Abstract
Introduction
Lurasidone is an atypical antipsychotic that was approved in Europe in 2014 for the treatment of schizophrenia in adults aged ≥ 18 years. Clinical experience with lurasidone in Europe is currently limited, and there is therefore a need to provide practical guidance on using lurasidone for the treatment of adults with schizophrenia.
Methods
A panel of European psychiatrists with extensive experience of prescribing lurasidone was convened to provide recommendations on using lurasidone to treat adults with schizophrenia.
Results
Extensive evidence from clinical trials and the panel’s clinical experience suggest that lurasidone is as effective as other atypical agents, with the possible exception of clozapine. Lurasidone is associated with a lower propensity for metabolic side effects (in particular, weight gain) and hyperprolactinaemia than most other atypical antipsychotics and has a relatively benign neurocognitive side effect profile. Patients switching to lurasidone from another antipsychotic may experience weight reduction and/or improvements in the ability to focus/concentrate. Most side effects with lurasidone (such as somnolence) are transitory, easily managed and/or ameliorated by dose adjustment. Akathisia and extrapyramidal symptoms may occur in a minority of patients, but these can be managed effectively with dose adjustment, adjunctive therapy and/or psychosocial intervention.
Conclusions
Given the crucial importance of addressing the physical as well as mental healthcare needs of patients, lurasidone is a rational therapeutic choice for adults with schizophrenia, both in the acute setting and over the long term.
Funding
Sunovion Pharmaceuticals Europe Ltd.
Electronic supplementary material
The online version of this article (10.1007/s40120-019-0138-z) contains supplementary material, which is available to authorized users.
Keywords: Antipsychotic, Cardiometabolic side effects, Lurasidone, Schizophrenia, Weight gain
Introduction
Several professional international and national organisations have published guidelines for the treatment of adults with schizophrenia, including the World Federation of Societies of Biological Psychiatry [1, 2], the American Psychiatric Association [3], the National Institute for Health and Care Excellence [4] and the British Association of Psychopharmacology [5, 6]. Key goals of treatment during the acute phase of schizophrenia are to prevent harm, control disturbed behaviour, reduce the severity of psychosis and its associated symptoms, determine and address the factors that led to the occurrence of the acute episode, achieve a rapid return to the best level of functioning and develop an effective alliance with the patient and family [1, 3]. During the stabilisation and maintenance phases, the main goals are to facilitate continued symptom reduction, consolidate and maintain remission, promote the process of recovery, maintain or improve functioning and quality of life and monitor for adverse treatment effects [2, 3].
A common theme among current guidelines is the importance of addressing the physical as well as mental healthcare needs of patients [1–6], in order to ensure that overall health, wellbeing and quality of life are optimised over the long term. Since antipsychotic agents are associated with differential risks of a variety of adverse effects, including extrapyramidal symptoms (EPS) and cardiometabolic, neurocognitive, sexual and endocrinological side effects, there has been an increasing focus on the importance of monitoring for such side effects and of choosing and adapting treatment based on each individual patient’s specific needs, priorities and preferences [1–6].
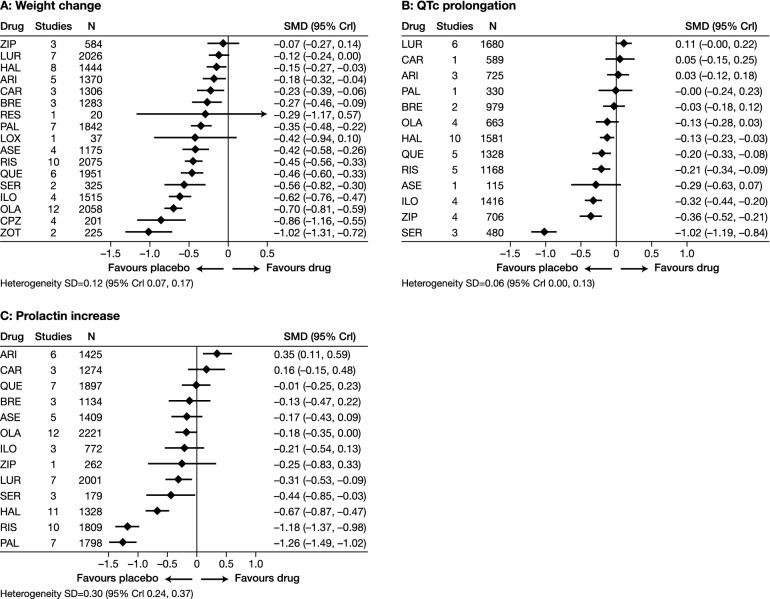
Meta-analysis of available evidence has indicated that, with the exception of clozapine, the efficacy of atypical antipsychotics is broadly similar [7]. Moreover, direct head-to-head evidence from clinical trials, such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study and the Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study (CUtLASS), has demonstrated that the efficacy of atypical agents is also similar to that of typical agents [8, 9]. Atypical antipsychotics differ pharmacologically from typical agents in their lower affinity for dopamine D2 receptors and greater affinities for other neuroreceptors, including the serotonin and noradrenaline receptors [10]. Although this lower affinity for D2 receptors has resulted in atypical antipsychotics being less likely to cause EPS than typical agents, the side effect profiles of available atypical agents differ greatly, particularly with regards to their relative propensities for causing cardiometabolic and endocrinological adverse effects (Fig. 1) [7]. There is also considerable heterogeneity among atypical antipsychotics regarding activating or sedating properties [11]. Predominantly activating medications include lurasidone, and predominantly sedating agents include olanzapine and quetiapine [11]. Selecting the optimal antipsychotic for a given individual therefore needs to be based on the clinical profile of that individual in terms of positive and negative symptoms, as well as on the side effect profiles of the available antipsychotic agents.
