs have emerged for the treatment of epilepsy. Regulatory authorities have recognized the need for early assessment of the efficacy of these drugs for the treatment of paediatric epilepsy and, consequently, an increasing number of AEDs are becoming available for the treatment of a younger population. Although some were initially licensed for adults and subsequently for children (for example levetiracetam and topiramate), an increasing number have been licensed for children from the outset (for example rufinamide and perampanel).14
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use has produced guidelines on the clinical investigation of medicinal products in the paediatric population, and it is also recognized that extrapolation of efficacy data to children may be possible when the condition is similar to that in adults; for example, in focal epilepsies.71,72 There has been welcome recognition of the need to use a syndrome-specific efficacy profile as opposed to seizure type to assess AED efficacy in children.73
The study of stiripentol in Dravet syndrome highlights the potential of a small well-designed study in a defined population to demonstrate therapeutic efficacy.74 Stiripentol therapy significantly reduced the number of major convulsive seizures and demonstrated sustained effect in an open-label extension of the trial.75,76 The assessment of rufinamide in Lennox–Gastaut syndrome is another example that might be relevant to first-generation AEDs.77 AEDs in childhood absence epilepsy were assessed in a well-designed randomized controlled trial (RCT), which revealed that ethosuximide was superior to valproate and lamotrigine in terms of therapeutic efficacy and tolerability.73
The identification of genetic mutations has led to the prospect of aetiologically driven treatments. The pathophysiological mechanisms underlying the efficacy of stiripentol in the treatment of Dravet syndrome—that is, whether or not it acts directly or through co-medication with valproate and clobazam—remain unclear. Mutations in the potassium channel gene KCNQ2 result in early-onset epilepsy syndromes ranging from benign neonatal convulsions to severe epileptic encephalopathies.59,78 Retigabine is an AED that modulates the cellular efflux of potassium, rendering the cell less excitable.15,79 Retigabine should, therefore, be considered in these syndromes as a means of restoring normal potassium balance. These AEDs are likely to benefit only a small number of patients and might, therefore, warrant their designation as ‘orphan drugs’ used in the treatment of rare diseases, as defined by the European Medicines Agency.80
Other medications are also reported as being effective with regard to specific epilepsy syndromes. Low-dose fenfluramine—an anti-obesity drug with known adverse cardiac events when used at high doses—has demonstrated long-lasting therapeutic efficacy in Dravet syndrome.81 Verapamil has also been reported to have beneficial effects in patients with Dravet syndrome as an add-on therapy, as have bromides.82,83 There are also anecdotal reports about the efficacious use of cannabinoids in refractory epilepsies.84 Whether or not these therapies are universally effective or only target epilepsies of specific aetiology through their modes of action requires further study. New animal models to evaluate AED modes of action in epilepsies with an underlying genetic cause may help to identify novel therapeutic agents in the future.12,13
Surgical and dietary interventions
Resective surgery is now a well-established option for selected children with drug-resistant focal epilepsy. Surgical candidates in early-onset epilepsy should be readily identified and referred to an appropriate centre with surgical expertise.22 Early surgery in properly evaluated and selected candidates will probably to lead to improved long-term outcomes with regard to cognition and quality of life.23,24,85,86 Epilepsies with certain aetiologies; for example, Rasmussen encephalitis,87 tuberous sclerosis complex,88 Sturge–Weber syndrome, and hypothalamic hamartoma, require specialist evaluation, so as to enable individualized medical decisions regarding if and when surgery might become an option. Technological advances such as improvements in imaging are widening the range of surgical candidates who are being identified by clinicians, although the use of these technologies in children requires continual evaluation, careful thought and expertise.56 Stereotactic laser ablation is reportedly safe and minimally invasive when used to treat patients with hypothalamic-hamartoma-related refractory gelastic epilepsy.89
For drug-resistant paediatric epilepsies that are not amenable to surgical resection, other options include the ketogenic diet. Although this diet has been around for many years, it has recently been demonstrated to be as effective as any new AED in therapy,16 and should be considered earlier rather than later in patients with certain types of epilepsy.17,19 A concern, however, is the availability of dietetic resources required to implement this treatment. Consequently, more-relaxed forms of the diet; for example, a low-glycaemic-index diet, or a modified Atkins diet, have been assessed in retrospective studies and a clinical trial, and were reportedly effective.90,91
Epilepsy is also increasingly recognized as a presentation of glucose transporter defects (and of some mitochondrial disorders such as those related to mitochondrial respiratory chain complex defects),92 for which the ketogenic diet should be considered as the treatment of choice.93,94 Interest is growing in identifying the component responsible for the therapeutic efficacy of dietary modification, perhaps ultimately leading to a less complex dietary change with similar efficacy.95 Many different underlying mechanisms of diet efficacy have been put forward, including reduction of glycolytic flux (leading to decreased cytosolic ATP and disinhibition of ATP-sensitive potassium channels, thereby facilitating potassium conductance), effects on AMPA-receptor trafficking, effects on mTOR signalling pathways, and enhanced purinergic (adenosine) inhibitory transmission.96,97
In patients with refractory epilepsy, vagus nerve stimulation (VNS) is known to have at least the same efficacy as introducing a new AED.98,99 In the large European Cybernics E-102 study of VNS in children with drug-resistant epilepsy, the responder rate after more than 1 year, even when concomitant AEDs were unchanged, was 40% (L. Lagae, unpublished work). The ketogenic diet and VNS should no longer be considered as last-resort treatment options, but can be considered early in the natural history of an epilepsy syndrome, especially if resective surgery is not possible.
