Abstract
Background
Despite advances in understanding the pathophysiology of acute respiratory distress syndrome (ARDS), effective pharmacological interventions have proven elusive. We believe this is a consequence of existing preclinical models being designed primarily to explore biological pathways, rather than predict treatment effects. Here we describe a mouse model in which both therapeutic intervention and ventilation were superimposed onto existing injury and explored the impact of β-agonist treatment, which is effective in simple models but not clinically.
Methods
Mice had lung injury induced by intranasal lipopolysaccharide (LPS), which peaked at 48 hours post-LPS based on clinically relevant parameters including hypoxaemia and impaired mechanics. At this peak of injury mice were treated intratracheally with either terbutaline or TNF receptor 1-targeting domain antibody, and ventilated with moderate tidal volume (20ml/kg) to induce secondary ventilator-induced lung injury (VILI).
Results
Ventilation of LPS-injured mice at 20ml/kg exacerbated injury compared to low tidal volume (8ml/kg). While terbutaline attenuated VILI within non-LPS treated animals, it was ineffective to reduce VILI in pre-injured mice, mimicking its lack of clinical efficacy. In contrast, anti-TNF receptor 1 antibody attenuated secondary VILI within pre-injured lungs, indicating that the model was treatable.
Conclusions
We propose adoption of a practical framework like that described here to reduce the number of ultimately ineffective drugs reaching clinical trials. Novel targets should be evaluated alongside interventions which have been previously tested clinically, using models that recapitulate the (lack of) clinical efficacy. Within such a framework, outperforming a failed pharmacologic should be a prerequisite for drugs entering trials.
Introduction
Acute respiratory distress syndrome (ARDS) is a frequently fatal condition caused by an overwhelming inflammatory response within the lung to a variety of insults. Despite widespread acceptance that inflammation is intimately linked to pathophysiology, none of the numerous pharmacological targets identified from preclinical studies have translated to patient benefit.
This lack of progress has led to much discussion regarding development of better paradigms in animal studies that may provide closer predictions of patient outcomes. Thus a host of models have been reported or proposed (1–3) utilising systems ranging from rodents to human tissue, varying in complexity from simple ‘single-hit’ models to those involving multiple challenges. Logically, the more closely a model mimics the patient scenario, the more reliable any predictions are likely to be. Practically, the numerous aetiologies of clinical ARDS means that no single model will ever truly capture the situation in all sufferers. However, there are factors common to the majority of patients including i) mechanical ventilation, an essential common denominator of ARDS treatment (indeed perhaps the main thing patients have in common); ii) deterioration in oxygenation and respiratory mechanics, and iii) the fact that patients primarily present for treatments only once they are sick. Thus we would propose that ‘predictive’ models (as opposed to those exploring biological processes) should examine therapeutic delivery into physiologically injured, mechanically ventilated lungs. Therefore, we developed a ‘two-hit’ model in which ARDS-like symptoms were induced in mice by intranasal lipopolysaccharide (LPS), and at the peak of injury intervened therapeutically and superimposed a secondary ventilator-induced lung injury (VILI).
A second, more complicated issue is, having developed any model, how does one evaluate whether it is ‘better’ than existing ones? Given the lack of specific biomarkers in ARDS there is no single readout one can measure in models that would determine their validity. We have considered an alternative approach to this question. The fact that many biological targets appear effective in animal models but not in patients indicates that existing models are susceptible to producing false positive results. Therefore, to validate our model we explored the response to β-agonist treatment which may be considered a clear example of a false positive, effective in various preclinical models (4–7), but ineffective in patients (8, 9).
We show here that the two-hit model was insensitive to intratracheal β-agonist (terbutaline). This contrasted with the clear beneficial effect of terbutaline within a one-hit pure VILI model, indicating that the response of the two-hit model was closer to that observed in patients. Importantly, we also showed that the model was amenable to intervention by an alternative approach, TNF-receptor-1 specific blockade. We propose a general framework in which new models and pharmacological targets are evaluated preclinically alongside mediators that have been previously clinically trialled. We feel that such an approach currently represents the best way to validate predictions from preclinical models, and should increase our ability to identify false positives before they enter patient trials.
Methods
Experiments were carried out according to the GSK Policy on the Care, Welfare and Treatment of Animals and the ARRIVE guidelines for the use and reporting of animals in research, under the Animals (Scientific Procedures) Act 1986 UK. 80 male C57BL/6 mice (Charles River) aged 9-12 weeks (25-31g) were used. Mice were housed in environmentally enriched, individually ventilated cages, maximum 5 mice/cage. Mice had free access to food and water, were maintained under a 12h light:dark cycle and had welfare assessed daily.
LPS-induced lung injury model
Mice were intranasally dosed with 25µg UltraPure LPS (E. coli O111:B4) under 2% isoflurane. At predetermined time points, respiratory mechanics and arterial blood gases were assessed as described previously (1). In brief, mice were anaesthetised (intraperitoneal ketamine 80mg/kg, xylazine 8mg/kg) and an endotracheal tube and carotid artery cannula introduced. Animals were ventilated using a custom-made ventilator (10) with 8ml/kg tidal volume (VT), 3cmH2O positive end-expiratory pressure (PEEP), using 100%O2. Following a recruitment manoeuvre (30cmH2O, 5s) ventilation was continued for 30 minutes to standardise lung history. Respiratory mechanics were then assessed by end-inflation occlusion and PaO2 determined.
Bronchoalveolar lavage fluid was evaluated for cytokines by ELISA and protein content by Bradford assay. Lung leukocytes were investigated using flow cytometry (11). Lungs were fixed and disrupted using a tissue dissociator. Samples were filtered (40µm), incubated with fluorescent antibodies against CD45, CD11b, CD11c, Ly6C, Ly6G, F4/80, CD206, ICAM-1 and NOS2, and analysed using a CyAn flow cytometer with Summit software.
Ventilator-induced lung injury
Two models of VILI were used within the current study, to evaluate the effects of interventions delivered into either healthy or injured lungs. Mice were anaesthetised and instrumented as described above. To model ‘pure’ VILI, healthy mice were ventilated with a high VT of 30-35ml/kg (PEEP 4cmH2O, 90 breaths/minute, 1:2 inspiratory:expiratory ratio, using 96%O2/4%CO2 to maintain normocapnia). Ventilation was continued for 3 hours (without recruitments) or until mice reached a mortality surrogate (arterial pressure <40mmHg). As a two-hit model, 48 hours after LPS (identified as peak injury) mice were ventilated using a moderately high VT of 20ml/kg (PEEP 4-5cmH2O, 90 breaths/minute, 1:2 inspiratory:expiratory ratio). Inspired gas was 99%O2/1% CO2. Recruitment manoeuvres (30cmH2O, 5s) were carried out every 15 minutes as we have previously shown that derecruitment occurs even with 20ml/kg in mice (10), and ventilation was continued for 3 hours or until mortality surrogate was reached.
