Abstract
Objective
Acute gastroenteritis (AGE) is a major cause of child mortality and morbidity. This study aimed at systematically reviewing clinical practice guidelines (CPGs) on AGE to compare recommendations and provide the basis for developing single universal guidelines.
Methods
CPGs were identified by searching MEDLINE, Cochrane-Library, National Guideline Clearinghouse and Web sites of relevant societies/organizations producing and/or endorsing CPGs.
Results
The definition of AGE varies among the 15 CPGs identified. The parameters most frequently recommended to assess dehydration are skin turgor and sunken eyes (11/15, 73.3%), general appearance (11/15, 66.6%), capillary refill time, and mucous membranes appearance (9/15, 60%). Oral rehydration solution is universally recognized as first-line treatment. The majority of CPGs recommend hypo-osmolar (Na+ 45–60 mmol/L, 11/15, 66.6 %) or low-osmolality (Na+ 75 mmol/L, 9/15, 60%) solutions. In children who fail oral rehydration, most CPGs suggest intravenous rehydration (66.6%). However, nasogastric tube insertion for fluid administration is preferred according by 5/15 CPGs (33.3%). Changes in diet and withdrawal of food are discouraged by all CPGs, and early refeeding is strongly recommended in 13 of 15 (86.7%). Zinc is recommended as an adjunct to ORS by 10 of 15 (66.6%) CPGs, most of them from low-income countries. Probiotics are considered by 9 of 15 (60%) CPGs, 5 from high-income countries. Antiemetics are not recommended in 9 of 15 (60%) CPGs. Routine use of antibiotics is discouraged.
Conclusions
Key recommendations for the management of AGE in children are similar in CPGs. Together with accurate review of evidence-base this may represent a starting point for developing universal recommendations for the management of children with AGE worldwide.
Keywords: acute diarrhea, children, guidelines
Acute gastroenteritis (AGE) is a major cause of child mortality and morbidity globally, with 760,000 deaths per year in infants and children under 5 years of age, especially in low-income countries (LIC) (1–3). Of all child deaths from diarrhea, 78% occur in Africa and South-East Asia. The United Nations (UN) developed the Millennium Development Goal 4 (MDG 4) with the aim of reducing mortality of children below 5 years by two thirds by 2015 but many countries, especially in south Asia and sub-Saharan Africa, are not on track to meet this target (4). Therefore, the Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea has recently outlined necessary actions for the elimination of preventable child deaths due to pneumonia and diarrhea by 2025 as part of the MDG5 (5).
Even if in high-income countries (HIC) mortality due to AGE is a rare event, the health care and economic burden of acute diarrhea remains high (6). AGE is a major cause of medical visits and hospitalization and leads to approximately 240,000 emergency department (ED) visits annually, and the hospitalization of 1 in every 10-25 children (7–8). In Europe, AGE is among the 3 most frequent causes of hospital admission, accounting for at least 4% to 17% of all hospitalizations (7–8).
Although the management of AGE is based on simple and straightforward interventions, there remains a high variability in practice with several unresolved issues that are strongly related to local traditions (ie, nutritional interventions) and the availability of drugs. New treatments and management strategies have also been proposed that remain controversial, such as “rapid intravenous rehydration” schemes (9) or specific medical therapies, including anti-emetics and antidiarrheal drugs (10).
Evidence-based guidelines are the standard reference for clinical practice that can empower health care providers to employ effective management strategies and improve quality of care (11). Several high quality Clinical Practice Guidelines (CPGs) for the management of AGE have been published in the last 15 years, but considerable variability in clinical practice persists (12–16). Given this scenario, only a minority of health care providers fully comply with recommendations in CPGs. Low adherence to guidelines for AGE has been reported both in developed and developing countries (13,17–19).
Members of the Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition (FISPGHAN) Working Group for AGE identified in 2012 priorities in medical intervention, education, and research that could reduce the burden of acute diarrhea in children worldwide (20). The Working Group agreed that developing universal evidence-based guidelines for the management of AGE would be of value to health care providers. Such guidelines should take into consideration differences in epidemiology and availability of local and regional resources. Agents of AGE (including cholera), local traditions and beliefs, and the costs involved in delivery of care all should be considered. The availability of global guidelines supported by public health authorities and pediatric societies (general pediatrics and pediatric gastroenterology, emergency care, and infectious diseases) would fill an unmet need in improving the outcomes for children with AGE and increase the likelihood of meeting goals set by MDG4.
The aim of this study was to systematically review published guidelines on AGE and compare the major recommendations for the management of AGE in children. This information, together with accurate revision of local evidence, will provide the basis for developing and producing universal guidelines for the management of AGE.
Methods
Literature Search
Relevant guidelines published in the last 10 years (January 2005–May 2015) were identified through MEDLINE (www.ncbi.nlm.nih.gov/pubmed), the Cochrane Library (www.cochranelibrary.com), and the National Guideline Clearinghouse (NGC) (www.guideline.gov). The following keywords were used for the search strategy for PubMed: (“Child, Preschool”[MeSH] OR “Infant”[MeSH:NoExp] OR “Infant, Newborn”[MeSH:NoExp] OR child* [tw] OR infant* [tw] OR newborn* [tw] OR neonate* [tw]) AND ((Gastroenteritis [mh:noexp] OR “Cholera Morbus”[MeSH] OR Colitis [mh] OR Dysentery [mh] OR enteritis [mh] OR enterocolitis [mh] OR Mucositis [mh] OR proctitis [mh] NOT “Appendicitis”[MeSH] NOT “Esophagitis”[MeSH] NOT “Gastritis”[MeSH] NOT “Inflammatory Bowel Diseases” [MeSH]) OR Diarrhea [MeSH]) AND vomiting [MeSH or tw]).
We also looked at Web sites of societies that produce and/or endorse CPGs, including the American Academy of Pediatrics—AAP (www.aap.org), Morbidity and Mortality Weekly Report (www.cdc.gov/mmwr), European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (www.espghan.org), North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (www.naspghan.org), Canadian Paediatric Society and Pediatric Emergency Medicine section (www.cps.ca), Common-wealth Association of Paediatric Gastroenterology & Nutrition (http://www.capgan.org/), Latin American Society of Pediatric Gastroenterology, Hepatology and Nutrition (http://www.laspghan.org/), Asian Pan Pacific Society for Pediatric Gastroenterology, Hepatology, and Nutrition (http://appspghan.org/), Indian Academy of Pediatrics (www.iapindia.org), American College of Emergency Physicians, Pediatric section of the European Society of Emergency Medicine, Society of Pediatric Urgent Care, Association of pediatric emergency Medicine, Pediatric Emergency Medicine of Australia & New Zeland. In addition, to obtain the largest possible number of documents, members of the FISPGHAN Working Group on Acute Diarrhea directly contacted (by e-mail) experts in all continents to obtain local guidelines, and some members personally discussed the initiatives during an international congress and at scientific meetings and workshops.