Fig. 1.
Effect of single antipsychotics, compared with placebo, on weight change (a), prolongation of the corrected QT interval (QTc) (b) and prolactin increase (c). For some drugs, little data are available, making the results unreliable. For example, the results for weight gain with reserpine are based on only one study with 20 patients. This caused uncertainty about the true effect, which is expressed by a large 95% credible interval (CrI). The effect sizes of the single drugs have not been compared with each other, but 95% CrIs that do not overlap with the y-axis indicate statistically significant differences compared with placebo. ARI Aripiprazole, ASE asenapine, BRE brexpiprazole, CAR cariprazine, CPZ chlorpromazine, HAL haloperidol, ILO iloperidone, LOX loxapine, LUR lurasidone, OLA olanzapine, PAL paliperidone, QUE quetiapine, RES reserpine, RIS risperidone, SD standard deviation, SER sertindole, SMD standardised mean difference, ZIP ziprasidone, ZOT zotepine.
Reproduced from [7] with permission from the American Journal of Psychiatry (copyright© 2017; American Psychiatric Association. All rights reserved)
Lurasidone is an atypical antipsychotic that is approved in Europe for the treatment of schizophrenia in adults aged ≥ 18 years, having received approval for this indication in 2014 [12]. In the USA, lurasidone is approved for the treatment of schizophrenia in adults and adolescents (aged 13–17 years) and for the treatment of bipolar depression in adults, as monotherapy and as adjunctive therapy with lithium or valproate [13]. In comparison with most other atypical antipsychotics, lurasidone has a lower propensity to cause cardiometabolic side effects, such as weight gain and QTc prolongation (Fig. 1) [7]. It is, therefore, a potentially useful treatment option to address both the physical and mental health of patients with schizophrenia.
Since clinical experience with lurasidone in Europe is currently limited, a panel of European psychiatrists with extensive experience of prescribing lurasidone was convened in London in October 2017 to share and discuss their experience and provide practical guidance for using lurasidone for the treatment of adults with schizophrenia. In this article, we outline the recommendations of the panel, within the context of available evidence for the use of lurasidone in this setting. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Pharmacology and Pharmacokinetics of Lurasidone
Lurasidone is a benzisothiazol derivative, with the molecular formula C28H36N4O2S·HCl and molecular weight 529.14 [13]. Similar to most other atypical antipsychotics, lurasidone is an antagonist, with a high affinity for the dopamine D2 and serotonin 5-HT2A (5-hydroxytryptamine 2A) receptors (Table 1) [14, 15] and a slightly lower affinity for the dopamine D3 receptors [15]. As with some other atypical agents (such as aripiprazole), it is also a partial agonist with a high affinity for 5-HT1A receptors [15]. However, lurasidone differs from other atypical antipsychotics in being an antagonist with a high affinity for 5-HT7 receptors [15]. Lurasidone has negligible affinity at the histamine H1 and muscarinic M1 receptors [15], thereby reducing the likelihood of sedation and weight gain [16], but also possibly increasing the risk of inducing EPS [17].
Table 1.
In vitro receptor binding profile of lurasidone compared with other atypical antipsychotics [14]
| Receptora | Antipsychotic | ||||
|---|---|---|---|---|---|
| Lurasidone | Olanzapine | Quetiapine | Risperidone | Aripiprazole | |
| D2 | +++ | ++ | + | +++ |
+++ PA |
| 5-HT1A |
+++ PA |
– |
+ PA |
+ |
+++ PA |
| 5-HT2A | +++ | +++ | ++ | ++++ | ++ |
| 5-HT1C | + | ++ | + | ++ | + |
| 5-HT7 | ++++ | + | ++ | +++ | +++ |
| α1 | ++ | ++ | +++ | +++ | ++ |
| α2a/2c | ++ | +/++ | + | ++/+++ | ++ |
| M1 | – | ++ | ++ | – | – |
| H1 | – | +++ | +++ | ++ | ++ |
Binding strengths are shown for receptor antagonism, unless indicated otherwise: +: weak; ++: moderately strong; +++: strong; ++++ : very strong; PA partial agonist
aD Dopamine receptor, 5-HT 5-hydroxytryptamine (serotonin) receptor, α alpha adrenergic receptor, M muscarinic acetylcholine receptor, H histamine receptor
Lurasidone has an elimination half-life of 20–40 h, consistent with once-daily oral dosing (Table 2) [12]. It reaches peak serum concentrations in approximately 1–3 h and steady state within 7 days [12]. In a food-effect study, lurasidone’s mean maximum plasma concentration (Cmax) and exposure (area under the time–concentration curve) were approximately three- and two-fold greater, respectively, when administered with food versus without food [18]. Lurasidone undergoes hepatic metabolism, mediated primarily by cytochrome P450 (CYP) 3A4 [12]. Although lurasidone is not an inducer or inhibitor of CYP3A4, its plasma levels could be affected by co-administration with inhibitors or inducers of this enzyme [12]. Further details of lurasidone’s pharmacokinetic profile are summarised in Table 2.