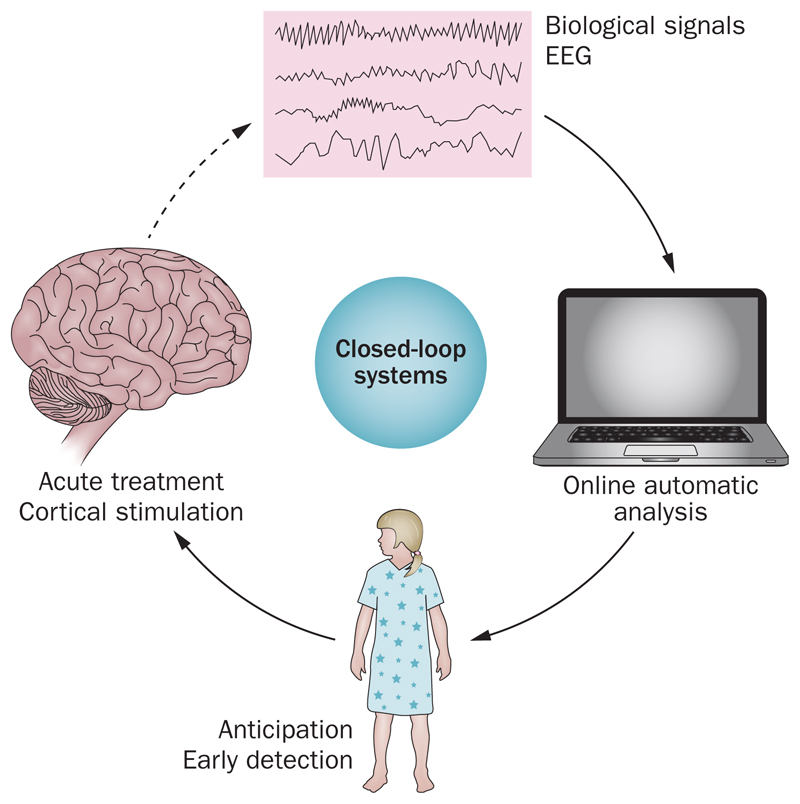
In the future, it is possible that miniature ‘closed-loop systems’ will be developed for the treatment of children with refractory epilepsy (Figure 1). These ‘responsive cortical stimulation’ devices use continuous measurement of intracranial EEG signals so that an imminent seizure is detected with individualized algorithms. After the onset of a seizure is detected, electrical stimulation is applied to the epileptogenic region to abort the seizure.20,22,23 The field of closed-loop treatment for epilepsy in adults is rapidly evolving after the pivotal SANTE trial and the Neuropace trial.18,20,21 These on-demand systems might reduce the need for chronic AED treatment in selected patients.
Figure 1.
Illustration of the mechanism for closed-loop systems. These ‘responsive cortical stimulation’ devices use continuous measurement of intracranial EEG signals so that an imminent seizure is detected with individualized algorithms. After the onset of a seizure is detected electrical stimulation is applied to the epileptogenic region to abort the seizure20,22,23 with immediate effect.
Optogenetic treatment options for seizures are also on the horizon. For example, after transfection of pyramidal cells with inhibitory halorhodopsin (a light-driven microbial chloride pump), a starting seizure can be stopped by light-induced stimulation of these specific cells.100,101 Although only possible in rodents at present, this research tool will probably influence our future therapeutic options.
Concerns in children and adolescents
Comorbidities
Children with epilepsy are at an increased risk of behavioural and cognitive symptoms, which have a prevalence of 23–34% in this patient group.97–102 The factors that contribute to comorbidity are unclear,102–107 but possibilities include preschool-age onset of seizures (especially in infancy), as well as the underlying cause, specific epilepsy syndrome, seizure localization at onset, seizure frequency, EEG abnormalities and treatment.108–111 Comorbidities are more difficult to treat in children with refractory epilepsy, although children whose seizures are controlled are not excluded from such complications.
Poor concentration is common in children with epilepsy: attention deficit hyperactivity disorder (ADHD) affects 12–20% of these individuals.112,113 Data suggest that methylphenidate can effectively be used in the management of ADHD for children with learning disability and ‘difficult-to-treat’ epilepsy, without exacerbation of seizures.108 Approximately 8% of children with autistic spectrum disorders (ASDs) have epilepsy; when combined with intellectual disability, this figure increases to 20%.114,115 Many children with difficult-to-treat epilepsy have characteristic traits of ASDs,116 and shared underlying causes, neuronal circuits and molecular pathways in epilepsy and ASDs are increasingly evident.117–119 Rapamycin therapy in patients with tuberous sclerosis complex blocks the mTOR signalling pathway, altering epileptogenesis and social behaviour.112,118,120
Some behavioural phenotypes are more obvious in some epilepsy syndromes or regional epilepsies than in others, and the link between the epilepsy syndrome and behavioural dysfunction is far from clear in many children. Frontal lobe epilepsy is associated with a high incidence of ADHD,121 whereas patients with juvenile myoclonic epilepsy are more likely to exhibit risk-taking behaviour, which implies frontal lobe dysfunction.122 Functional neuroimaging in these latter patients indicated interictal dysfunction in the dorsolateral prefrontal cortex and the medial prefrontal cortex, which seemed to affect the ability of these patients to learn and regulate their behaviour.122
Another study confirmed that cognitive deficits are frequently evident by the time of onset of childhood epilepsy, but no specific relationship was observed between the epilepsy syndrome and the cognitive profile.123 Depression and anxiety are also recorded in a substantial proportion of children with epilepsy (prevalence in this patient group 23–34% and 16–36%, respectively).124–128 These comorbidities potentially influence therapeutic seizure control and management, as well as prognosis and quality of life.129 A need exists, therefore, for early screening and identification of psychological comorbidities in children with epilepsy.130,131
Multidisciplinary care
Paediatric epilepsies comprise a large number of rare and serious disorders with onset usually in the first few years of life, which are not seen de novo in adult patients. Consequently, the diagnosis and care of these young patients requires a highly specialized level of expertise.132 The therapeutic management of children with epilepsy should be individualized and flexible, should follow an innovative approach for the patient who might present with epilepsy at any time from birth to adolescence, and should be supported by a multidisciplinary team.1,133,134 Seizures at any age, and the medications used to control them, can take their toll on cognitive functions. Seizures in the very young carry an additional set of risks owing to the possibility of interference with the normal processes of brain development during critical periods. This concern relates not only to seizures and seizure activity, but also to many of the medications used to treat seizures.
Children with epilepsy have a range of developmental, cognitive and behavioural comorbidities that can be present from the outset of their epilepsy. Although some of these conditions are also seen in children who are otherwise neurotypical;102,110,135 the risk is increased in children with epilepsy, and has substantial implications for screening in the epilepsy clinic and for the types of services that are included in a comprehensive care model.134,136 The transition of adolescent patients into the adult sector is a huge challenge since they have unique needs, and planning for the transfer should ideally be initiated in late childhood or early adolescence.11,137,138 Patients with intellectual disability are one of the greatest challenges in terms of adaptation to a new health-care environment.139 The transfer process to adult services is poorly established in most centres,137 with some reporting that up to 27% of their paediatric epilepsy service attendees are adults, including elderly patients.140 Adolescent epilepsy services require a multidisciplinary approach to address the legal and psychosocial issues that these young adults face.139 For these clinics to be successful, an epilepsy nurse specialist is essential (Box 1).141,142
Box 1. Model for an adolescent epilepsy clinic*139,141.