Inclusion/Exclusion Criteria
Starting from the definition of CPGs as “systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances” (21), the Working Group selected guidelines, consensus statements or care protocols on the management of acute diarrhea in infants and children between 1 month and 18 years of age. CPGs published in 1 of the 3 most spoken languages (Chinese, Spanish, and English) (22) were included. In cases of studies that referred to or endorsed previous publications, we evaluated the original document. Guidelines focusing on diarrhea prevention, vaccination, surgery, or other rare diseases and documents based on adult populations were excluded.
Comparisons
CPGs were categorized according to the following domains:
Definition of acute diarrhea and gastroenteritis
Assessment of dehydration
Nutritional interventions
Rehydration in outpatient and inpatient settings
Antidiarrheal treatment
Anti-infectious therapy
The recommendations of each article were reported in a table of evidence according to each domain. The quality of supporting evidence was also included when reported in the original document.
Because clinical recommendations may slightly vary according to the local setting and to better compare recommendations for children living in HIC and LIC, CPGs were classified according to the International Monetary Fund list of countries with advanced economy (http://www.imf.org/external/pubs/ft/weo/2015/01/pdf/text.pdf).
Results
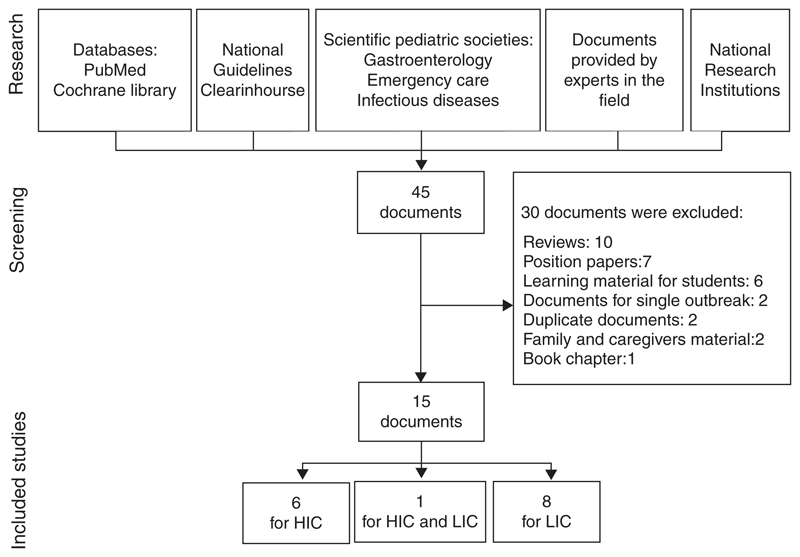
After application of inclusion and exclusion criteria and elimination of duplicates, 15 CPGs were included in this analysis (Fig. 1) (10,23–37). Seven CPGs were developed in HIC, 8 in LIC, and 1 developed by the World Gastroenterology Organization (28) referred to children living either in HIC or LIC. The main characteristics of CPGs are summarized in the Table 1.
Figure 1.
Literature sources for retrievement of guidelines. HIC = high-income countries; LIC = low-income countries.
Table 1. Guidelines included in the comparative analysis.
| Guidelines | Year | Country/geographical area | Language | Country (H/L) | Setting (O/I) | Target population |
|---|---|---|---|---|---|---|
| NSW Ministry of Health—Australia guidelines (23) | 2014 | Australia | English | H | O/I | Infants and children |
| ESPGHAN (24) | 2014 | Europe | English | H | O/I | <5 y |
| Salazar-Lindo et al (25) | 2014 | Latin America | Spanish | L | O/I | <5 y |
| Princess Marina Hospital (26) | 2012 | Botswana | English | L | O/I | NS |
| Wittenberg (27) | 2012 | South Africa | English | L | O/I | Infants |
| WGO (28) | 2012 | World | English | H/L | O/I | NS |
| Ministry of Health and Sanitation—Kenya (29) | 2011 | Kenya | English | L | O/I | <5 y |
| Cincinnati Children’s Hospital Medical Center (30) | 2011 | USA | English | H | O/I | <18 y |
| Malaysian Pediatric Association (31) | 2011 | Malaysia | English | L | O/I | <5 y |
| Chinese Guidelines Group (32) | 2009 | China | Chinese | L | O/I | NS |
| NICE (10) | 2009 | UK | English | H | O/I | <5 y |
| Harris et al (33) | 2008 | Australia | English | H | O/I | >3 mo |
| Bhatnagar et al (34) | 2007 | India | English | L | O | <5 y |
| Canadian Paediatric Society (35,36) | 2006, 2011 | Canada | English | H | O/I | <5 y |
| WHO (37) | 2005 | Developing | English | L | O/I | <5 y |
ESPGHAN = European Society for Paediatric Gastroenterology Hepatology and Nutrition; H = high income; I = inpatient; L = low income; NICE = National Institute for Health and Care Excellence; NS = not specified; O = outpatients.
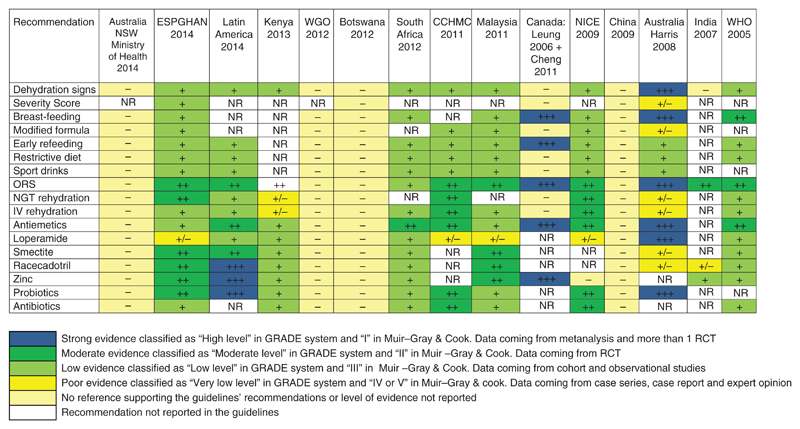
The level of evidence supporting the clinical recommendations included in single CPGs is reported in Fig. 2 for each of the guidelines and according to single domains. Different grading systems were used to assess the level of evidence and the strength of recommendations, and 4 guidelines provided recommendations without reporting the supporting evidence.
Figure 2.
Level of evidence supporting recommendations included in CPGs for AGE. ESPGHAN = European Society for Paediatric Gastroenterology Hepatology and Nutrition; CCHMC = Cincinnati Children’s Hospital Medical Center; IV = intravenous; NGT = nasogastric tube; NR = not reported; NICE = National Institute for Health and Care Excellence; NSW = New South Wales Government; ORS = oral rehydration solution; WGO = World Gastroenterology Organisation; WHO = World Health Organization.