Table 2.
| Pharmacokinetic property | Details |
|---|---|
| Absorption | |
| Bioavailability | 9–19% |
| Time to peak plasma concentration | 1–3 h |
| Time to reach steady state | 7 days |
| Distribution | |
| Protein binding (serum proteins) | ~ 99% |
| Metabolism | |
| Route | Hepatic; CYP3A4-mediated |
| Elimination | |
| Route | Faeces (80%); urine (9%) |
| Elimination half-life | 20–40 h |
CYP Cytochrome P450
Clinical Trials of Lurasidone in Adults with Schizophrenia
Lurasidone’s approval as a treatment for schizophrenia was based on the results of a clinical development programme that included five similarly designed, 6-week-long, fixed-dose, placebo-controlled trials and several long-term, open-label maintenance studies (see Electronic Supplementary Material [ESM] Table S1 for details) [19–27]. Details of these clinical trials have been extensively published and reviewed elsewhere [28, 29]; therefore, only a brief overview of key data is provided here.
In the acute treatment setting, lurasidone demonstrated significant reductions in the Positive and Negative Syndrome Scale (PANSS) or Brief Psychiatric Rating Scale (derived from PANSS) total scores, compared with placebo, across the dose range 37–148 mg/day (ESM Table S1) [19–23]. Lurasidone also demonstrated significant improvements versus placebo across a range of secondary outcome measures, including the Clinical Global Impression of Severity (CGI-S) (ESM Table S1). In a post hoc analysis of the PEARL 2 trial, lurasidone was shown to be not significantly different from olanzapine in terms of both PANSS total score and CGI-S score [22]. The most commonly observed side effects observed in adults with schizophrenia in the short-term trials (incidence ≥ 5% and at least twice the rate for placebo) were somnolence, akathisia, EPS and nausea [13].
The effectiveness and safety/tolerability of lurasidone as a maintenance treatment for adults with schizophrenia were established in an open-label extension study of an initial phase III trial [24]; a 12-month, double-blind, non-inferiority study versus quetiapine extended-release (XR) [25]; a double-blind, placebo-controlled, randomised withdrawal study [26]; and a double-blind, active-controlled safety study versus risperidone [27] (ESM Table S1). In these studies, lurasidone demonstrated sustained antipsychotic efficacy for up to 12 months of treatment. In the 12-month PEARL 3 extension study, lurasidone was shown to be non-inferior to quetiapine XR in terms of risk of relapse and significantly superior to quetiapine XR in terms of risk of rehospitalisation [25]. In the 12-month safety study versus risperidone, a comparable improvement in efficacy measures was observed with lurasidone and risperidone, and the rates of relapse were similar [27]. However, the proportion of patients who experienced ≥ 7% increase in body weight with lurasidone was half that observed with risperidone (7 vs. 14%), and the median increase in prolactin from baseline was significantly higher for risperidone than for lurasidone (p < 0.001) [27]. In the PEARL 2 extension study, patients who gained weight following treatment with olanzapine in the initial acute trial experienced decreased weight and improvements in lipid levels after switching to lurasidone, while those treated with lurasidone or placebo in the acute trial experienced minimal changes during maintenance treatment with lurasidone [24]. Overall, lurasidone was not associated with clinically significant changes in cardiometabolic parameters following acute and maintenance treatment in its clinical development programme.
Choosing Lurasidone Versus Other Agents to Treat Adults with Schizophrenia
Clinical trials are essential for the development and approval of new antipsychotics, but the results do not necessarily reflect the effectiveness and tolerability of an agent when used in clinical practice. In particular, clinical trials actively select patient populations that are relatively homogeneous, and they typically employ rigid dosing and titration schedules, whereas patients encountered in clinical practice have diverse clinical characteristics, necessitating individualised treatment. Real-world studies therefore provide useful complementary evidence by demonstrating an agent’s effectiveness when used under everyday clinical practice conditions. In the case of lurasidone, there is currently a lack of published real-world data and, consequently, a need for guidance from those experts with clinical experience of using lurasidone in this setting.
Efficacy
The opinion of the panel is that lurasidone is efficacious in both the acute and maintenance phases of treatment of schizophrenia in adults. Extensive evidence from lurasidone’s clinical development programme (ESM Table S1) and the panel’s clinical experience suggest that it is as effective as other atypical agents, possibly with the exception of clozapine. In addition, lurasidone may be an option in patients who have previously not responded to other atypical antipsychotics. Since other approved antipsychotics are also effective in treating adults with schizophrenia, it is primarily the side effect profile of lurasidone that differentiates it from other agents.
Low Propensity for Metabolic Side Effects
Individuals with schizophrenia already have disease-related increased risks of metabolic problems, including obesity, hyperlipidaemia and diabetes, which are frequently exacerbated by the adverse metabolic side effects of antipsychotic therapy [30, 31]. Antipsychotic-induced weight gain is particularly distressing for patients, decreasing self-esteem and treatment adherence and increasing the likelihood of depression.
The opinion of the panel is that lurasidone is associated with a lower propensity for metabolic side effects (in particular, weight gain) than most other atypical antipsychotics. Furthermore, overweight patients may lose weight when treated with lurasidone, although the panel also acknowledges that a minority of patients may gain weight while taking lurasidone. During the 6-week lurasidone clinical studies, approximately 5% of patients experienced a ≥ 7% weight gain (Sunovion Pharmaceuticals Europe Ltd, data on file).
Drug-induced prolongation of the QT interval is associated with an increased risk of cardiovascular mortality, and evidence has shown that there is considerable variation between atypical antipsychotics regarding their propensities for causing QT interval prolongation [7, 32]. In a meta-analysis of evidence from randomised controlled trials (RCTs), observational studies and post-marketing surveillance studies, all conducted in patients with mental disorders treated with six atypical antipsychotics (aripiprazole, brexpiprazole, olanzapine, quetiapine, risperidone and ziprasidone), aripiprazole, brexpiprazole and olanzapine were found not to increase the QT interval [32]. In another meta-analysis of RCTs that included a wider range of antipsychotics, lurasidone was found to have the most favourable profile in terms of prolongation of the corrected QT interval (QTc) (Fig. 1) [7]. Lurasidone therefore does not appear to have a detrimental impact on QTc [7, 12], and none of the panel has observed clinically significant QTc prolongation with lurasidone treatment to date. As with all antipsychotics, however, the QTc should be monitored before and after initiating treatment with lurasidone (see section Monitoring of Physical Health).