Attendees
-
▪
Affected adolescent
-
▪
Primary caregiver—if invited by the patient
Clinicians
-
▪
Paediatric neurologist
-
▪
Adult neurologist
-
▪
Nurse specialist in paediatric epilepsy; an essential role in therapeutic management of epilepsy and life-skills counselling, and crucial to the success of the programme
Venue
-
▪
Adult health-care setting
Clinic frequency
-
▪
Every 2 months—aim for transition to adult care centre after two visits
Referrals
-
▪
Paediatric neurologists, general paediatricians
-
▪
General practitioners only in specific circumstances
Additional support
-
▪
Social workers; community agencies
-
▪
Additional medical advice may be needed from psychologists, pharmacologists and ancillary rehabilitation services
Addendum activities
-
▪
Adolescent group
-
▪
Encourage to meet outside the hospital setting
A large population-based study in Europe and North America, indicated that most deaths in children with epilepsy were not seizure-related.143 However, sudden and seizure-related deaths alone accounted for a doubling of mortality in children with epilepsy relative to the general population. In those with uncomplicated epilepsy, such deaths occurred at rates comparable with leading causes of death in young people, such as road traffic accidents. The need to address sudden unexpected death in epilepsy with carers and patients (where appropriate) was highlighted in this study.
Although many epilepsies do not present in adulthood, patients do eventually enter the adult arena of health care because these conditions are associated with life-long seizures and disability.139,144 Psychiatric and psychological comorbidities in adult patients were noted in one study as major issues in health-care provision.140 Neurologists who treat adult patients should be aware of the possible comorbidities in patients with epilepsy. Many adult patients, worldwide, with early-onset epilepsies will not have benefitted from the emerging era of genetic testing or high-quality neuroimaging. These patients should be considered for further testing if the cause of their epilepsy is unknown. Although the effects of decades of seizures are unlikely to be fully corrected, some improvement in seizure control might be possible if treatments are optimized on the basis of diagnostic findings. At the very least, knowing the underlying cause of epilepsy may help families to understand what has happened and gain some relief from the grief or anxiety that caregivers experience due to the lack of diagnosis.
Guidelines for children with epilepsy
Guidelines are potentially powerful tools to enable informed decisions, to improve patient outcomes and to maximize effective use of limited resources. They can be used to lobby local government representatives for better facilities, and to deliver appropriate health care. The content of guidelines should, therefore, be useful, accurate, and viable across private and public health-care sectors and regionally. The lack of Class I evidence in the paediatric epilepsies means that either no recommendation can be made at all, or working groups must revert to the less accepted method of consensus statements based on ‘expert-opinion’. Some reports only present data, and the reader is encouraged to decide how the findings can influence their best practice.145 This latter approach reduces concerns about the medicolegal implications resulting from rigid guidelines.
Treatment guidelines are irrelevant if they are not read, cannot be implemented or are not appropriate to a specific region.146,147 The carefully devised National Institute for Health and Care Excellence guidelines for the provision and care of children and adults with epilepsy and their carers have received substantial scrutiny and, as a result, adaptations to the implementation of these guidelines have been reviewed (Box 2).50,148
Box 2. Strategies to improve the therapeutic management of epilepsy in children.
-
▪
Promote guidelines to decision makers:166 clinicians, public health officers, opinion leaders and policymakers.156 Promote guidelines as an ongoing process:167 educational materials, educational meetings with care providers, educational outreach and consensus processes
-
▪
Patient-directed interventions, financial interventions, organizational interventions, structural interventions and regulatory interventions
-
▪
Audit and feedback, reminders to care providers, mass media and marketing
-
▪
Implementation of the guidelines at a local level:168 local adaptation of guidelines, implementation, impact-assessment and programme revision
-
▪
Identify and ameliorate sociocultural and financial barriers
-
▪
Health-care authorities at the national, provincial, district and institutional levels strongly advocate adoption of guidelines
-
▪
Collaboration with local stakeholders; for example, traditional healers in resource-poor countries, are essential to develop programmes and evaluate progress
-
▪
Address barriers at the patient, health-care worker and population level that threaten the implementation of guidelines
RCTs are frequently funded by pharmaceutical companies and focus on next-generation drugs. Trials of drug efficacy have been undertaken primarily to attain a licence for clinical use and, are therefore, superiority trials assessing drug efficacy against a placebo. Novel therapeutic agents tend not to have been compared with the agents used in routine practice, although regulatory bodies are beginning to address this deficit. Although there are a lack of studies comparing next-generation AEDs with phenobarbitone or phenytoin there are a few high-quality studies examing the roles of the latter two agents.149,150
Informed decisions about therapy are driven by evidence-based medicine, which is established from the current RCTs. These data, however, do not necessarily reflect optimal interventions, or the day-to-day clinical situations and challenges that most clinicians face. For example, most RCTs use a 50% reduction in seizures as the primary end point, but the treating clinician must adjust these outcomes to include the various comorbidities in their patients. New methods and rigorously designed studies are essential to answer key therapeutic questions in relation to paediatric epilepsies. RCTs are severely limited due to highly selected study groups that must meet strict entry criteria and, as such, are not representative of typical patients in whom treatments might be applicable. In addition, the focus of studies is on the short-term proxy outcome (for example, a 50% reduction in seizure frequency) and not on the outcomes that might be more meaningful to patients (for example, complete freedom from seizures). In other rare diseases, different methods, such as genotyping, have provided high-quality data, as well as generating the information needed to address complex clinical issues and to find the underlying mechanisms of disease.146,151
In many resource-poor countries, treatment guidelines are impossible to follow, owing to a lack of clinical skills (training), and the fact that many recommend the use of tools that are scare, such as EEG or neuroimaging.152,153 The recommended AEDs are often not available, or the supply is unreliable. This ‘treatment gap’ for epilepsy can be defined as the number of people with the disease who need treatment but do not get it, expressed as a percentage of the number of people with active epilepsy.154 The treatment gap in rural areas of resource-poor countries is 73.3%, and in urban regions is 46.8%.25,155 Potentially, this dichotomy leads to two sets of clinical practice guidelines and recommendations, one for a well-equipped and the other for a resource-poor health-care system. Ethically, this situation places children in resource-poor settings at an unacceptable disadvantage in terms of health care. Flexible guidelines are required in these settings, and the cautious incorporation of low-quality evidence and adaptation of the existing guidelines to be in line with local capacity have been proposed as a measures to improve health care.156
Conclusions
Children bear the brunt of epilepsy, with most epilepsies starting in childhood and the highest incidence being observed during infancy. The burden is greater for patients in low-income and middle-income countries due to a lack of education opportunities, overt stigma and high mortality. Many epilepsy syndromes are found only in children and, therefore, require paediatric-specific interventions. The explosion in development of genomic approaches and more-sophisticated neuroimaging techniques has enabled the cause of epilepsy to be elucidated in many instances, and these findings might lead to more-effective therapies for different forms of epilepsy targeted to the individual child. Further research to prevent epilepsy in children in low-income and middle-income countries and to reduce the treatment gap is urgently needed. The psychological and social implications of epilepsy also require further research to improve quality of life for patients and their carers. In particular, multidisciplinary care needs to be addressed. Guidelines for the therapeutic management of patients with epilepsy must be effective and feasible across resource-equipped and resource-poor settings. Ultimately, most children with epilepsy grow into adults with epilepsy; therefore, the approach to the treatment of epilepsies in children is applicable to these patients as adults. In general, the challenges of therapeutic management in paediatric epilepsy provide valuable lessons that can be applied to the adult population.