Definition of Acute Diarrhea and Gastroenteritis
Acute diarrhea is defined in all included CPGs as a decrease in consistency of stools (loose or liquid) and/or an increase in frequency of evacuations (typically ≥3 in 24 hours). Some CPGs specifically focused on AGE and reported the presence of either fever or vomiting (10,24) whereas others did not. Acute diarrhea is defined as diarrhea lasting 7 days or less by 4 of the 17 CPGs, all developed in HIC (10,24,30,33). Seven CPGs define acute diarrhea as lasting 14 days or less, all these documents were developed in LIC (25–29,32,37). Four CPGs do not report a specific definition of diarrhea (23,31,34–36).
Assessment of Dehydration
Most guidelines identified loss of body weight as the most reliable parameter to assess the presence and severity of dehydration. Several clinical signs and symptoms have been used to indirectly estimate the degree of dehydration. The most recommended parameters reported by the 15 CPGs were skin turgor and sunken eyes (11/15, 73.3%), general appearance (11/15, 66.6%), capillary refill time, and mucous membranes (9/15, 60%) (Table 2).
Table 2. Clinical signs to estimate the degree of dehydration.
| Clinical sign | High income | Low income | Total (% on 15 CPGs) |
|---|---|---|---|
| Skin turgor | 7 | 5 | 12 (80) |
| Sunken eyes | 5 | 6 | 11 (73.3) |
| Consciousness/general appearance | 4 | 6 | 10 (66.6) |
| Capillary refill time | 5 | 4 | 9 (60) |
| Mucous membranes | 5 | 4 | 9 (60) |
| Respiratory pattern | 4 | 2 | 6 (40) |
| Thirst | 3 | 3 | 6 (40) |
| Tears | 3 | 3 | 6 (40) |
| Urine output | 3 | 1 | 4 (26.6) |
| Radial pulse | 1 | 2 | 3 (20) |
| Heart rate | 2 | 1 | 3 (20) |
| Extremities | 1 | 1 | 2 (13.3) |
| Metabolic acidosis | 0 | 1 | 1 (6.6) |
| Sunken anterior fontanelle | 1 | 0 | 1 (6.6) |
CPGs = clinical practice guidelines.
Different scores or scales, combining >1 symptom or sign, have been studied and proposed for clinical practice. Four CPGs reported standardized clinical scores for use by practitioners managing children with AGE. The most commonly recommended score is the Clinical Dehydration Scale (CDS) (38) recommended by 3 of 4 CPGs.
Rehydration
Oral rehydration solution (ORS) is universally recognized as first-line treatment of AGE and is recommended by all CPGs. Several ORS formulations are available worldwide: the majority of CPGs recommend hypo-osmolar (Na+ concentration 45–60 mmol/L, 11/15, 66.6%) or low-osmolality (Na+ concentration 75 mmol/L, 9/15, 60%) ORS. Only a minority of CPGs considers the standard WHO solution containing 90 mmol/L of sodium (4/15, 26.6%). No significant difference was observed between the formulations used in HIC and LIC according to the Na+ concentration (P = 0.56). It should be, however, noted that 3 CPGs recommend the use of a specific ORS for malnourished children ReSoMal (Rehydration Solution for Malnutrition) containing 45 mmol/L Na+ and 40 mmol/L K+. Such variability may reflect local variations in etiologic agents causing enteritis (39).
Micronutrients can be added to ORS to improve efficacy, and these preparations are referred to as “Super ORS.” Only 2 CPGs consider the use of Super ORS (24–25) and conclude that they are not routinely recommended in clinical practice, except for zinc-containing solutions recommended in children living in developed areas.
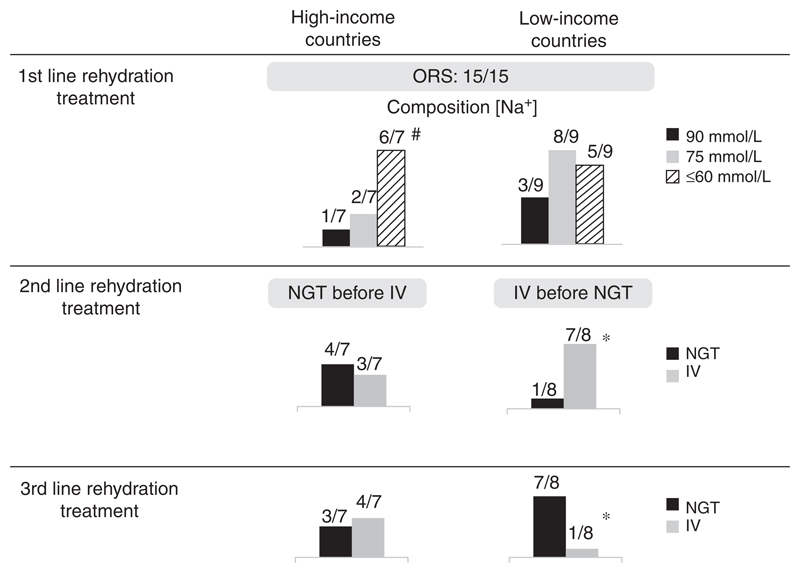
In children who fail on oral rehydration, other rehydration options need to be attempted such as administering fluids either by nasogastric tube (NGT) or intravenously (IV). NGT is preferred to IV rehydration as second-line rehydration treatment by 5 of 15 CPGs (33.3%), whereas 10 of 15 (66.6%) recommend IV rehydration rather than NGT (Fig. 3). NGT is more commonly recommended in guidelines arising from HIC and IV rehydration in LIC. One guideline in LIC does not report recommendations for hospital management (34).
Figure 3.
Schemes of rehydration recommended in CPGs according to settings. CPGs = clinical practice guidelines; HIC = high-income countries; IV = intravenous; LIC = low-income countries; NGT = nasogastric tube; ORS = oral rehydration solution. #Two guidelines suggest >1 ORS formulation. *Bhatnagar et al (34) did not include hospital rehydration.
Nutritional Interventions
Changes in diet and milk (lactose) withdrawal are generally discouraged by all CPGs. Early refeeding is strongly recommended in 13 of 15 documents (86.7%). Most CPGs recommend that infants younger than 6 months should not interrupt breast-feeding (10/15, 66.6%) or introduce diluted or modified formula (8/15, 53.3%) (Table 3). Lactose-free formula may be indicated in the in-patient setting (2/15, 13.3%) and in cases of diarrhea lasting for more than 7 days (1/15, 6.6%). Chinese, Peruvian, and Botswanian CPGs recommend the use of lactose-free milk to shorten the duration of AGE (3/15, 20%). Table 3 summarizes other recommendations for nutritional management recommended by CPGs produced in both HIC and LIC.
Table 3. Nutritional interventions included in CPGs according to the setting.
| Nutritional intervention | High-income countries | Low-income countries | Total |
|---|---|---|---|
| Milk | |||
| Continue breast-feeding | 5/7 | 6/9 | 12/15* |
| No indication for modified formula | 6/7 | 3/9 | 9/15* |
| Diet | |||
| Early refeeding | 7/7 | 7/9 | 14/15* |
| No restrictive diet | 7/7 | 7/9 | 14/15* |
| Avoidance of sport drinks | 5/7 | 5/9 | 10/15* |
CPGs=clinical practice guidelines.