We additionally incorporated various control experiments to clarify that the model was truly two-hit in nature, i.e. that both insults (LPS pre-injury and moderate VT ventilation) were required to produce substantial physiological injury. Firstly, a group of 48hour LPS-injured animals were ventilated with low VT (8ml/kg, PEEP 4cmH2O, 100% O2). Secondly, healthy uninjured mice were ventilated with the moderate 20ml/kg VT. Both control groups were ventilated for 3 hours with recruitment manoeuvres every 15 minutes.
Interventions
Mice within both the one-hit and two-hit VILI strategies were randomly allocated to receive 50µl of saline or 10-4M terbutaline intratracheally via the endotracheal tube. This was delivered immediately before the onset of high or moderate VT ventilation, with recruitment manoeuvres (4x30cmH2O, 5s) to aid distribution. In addition, mice within the two-hit model only were randomly allocated to receive 100µg TNF receptor 1-targeting domain antibody (dAb™; Dom-1m-15-12; Biopharmaceuticals R&D, GlaxoSmithKline, Stevenage, UK) or non-targeting ‘dummy’ antibody, delivered intratracheally in the same way as described for terbutaline.
Statistics
Model assumptions of normality were determined by analysis of residuals by Shapiro-Wilk test. Non-normally distributed data were transformed to normality where possible. Normally distributed data were evaluated by two-way ANOVA (time course analysis) and Student’s t-test (end-point studies). Non-normally distributed data were evaluated by Mann-Whitney U-test or Kruskal-Wallis test followed by Dunn’s multiple comparisons test.
Results
LPS-induced lung injury model
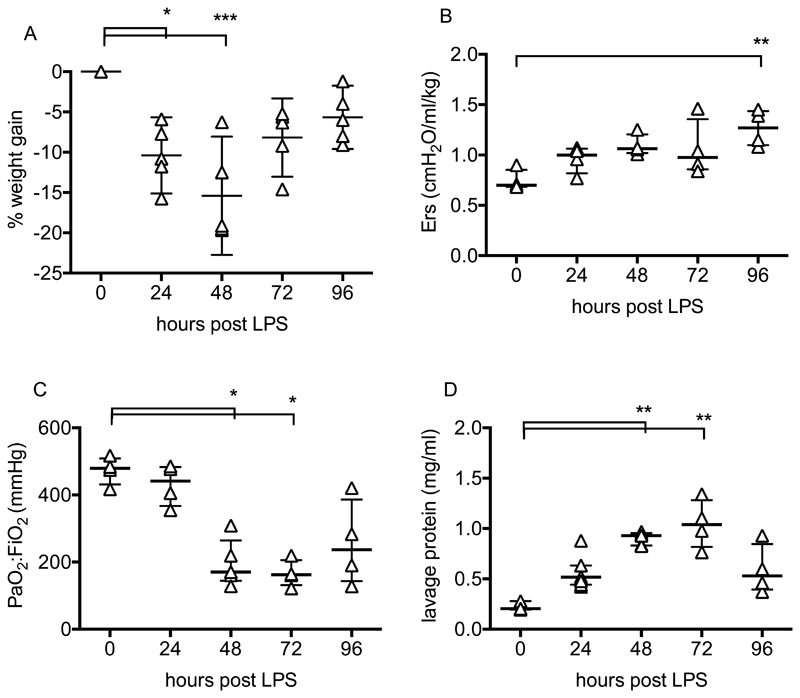
Intranasal LPS induced weight loss peaking at 48 hours before returning towards pre-injury levels (Fig 1A). At each time point, respiratory system elastance and arterial oxygenation were determined after 30 minutes of 8ml/kg VT ventilation (with 100% O2) to standardise measurements. Elastance (Fig 1B) increased during the course of injury. Arterial pO2 (Fig 1C) was relatively normal at 24 hours after LPS but fell thereafter, attaining levels at 48-72 hours consistent with mild-moderate clinical ARDS (~200 mmHg). Alveolar epithelial barrier permeability, evaluated by lavage fluid protein was increased throughout, peaking at 48-72 hours (Fig 1D).
Figure 1.
Time course of LPS-induced lung injury. Body weight (A) is expressed as % increase from pre-injured levels. Elastance (Ers, B) and arterial oxygenation (PaO2/FiO2, C) were evaluated following a 30 minute period of non-injurious ventilation. Total protein (D) was determined in lung lavage fluid. N= 4-6 for each readout at each time point, so all were treated as non-normally distributed data. Data are displayed as individual points with median and interquartile range, and were analysed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. *p<0.05, **p<0.01, ***p<0.001 vs 0h (untreated mice). Exact p-values were as follows: Panel A - 24h p=0.0134, 48h p=0.0005, 72h p=0.0890, 96h p=0.3379; Panel B - 24h p=0.5720, 48h p=0.0789, 72h p=0.2730, 96h p=0.0033; Panel C - 24h p>0.9999, 48h p=0.0377, 72h p=0.0146, 96h p=0.1839; Panel D - 24h p=0.4495, 48h p=0.0092, 72h p=0.0024, 96h p=0.5134.