Key points.
-
▪
Information on the aetiology of epilepsies in children can be used to direct optimal treatments
-
▪
Genetic analysis, neuroimaging and well-designed clinical trials can help to inform clinicians about therapeutic management of paediatric epilepsy
-
▪
The availability of therapies and implementation of treatment guidelines differs between resource-poor and resource-rich countries
-
▪
Comprehensive treatment options for paediatric patients with epilepsy should include antiepileptic drugs, a ketogenic diet, vagus nerve stimulation with surgery
-
▪
Behavioural and cognitive problems frequently occur as comorbidities in children with epilepsy
Footnotes
Author contributions
J.M.W., A.T.B. and J.H.C. researched the data for the manuscript, and reviewed and edited the manuscript before final submission. J.M.W., A.T.B., L.L. and J.H.C. provided substantial contributions to discussion and writing of the content. C.R.N. contributed to writing of the article.
Competing interests
The authors declare no competing interests.
Contributor Information
Jo M. Wilmshurst, Red Cross War Memorial Children’s Hospital, University of Cape Town, Rondebosch 7700, South Africa
Anne T. Berg, Ann & Robert H. Lurie Children’s Hospital of Chicago, 225 East Chicago Avenue, Chicago, IL 60611, USA
Lieven Lagae, Department of Pediatric Neurology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Charles R. Newton, Centre for Geographic Medicine Research–Coast, Kenya Medical Research Institute, PO Box 230, Kilifi 80108, Kenya
J. Helen Cross, UCL Institute of Child Health, 4/5 Long Yard, London WC1N 3LU, UK.
References
- 1.Cross JH, Kluger G, Lagae L. Advancing the management of childhood epilepsies. Eur J Paediatr Neurol. 2013;17:334–347. doi: 10.1016/j.ejpn.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Mlsna LM, Koh S. Maturation-dependent behavioral deficits and cell injury in developing animals during the subacute postictal period. Epilepsy Behav. 2013;29:190–197. doi: 10.1016/j.yebeh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paolicchi JM. The timing of pediatric epilepsy syndromes: what are the developmental triggers? Ann N Y Acad Sci. 2013;1304:45–51. doi: 10.1111/nyas.12307. [DOI] [PubMed] [Google Scholar]
- 4.Sankar R, Rho JM. Do seizures affect the developing brain? Lessons from the laboratory. J Child Neurol. 2007;22:21S–29S. doi: 10.1177/0883073807303072. [DOI] [PubMed] [Google Scholar]
- 5.Holmes GL. EEG abnormalities as a biomarker for cognitive comorbidities in pharmacoresistant epilepsy. Epilepsia. 2013;54(Suppl. 2):60–62. doi: 10.1111/epi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldenkamp AP. Effect of seizures and epileptiform discharges on cognitive function. Epilepsia. 1997;38(Suppl 1):S52–S55. doi: 10.1111/j.1528-1157.1997.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 7.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–730. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 8.Fournier NM, Botterill JJ, Marks WN, Guskjolen AJ, Kalynchuk LE. Impaired recruitment of seizure-generated neurons into functional memory networks of the adult dentate gyrus following long-term amygdala kindling. Exp Neurol. 2013;244:96–104. doi: 10.1016/j.expneurol.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Berg AT, et al. Longitudinal assessment of adaptive behavior in infants and young children with newly diagnosed epilepsy: influences of etiology, syndrome, and seizure control. Pediatrics. 2004;114:645–650. doi: 10.1542/peds.2003-1151-L. [DOI] [PubMed] [Google Scholar]
- 10.Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012;79:1384–1391. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camfield P, Camfield C, Pohlmann-Eden B. Transition from pediatric to adult epilepsy care: a difficult process marked by medical and social crisis. Epilepsy Curr. 2012;12:13–21. doi: 10.5698/1535-7511-12.4s.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4 doi: 10.1038/ncomms3410. 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortopan GA, Dinday MT, Baraban SC. Zebrafish as a model for studying genetic aspects of epilepsy. Dis Model Mech. 2010;3:144–148. doi: 10.1242/dmm.002139. [DOI] [PubMed] [Google Scholar]
- 14.Kayani S, Sirsi D. The safety and tolerability of newer antiepileptic drugs in children and adolescents. J Cent Nerv Syst Dis. 2012;4:51–63. doi: 10.4137/JCNSD.S5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Large CH, et al. The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia. 2012;53:425–436. doi: 10.1111/j.1528-1167.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 16.Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 17.Oguni H, et al. Treatment and long-term prognosis of myoclonic-astatic epilepsy of early childhood. Neuropediatrics. 2002;33:122–132. doi: 10.1055/s-2002-33675. [DOI] [PubMed] [Google Scholar]