WGO guidelines are intended for both high- and low-income countries.
Active Treatment of Diarrhea
Many different pharmacological interventions have been proposed as an adjunct to ORS to reduce the severity of symptoms and the duration of illness. Active treatment with selected probiotics or drugs was considered by some CPGs (Table 4).
Table 4. Recommendations to antidiarrheal and antibiotic treatment according to included CPGs.
| Antibiotics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Guidelines | Probiotics | Antimotility drugs | Adsorbents | Racecadotril | Zinc | Based on etiology* | Dysentery | Septicemia | Malnutrition/chronic conditions |
| Australia—NSW 2014 (23) | + | − | − | − | + | NA | NA | NA | NA |
| ESPGHAN 2014 (24) | + | − | + | + | − | + | + | NA | NA |
| Latin America 2014 (25) | + | − | + | + | + | NA | NA | NA | NA |
| Kenya 2013 (26) | − | − | − | − | + | NA | NA | NA | NA |
| Botswana 2012 (27) | − | NA | NA | NA | + | NA | NA | NA | NA |
| WGO 2012 (28) | + | − | + | + | + | + | NA | NA | |
| South Africa 2012 (29) | † | − | − | − | + | NA | NA | NA | NA |
| CCHMC 2011 (30) | † | − | − | − | NA | + | NA | NA | NA |
| Malaysian Pediatric Association 2011 (31) | + | − | + | + | + | + | + | NA | NA |
| NICE 2009 (10) | − | − | NA | NA | NA | + | + | + | NA |
| China 2009 (32) | + | NA | + | + | + | + | NA | NA | NA |
| Australia Harris 2008 (33) | + | − | − | − | NA | NA | NA | NA | NA |
| Indian Pediatric Society 2007 (34) | − | NA | NA | − | + | NA | + | NA | NA |
| Canadian Paediatric Society 2006 (35–36) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| WHO 2005 (37) | − | − | − | − | + | + | + | NA | + |
CCHMC = Cincinnati Children’s Hospital Medical Center; CPGs = clinical practice guidelines; ESPGHAN = European Society for Paediatric Gastroenterology Hepatology and Nutrition; NA = not assessed; NICE = National Institute for Health and Care Excellence; WGO = World Gastroenterology Organisation; WHO = World Health Organization.
Shigella, Campylobacter (within 72 h), E coli ETEC, Clostridium, parasitic infections, Salmonella non-typhi in high-risk categories.
See the text.
Zinc was recommended as an adjunct to oral rehydration therapy by 10 of 15 CPGs, most of all from LIC. The other 5 CPGs developed in HIC discussed its use for the management of children with AGE, but did not recommend routine supplementation with Zinc in nondeficient children.
Probiotics were considered by 9 CPGs (4 in HIC and 4 in LIC and WGO addressed to both LIC and HIC). One CPG developed in United States recommended discussing the use of selected probiotics with families (30). South African CPGs did not recommend probiotics for routine practice, but considered the use of probiotics only for inpatient children developing nosocomial diarrhea (27). Probiotics strains most commonly recommended are Lactobacillus rhamnosus GG and Saccharomyces boulardii. Clinical recommendation also varies according to availability of products. More specifically, Australian CPGs recognize the efficacy of Lactobacillus strain GG and would recommend its use, but underline that probiotics are not available in the country (33). Four other CPGs from LIC do not recommend probiotics. Two guidelines do not discuss the use of probiotics for children with diarrhea.
Antimotility drugs, in particular loperamide, are explicitly discouraged by 11 of 15 CPGs. Canadian, Botswanian, Chinese, and Indian guidelines do not discuss their use for children with AGE.
Recommendations concerning other antidiarrheal agents are controversial. Racecadotril/acetorphan was recommended as a useful active treatment for diarrhea by 5 of 15 CPGs (1 HIC, 3 LIC, and the WGO). On the contrary, it was not recommended by 8 of 15 CPGs (4 HIC and 4 LIC) and it is not discussed by 2 CPGs (10,26,35–36). Similarly, smectite was judged useful for AGE by 4 of 15 CPGs (1 HIC and 3 LIC), but not recommended by 5 of 15 CPGs (2 HIC and 3 LIC), and it was not included in the 6 other documents.
Antiemetics were not recommended for use in most cases of AGE (9/15 CPGs). Five CPGs, including Canadian, Australian, Chinese, Indian and Peruvian guidelines, however, consider their use for persistent vomiting. Recent ESPGHAN/ESPID CPGs discuss the use of selected drugs, principally ondansetron, for specific conditions, such as use in the emergency department with the aim of reducing hospital admissions. Warnings released by FDA and EMA on potentially severe side effects, however, significantly limit these indications for use (40).
Anti-infectious Therapy
Routine use of antibiotics is not recommended by all CPGs included in this analysis. The use of antibiotics is discussed by 11 of 15 CPGs (73.3%) and considered only in specific situations: young infants (especially those <3 months of age), children with underlying chronic conditions or immunodeficiency at risk of developing severe or extraintestinal dissemination, and children in the community to reduce the risk of spreading the infection. The pattern of specific indications to use antibiotic therapy is, however, wide and varies in each of the CPGs.
Table 4 summarizes major recommendations for antiinfectious treatment in the setting of pediatric AGE. Most guidelines recommend a pathogen-based approach (also in association with local epidemiological patterns of intestinal infection), other consider an empiric antibiotic treatment in children with dysentery.
Discussion
There is consensus that the main pillars for the management of AGE namely definition of the problem, assessment of the degree of dehydration, rehydration through the oral route, age-appropriate diet, and possible use of selected products that could reduce the severity and duration of diarrhea are common to all settings and may be applicable to most children living either in HIC or LIC. The approach to severely undernourished children is not specifically addressed in the present article, and it is likely to require a more specific and tailored clinical approach (41,42). Taking all of these considerations into account, the FISPGHAN Working Group for AGE started by comparing presently available CPGs.