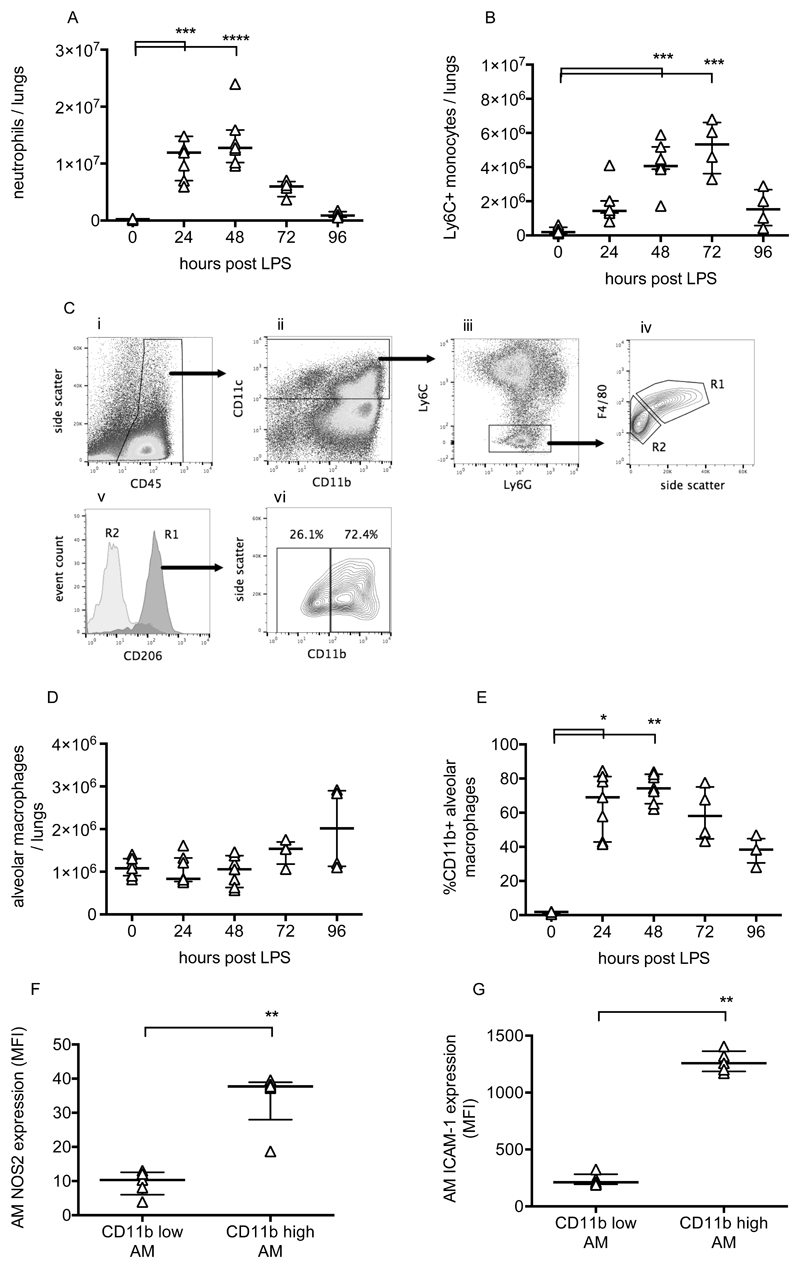
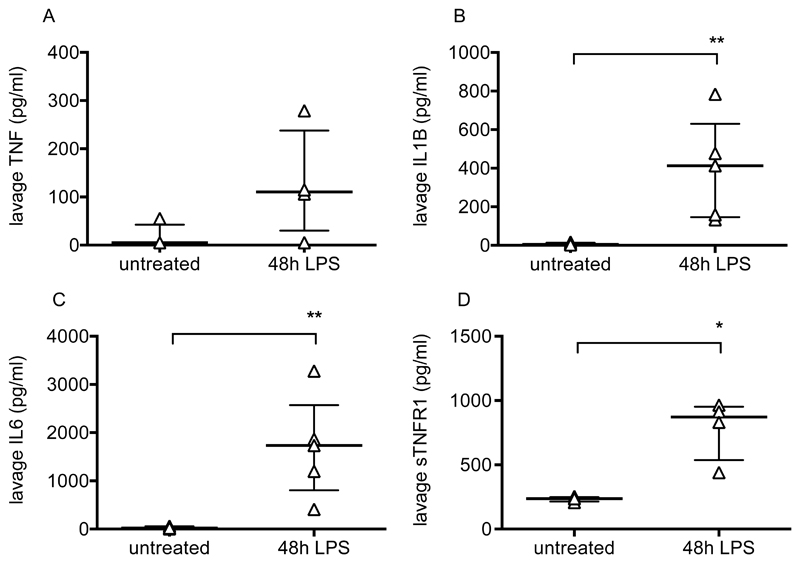
Pulmonary inflammation was determined by flow cytometric evaluation of leukocytes. Neutrophils and monocytes were defined as CD45+, CD11b+ and differentiated based on expression of Ly6C and Ly6G (11). Lung tissue neutrophil and Ly6C+ monocyte numbers changed dramatically during the progression of injury (Fig 2A/B), peaking at 48-72 hours before returning to near normal levels. Alveolar macrophages were also investigated as they play a major role in the regulation of inflammation. These were defined as CD45+, CD11c+, Ly6C-, F4/80 high cells, and confirmed as having positive CD206 (mannose receptor) expression (Fig 2C). While alveolar macrophage numbers did not change substantially (Fig 2D), they dramatically upregulated their expression of CD11b, NOS2 and ICAM-1 (Fig 2E-G). These data are consistent with previous findings (12–14) suggesting polarisation of a subset of macrophages towards a more inflammatory phenotype. Finally, lavage fluid proinflammatory cytokines TNF, IL-1β and IL-6, along with soluble TNF receptor 1, were substantially elevated at 48 hours post LPS (Fig 3).
Figure 2.
Leukocyte changes during LPS-induced lung injury. Lung tissue neutrophils (A) and Ly6C+ inflammatory monocytes (B) were quantified using flow cytometry of lung cell suspensions. Identification of resident alveolar macrophages in lungs 48 hours after LPS treatment is shown in panel C. Macrophages were identified as CD45+ (Ci), CD11c+ (Cii), Ly6C and Ly6G low (Ciii), and F4/80 high (Civ, gate R1). Gate R1 cells were also confirmed as being CD206 (mannose receptor) positive (Cv), while no other cells were. Panel Cvi demonstrates that these cells, definitively identified as resident alveolar macrophages, existed in both CD11b positive and negative populations. Panel D shows alveolar macrophage number in lung tissue, and panel E shows % of resident macrophages positive for CD11b expression. Expression of nitric oxide synthase 2 (NOS2; panel F) and ICAM-1 (Panel G) were evaluated in CD11b+ and CD11b- alveolar macrophages at 48 hours after LPS. N=4-7 for each readout at each time point, so all were treated as non-normally distributed data and are displayed as individual points with median and interquartile range. Data in Panels A, B, D & E were analysed by Kruskal-Wallis test followed by Dunn’s multiple comparisons test; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs 0h (untreated mice). Data in Panels F & G were analysed by Mann-Whitney U-test; **p<0.01. Exact p-values were as follows: Panel A - 24h p=0.0008, 48h p<0.0001, 72h p=0.2191, 96h p>0.9999; Panel B - 24h p=0.1213, 48h p=0.0003, 72h p=0.0003, 96h p=0.4951; Panel D - 24h p>0.9999, 48h p>0.9999, 72h p=0.4571, 96h p=0.4571; Panel E - 24h p=0.0121, 48h p=0.0015, 72h p=0.1059, 96h p>0.9999; Panel F p=0.0079; Panel G p=0.0079.
Figure 3.