In accordance with current guidelines regarding the core importance of physical health [1–6], the panel is in agreement that lurasidone’s favourable metabolic profile, in comparison with those of most other antipsychotics, is a major consideration for long-term maintenance treatment and is therefore a key reason for considering lurasidone, for both clinicians and patients. Choosing an agent with a favourable metabolic profile can lead to improvements in physical health and wellbeing, self-esteem, quality of life and treatment satisfaction, which in turn increase the likelihood of treatment adherence and thereby reduce the risk of relapse and rehospitalisation.
Low Propensity for Hyperprolactinaemia
Hyperprolactinaemia is associated with a range of adverse effects, including sexual dysfunction [33]. Moreover, sexual dysfunction related to antipsychotic-induced hyperprolactinaemia is an important driver of treatment non-adherence in schizophrenia [34]. Lurasidone has a relatively neutral impact on prolactin levels in comparison with other antipsychotics [7]. Experience with lurasidone did not show the presence of elevated levels of prolactin in a case series of over 30 patients in clinical practice (Afzal Javed, personal communication, 2019).
Low Propensity for Neurocognitive Side Effects
As with metabolic problems, neurocognitive dysfunction in schizophrenia comprises both illness- and medication-related effects. Many antipsychotics have detrimental neurocognitive side effects [35], and neurocognitive side effects are a major cause of antipsychotic non-adherence [36]. Importantly, neurocognition is a key mediator of functional outcome in schizophrenia [37–39].
The opinion of the panel is that patients who switch to lurasidone may experience improvements in the ability to focus/concentrate/‘start their thoughts’. In the panel’s clinical experience, in some patients, such improvements have been confirmed using objective tests, supporting some limited evidence from clinical trials suggesting that lurasidone treatment may be associated with improvements in executive functioning [40, 41]. However, such limited data must be viewed with caution. Since neurocognitive side effects are a common complaint of patients, particularly young patients, lurasidone’s relatively benign neurocognitive side effect profile may potentially be an important factor to consider when choosing treatment.
Long-Term Wellbeing
The opinion of the panel is that the focus of treatment should, from its outset, be the long-term wellbeing of the patient. The overall effectiveness of an agent is not just dependent on its efficacy and tolerability, but on a global impression of treatment satisfaction that also encompasses a patient’s functional status (social life, ability to work, etc.), wellbeing and quality of life. The panel would like to see quality of life as a primary outcome that is systematically measured in future research.
The overall prognosis of schizophrenia is dependent on long-term treatment adherence. It is important to ensure that the initial choice of treatment is correct, since patients often remain on medication that gives a good initial response. Moreover, a negative experience in the early stage of treatment can damage the long-term patient–doctor relationship. Appropriate choice of initial treatment will help optimise the likelihood of long-term adherence. Good cooperation and communication between acute and community services is required to ensure that patients receive the most appropriate treatment from the outset and do not continue to take medication over the long term that has adverse metabolic sequelae. Features of lurasidone considered most likely to encourage adherence and help optimise the long-term wellbeing of patients are its long-term efficacy, low propensity for metabolic side effects (particularly weight gain) and hyperprolactinaemia and relatively benign neurocognitive side effect profile. Naturalistic studies have provided further insights into adherence to lurasidone treatment. A real-world assessment of treatment adherence in patients with schizophrenia, based on insurance claims, demonstrated that adherence to lurasidone was better than adherence to other oral atypical antipsychotics [42]. In a subsequent prospective, non-interventional, observational study of patients consecutively prescribed lurasidone in a UK mental health trust, three variables were found to be significantly associated with treatment discontinuation: treatment resistance at initiation of lurasidone, poor tolerability to the antipsychotic prescribed immediately prior to initiating lurasidone and low lurasidone doses, compared with medium and high doses [43]. The authors concluded that adherence to lurasidone is likely to be improved by early dose optimisation and by targeting patients who are most likely to benefit from treatment (i.e. those without evidence of treatment resistance) [43] (see section Dosing for more information on the importance of early dose optimisation).
Given lurasidone’s efficacy (as acute and maintenance therapy) and side effect profile—in particular its relatively low propensity for causing adverse metabolic effects—the opinion of the panel is that it is appropriate to consider lurasidone early in the course of treatment for adults with schizophrenia. First-episode patients can be treated effectively with lurasidone and continue to receive lurasidone as long-term maintenance therapy.
Considerations when Using Lurasidone to Treat Adults with Schizophrenia
Dosing
Lurasidone is formulated as film-coated tablets at dose strengths of 18.5, 37 and 74 mg [12]. The opinion of the panel is that, for the majority of patients, lurasidone should be initiated without titration at a starting dose of 37 mg/day and increased as appropriate, according to response and tolerability, within the dose range 37–148 mg/day. Dosing during maintenance treatment should continue in the dose range 37–148 mg/day, according to response and tolerability. This applies to both previously untreated patients and those switching from other antipsychotics. The panel also acknowledges that if there are tolerability concerns, such as a previous history of EPS or akathisia, then it may be appropriate to adjust dosing for a short period of time before up-titrating again. In patients with moderate or severe hepatic impairment, moderate or severe renal impairment or end-stage renal disease, and/or those taking moderate CYP3A4 inhibitors, a lurasidone starting dose of 18.5 mg/day is recommended and the maximum dose should not exceed 74 mg/day (37 mg/day in patients with severe hepatic impairment) [12].