A prior article on RCTs in acute diarrhea found 64 different definitions of diarrheal illnesses, based on the inclusion criteria (43). By contrast, we found a quite homogeneous definition of AGE. Gastroenteritis is usually defined by the presence of diarrhea, “a decrease in the consistency of stools (loose or liquid) and/or an increase in the frequency of evacuations (typically ≥3 in 24 hours)”, with or without fever or vomiting. This quantitative definition, validated by a prospective community-based surveillance study (44), has become the most widely accepted definition of AGE. When we looked at the definition of acute diarrhea, some differences, however, emerged among CPGs, with most of the heterogeneity associated to the setting. CPGs produced in HIC define “acute” as diarrhea lasting 7 days or less, whereas guidelines produced in LIC, diarrhea was considered “acute” for up to 14 days duration. Although all of the CPGs agree on the definition of chronic diarrhea after 14 days of symptoms, the difference could be explained by the introduction of a third, intermediate condition, defined as either acute protracted or prolonged diarrhea that lasts 7 to 14 days (45–48). The difference in definition has potential importance in practical terms because of the application of a number of medical interventions in those children experiencing diarrhea that overcome the definition of acute (>7 or 14 days): including changes in approach to alimentation, requests for additional diagnostic or microbiological tests, and the use of antidiarrheal or antiinfectious drugs. The identification and use of a standardized definition of diarrhea also makes possible an adequate synthesis of trial results and the application of synthesized measures of outcomes in a meaningful way (49).
The assessment of the degree of dehydration is the first step for the management of children with AGE, and all CPGs, both in HIC and LIC, identify the percentage of body weight loss as the best measure to assess the presence and severity of dehydration. In practice, preillness weight is, however, rarely available, and some authors have challenged its reliability (50). Several scoring systems have been proposed to assess dehydration according to clinical signs and symptoms (24). There is, however, no single standard method, and the assessment of dehydration usually derives from a compromise between accuracy and reliability on the one hand, and operators in a specific clinical setting on the other. Even if there are significant differences between parameters suggested by individual CPG, the 5 most commonly recommended signs are capillary refill time, skin turgor, sunken eyes, general appearance, and assessment of mucous membranes. It should be noted that the last 3 parameters are part of the 4 item-based CDS, together with the presence of tears (38,51).
Several studies have been conducted to validate the CDS for children 1 to 36 months of age with AGE in the ED. Although most studies were produced by the same group, CDS was characterized by moderate to good interobserver reliability and found to be useful in predicting the need for intravenous rehydration, weight gain, need for blood tests, need for hospitalization, and length of stay in the hospital and in the ED (51–54). CDS, therefore, may be considered a reliable instrument to assess dehydration in children with AGE. It should be, however, noted that capillary refill time is the single most sensitive and predictive sign to rule out severe dehydration (24,30). This easy-to-use parameter may be used to entrust the estimation to nonmedical personnel and to field workers. It also should be emphasized that some parameters, such as skin turgor and sunken eyes, may be difficult to be assessed in severely malnourished subjects (55). The dehydration assessment strategy needs to be a 2-prong tool, to correctly identify those who are not dehydrated and those who are severely dehydrated. The first group could be easily managed at home using ORS to prevent dehydration. The severely dehydrated group needs to be eagerly treated in hospital setting. The group in between (some level of dehydration) is, however, the larger in clinical practice and needs to receive a period of intense and supervised oral rehydration.
ORS is globally accepted as the first-line treatment of AGE in children. All of the CPGs agree on this statement, but the composition and osmolality of recommended solutions is different between CPGs: reduced osmolality and hypotonic solutions containing [Na+] 75 mmol/L and [Na+] 60 mmol/L, respectively, are generally recommended in HIC. In this setting, some guidelines have also introduced lower Na concentration ([Na+] 45–50 mmol/L) that has a better palatability and increases child compliance to oral rehydration (30).
On the contrary, CPGs developed in LIC usually refer to WHO ORS with [Na+] 75 mmol/L ORS (37) for otherwise healthy children with AGE (25–26,28–29,31–32,34). The so-called standard WHO ORS containing 90 mmol/L Na+ is no longer commercially available in many HIC and is presently recommended only for children living in LIC with high purging diarrhea such as cholera. When compared with WHO standard ORS, reduced osmolality ORS is associated with fewer unscheduled intravenous fluid infusions, reduced stool volume, and less vomiting (56). Therefore, the high Na+ concentration ORS should be reserved only to severe cholera diarrhea.
Children with severe acute malnutrition and diarrhea need to be rehydrated with specific ORS containing low sodium and high potassium concentration. Although supporting evidence is still weak (57), rehydration solution for malnutrition containing [Na+] 45 mmol/L and [K+]40 mmol/L is presently recommended by CPGs for the management of malnourished children in LIC (26–27,29,58).
In children unable to receive ORS, rehydration can be pursued by either the enteral or parenteral routes. Enteral rehydration by nasogastric route appears to be as effective as IV rehydration, it is associated with significantly fewer major adverse events, and results in a shorter hospital stay compared with IV therapy (24). According to this recent evidence, a third of CPGs considered enteral rehydration through NGT as a valid alternative to IV rehydration, although some physicians and families regard it as a more invasive and painful procedure. Surprisingly, almost all CPGs developed in LIC consider NG rehydration as a third-line option for children who failed on oral and IV rehydration and for severely dehydrated children. This recommendation is, however, in contrast with evidence deriving from a recent systematic review, including 12 studies (in either HIC or LIC) on the efficacy of NG rehydration compared with IV treatment. The authors conclude that NG rehydration should be considered as second-line therapy, after oral rehydration, particularly in resource-limited settings in which children frequently present with severe dehydration and intravascular access is technically challenging or impossible (59–60).
Overall, it could be useful to reconsider these recommendations, in preparation of a universal guideline paper in which NG intervention could be a preferred method over IV rehydration, either in HIC and LIC. Only a few CPGs specifically reported IV rehydration regimens with substantial agreement on the treatment of children in hypovolemic shock and relevant variability in the management of moderate to severe dehydration. The composition and route of rehydration, however, still remains a matter of debate (61).
A further cornerstone in the management of AGE is nutritional intervention. We recorded homogeneity among recommendations given by CPGs both in HIC and LIC. Most documents recommend that children younger than 6 months should not interrupt breast-feeding and should not introduce modified formula. In addition, children should be refed early after rapid oral rehydration therapy (4–6 hours), with no prescription of restricted diets. Beverages with high sugar content should be avoided.
On the contrary, the use of lactose-free formula is still controversial. Some guidelines recommend lactose withdrawal to shorten the duration of diarrhea. A Cochrane review evaluating the efficacy of lactose-free versus lactose-containing diets in children younger than 5 years reported that lactose-free products were associated with a reduction in the duration of diarrhea in hospitalized children by approximately 18 hours (62). Results were, however, different in outpatients setting, suggesting that there is no need to prescribe lactose-free formula in nonhospitalized children.
Even if AGE is generally a self-limiting disease not requiring specific therapies in addition to rehydration, active pharmacological treatments have been extensively evaluated with the aim of reducing either the duration or severity of symptoms. There is no agreement, with various CPGs providing different recommendations.
Zinc, which is recommended by WHO as first line treatment together with ORS, is the only therapy recommended for LIC by almost all CPGs. According to a recent Cochrane metanalysis, zinc supplementation may shorten by approximately 10 hours the duration of diarrhea in children older than 6 months with AGE and probably reduces the risk of prolonged diarrhea. Greater efficacy has been demonstrated in children with signs of moderate malnutrition (27 hours reduction of diarrhea) (63).