Intra-alveolar cytokines after LPS-induced lung injury. Bronchoalveolar lavage fluid levels of TNF (A), IL1-β (B), IL-6 (C) and soluble TNF receptor 1 (D) in untreated mice and mice 48 hours after intranasal LPS. N=4-5 for each group, so all were treated as non-normally distributed data. Data are displayed as individual points with median and interquartile range and were analysed by Mann-Whitney U-test. *p<0.05, **p<0.01. Exact p-values were as follows: Panel A p=0.1429; Panel B p=0.0079; Panel C p=0.0079; Panel D p=0.0286.
Effects of terbutaline in one–hit model of VILI
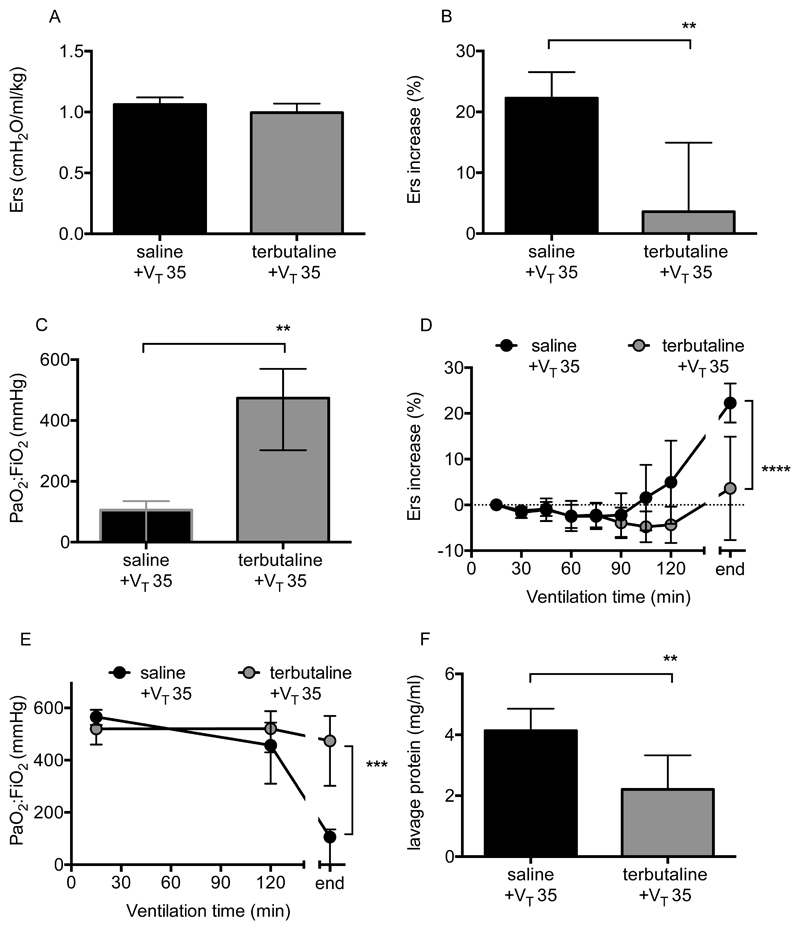
The impact of the β-agonist terbutaline was explored within the one-hit and two-hit models of VILI, specifically because β-agonists have shown contrasting outcomes in simple preclinical models versus ARDS patients. The one-hit model was evaluated to confirm that the dose, route and timing of administration used would produce the anticipated protection within healthy lungs, based on previous studies (6, 7). The initial respiratory system elastance was similar between the vehicle (saline) and terbutaline-treated groups (Fig 4A) but as expected, terbutaline-treated animals were protected from the development of high VT (35ml/kg) induced VILI. The overall increase in elastance (Fig 4B) and deterioration in oxygenation (Fig 4C) were substantially attenuated by terbutaline (p=0.003, p=0.006 respectively). As animals develop injury at different rates we also evaluated the time course of changes in elastance and oxygenation (Fig 4D/E), which were both attenuated by terbutaline (p<0.0001, p=0.0004 respectively). Finally, lavage fluid protein levels were also reduced (Fig 4F; p=0.0041).
Figure 4.
Physiological parameters during pure VILI model following terbutaline treatment. Initial elastance immediately after instillation and setting high VT was no different between saline and terbutaline-instilled animals (A). Panel B shows overall elastance change and Panel C shows final arterial oxygen levels in animals following ventilation with 35ml/kg tidal volume (VT 35). As animals develop injury at different rates, time courses of elastance (D) and oxygenation (E) were also evaluated. Note that ‘end’ relates to the final value, either after 3 hours of ventilation or whenever animals met the mortality surrogate. Panel F shows protein concentration in lavage fluid taken at the end of experiments. N=6/group for all datasets. End-point data in panels A, B & F were normally distributed, analysed by t-test and displayed as mean±SD. Data in Panel C required transformation before analysis and are displayed as back-transformed mean with 95% confidence intervals. *p<0.05, **p<0.01 vs vehicle (saline) by Students t-test. Time-course data in panel D were normally distributed and displayed as mean±SD while data in panel E required transformation before analysis and are displayed as back-transformed mean with 95% confidence intervals. Time courses were analysed by 2-way ANOVA. ***p<0.001, ****p<0.0001 for interaction. Exact p-values were as follows: Panel A p=0.1432; Panel B p=0.0030; Panel C p=0.0064; Panel D p<0.0001 (interaction); Panel E p=0.0004 (interaction); Panel F p=0.0041.
Effects of terbutaline in two–hit model
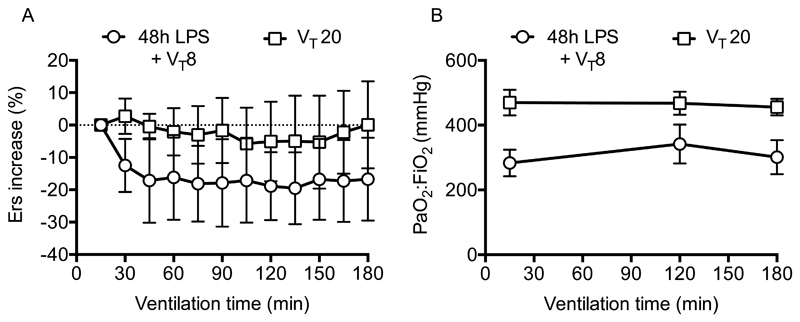
To explore whether the two-hit model provided a closer approximation to the clinical scenario, we chose 48 hours post-LPS as our pre-injury, as this was where most parameters peaked in severity. While patients may not necessarily present at peak injury we felt this best represented an ‘ARDS-like’ dysfunction that would necessitate ventilation. At this point, mice were instrumented and ventilated with a low (8ml/kg) or moderate (20ml/kg) VT. Importantly neither LPS-injured animals ventilated with 8ml/kg, nor healthy animals (without LPS-injury) ventilated with 20ml/kg VT displayed any worsening of respiratory mechanics or oxygenation (Fig 5).