- 18.Morrell MJ, RNS System in Epilepsy Study Group Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 19.Caraballo RH, et al. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005;46:1539–1544. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 20.Cook MJ, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12:563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- 21.Fisher R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 22.Cross JH, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the subcommission for pediatric epilepsy surgery. Epilepsia. 2006;47:952–959. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 23.Loddenkemper T, et al. Developmental outcome after epilepsy surgery in infancy. Pediatrics. 2007;119:930–935. doi: 10.1542/peds.2006-2530. [DOI] [PubMed] [Google Scholar]
- 24.Moosa AN, et al. Long-term functional outcomes and their predictors after hemispherectomy in 115 children. Epilepsia. 2013;54:1771–1779. doi: 10.1111/epi.12342. [DOI] [PubMed] [Google Scholar]
- 25.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whorf BL. Language, Thought, and Reality: Selected Writings of Benjamin Lee Whorf. The Technology Press of Massachusetts Institute of Technology/John Wiley & Sons, Inc; 1956. [Google Scholar]
- 27.Merlis JK. Proposal for an international classification of the epilepsies. Epilepsia. 1970;11:114–119. doi: 10.1111/j.1528-1157.1970.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 28.Dreifuss FE. Classification of the epilepsies: influence on management. Rev Neurol (Paris) 1987;143:375–380. [PubMed] [Google Scholar]
- 29.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 30.Berg AT, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 31.Berg AT, Cross JH. Towards a modern classification of the epilepsies? Lancet Neurol. 2010;9:459–461. doi: 10.1016/S1474-4422(10)70024-7. [DOI] [PubMed] [Google Scholar]
- 32.Blume WT, et al. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 33.Dura-Trave T, Yoldi-Petri ME, Gallinas-Victoriano F. Incidence of epilepsies and epileptic syndromes among children in Navarre, Spain: 2002 through 2005. J Child Neurol. 2008;23:878–882. doi: 10.1177/0883073808314898. [DOI] [PubMed] [Google Scholar]
- 34.Eltze CM, et al. A population-based study of newly diagnosed epilepsy in infants. Epilepsia. 2013;54:437–445. doi: 10.1111/epi.12046. [DOI] [PubMed] [Google Scholar]
- 35.Freitag CM, May TW, Pfafflin M, Konig S, Rating D. Incidence of epilepsies and epileptic syndromes in children and adolescents: a population-based prospective study in Germany. Epilepsia. 2001;42:979–985. doi: 10.1046/j.1528-1157.2001.042008979.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz Z, Tookey P, Ross E. Epilepsy in young people: 23 year follow up of the British National Child Development Study. BMJ. 1998;316:339–342. doi: 10.1136/bmj.316.7128.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 38.Doose H, Sitepu B. Childhood epilepsy in a German city. Neuropediatrics. 1983;14:220–224. doi: 10.1055/s-2008-1059582. [DOI] [PubMed] [Google Scholar]
- 39.Granieri E, et al. A descriptive study of epilepsy in the district of Copparo, Italy, 1964–1978. Epilepsia. 1983;24:502–514. doi: 10.1111/j.1528-1157.1983.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 40.Olafsson E, et al. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4:627–634. doi: 10.1016/S1474-4422(05)70172-1. [DOI] [PubMed] [Google Scholar]
- 41.Verity CM, Ross EM, Golding J. Epilepsy in the first 10 years of life: findings of the child health and education study. BMJ. 1992;305:857–861. doi: 10.1136/bmj.305.6858.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 43.GBD Compare. Institute for Health Metrics and Evaluation. 2013 [online], http://viz.healthmetricsandevaluation.org/gbd-compare/
- 44.Diop AG, Hesdorffer DC, Logroscino G, Hauser WA. Epilepsy and mortality in Africa: a review of the literature. Epilepsia. 2005;46(Suppl 11):33–35. doi: 10.1111/j.1528-1167.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 45.Ding D, et al. Premature mortality risk in people with convulsive epilepsy: long follow-up of a cohort in rural China. Epilepsia. 2013;54:512–517. doi: 10.1111/epi.12048. [DOI] [PubMed] [Google Scholar]
- 46.Ngugi AK, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–263. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mung’ala-Odera V, et al. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure. 2008;17:396–404. doi: 10.1016/j.seizure.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter JA, et al. The reasons for the epilepsy treatment gap in Kilifi, Kenya: using formative research to identify interventions to improve adherence to antiepileptic drugs. Epilepsy Behav. 2012;25:614–621. doi: 10.1016/j.yebeh.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. NICE Clinical Guidelines. 2013 [online], http://www.nice.org.uk/nicemedia/live/13635/57779/57779.pdf.