Presently, it has, however, no indication in CPGs from HIC. This discrepancy is probably related to proven efficacy of zinc in children with severe malnutrition because of the effects that zinc deficiency have on severity and duration of diarrhea (64–65). Administration of zinc in malnourished children may be seen as a nutritional intervention or a micronutrient replacement rather than as an active treatment of diarrhea.
Antiemetics are not recommended in the majority of guidelines. The use of antiemetic drugs (3/5 ondansetron) in children with persistent vomiting is considered in CPGs produced in Australia, Canada, China, Latin America, and India (23,25,32,34–36). In several studies and in Cochrane reviews, it was found there is some evidence, albeit weak evidence, that antiemetics such as ondansetron (a 5-HT3 serotonin antagonist) and the prokinetic metoclopramide (a dopamine antagonist) reduce the number of episodes of vomiting and may reduce the need for hospital admission (35,66–68). The increase of diarrhea noted with both ondansetron and metoclopramide and the potentially dangerous side effects of these drugs, in particular metoclopramide, limit their use in pediatric patients. In addition, it should be noted that the United States Food and Drug Administration released in the last years 2 warnings for both ondansetron and domperidone, reporting the risk of prolongation of the QT interval, which can lead to an abnormal and potentially fatal heart rhythms, including Torsade de Pointes (69).
Active therapies include adsorbents (smectite) and antisecretory drugs (racecadotril) and selected probiotic strains, their use may vary according to evidence collected in developed or developing countries (70). Some CPGs promote the use of selected probiotics strains (24,25,31); however, selected products are available in some countries but not in others. As an example, the probiotic L rhamnosus GG is formally recommended for use in Australian guidelines as an effective treatment as an adjunct to ORS. The absence of the product in the local marketplace, however, limits its use in clinical practice. Similarly, guidelines from India that critically looked at Western recommendations consider the use of probiotics effective in developed areas but not in India because of lack of supporting evidence in developing settings.
Routine use of antibiotics for uncomplicated AGE is always discouraged, but in specific host-related and epidemiological conditions, and in children in which AGE is accompanied by signs of sepsis an empiric antibiotic treatment may well be necessary and appropriate. Although CPGs usually recommend an etiologic approach, this recommendation is hampered by the lack of indications to microbiological investigations. The presence of dysentery (bloody diarrhea), however, represents an indication to empiric antibiotic therapy in some contexts, such as where Shigella is common and enterohemorrhagic Escherichia coli O157:H7 and other serotypes are uncommon. Broad-spectrum antibiotics also should be used in children with underlying immunodeficiency and severe malnutrition (71–73). Secondary Salmonella bacteremia—with extraintestinal focal infections—occurs more often in children with sickle cell anemia, and in neonates or young infants; therefore, antibiotic therapy is suggested in these children (74).
Our work has some limitations. The first is the selection of languages in research strategy. Although we referred to the 3 most widely used languages in the world, some documents published in different language spoken by million of people (such as Hindi-Urdu, Arabic, French) were not included limiting the comparison of recommendations. Secondly, we searched for guidelines produced in the last 10 years. Several guidelines are updated every 5 years (estimated of scientific knowledge half-life); however, it should be noted that the World health Organization did not retire or revise their last guidelines published 10 years ago (although integrated with other documents), indicating that the main pillars for management of AGE are not radically changed in the last decade. Similarly the Centre for Diseases Control and Prevention in United States produced the last guidelines in 2003 (75) and did not provide any recent update to the document. It may indicate that practitioners living all around the world may have access to these documents to support their practice. We believe that 10 years is a reasonable time to include documents that are presently applied.
Conclusions
According to this comparative analysis of recommendations, there are 6 major interventions for the management of children with AGE that are common to all countries: definition of acute diarrhea, evaluation of the degree of dehydration through standardized and validated scores, early oral rehydration with ORS, early refeeding with breast milk or age-appropriate diet, use of enteral rehydration in children who do not tolerate oral rehydration, and limiting diagnostic work up and microbiological investigations to selected cases. These indications are common to all CPGs and could form the main pillars for the development of global recommendations for the management of AGE in children.
In keeping with the mission of FISPGHAN Working Group for acute diarrhea, and according to previously identified priorities, the findings presented in the present article can form the basis for producing a document that outlines global recommendations for the management of children with AGE. The specific recommendations, however, will need to be discussed with experts and members of FISPGHAN societies, and relevant agencies and institutions worldwide. The following issues in particular need to be discussed and resolved at a global level:
Universal definition of diarrhea and gastroenteritis
Score symptom versus single signs to rule out severe dehydration or sepsis
Limit the use of lactose-free formulas/diet to selected conditions
ORS composition and indications and rates of enteral and intravenous rehydration, including in severely malnourished children
Indications and settings for active antidiarrheal treatment and anti-infectious therapy.
Because malnutrition may significantly change the overall approach to children with AGE for all the points reported below, global recommendations should accurately differentiate between malnourished and nonmalnourished children, reporting evidence supporting single intervention in the appropriate population.
What Is Known
Gastroenteritis is a major cause of child mortality and morbidity worldwide.
Management is affected by high heterogeneity and low adherence to standard recommendations.
Optimal and shared management could reduce the burden of gastroenteritis.
What Is New
The comparison of guidelines from different parts of the world shows a high grade of consistency, however there is some inconsistency in the use of antiemetics and selected antidiarrheal drugs.
The assessment of dehydration, indications for rehydration, dietary and active treatment are similar in low- and high-income countries.
These findings form the basis for developing universal recommendations for children with gastroenteritis.
Acknowledgments
The working group warmly thanks Dr Gong Si Tang (China), Dr Geng Lanlan (China), Dr Nazrul Neezam (Malaysia), and Dr Ahmed Laving (Kenya) for their kind collaboration in providing local guidelines and discussing recommendations developed in their countries. The project was partially supported by the United European Gastroenterology under a “Mono-thematic Initiative” educational grant.