Figure 5.
Time courses of elastance (A) and oxygenation (B) showed no deterioration in either 48h LPS-treated mice ventilated with 8ml/kg (VT 8), or in healthy mice ventilated with 20ml/kg (VT 20). N=6/group for both datasets. Data were normally distributed and displayed as mean±SD.
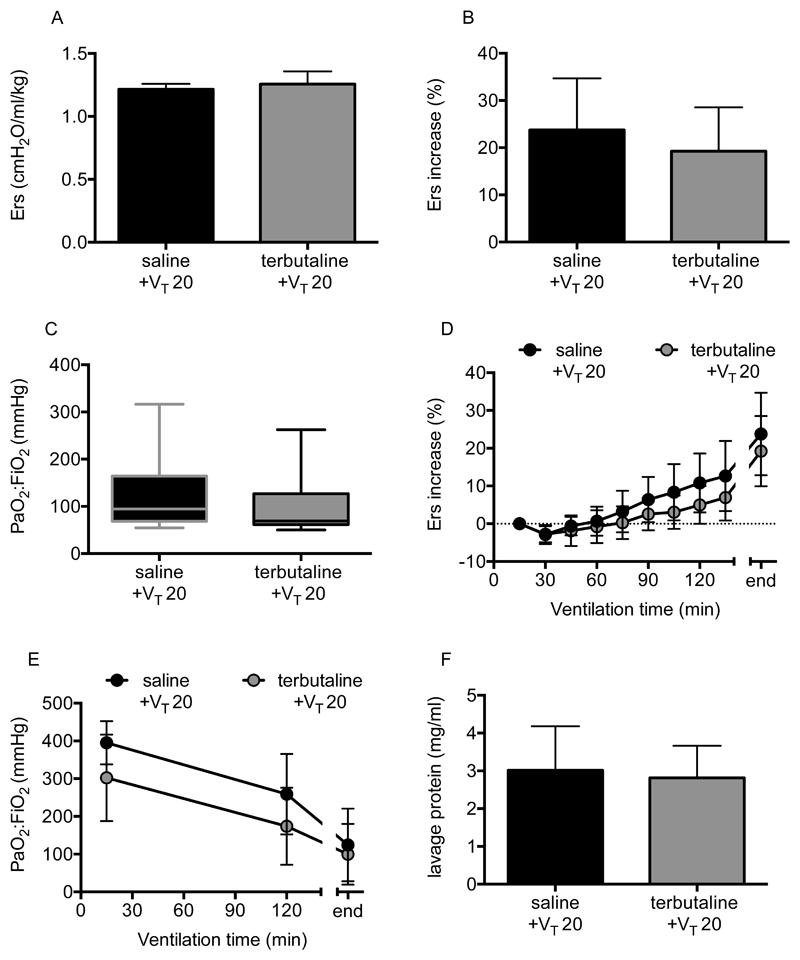
As with the one-hit model, the initial elastance following saline or terbutaline instillation was similar, indicating no immediate effect of the β-agonist within pre-injured lungs (Fig 6A). As anticipated, LPS-injured mice instilled with saline and ventilated with 20ml/kg displayed a substantial deterioration in elastance and oxygenation (Fig 6B-E), demonstrating that pre-injury sensitised the lungs to moderate VT ventilation. Crucially, completely unlike the one-hit model, delivery of terbutaline into pre-injured lungs had no effect to attenuate secondary VILI, evaluated either by physiological parameters (Fig 6B-E) or lavage fluid protein (Fig 6F). These data reflect the lack of effect of β-agonists in clinical ARDS (8, 9), suggesting that this model was less likely to report a false positive result than the simple one-hit model.
Figure 6.
Physiological parameters following terbutaline treatment in two-hit LPS+VILI model. Initial elastance immediately after instillation and setting moderate VT (VT 20) was no different between saline and terbutaline-instilled animals (A). There was no impact of terbutaline treatment on respiratory mechanics or oxygenation, either in terms of overall end-point changes (B, C) or time courses (D, E). Similarly, lavage fluid protein at the end of ventilation was no different (F). N=6/group for all datasets. Data were all normally distributed and displayed as mean±SD, except for panel C which were non-normally distributed and displayed as box-whisker plot. Data were analysed by Students t-test (A, B, F), Mann-Whitney U-test (C) and 2-way ANOVA (D, E) as appropriate. Exact p-values were as follows: Panel A p=0.3798; Panel B p=0.4580; Panel C p=0.2381; Panel D p=0.3711 (interaction); Panel E p=0.3102 (interaction); Panel F p=0.7526.
Effects of TNF receptor-1 blockade in two-hit model of VILI
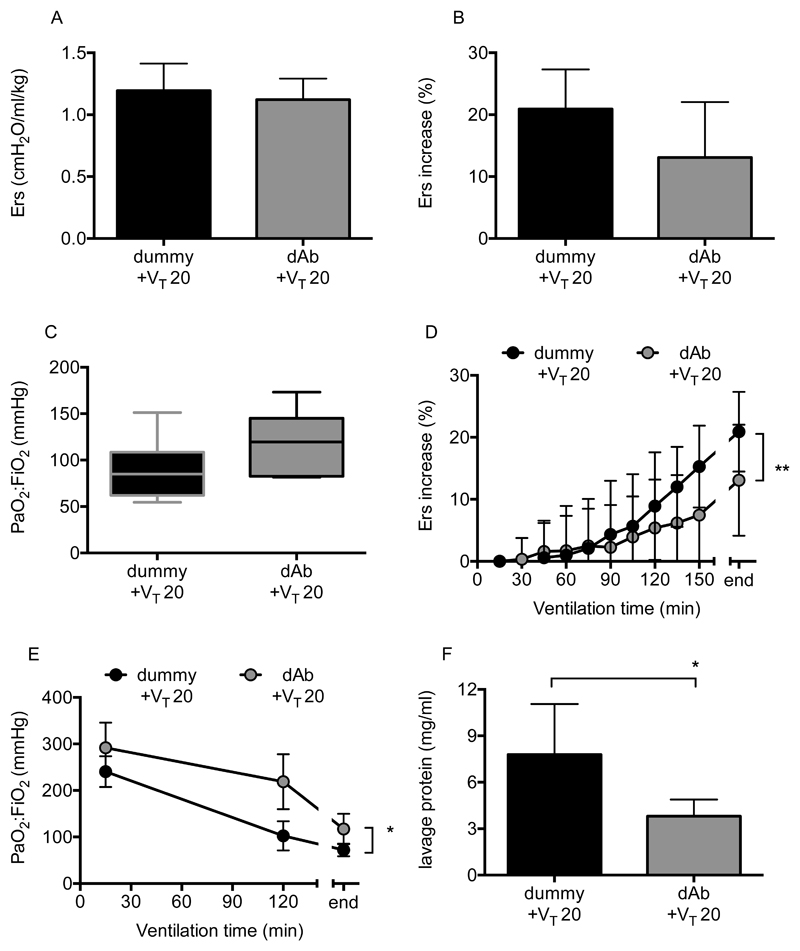
In order to evaluate whether the two-hit model was attenuable with a different pharmacological approach, mice with pre-existing lung injury were randomly instilled with either TNF receptor 1-targeting domain antibody (dAb) or dummy non-targeting antibody, and ventilated using 20ml/kg VT. We have previously shown that this antibody attenuates progression of injury when delivered prophylactically into healthy lungs using various one-hit models (15, 16). Again, elastance immediately after drug instillation (Fig 7A) was no different between groups (p=0.4858). Ventilation of dummy-treated mice with 20ml/kg VT induced substantial deterioration of elastance and oxygenation, but unlike the situation with terbutaline, this secondary VILI was attenuated by TNF receptor 1-blockade. While there was not a major difference (Fig 7B/C) in end-point elastance (p=0.0712) or oxygenation (p=0.0906) following dAb treatment, time-course evaluation (Fig 7D/E) indicated clear attenuation of injury progression (p=0.0089 for elastance, p=0.0399 for oxygenation). Finally, TNF receptor 1-blockade reduced protein permeability (Fig 7F, p=0.0295).