- 51.Visser AM, et al. Paroxysmal disorders in infancy and their risk factors in a population-based cohort: the Generation R. Study. Dev Med Child Neurol. 2010;52:1014–1020. doi: 10.1111/j.1469-8749.2010.03689.x. [DOI] [PubMed] [Google Scholar]
- 52.Uldall P, Alving J, Hansen LK, Kibaek M, Buchholt J. The misdiagnosis of epilepsy in children admitted to a tertiary epilepsy centre with paroxysmal events. Arch Dis Child. 2006;91:219–221. doi: 10.1136/adc.2004.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeiler SR, Kaplan PW. Our digital world: camera phones and the diagnosis of a seizure. Lancet. 2009;373:2136. doi: 10.1016/S0140-6736(09)60304-4. [DOI] [PubMed] [Google Scholar]
- 54.Gaillard WD, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50:2147–2153. doi: 10.1111/j.1528-1167.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- 55.Craven IJ, et al. 3.0 T MRI of 2000 consecutive patients with localisation-related epilepsy. Br J Radiol. 2012;85:1236–1242. doi: 10.1259/bjr/30177037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayakar P, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. doi: 10.1111/epi.12544. [DOI] [PubMed] [Google Scholar]
- 57.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahi-Buisson N, et al. Recurrent mutations in the CDKL5 gene: genotype–phenotype relationships. Am J Med Genet A. 2012;158A:1612–1619. doi: 10.1002/ajmg.a.35401. [DOI] [PubMed] [Google Scholar]
- 59.Weckhuysen S, et al. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81:1697–1703. doi: 10.1212/01.wnl.0000435296.72400.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carvill GL, et al. GRIN2A mutations cause epilepsy–aphasia spectrum disorders. Nat Genet. 2013;45:1073–1076. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harkin LA, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–852. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 62.Brunklaus A, et al. The clinical utility of an SCN1A genetic diagnosis in infantile-onset epilepsy. Dev Med Child Neurol. 2013;55:154–161. doi: 10.1111/dmcn.12030. [DOI] [PubMed] [Google Scholar]
- 63.Zuberi SM, et al. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology. 2011;76:594–600. doi: 10.1212/WNL.0b013e31820c309b. [DOI] [PubMed] [Google Scholar]
- 64.Klepper J. GLUT1 deficiency syndrome in clinical practice. Epilepsy Res. 2012;100:272–277. doi: 10.1016/j.eplepsyres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hacohen Y, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84:748–755. doi: 10.1136/jnnp-2012-303807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irani SR, Bien CG, Lang B. Autoimmune epilepsies. Curr Opin Neurol. 2011;24:146–153. doi: 10.1097/WCO.0b013e3283446f05. [DOI] [PubMed] [Google Scholar]
- 68.Illingworth MA, et al. Elevated VGKC-complex antibodies in a boy with fever-induced refractory epileptic encephalopathy in school-age children (FIRES) Dev Med Child Neurol. 2011;53:1053–1057. doi: 10.1111/j.1469-8749.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 69.Nabbout R. Autoimmune and inflammatory epilepsies. Epilepsia. 2012;53(Suppl 4):58–62. doi: 10.1111/j.1528-1167.2012.03614.x. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi Y, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRε2 in patients with Rasmussen’s encephalitis and chronic progressive epilepsia partialis continua. Epilepsia. 2005;46(Suppl 5):152–158. doi: 10.1111/j.1528-1167.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 71.Guidance for industry. FDA. 2000. E11 clinical investigation of medicinal products in the pediatric population. [online], http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073143.pdf. [PubMed] [Google Scholar]
- 72.Chiron C, Dulac O, Pons G. Antiepileptic drug development in children: considerations for a revisited strategy. Drugs. 2008;68:17–25. doi: 10.2165/00003495-200868010-00002. [DOI] [PubMed] [Google Scholar]
- 73.Glauser TA, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–799. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiron C, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356:1638–1642. doi: 10.1016/s0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- 75.Wirrell EC, et al. Stiripentol in Dravet syndrome: results of a retrospective U. S. study. Epilepsia. 2013;54:1595–1604. doi: 10.1111/epi.12303. [DOI] [PubMed] [Google Scholar]
- 76.Thanh TN, et al. Long-term efficacy and tolerance of stiripentaol in severe myoclonic epilepsy of infancy (Dravet’s syndrome) Arch Pediatr. 2002;9:1120–1127. doi: 10.1016/s0929-693x(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 77.Glauser T, et al. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70:1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 78.Kato M, et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–1287. doi: 10.1111/epi.12200. [DOI] [PubMed] [Google Scholar]
- 79.Miceli F, et al. Genotype–phenotype correlations in neonatal epilepsies caused by mutations in the voltage sensor of Kv7.2 potassium channel subunits. Proc Natl Acad Sci USA. 2013;110:4386–4391. doi: 10.1073/pnas.1216867110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.European Medicines Agency. [online], http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000029.jsp.
- 81.Ceulemans B, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53:1131–1139. doi: 10.1111/j.1528-1167.2012.03495.x. [DOI] [PubMed] [Google Scholar]
- 82.Lotte J, Haberlandt E, Neubauer B, Staudt M, Kluger GJ. Bromide in patients with SCN1A-mutations manifesting as Dravet syndrome. Neuropediatrics. 2012;43:17–21. doi: 10.1055/s-0032-1307454. [DOI] [PubMed] [Google Scholar]
- 83.Nicita F, et al. Efficacy of verapamil as an adjunctive treatment in children with drug-resistant epilepsy: a pilot study. Seizure. 2014;23:36–40. doi: 10.1016/j.seizure.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–577. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46:561–567. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- 86.Jonas R, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–750. doi: 10.1212/01.WNL.0000151970.29205.70. [DOI] [PubMed] [Google Scholar]
- 87.Varadkar S, et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jansen FE, van Huffelen AC, Algra A, van Nieuwenhuizen O. Epilepsy surgery in tuberous sclerosis: a systematic review. Epilepsia. 2007;48:1477–1484. doi: 10.1111/j.1528-1167.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 89.Wilfong AA, Curry DJ. Hypothalamic hamartomas: optimal approach to clinical evaluation and diagnosis. Epilepsia. 2013;54(Suppl. 9):109–114. doi: 10.1111/epi.12454. [DOI] [PubMed] [Google Scholar]
- 90.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: a randomized controlled trial. Epilepsia. 2013;54:481–486. doi: 10.1111/epi.12069. [DOI] [PubMed] [Google Scholar]
- 91.Muzykewicz DA, et al. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–1126. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 92.Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–88. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 93.Suls A, et al. Early-onset absence epilepsy caused by mutations in the glucose transporter GLUT1. Ann Neurol. 2009;66:415–419. doi: 10.1002/ana.21724. [DOI] [PubMed] [Google Scholar]