A randomised, double-blind, placebo-controlled trial conducted in adults with schizophrenia demonstrated that, in patients showing non-response to 2 weeks of treatment with lurasidone 74 mg/day (defined as < 20% improvement from baseline in the PANSS total score), a dose increase to 148 mg/day resulted in significant symptom improvement, compared with continuing treatment with lurasidone 74 mg/day [44]. Patients showing an insufficient response to treatment within 2 weeks should therefore be up-titrated gradually to a maximum dose of 148 mg/day, consistent with this clinical trial evidence [44]. This approach should be adopted before a switch to an alternative treatment is considered.
Switching to Lurasidone from Other Antipsychotics
The opinion of the panel is that, in general, a cross-titration approach should be used when switching to lurasidone from another antipsychotic. The previous antipsychotic should be gradually down-titrated as lurasidone is gradually up-titrated over a period of approximately 2 weeks. Since the elimination half-life of lurasidone is 20–40 h [12], its full clinical effect may not be observed until after 1 week of treatment with an adequate dose. In patients switching to lurasidone due to tolerability problems, a faster cross-titration may be used. Conversely, in those switching from an antipsychotic that is particularly sedating (e.g. olanzapine, quetiapine), a longer cross-titration may be preferable in order to minimise the likelihood of rebound effects. In patients switching from aripiprazole to lurasidone, cross-titration or an abrupt switch approach can be used, since aripiprazole has a long half-life and can take up to 16–19 days to be fully eliminated. For this reason, patients switching from aripiprazole due to side effects should be informed that they may continue to experience these side effects for some time after stopping aripiprazole treatment.
Requirement for Food and Timing of Administration
Lurasidone should be taken once daily with a meal [12], since food improves its absorption. If taken without a meal of at least 350 calories, exposure to lurasidone is substantially decreased [13, 18] and the patient will therefore not receive the appropriate dose.
In the panel’s clinical experience, lurasidone can be taken at any time during the day, as long as it is taken with a meal. The optimal timing of administration will depend on patient choice. Some patients find that taking lurasidone with their evening meal decreases the impact of side effects, such as akathisia and somnolence. However, since lurasidone may cause insomnia in some patients, others find that morning dosing is preferable. Changing the timing of administration can therefore be helpful in improving a patient’s experience of taking lurasidone and may help avoid treatment non-adherence. The requirement to take lurasidone with a meal can help patients establish a daily routine, which may also improve adherence.
Management of Extrapyramidal Symptoms
Initial data and clinical experience suggest that the risk for EPS (acute dystonic reaction, akathisia, parkinsonism and tardive dyskinesia) with lurasidone, although low, may be at least equivalent and perhaps slightly higher than for the ‘average’ atypical antipsychotic [7, 17]. As such, the risk of EPS with lurasidone should be considered and the potential development of EPS assessed for and monitored. If EPS develop, they should be managed by reducing the dose of lurasidone, in accordance with existing guidelines [3, 45, 46]. Nevertheless, evidence from clinical trials and extensive clinical practice experience in the USA indicates that the risk of tardive dyskinesia with lurasidone is low [12]. The members of the panel have not observed any cases of tardive dyskinesia with lurasidone to date, but it is too early to comment on this possibility.
As with other antipsychotics, lurasidone treatment may be associated with akathisia. In clinical trials, the incidence of akathisia was 12.9% with lurasidone versus 3.0% with placebo [12]. The opinion of the panel is that, in clinical practice, akathisia may be observed with lurasidone treatment in a minority of patients, particularly when first initiated. However, approaches can be taken to minimise the risk of akathisia occurring and effectively manage it in the event of its occurrence.
In patients at risk of developing akathisia (such as young adults, the elderly, those with a history of drug/substance abuse and those with a previous history of akathisia with other agents), the panel recommends adjusting the dose for a short period of time before up-titrating again. Akathisia is also reported less frequently in patients who take lurasidone in the evening, as opposed to earlier in the day. The panel also recommends using the Barnes rating scale [47] if akathisia is suspected, since this scale is useful in differentiating akathisia from other conditions (e.g. anxiety, agitation), effective in identifying mild cases of akathisia (when it can manifest as an ‘inner urge to move’, which is often distressing for patients but difficult for them to describe) and helpful in benchmarking and monitoring progress. Proactively asking a patient’s opinion about the causality of their symptoms can be useful in differentiating akathisia from conditions such as anxiety.
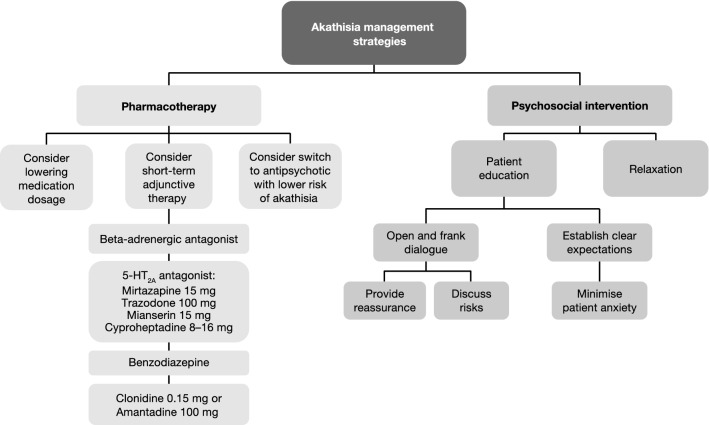
The panel highlights that akathisia is unlikely to respond to treatment with anticholinergic agents, unless patients experience concomitant parkinsonism [48]. If akathisia occurs, it can, however, be effectively managed in a variety of ways (Fig. 2). The lurasidone dose may be decreased for a number of weeks and slowly up-titrated again. Short-term adjunctive therapy can also be considered, in the following order:
a β-adrenergic antagonist (‘beta blocker’);
a 5-HT2A antagonist, such as mirtazapine (15 mg) [49], trazodone (titrate up to 100 mg over 5 days), mianserin (15 mg) or cyproheptadine (8–16 mg);
a benzodiazepine;
clonidine (0.15 mg) or amantadine (100 mg).