Figure 7.
Physiological parameters following TNF receptor-1 domain antibody (dAb) treatment in two-hit LPS+VILI model. Initial elastance immediately after instillation and setting moderate VT was no different between groups (A). Overall elastance change (B) and final oxygenation (C) showed trends towards improvement with dAb treatment (P<0.1) in mice ventilated with 20ml/kg VT post-LPS. Time courses of elastance change (D) and oxygenation (E) were both significantly attenuated, as was final lavage fluid protein (F). N=6-7/group for all datasets. Data were all normally distributed and displayed as mean±SD, except for panel C which were non-normally distributed and displayed as box-whisker plot. End-point data were analysed by Students t-test (A, B, F) or Mann-Whitney U-test (C). *p<0.05 vs vehicle (dummy). Time course data (D, E) were analysed by 2-way ANOVA. *p<0.05, **p<0.01 for interaction. Exact p-values were as follows: Panel A p=0.4858; Panel B p=0.0712; Panel C p=0.0906; Panel D p=0.0089 (interaction); Panel E p=0.0399 (interaction); Panel F p=0.0295.
Discussion
Despite identification of many therapeutic targets in preclinical models, clinically effective pharmacological treatments for ARDS have remained elusive. This does not mean that animal models are without value – indeed the development of lung protective ventilation, the primary ‘therapy’ for ARDS patients, was based ultimately on animal studies. However it seems clear that while current models are useful for hypothesis generation and exploring biological processes, they appear suboptimal to predict outcomes of pharmacological intervention. Here we describe a model which we feel contains the major components of a predictive scenario. We then explored a potential framework within which to determine the validity of this, and other models.
Due to the heterogeneous nature of ARDS aetiology a range of preclinical models, of varying complexity have been developed. While such variety of models is doubtless useful for exploring aspects of pathobiology, and while none could be thought perfect under all conditions, we would suggest that models intending to predict clinical efficacy should incorporate two particular considerations. Firstly, models should incorporate mechanical ventilation. This is effectively obligatory for ARDS patients, and thus any predictions which do not consider possible interactions between pharmacological intervention and ventilation are likely lacking. Secondly, models must utilise therapeutic dosing into injured lungs. The environment within which agents act in injured lungs is entirely different to that within healthy lungs. The differences are likely to be numerous, but we would envisage three crucial factors. Firstly, alveolar flooding and congestion could be anticipated to impede delivery of inhaled drugs to the site of injury, while intravenous delivery may be complicated by unpredictable regional blood flow distribution (17). Secondly, major changes in the environment within injured lungs, including increases in proinflammatory cytokines, leukocyte influx and changes in the phenotype of resident cells, could all influence the outcome of manipulating pathways. Finally, pre-existing injury leads to initiation of mechanisms which may desensitise pathways either in a protective way, e.g. upregulation of endogenous antagonists and receptor shedding, or as a result of cell damage. Such a consideration was proposed to explain the ‘failure’ of a recent clinical trial of KGF in ARDS patients (18) despite it being beneficial in a range of animal and human preclinical models (19). Crucially, preclinical studies informing the trial demonstrated efficacy of KGF when used as a pretreatment or very early (1hour) post-injury treatment (19–21). Ultimately, authors concluded that KGF may be ineffective in patients as receptors that are present in healthy lungs may not be present on injured epithelium (18). The presence or absence of pre-existing injury will certainly therefore influence the biology of pharmacological agents, and we should not expect similar outcomes. Moreover, modulation of pathways that play important roles in the initiating phases of ARDS may be counterproductive or even deleterious in established disease.
In the current study we attempted to develop and validate a model which incorporates the important considerations outlined above. We chose to induce injury by intranasal administration of LPS, as a direct pulmonary injury/pneumonia-type model. Based on initial development studies, we utilised a time point of 48 hours post-LPS for our two-hit injury model, when mice most resembled a clinical picture of ARDS. They had lost weight, respiratory system elastance was increased, arterial oxygenation deteriorated and alveolar epithelial barrier permeability was increased. There were also dramatic changes in pulmonary leukocyte population and soluble pro-/anti-inflammatory milieu. Onto this we superimposed a relatively moderate (for a mouse) VT of 20ml/kg, which in agreement with previous data (10), did not induce injury within healthy mice over 3 hours. Here however, when applied to mice with established injury, this protocol induced substantial worsening of lung function, indicating sensitisation of the lungs to ventilation. It is worth pointing out that the model does have limitations. Bolus administration of a very high dose of LPS was used as a reproducible, well-studied model but is unlikely to occur clinically. Additionally, the VT used was much higher than would be used in patients, partly as a reflection of the different respiratory mechanics between species (10). However, the model does allow us to explore the impact of pharmacological intervention within an environment that displayed heightened sensitivity to ventilation and was dramatically deviated from normal, both in terms of physiology and inflammation, as is the case within ARDS patients.