- 94.Mullen SA, et al. Glucose transporter 1 deficiency as a treatable cause of myoclonic astatic epilepsy. Arch Neurol. 2011;68:1152–1155. doi: 10.1001/archneurol.2011.102. [DOI] [PubMed] [Google Scholar]
- 95.Chang P, et al. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology. 2013;69:105–114. doi: 10.1016/j.neuropharm.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28:1027–1033. doi: 10.1177/0883073813487598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masino SA, Rho JM. Mechanisms of ketogenic diet action. In: Noebels JL, et al., editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th edn. Bethesda National Center for Biotechnology Information; 2012. [PubMed] [Google Scholar]
- 98.Morris GL, 3rd, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:1453–1459. doi: 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terra VC, et al. Vagus nerve stimulation in pediatric patients: is it really worthwhile? Epilepsy Behav. 2013;31:329–333. doi: 10.1016/j.yebeh.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 100.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4 doi: 10.1038/ncomms2376. 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paz JT, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Austin JK, et al. Behavior problems in children at time of first recognized seizure and changes over the following 3 years. Epilepsy Behav. 2011;21:373–381. doi: 10.1016/j.yebeh.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caplan R, et al. Psychopathology and pediatric complex partial seizures: seizure-related, cognitive, and linguistic variables. Epilepsia. 2004;45:1273–1281. doi: 10.1111/j.0013-9580.2004.58703.x. [DOI] [PubMed] [Google Scholar]
- 104.Ott D, et al. Behavioral disorders in pediatric epilepsy: unmet psychiatric need. Epilepsia. 2003;44:591–597. doi: 10.1046/j.1528-1157.2003.25002.x. [DOI] [PubMed] [Google Scholar]
- 105.Oostrom KJ, et al. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 106.Oostrom KJ, et al. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only” —a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 107.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Cognition, academic achievement, language, and psychopathology in pediatric chronic epilepsy: short-term outcomes. Epilepsy Behav. 2010;18:211–217. doi: 10.1016/j.yebeh.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fosi T, Lax-Pericall MT, Scott RC, Neville BG, Aylett SE. Methylphenidate treatment of attention deficit hyperactivity disorder in young people with learning disability and difficult-to-treat epilepsy: evidence of clinical benefit. Epilepsia. 2013;54:2071–2081. doi: 10.1111/epi.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl. 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- 110.Berg AT. Epilepsy, cognition, and behavior: the clinical picture. Epilepsia. 2011;52(Suppl. 1):7–12. doi: 10.1111/j.1528-1167.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Helmstaedter C, et al. Disentangling the relationship between epilepsy and its behavioral comorbidities—the need for prospective studies in new-onset epilepsies. Epilepsy Behav. 2014;31:43–47. doi: 10.1016/j.yebeh.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 112.Pineda E, et al. Behavioral impairments in rats with chronic epilepsy suggest comorbidity between epilepsy and attention deficit/hyperactivity disorder. Epilepsy Behav. 2014;31:267–275. doi: 10.1016/j.yebeh.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaufmann R, Goldberg-Stern H, Shuper A. Attention-deficit disorders and epilepsy in childhood: incidence, causative relations and treatment possibilities. J Child Neurol. 2009;24:727–733. doi: 10.1177/0883073808330165. [DOI] [PubMed] [Google Scholar]
- 114.Kantzer AK, Fernell E, Gillberg C, Miniscalco C. Autism in community pre-schoolers: developmental profiles. Res Dev Disabil. 2013;34:2900–2908. doi: 10.1016/j.ridd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 115.Tuchman R. Autism and social cognition in epilepsy: implications for comprehensive epilepsy care. Curr Opin Neurol. 2013;26:214–218. doi: 10.1097/WCO.0b013e32835ee64f. [DOI] [PubMed] [Google Scholar]
- 116.Matsuo M, Maeda T, Sasaki K, Ishii K, Hamasaki Y. Frequent association of autism spectrum disorder in patients with childhood onset epilepsy. Brain Dev. 2010;32:759–763. doi: 10.1016/j.braindev.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Tuchman R, Moshe SL, Rapin I. Convulsing toward the pathophysiology of autism. Brain Dev. 2009;31:95–103. doi: 10.1016/j.braindev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang JQ, Barnes G. A common susceptibility factor of both autism and epilepsy: functional deficiency of GABAA receptors. J Autism Dev Disord. 2013;43:68–79. doi: 10.1007/s10803-012-1543-7. [DOI] [PubMed] [Google Scholar]
- 119.Pineda E, et al. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann Neurol. 2013;74:11–19. doi: 10.1002/ana.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol. 2012;22:895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang DQ, Li FH, Zhu XB, Sun RP. Clinical observations on attention-deficit hyperactivity disorder (ADHD) in children with frontal lobe epilepsy. J Child Neurol. 2014;29:54–57. doi: 10.1177/0883073812470004. [DOI] [PubMed] [Google Scholar]
- 122.Wandschneider B, et al. Risk-taking behavior in juvenile myoclonic epilepsy. Epilepsia. 2013;54:2158–2165. doi: 10.1111/epi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson DC, et al. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr. 2013;162:1047–1053.e1. doi: 10.1016/j.jpeds.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunn DW, Austin JK, Huster GA. Symptoms of depression in adolescents with epilepsy. J Am Acad Child Adolesc Psychiatry. 1999;38:1132–1138. doi: 10.1097/00004583-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 125.Ettinger AB, et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39:595–599. doi: 10.1111/j.1528-1157.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 126.Jones JE, et al. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 127.Williams J, et al. Anxiety in children with epilepsy. Epilepsy Behav. 2003;4:729–732. doi: 10.1016/j.yebeh.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 128.Mazarati A, Shin D, Auvin S, Caplan R, Sankar R. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007;10:377–383. doi: 10.1016/j.yebeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guilfoyle SM, Wagner JL, Smith G, Modi AC. Early screening and identification of psychological comorbidities in pediatric epilepsy is necessary. Epilepsy Behav. 2012;25:495–500. doi: 10.1016/j.yebeh.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 130.Hesdorffer DC, et al. Risk factors for febrile status epilepticus: a case–control study. J Pediatr. 2013;163:1147–1151.e1. doi: 10.1016/j.jpeds.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Screening for suicidal ideation in children with epilepsy. Epilepsy Behav. 2013;29:521–526. doi: 10.1016/j.yebeh.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 133.Berg AT, Baca CB, Loddenkemper T, Vickrey BG, Dlugos D. Priorities in pediatric epilepsy research: improving children’s futures today. Neurology. 2013;81:1166–1175. doi: 10.1212/WNL.0b013e3182a55fb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hamiwka LD, Singh N, Niosi J, Wirrell EC. Diagnostic inaccuracy in children referred with “first seizure”: role for a first seizure clinic. Epilepsia. 2007;48:1062–1066. doi: 10.1111/j.1528-1167.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 135.Austin JK, et al. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- 136.Fisher RS, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24:304–310. doi: 10.1016/j.yebeh.2012.04.128. [DOI] [PubMed] [Google Scholar]