Fig. 2.
Approaches for managing akathisia (Lars Hansen, personal communication, 2019). 5-HT2A 5-Hydroxytryptamine (serotonin) receptor 2A
Concomitant with these pharmacological approaches, psychosocial intervention should be employed. Patients should be proactively informed of the potential risk of akathisia and reassured that, if it occurs, it is likely to be of short duration and can be managed effectively. Management of patient expectations can be very effective in alleviating the impact of akathisia, if it occurs. The use of a relaxation/stretching technique [50] can also be beneficial in addition to pharmacological and psychosocial intervention. If the above approaches are ineffective, it may be appropriate to consider switching to an antipsychotic with a lower risk of akathisia.
Management of Other Side Effects
In clinical studies of lurasidone 18.5–148 mg/day in patients with schizophrenia treated for up to 52 weeks, and in the post-marketing setting, the most common side effects (≥ 10% patients) were akathisia and somnolence [12]. These side effects were dose related up to 111 mg/day. Other side effects reported by patients in lurasidone clinical studies with an incidence of ≥ 5% and at least twice the rate observed in patients treated with placebo were nausea and EPS [13].
In the panel’s clinical experience, most side effects with lurasidone are transitory, easily managed and/or ameliorated by adjusting the patient’s dose. Somnolence/sedation is associated with many antipsychotic treatments, and lurasidone is less sedating than other atypical antipsychotics, such as quetiapine, olanzapine and risperidone [7], even at a high dose level. As mentioned previously (see section Dosing), in patients switching to lurasidone from an antipsychotic that is particularly sedating, a longer cross-titration may help to minimise the likelihood of rebound effects, such as insomnia. In newly treated patients, evening dosing with lurasidone may help minimise the impact of somnolence. In the panel’s clinical experience, nausea is very rarely reported with lurasidone treatment. This may in part be due to the requirement to take lurasidone with a meal.
Monitoring of Physical Health
The panel is in agreement that guidelines relating to the monitoring of patients’ physical health, such as those formulated by the British Association of Psychopharmacology (Table 3) [6] and The Maudsley Group [51], should be followed. These include the measurement of body mass index, glycated haemoglobin, lipids and blood pressure before the patient is started on antipsychotic treatment and at regular intervals after treatment is commenced [6, 51]. Although there is no signal for QTc prolongation with lurasidone treatment, as with all antipsychotics, QTc should be measured before and after starting lurasidone treatment, in line with existing guidelines (such as The Maudsley Prescribing Guidelines [51]).
Table 3.
Summary of British Association of Psychopharmacology recommendations for monitoring physical health risk factors [6]
| • The measurements below should be assessed before starting an antipsychotic medication, or as soon as possible afterwards, and then at the intervals indicated • If there is a change in antipsychotic medication then, when clinically relevant, it is appropriate to revisit all the steps outlined below | ||
|---|---|---|
| Measurement | Monitoring | Key interventions |
| Weight and BMI |
• BMI should be used to monitor whether an individual is becoming overweight or obese • Weight should be measured frequently during the early stage of treatment: ideally weekly for first 4–6 weeks and then every 2–4 weeks up to 12 weeks (at a minimum, once every 4 weeks for first 12 weeks of treatment) • Weight and BMI should then be assessed at 6 months and at least annually thereafter, unless clinical situation demands more frequent assessment • It is important to take ethnicity into account when evaluating BMI results |
• Lifestyle interventions: these should always be part of the first-line approach and, in most cases, continued with any additional intervention • Antipsychotic switching: switching to an antipsychotic with a lower propensity for weight gain should be considered • Adjunctive aripiprazole: recommended as a possible intervention for weight gain associated with clozapine and olanzapine • Adjunctive metformin: should be considered to attenuate or reduce weight gain following antipsychotic treatment |
| Glucose control |
• Long-term blood glucose control should be monitored using HbA1c • In the early weeks of treatment, fasting or random plasma glucose may provide a more appropriate measure of glucose control • Glucose control should be further assessed at 12 weeks, 6 months and then annually |
• Annual screening for pre-diabetic states is recommended for individuals with psychosis receiving antipsychotic medications • Diabetes should be managed as per NICE guidelines |
| Lipid profile |
• Lipid profile should be assessed at 12 weeks, 6 months and then annually • Total cholesterol/HDL cholesterol ratio will be required to assess cardiovascular risk • Random rather than fasting sampling can be used if a fasting sample cannot be obtained |
• Dyslipidaemia should be actively managed according to NICE guidelines • Use of statins is not contraindicated in people treated with antipsychotics |
| Blood pressure | • Blood pressure should be monitored at 12 weeks, 6 months and then annually | • Hypertension should be managed according to NICE guidelines |
| Tobacco smoking and alcohol use | • Tobacco smoking and alcohol use should be enquired about at all opportunities |
• Those who smoke should be referred to smoking cessation services • Alcohol and other substance use should be assessed and treatment focussed on any harmful substance use, abuse or dependence • Optimisation of antipsychotic treatment may play a role in reducing substance misuse |
BMI Body mass index, HbA1c glycated haemoglobin, HDL high-density lipoprotein, NICE National Institute of Health and Care Excellence
As previously mentioned (see section Low Propensity for Hyperprolactinaemia), lurasidone has a modest impact on prolactin levels in comparison with other antipsychotics [7]. As with all antipsychotics, it is nevertheless prudent to monitor prolactin levels in patients treated with lurasidone, particularly since apparently asymptomatic patients may have increased prolactin levels that can have a detrimental impact over the long term (e.g. decreased bone mineral density, increased risk of breast cancer) [33]. Hyperprolactinaemia is associated with a range of adverse effects, including sexual dysfunction, irregular menstrual cycle, abnormal semen production, infertility, galactorrhoea and hirsutism [33]. In the panel’s clinical experience, sexual dysfunction is one of the most important drivers of treatment non-adherence. Patients should therefore be proactively asked about sexual side effects, since they are unlikely to spontaneously volunteer the information.