We propose that such a ‘substantial deviation from normal’ should be a crucial aspect of models designed to predict or inform clinical use of pharmacological targets, and should be distinguished from more subtle scenarios. For example, a number of studies have utilised two-hit models of intrapulmonary LPS plus ventilation, but have initiated ventilation acutely (<4h) after LPS (22–27) which may be informative but is unlikely to reproduce the biological derangement occurring within ARDS. Even with longer term (up to 24h) LPS exposure, our data and that of others suggests that deterioration of important physiological parameters may not yet be present (28, 29). In studies which explicitly claim that lungs were actually injured before ventilation was initiated (30–34), the primary marker evaluated is lavage fluid protein. However, this alone does not prove a physiologically meaningful lung injury (35). Thus without clear evaluation of the degree of injury, most experimental paradigms exploring biological/immunological mechanisms of ARDS likely utilise regimes equivalent to very early intervention delivered into effectively healthy animals (3).
While logic implies that better prediction of therapeutic outcomes would be achieved within the context of pre-existing injury, it remains difficult to prove this idea as there are no specific biomarkers for VILI or ARDS progression against which a model can be judged. We propose that the best current way to address this is to test a model against interventions which have been evaluated clinically. Here, we took the approach of exploring β-agonist therapy, specifically because this has produced disparate outcomes in clinical trials versus simple preclinical models. β-agonists improve alveolar fluid clearance, reduce permeability and attenuate oedema in models of acute P. aeruginosa pneumonia (4), hyperoxia (5) and VILI (6, 7). However both systemic and intrapulmonary β-agonists were ineffective in clinical trials (8, 9). A model with better predictive power should therefore mirror the lack of efficacy seen clinically, rather than the false-positive findings of simple models. Here we showed that while intratracheal terbutaline reduced injury in a one-hit pure VILI model as expected, it was ineffective when delivered into lungs with established injury. One suggestion for the failure of clinical β-agonist therapy is that the inflammatory environment within ARDS patients may cause β-receptor desensitization (36). Regardless of the mechanism underlying the differences in efficacy between healthy and pre-injured lungs, it is clear that the two-hit model produced a much less ‘misleading’ result compared to simpler models. It is worth clarifying though that for other scenarios, e.g. ventilation of surgical patients without underlying injury, predictions from the pure VILI model may be more valid.
Finally, but importantly, we showed that the lack of efficacy of terbutaline within the two-hit model was not simply because the model was too injurious to be amenable to treatment. We have previously shown efficacy of prophylactic TNF-receptor 1 inhibition using domain antibodies in models of VILI and acid aspiration (15, 16), but here demonstrate that this remains effective when delivered into pre-injured lungs. Interestingly this occurred despite the finding that soluble TNF receptor 1 levels were increased in lavage fluid following LPS (Fig 3). It is not clear whether this soluble TNF receptor-1 upregulation was due to cell-surface receptor shedding (37) or non-specific translocation from plasma (38) but the efficacy of TNF receptor 1 inhibition indicates that receptors remained present on crucial target cells. The mechanisms by which TNF receptor-1 inhibition works remain unclear, but the fact that it was effective within a model which could not be attenuated by β-agonist therapy demonstrates that the mode of action of the two pharmacological agents are different, and that TNF signalling remains important during the progression of VILI even in the context of established injury.
In conclusion, a major reason for the lack of bench to bedside translation in ARDS research is likely to be misplaced confidence in targets. This comes partly from simple models using prophylactic administration of agents, as well as conflation of the concepts of statistical significance and biological importance. There has been much focus on development of models, but in our opinion a greater question relates to their validation. Developing more models without a system in which they can be judged is likely to perpetuate the current stagnation. We would propose a framework wherein knowledge of failed clinical pharmacologics represents an opportunity to validate new models and targets. A good model will be amenable to treatment but provide predictions of failure where that has been shown to happen clinically. A good target molecule will outperform a failed pharmacologic within a good model. We believe that the model described herein fulfils the criteria of a good model in that it incorporates crucial components of ventilation and pre-existing injury, and provided a better prediction of clinical β-agonist outcome than observed in simpler models. Given the heterogeneity of ARDS we would not claim that this particular model would be the most appropriate under all conditions. Indeed it may be considered one step on a pathway including varying strains, sex, age and comorbidities of animals to better reflect the human situation (3). While this will have substantial resource implications, moving quickly from simple models to clinical trials seems to be a false economy. We believe that adopting a framework wherein new models and targets are evaluated alongside interventions with a known clinical outcome, would represent a crucial step towards reducing the likelihood of ineffective treatments being tested in patients.
What is the key question
How can we design models of acute lung injury/acute respiratory distress syndrome to have better predictive power, and evaluate them, in a preclinical setting?
What is the bottom line
A ‘two-hit’ mouse model of acute lung injury, in which therapeutic intervention and ventilation were superimposed onto existing injury, provided better predictions of clinical β-agonist efficacy than when treatment was delivered into healthy lungs in a simple one-hit model.
Why read on
In our study we propose a framework whereby knowledge of failed clinical pharmacologics is used to validate new experimental models and targets, in order to reduce the likelihood of ineffective treatments being tested in patients.
Acknowledgements
The authors would like to acknowledge the support and input from Biopharm Molecular Discovery, GlaxoSmithKline R&D, in particular Dr Joanna Cordy, Dr Tracey Wright, Dr Peter Morley and Dr Andrew Bayliffe.
Funding
This study was supported by funding from GlaxoSmithKline (STU100031322; STU3000031807) and the Biotechnology and Biological Sciences Research Council (BB/L502261; BB/R505274/1). SS was recipient of a clinical research training fellowship from the Medical Research Council/British Journal of Anaesthesia (MR/M018164/1). MWK is supported by the Agency for Science, Technology and Research (A*STAR) NSS (MBBS-PhD) scholarship.
Footnotes
Conflict of Interest Statement
MW and MT received research funding from GlaxoSmithKline to carry out this work. GSK have a financial interest in the use of domain antibodies, including those targeting p55 TNF receptor, in the treatment of pulmonary and other diseases. GSK were involved in the initial design of the study but had no other involvement in collection, analysis, and interpretation of data, or in the decision to submit the report for publication. Other authors declare they have no potential conflicts of interest.
Author Contributions
Conception and design; CMO, KPO, MT, MRW. Data collection, analysis and interpretation; CMO, MWK, SS, RFB, MRW. All authors participated in manuscript revisions and approved the final version.