- 137.Iyer A, Appleton R. Transitional services for adolescents with epilepsy in the U.K.: a survey. Seizure. 2013;22:433–437. doi: 10.1016/j.seizure.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 138.Thomson L, Fayed N, Sedarous F, Ronen GM. Life quality and health in adolescents and emerging adults with epilepsy during the years of transition: a scoping review. Dev Med Child Neurol. doi: 10.1111/dmcn.12335. [DOI] [PubMed] [Google Scholar]
- 139.Camfield PR, Gibson PA, Douglass LM. Strategies for transitioning to adult care for youth with Lennox–Gastaut syndrome and related disorders. Epilepsia. 2011;52(Suppl. 5):21–27. doi: 10.1111/j.1528-1167.2011.03179.x. [DOI] [PubMed] [Google Scholar]
- 140.Taniguchi G, Watanabe M, Watanabe Y, Okazaki M, Murata Y. Report of study on transitional medicine for epilepsy: questionnaire survey of pediatric neurologists. No To Hattatsu. 2012;44:311–314. [PubMed] [Google Scholar]
- 141.Appleton RE, Chadwick D, Sweeney A. Managing the teenager with epilepsy: paediatric to adult care. Seizure. 1997;6:27–30. doi: 10.1016/s1059-1311(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 142.Jurasek L, Ray L, Quigley D. Development and implementation of an adolescent epilepsy transition clinic. J Neurosci Nurs. 2010;42:181–189. doi: 10.1097/jnn.0b013e3181e26be6. [DOI] [PubMed] [Google Scholar]
- 143.Berg AT, et al. Mortality risks in new-onset childhood epilepsy. Pediatrics. 2013;132:124–131. doi: 10.1542/peds.2012-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nolan K, Camfield CS, Camfield PR. Coping with a child with Dravet syndrome: insights from families. J Child Neurol. 2008;23:690–694. doi: 10.1177/0883073808314162. [DOI] [PubMed] [Google Scholar]
- 145.Glauser T, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 146.Vickrey BG, Hirtz D, Waddy S, Cheng EM, Johnston SC. Comparative effectiveness and implementation research: directions for neurology. Ann Neurol. 2012;71:732–742. doi: 10.1002/ana.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leviton A, Loddenkemper T, Pomeroy SL. Clinical practice guidelines and practice parameters for the child neurologist. J Child Neurol. 2013;28:917–925. doi: 10.1177/0883073813483362. [DOI] [PubMed] [Google Scholar]
- 148.Dunkley C, Cross JH. NICE guidelines and the epilepsies: how should practice change? Arch Dis Child. 2006;91:525–528. doi: 10.1136/adc.2005.080036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nolan SJ, Tudur Smith C, Pulman J, Marson AG. Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalised onset tonic–clonic seizures. Cochrane Database of Systematic Reviews. 2013;(1) doi: 10.1002/14651858.CD002217.pub2. Art. No.: CD002217. [DOI] [PubMed] [Google Scholar]
- 150.Pal DK, Das T, Chaudhury G, Johnson AL, Neville BG. Randomised controlled trial to assess acceptability of phenobarbital for childhood epilepsy in rural India. Lancet. 1998;351:19–23. doi: 10.1016/S0140-6736(97)06250-8. [DOI] [PubMed] [Google Scholar]
- 151.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirtz D, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, The Child Neurology Society, and The American Epilepsy Society. Neurology. 2000;55:616–623. doi: 10.1212/wnl.55.5.616. [DOI] [PubMed] [Google Scholar]
- 153.Gaillard WD, et al. Epilepsy imaging study guideline criteria: commentary on diagnostic testing study guidelines and practice parameters. Epilepsia. 2011;52:1750–1756. doi: 10.1111/j.1528-1167.2011.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kale R. Global campaign against epilepsy: the treatment gap. Epilepsia. 2002;43(Suppl. 6):31–33. doi: 10.1046/j.1528-1157.43.s.6.13.x. [DOI] [PubMed] [Google Scholar]
- 155.Wilmshurst JM, et al. Child neurology services in Africa. J Child Neurol. 2011;26:1555–1563. doi: 10.1177/0883073811420601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.English M, Opiyo N. Getting to grips with GRADE-perspective from a low-income setting. J Clin Epidemiol. 2011;64:708–710. doi: 10.1016/j.jclinepi.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.van Harssel JJ, et al. Clinical and genetic aspects of PCDH19-related epilepsy syndromes and the possible role of PCDH19 mutations in males with autism spectrum disorders. Neurogenetics. 2013;14:23–34. doi: 10.1007/s10048-013-0353-1. [DOI] [PubMed] [Google Scholar]
- 158.Bahi-Buisson N, Bienvenu T. CDKL5-related disorders: from clinical description to molecular genetics. Mol Syndromol. 2012;2:137–152. doi: 10.1159/000331333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kilstrup-Nielsen C, et al. What we know and would like to know about CDKL5 and its involvement in epileptic encephalopathy. Neural Plast. 2012;2012 doi: 10.1155/2012/728267. 728267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barcia G, et al. Early epileptic encephalopathies associated with STXBP1 mutations: could we better delineate the phenotype? Eur J Med Genet. 2014;57:15–20. doi: 10.1016/j.ejmg.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 161.Kato M, Koyama N, Ohta M, Miura K, Hayasaka K. Frameshift mutations of the ARX gene in familial Ohtahara syndrome. Epilepsia. 2010;51:1679–1684. doi: 10.1111/j.1528-1167.2010.02559.x. [DOI] [PubMed] [Google Scholar]
- 162.Kato M, et al. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome) Am J Hum Genet. 2007;81:361–366. doi: 10.1086/518903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Striano P, Coppola G, Zara F, Nabbout R. Genetic heterogeneity in malignant migrating partial seizures of infancy. Ann Neurol. doi: 10.1002/ana.24061. [DOI] [PubMed] [Google Scholar]
- 164.Barcia G, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McTague A, et al. Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum. Brain. 2013;136:1578–1591. doi: 10.1093/brain/awt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fretheim A, Schunemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 15. Disseminating and implementing guidelines. Health Res Policy Syst. 2006;4:27. doi: 10.1186/1478-4505-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grimshaw J, Eccles M, Tetroe J. Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof. 2004;24(Suppl. 1):S31–S37. doi: 10.1002/chp.1340240506. [DOI] [PubMed] [Google Scholar]
- 168.Katchanov J, Birbeck GL. Epilepsy care guidelines for low- and middle-income countries: from WHO mental health GAP to national programs. BMC Med. 2012;10:107. doi: 10.1186/1741-7015-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]