Shared Decision Making
The panel highlights that, wherever possible, patients should be involved in decisions relating to their treatment. The clinical team should make efforts to inform and support patients/families/carers in the decision-making process in order to ensure that their expectations are managed and their needs and wishes are taken into account. In addition to its proven efficacy, lurasidone’s low propensities for metabolic side effects (particularly weight gain), hyperprolactinaemia and neurocognitive side effects are key reasons why patients may choose this medication, if they are educated on these features of lurasidone. Patients/families/carers should also be fully informed about the potential side effects that can occur with lurasidone treatment and educated on how to manage such side effects, if they occur. Long-term adherence is dependent on addressing the specific individual needs of the patient. Management of expectations is a key aspect of care that can greatly impact patients’ sense of treatment satisfaction and reduce the likelihood of non-adherence.
Conclusions
Based on current evidence and clinical experience, the opinion of the panel is that lurasidone is an effective atypical antipsychotic for the treatment of adults with schizophrenia, with a favourable side effect profile, characterised by a low propensity for metabolic side effects and hyperprolactinaemia, and a benign neurocognitive side effect profile. Most of the side effects most commonly associated with lurasidone, such as somnolence, are transitory and easily managed. Akathisia and EPS may occur in a minority of patients, but these too can be effectively managed with dose adjustment, adjunctive therapy and/or psychosocial intervention. Given the increasing recognition of the importance of physical as well as mental health in the management of schizophrenia, lurasidone’s effectiveness and side effect profile make it a rational therapeutic choice for adults with schizophrenia, both in the acute setting and over the long term. Importantly, its favourable side effect profile addresses some of the key problems patients have with some other atypical antipsychotics—such as weight gain and neurocognitive adverse effects—and therefore may help encourage long-term treatment adherence, thereby reducing the likelihood of relapse and rehospitalisation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The panel meeting for the development of this article was organised and funded by Sunovion Pharmaceuticals Europe Ltd. Article processing charges were also funded by Sunovion Pharmaceuticals Europe Ltd.
Medical writing, Editorial, and Other Assistance
Editorial support for the preparation of the article was provided by John Scopes of mXm Medical Communications. Support for this assistance was funded by Sunovion Pharmaceuticals Europe Ltd.
Authorship
The article is based on the discussions from the panel meeting. The opinions expressed in the article are the independent views of the authors and have not been influenced by third-party sponsorship. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors received an honorarium for attendance of the panel meeting. Afzal Javed has received speaker fees from, and undertaken consultancy work and the organisation of scientific meetings for, Sunovion and Lundbeck, over the past 3 years. Holger Arthur has nothing to declare. Logos Curtis has received honoraria for advisory boards, talks and support to attend conferences from Lundbeck, Otsuka, Servier, Sunovion, Takeda and Vifor. Lars Hansen has received speaker fees from Sunovion, Lundbeck and Janssen, and acted as a consultant for Sunovion and Janssen, over the past 5 years. Sofia Pappa has received speaker fee and conference support from Janssen and Sunovion.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8057405.
References
- 1.Hasan A, Falkai P, Wobrock T, World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 2.Hasan A, Falkai P, Wobrock T, World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14:2–44. doi: 10.3109/15622975.2012.739708. [DOI] [PubMed] [Google Scholar]
- 3.Lehman AF, Lieberman JA, Dixon LB, American Psychiatric Association; Steering Committee on Practice Guidelines et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(Suppl 2):1–56. [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Clinical Guideline 178. Psychosis and schizophrenia in adults: prevention and management, https://www.nice.org.uk/guidance/cg178/resources/psychosis-and-schizophrenia-in-adults-prevention-and-management-pdf-35109758952133. 2014. Accessed 24 Apr 2019. [PubMed]
- 5.Barnes TR, Schizophrenia Consensus Group of British Association for Psychopharmacology Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:567–620. doi: 10.1177/0269881110391123. [DOI] [PubMed] [Google Scholar]
- 6.Cooper SJ, Reynolds GP, With expert co-authors (in alphabetical order) Barnes T, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016;30:717–748. doi: 10.1177/0269881116645254. [DOI] [PubMed] [Google Scholar]
- 7.Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–942. doi: 10.1176/appi.ajp.2017.16121358. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JA, Stroup TS, McEvoy JP, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 9.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto S, Miyake N, Jarskog LF, et al. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 11.Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37:138–147. doi: 10.1097/JCP.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 12.Sunovion Pharmaceuticals Europe Ltd. Latuda® summary of product characteristics. https://www.medicines.org.uk/emc/medicine/29125. 2019. Accessed 24 Apr 2019.