Footnotes
The authors report no conflicts of interest.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.United Nations. The Millenium Development Goals Report 2014. UN, New York: 2014. [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.United Nations. The Millennium Development Goals Report 2011. UN, New York: 2011. [Google Scholar]
- 5.Quazi S, Aboubaker S, MacLean R, et al. Ending preventable child deaths from pneumonia and diarrhea by 2025. Development of the integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea. Arch Dis Child. 2015;100:s23–8. doi: 10.1136/archdischild-2013-305429. [DOI] [PubMed] [Google Scholar]
- 6.Freedman SB, Steiner MJ, Chan KJ. Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogilvie I, Khoury H, Goetghebeur MM, et al. Burden of community acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis. 2012;12:62. doi: 10.1186/1471-2334-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegering V, Kaiser J, Tappe D, et al. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15:e401–7. doi: 10.1016/j.ijid.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Bruzzese E, Lo Vecchio A, Guarino A. Hospital management of children with acute gastroenteritis. Curr Opin Gastroenterol. 2013;29:23–30. doi: 10.1097/MOG.0b013e32835a352f. [DOI] [PubMed] [Google Scholar]
- 10.National Collaborating Centre for Women’s and Children’s Health (UK) Diarrhoea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment and Management in Children Younger Than 5 Years. London: RCOG Press; 2009. National Institute for Health and Clinical Excellence: Guidance. [PubMed] [Google Scholar]
- 11.Shekelle PG, Pronovost PJ, Wachter RM, et al. Advancing the science of patient safety. Ann Intern Med. 2011;154:693–6. doi: 10.7326/0003-4819-154-10-201105170-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lo Vecchio A, Giannattasio A, Duggan C, et al. Evaluation of the quality of guidelines for acute gastroenteritis in children with the AGREE instrument. J Pediatr Gastroenterol Nutr. 2011;52:183–9. doi: 10.1097/MPG.0b013e3181e233ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Vecchio A, Liguoro I, Bruzzese D, et al. Adherence to guidelines for management of children hospitalized for acute diarrhea. Pediatr Infect Dis J. 2014;33:1103–8. doi: 10.1097/INF.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 14.Powell EC, Hampers LC. Physician variation in test ordering in the management of gastroenteritis in children. Arch Pediatr Adolesc Med. 2003;157:978–83. doi: 10.1001/archpedi.157.10.978. [DOI] [PubMed] [Google Scholar]
- 15.Freedman SB, Gouin S, Bhatt M, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127:e287–95. doi: 10.1542/peds.2010-2214. [DOI] [PubMed] [Google Scholar]
- 16.Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124:e1081–7. doi: 10.1542/peds.2009-0473. [DOI] [PubMed] [Google Scholar]
- 17.Mangione-Smith R, DeCristofaro AH, Setodji CM, et al. The quality of ambulatory care delivered to children in the United States. New Engl J Med. 2007;357:1515–23. doi: 10.1056/NEJMsa064637. [DOI] [PubMed] [Google Scholar]
- 18.Freedman SB, Thull-Freedman JD, Rumantir M, et al. Emergency department revisits in children with gastroenteritis: a retrospective observational cohort study. J Pediatr Gastroenterol Nutr. 2013;57:612–8. doi: 10.1097/MPG.0b013e3182a1dd93. [DOI] [PubMed] [Google Scholar]
- 19.Pathak D, Pathak A, Marrone G, et al. Adherence to treatment guidelines for acute diarrhoea in children up to 12 years in Ujjain, India—a cross-sectional prescription analysis. BMC Infect Dis. 2011;11:32. doi: 10.1186/1471-2334-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarino A, Winter H, Sandhu B, et al. Acute gastroenteritis disease: Report of the FISPGHAN Working Group. J Pediatr Gastroenterol Nutr. 2012;55:621–6. doi: 10.1097/MPG.0b013e318272b5e2. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine, FMLohr K, editor. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academy Press; 1990. [PubMed] [Google Scholar]
- 22.Lewis MP. Ethnologue: Languages of the World. XVI edition. Dallas, TX: SIL International; 2009. [Google Scholar]
- 23.Ministry of Health, NSW. Infants and children: Management of Acute Gastroenteritis. Fourth edition. Dec, 2014. Clinical Practice Guideline–GL2014_024. [Google Scholar]
- 24.Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132–52. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 25.Salazar-Lindo E, Polanco Allué I, Gutiérrez-Castrellón P. Grupo Ibero-Latinoamericano sobre el Manejo de la Diarrea Aguda (GILA).Guía de prácticaclínica ibero-latinoamericana sobre el manejo de la gastroenteritis aguda en menores de 5 años. AnPediatr (Barc) 2014;80(suppl 1):1–22. doi: 10.1016/S1695-4033(14)75260-9. [DOI] [PubMed] [Google Scholar]
- 26.Princess Marina Hospital. Department of Paediatrics Protocols. 2012 Aug [Google Scholar]
- 27.Wittenberg DF. Management guidelines for acute infective diarrhoea/gastroenteritis in infants. SAMJ. 2012;102:2. doi: 10.7196/samj.5243. [DOI] [PubMed] [Google Scholar]
- 28.World Gastroenterology Organisation Global Guidelines. Acute diarrhea in adults and children: a global perspective. 2012 Feb [Google Scholar]
- 29.Ministry of Public Health and Sanitation (MOPHS) Scaling up strategy for essential treatments in children under five years in kenya: diarrhoea and pneumonia. Implementation Plan for the Period 2011/12–2015/16. [Accessed May 15, 2015]; www.publichealth.go.ke.
- 30.Acute Gastroenteritis Guideline Team, Cincinnati Children’s Hospital Medical Center. Evidence-based care guideline for prevention and management of acute gastroenteritis in children age 2 months to 18 years. [Accessed June 1, 2016]; http://www.guideline.gov.
- 31.College of Paediatrics, Academy of Medicine of Malaysia/Malaysian Paediatric Association. Guidelines on the management of acute diarrhea in children. 2011 [Google Scholar]
- 32.Subspecialty Groups of Gastroenterology and Infectious Diseases; Society of Pediatrics; Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Experts consensus on the principles of diagnosis and treatment of diarrheal diseases in children. Zhonghua Er Ke Za Zhi. 2009;47:634–6. [PubMed] [Google Scholar]
- 33.Harris C, Wilkinson F, Mazza D, et al. Evidence based guideline for the management of diarrhoea with or without vomiting in children. Aust Fam Physician. 2008;37:22–9. [PubMed] [Google Scholar]
- 34.Bhatnagar S, Lodha R, Choudhury P, et al. IAP Guidelines 2006 on management of acute diarrhea. Indian Pediatr. 2007;44:380–9. [PubMed] [Google Scholar]
- 35.Cheng A. Emergency department use of oral ondansetron for acute gastroenteritis-related vomiting in infants and children. Paediatr Child Health. 2011;16:177–82. doi: 10.1093/pch/16.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung A, Prince T. Canadian Paediatric Society, Nutrition and Gastroenterology Committee. Paediatr Child Health. 2006;11:527–31. [Google Scholar]
- 37.World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. 2005 4th rev. [Google Scholar]
- 38.Bailey B, Gravel J, Goldman RD, et al. External validation of the clinical dehydration scale for children with acute gastroenteritis. Acad Emerg Med. 2010;17:583–8. doi: 10.1111/j.1553-2712.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 39.Musekiwa A, Volmink J. Oral rehydration salt solution for treating cholera: ≤ 270 mOsm/L solutions vs ≥ 310 mOsm/L solutions. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD003754.pub3. CD003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Zofran (ondansetron): Drug Safety Communication—Risk of Abnormal Heart Rhythms. 2011 Posted 09/15/2011. [Google Scholar]