References
- 1.Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J. 2012;39:1162–70. doi: 10.1183/09031936.00093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol. Lung Cell Mol Physiol. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonniaud P, Fabre A, Frossard N, et al. Optimising experimental research in respiratory diseases: an ERS statement. Eur Respir J. 2018;51 doi: 10.1183/13993003.02133-2017. 1702133. [DOI] [PubMed] [Google Scholar]
- 4.Robriquet L, Kipnis E, Guery B. Beta-adrenergic modulation of lung fluid balance in acute P aeruginosa pneumonia in rats. Exp Lung Res. 2011;37:453–60. doi: 10.3109/01902148.2011.593092. [DOI] [PubMed] [Google Scholar]
- 5.Lasnier JM, Wangensteen OD, Schmitz LS, et al. Terbutaline stimulates alveolar fluid resorption in hyperoxic lung injury. J Appl Physiol. 1996;81:1723–9. doi: 10.1152/jappl.1996.81.4.1723. [DOI] [PubMed] [Google Scholar]
- 6.Saldias FJ, Lecuona E, Comellas AP, et al. beta-adrenergic stimulation restores rat lung ability to clear edema in ventilator-associated lung injury. Am J Respir Crit Care Med. 2000;162:282–7. doi: 10.1164/ajrccm.162.1.9809058. [DOI] [PubMed] [Google Scholar]
- 7.de Prost N, Dreyfuss D, Ricard JD, et al. Terbutaline lessens protein fluxes across the alveolo-capillary barrier during high-volume ventilation. Intensive Care Med. 2008;34:763–70. doi: 10.1007/s00134-007-0954-y. [DOI] [PubMed] [Google Scholar]
- 8.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson MR, Patel BV, Takata M. Ventilation with "clinically relevant" high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit Care Med. 2012;40:2850–7. doi: 10.1097/CCM.0b013e31825b91ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel BV, Wilson MR, O'Dea KP, et al. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol. 2013;190:4274–82. doi: 10.4049/jimmunol.1202437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen WJ, Barthel L, Muldrow A, et al. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mould KJ, Barthel L, Mohning MP, et al. Cell Origin Dictates Programming of Resident versus Recruited Macrophages during Acute Lung Injury. Am J Respir Cell Mol Biol. 2017;57:294–306. doi: 10.1165/rcmb.2017-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel BV, Tatham KC, Wilson MR, et al. In vivo compartmental analysis of leukocytes in mouse lungs. Am J Physiol. Lung Cell Mol Physiol. 2015;309:L639–52. doi: 10.1152/ajplung.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertok S, Wilson MR, Morley PJ, et al. Selective inhibition of intra-alveolar p55 TNF receptor attenuates ventilator-induced lung injury. Thorax. 2012;67:244–51. doi: 10.1136/thoraxjnl-2011-200590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MR, Wakabayashi K, Bertok S, et al. Inhibition of TNF Receptor p55 By a Domain Antibody Attenuates the Initial Phase of Acid-Induced Lung Injury in Mice. Front Immunol. 2017;8:128. doi: 10.3389/fimmu.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelosi P, de Abreu MG. Acute respiratory distress syndrome: we can't miss regional lung perfusion! BMC Anesthesiol. 2015;15:35. doi: 10.1186/s12871-015-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAuley DF, Cross LM, Hamid U, et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med. 2017;5:484–491. doi: 10.1016/S2213-2600(17)30171-6. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol. Lung Cell Mol Physiol. 2002;282:L924–40. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyamsundar M, McAuley DF, Ingram RJ, et al. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189:1520–9. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truscott EA, McCaig LA, Yao LJ, et al. Surfactant protein-A reduces translocation of mediators from the lung into the circulation. Exp Lung Res. 2010;36:431–9. doi: 10.3109/01902141003721440. [DOI] [PubMed] [Google Scholar]
- 23.Altemeier WA, Matute-Bello G, Gharib SA, et al. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol. 2005;175:3369–76. doi: 10.4049/jimmunol.175.5.3369. [DOI] [PubMed] [Google Scholar]
- 24.Yildiz C, Palaniyar N, Otulakowski G, et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology. 2015;122:864–75. doi: 10.1097/ALN.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Guo Y, Zhang L, et al. A Critical Role for P2X7 Receptor-Induced VCAM-1 Shedding and Neutrophil Infiltration during Acute Lung Injury. J Immunol. 2016;197:2828–37. doi: 10.4049/jimmunol.1501041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehead TC, Zhang H, Mullen B, et al. Effect of mechanical ventilation on cytokine response to intratracheal lipopolysaccharide. Anesthesiology. 2004;101:52–8. doi: 10.1097/00000542-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Jones HD, Crother TR, Gonzalez-Villalobos RA, et al. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol. 2014;50:270–80. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wosten-van Asperen RM, Lutter R, Specht PA, et al. Ventilator-induced inflammatory response in lipopolysaccharide-exposed rat lung is mediated by angiotensin-converting enzyme. Am J Pathol. 2010;176:2219–27. doi: 10.2353/ajpath.2010.090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Englert JA, Macias AA, Amador-Munoz D, et al. Isoflurane Ameliorates Acute Lung Injury by Preserving Epithelial Tight Junction Integrity. Anesthesiology. 2015;123:377–88. doi: 10.1097/ALN.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoegl S, Brodsky KS, Blackburn MR, et al. Alveolar Epithelial A2B Adenosine Receptors in Pulmonary Protection during Acute Lung Injury. J Immunol. 2015;195:1815–24. doi: 10.4049/jimmunol.1401957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo S, Shin YJ, Ahmad K, et al. Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury. Am J Physiol. Lung Cell Mol Physiol. 2017;312:L297–l308. doi: 10.1152/ajplung.00305.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzo AN, Sammani S, Esquinca AE, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol. Lung Cell Mol Physiol. 2015;309:L1294–304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rentsendorj O, Damarla M, Aggarwal NR, et al. Knockdown of lung phosphodiesterase 2A attenuates alveolar inflammation and protein leak in a two-hit mouse model of acute lung injury. Am J Physiol. Lung Cell Mol Physiol. 2011;301:L161–70. doi: 10.1152/ajplung.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman JL, Sammani S, Kempf C, et al. Pleiotropic effects of interleukin-6 in a "two-hit" murine model of acute respiratory distress syndrome. Pulm Circ. 2014;4:280–8. doi: 10.1086/675991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol. 2012;91:590–601. doi: 10.1016/j.ejcb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Eisenhut M. Inflammation-induced desensitization of beta-receptors in acute lung injury. Am J Respir Crit Care Med. 2012;185:894. doi: 10.1164/ajrccm.185.8.894. [DOI] [PubMed] [Google Scholar]
- 37.Bertok S, Wilson MR, Dorr AD, et al. Characterization of TNF receptor subtype expression and signaling on pulmonary endothelial cells in mice. Am J Physiol. Lung Cell Mol Physiol. 2011;300:L781–9. doi: 10.1152/ajplung.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorr AD, Wilson MR, Wakabayashi K, et al. Sources of alveolar soluble TNF receptors during acute lung injury of different etiologies. J Appl Physiol. 2011;111:177–84. doi: 10.1152/japplphysiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]