- 41.Iannotti LL, Trehan I, Clitheroe KL, et al. Diagnosis and treatment of severely malnourished children with diarrhoea. J Paediatr Child Health. 2015;51:387–95. doi: 10.1111/jpc.12711. [DOI] [PubMed] [Google Scholar]
- 42.Gaffey MF, Wazny K, Bassani DG, et al. Dietary management of childhood diarrhea in low- and middle-income countries: a systematic review. BMC Public Health. 2013;13(suppl 3):S17. doi: 10.1186/1471-2458-13-S3-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston BC, Shamseer L, da Costa BR, et al. Measurement issues in trials of pediatric acute diarrheal diseases: a systematic review. Pediatrics. 2010;126:222–31. doi: 10.1542/peds.2009-3667. [DOI] [PubMed] [Google Scholar]
- 44.Baqui AH, Black RE, Yunus M, et al. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol. 1991;20:1057–63. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 45.Lee KS, Kang DS, Yu J, et al. How to do in persistent diarrhea of children?: concepts and treatments of chronic diarrhea. Pediatr Gastroenterol Hepatol Nutr. 2012;15:229–36. doi: 10.5223/pghn.2012.15.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarino A, Lo Vecchio A, Berni Canani R. Chronic diarrhoea in children. Best Pract Res Clin Gastroenterol. 2012;26:649–61. doi: 10.1016/j.bpg.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Mathai J, Raju B, Bavdekar A, et al. Chronic and persistent diarrhea in infants and young children: status statement. Indian Pediatr. 2011;48:37–42. doi: 10.1007/s13312-011-0018-9. [DOI] [PubMed] [Google Scholar]
- 48.Moore SR. Update on prolonged and persistent diarrhea in children. Curr Opin Gastroenterol. 2011;27:19–23. doi: 10.1097/MOG.0b013e32833f215d. [DOI] [PubMed] [Google Scholar]
- 49.Karas J, Ashkenazi S, Guarino A, et al. A core outcome set for clinical trials in acute diarrhoea. Arch Dis Child. 2015;100:359–63. doi: 10.1136/archdischild-2014-307403. [DOI] [PubMed] [Google Scholar]
- 50.Pruvost I, Dubos F, Chazard E, et al. The value of body weight measurement to assess dehydration in children. PLoS One. 2013;8:e55063. doi: 10.1371/journal.pone.0055063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman JN, Goldman RD, Srivastava R, et al. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145:201–7. doi: 10.1016/j.jpeds.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 52.Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545–9. doi: 10.1542/peds.2007-3141. [DOI] [PubMed] [Google Scholar]
- 53.Gravel J, Manzano S, Guimont C, et al. Multicenter validation of the clinical dehydration scale for children. Arch Pediatr. 2010;17:1645–51. doi: 10.1016/j.arcped.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Kinlin LM, Freedman SB. Evaluation of a clinical dehydration scale in children requiring intravenous rehydration. Pediatrics. 2012;129:e1211–9. doi: 10.1542/peds.2011-2985. [DOI] [PubMed] [Google Scholar]
- 55.Dekate P, Jayashree M, Singhi SC. Management of acute diarrhea in emergency room. Indian J Pediatr. 2013;80:235–46. doi: 10.1007/s12098-012-0909-3. [DOI] [PubMed] [Google Scholar]
- 56.Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydrationsolution for treating dehydration caused by acute diarrhea in children. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002847. CD002847. [DOI] [PubMed] [Google Scholar]
- 57.Jones KDJ, Berkley JA. Severe acute malnutrition and infection. Paediatr Int Child Health. 2014;34:S1–29. doi: 10.1179/2046904714Z.000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. Management of severe acute malnutrition in infants and children. [Accessed June 1, 2016]; http://www.who.int/elena/titles/full_recommen dations/sam_management/en/ [PubMed]
- 59.Rouhani S, Meloney L, Ahn R, et al. Alternative rehydration methods: a systematic review and lessons for resource-limited care. Pediatrics. 2011;127:e748–57. doi: 10.1542/peds.2010-0952. [DOI] [PubMed] [Google Scholar]
- 60.Obonyo N, Maitland K. Fluid management of shock in severe malnutrition: what is the evidence for current guidelines and what lessons have been learned from clinical studies and trials in other pediatric populations? Food Nutr Bull. 2014;35(2 suppl):S71–8. doi: 10.1177/15648265140352S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan JA. Question 2: should 0.9% saline be used for maintenance fluids in hospitalised children? Arch Dis Child. 2015;100:715–7. doi: 10.1136/archdischild-2015-308821. [DOI] [PubMed] [Google Scholar]
- 62.Macgillivray S, Fahey T, McGuire W. Lactose avoidance for young children with acute diarrhoea. Cochrane Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD005433.pub2. CD005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazzerini M. Ronfani L Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD005436.pub4. CD005436. [DOI] [PubMed] [Google Scholar]
- 64.Alhashimi D, Alhashimi H, Fedorowicz Z. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005506.pub3. CD005506. [DOI] [PubMed] [Google Scholar]
- 65.Szajewska H, Gieruszczak-Bialek D, Dylag M. Meta-analysis: ondansetron for vomiting in acute gastroenteritis in children. Aliment Pharmacol Ther. 2007;25:393–400. doi: 10.1111/j.1365-2036.2006.03231.x. [DOI] [PubMed] [Google Scholar]
- 66.DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2008;162:858–65. doi: 10.1001/archpedi.162.9.858. [DOI] [PubMed] [Google Scholar]
- 67.Carter B, Fedorowicz Z. Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta-analysis and mixed treatment comparison in a Bayesian framework. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2011-000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freedman SB, Pasichnyk D, Black KJ, et al. Gastroenteritis therapies in developed countries: systematic review and meta-analysis. PLoS One. 2015;10:e0128754. doi: 10.1371/journal.pone.0128754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patro B, Golicki D, Szajewska H. Meta-analysis: zinc supplementation for acute gastroenteritis in children. Aliment Pharmacol Ther. 2008;28:713–23. doi: 10.1111/j.1365-2036.2008.03787.x. [DOI] [PubMed] [Google Scholar]
- 70.Patro B, Szymański H, Szajewska H. Oral zinc for the treatment of acute gastroenteritis in Polish children: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2010;157:984–988. doi: 10.1016/j.jpeds.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 71.Bandin F, Kwon T, Linas MD, et al. Cryptosporidiosis in paediatric renal transplantation. Pediatr Nephrol. 2009;24:2245–55. doi: 10.1007/s00467-009-1274-y. [DOI] [PubMed] [Google Scholar]
- 72.Sugata K, Taniguchi K, Yui A, et al. Analysis of rotavirus antigenemia in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2012;14:49–56. doi: 10.1111/j.1399-3062.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- 73.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–32. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shkalim V, Amir A, Samra Z, et al. Characteristics of non-typhi Salmonella gastroenteritis associated with bacteremia in infants and young children. Infection. 2012;40:285–9. doi: 10.1007/s15010-011-0231-4. [DOI] [PubMed] [Google Scholar]
- 75.King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1–16. [PubMed] [Google Scholar]