- 13.Sunovion Pharmaceuticals Inc. Latuda® prescribing information. http://www.latuda.com/LatudaPrescribingInformation.pdf. 2018. Accessed 24 Apr 2019.
- 14.Stahl SM. Stahl’s essential pharmacotherapy: neuroscientific basis and practical applications. 4. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 15.Greenberg WM, Citrome L. Pharmacokinetics and pharmacodynamics of lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet. 2017;56:493–503. doi: 10.1007/s40262-016-0465-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Maneen MJ, Stahl SM. Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics. 2009;6:78–85. doi: 10.1016/j.nurt.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. doi: 10.2147/TCRM.S117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preskorn S, Ereshefsky L, Chiu YY, et al. Effect of food on the pharmacokinetics of lurasidone: results of two randomized, open-label, crossover studies. Hum Psychopharmacol. 2013;28:495–505. doi: 10.1002/hup.2338. [DOI] [PubMed] [Google Scholar]
- 19.Ogasa M, Kimura T, Nakamura M, et al. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl) 2013;225:519–530. doi: 10.1007/s00213-012-2838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:829–836. doi: 10.4088/JCP.08m04905. [DOI] [PubMed] [Google Scholar]
- 21.Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47:670–677. doi: 10.1016/j.jpsychires.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168:957–967. doi: 10.1176/appi.ajp.2011.10060907. [DOI] [PubMed] [Google Scholar]
- 23.Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101–109. doi: 10.1016/j.schres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507–515. doi: 10.4088/JCP.12m08084. [DOI] [PubMed] [Google Scholar]
- 25.Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147:95–102. doi: 10.1016/j.schres.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30:69–77. doi: 10.1177/0269881115620460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27:165–176. doi: 10.1097/YIC.0b013e32835281ef. [DOI] [PubMed] [Google Scholar]
- 28.Harvey PD. The clinical utility of lurasidone in schizophrenia: patient considerations. Neuropsychiatr Dis Treat. 2015;11:1103–1109. doi: 10.2147/NDT.S68417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruijnzeel D, Yazdanpanah M, Suryadevara U, et al. Lurasidone in the treatment of schizophrenia: a critical evaluation. Expert Opin Pharmacother. 2015;16:1559–1565. doi: 10.1517/14656566.2015.1058780. [DOI] [PubMed] [Google Scholar]
- 30.Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007;68(Suppl 1):20–27. [PubMed] [Google Scholar]
- 31.Dayabandara M, Hanwella R, Ratnatunga S, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–2241. doi: 10.2147/NDT.S113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronow WS, Shamliyan TA. Effects of atypical antipsychotic drugs on QT interval in patients with mental disorders. Ann Transl Med. 2018;6:147. doi: 10.21037/atm.2018.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28:421–453. doi: 10.1007/s40263-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer MK, Castelein S, Wiersma D, et al. The facts about sexual (Dys)function in schizophrenia: an overview of clinically relevant findings. Schizophr Bull. 2015;41:674–686. doi: 10.1093/schbul/sbv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longden E, Read J. Assessing and reporting the adverse effects of antipsychotic medication: a systematic review of clinical studies, and prospective, retrospective, and cross-sectional research. Clin Neuropharmacol. 2016;39:29–39. doi: 10.1097/WNF.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 36.Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. doi: 10.2147/PPA.S124658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Green MF, Llerena K, Kern RS. The “Right Stuff” revisited: what have we learned about the determinants of daily functioning in schizophrenia? Schizophr Bull. 2015;41:781–785. doi: 10.1093/schbul/sbv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuechterlein KH, Subotnik KL, Green MF, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(Suppl 2):S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey PD, Ogasa M, Cucchiaro J, et al. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res. 2011;127:188–194. doi: 10.1016/j.schres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Harvey PD, Siu CO, Hsu J, et al. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;23:1373–1382. doi: 10.1016/j.euroneuro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Rajagopalan K, Wade S, Meyer N, Loebel A. Real-world adherence assessment of lurasidone and other oral atypical antipsychotics among patients with schizophrenia: an administrative claims analysis. Curr Med Res Opin. 2017;33:813–820. doi: 10.1080/03007995.2017.1284656. [DOI] [PubMed] [Google Scholar]
- 43.Osborne IJ, Mace S, Taylor D. A prospective year-long follow-up of lurasidone use in clinical practice: factors predicting treatment persistence. Ther Adv Psychopharmacol. 2018;8:117–125. doi: 10.1177/2045125317749740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loebel A, Silva R, Goldman R, et al. Lurasidone dose escalation in early nonresponding patients with schizophrenia: a randomized, placebo-controlled study. J Clin Psychiatry. 2016;77:1672–1680. doi: 10.4088/JCP.16m10698. [DOI] [PubMed] [Google Scholar]
- 45.Holloman LC, Marder SR. Management of acute extrapyramidal effects induced by antipsychotic drugs. Am J Health Syst Pharm. 1997;54:2461–2477. doi: 10.1093/ajhp/54.21.2461. [DOI] [PubMed] [Google Scholar]
- 46.Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf. 2005;28:191–208. doi: 10.2165/00002018-200528030-00002. [DOI] [PubMed] [Google Scholar]
- 47.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 48.Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196:89–91. doi: 10.1192/bjp.bp.109.070540. [DOI] [PubMed] [Google Scholar]
- 49.Poyurovsky M, Epshtein S, Fuchs C, et al. Efficacy of low-dose mirtazapine in neuroleptic-induced akathisia: a double-blind randomized placebo-controlled pilot study. J Clin Psychopharmacol. 2003;23:305–308. doi: 10.1097/01.jcp.0000084027.22282.16. [DOI] [PubMed] [Google Scholar]
- 50.Hansen LK, L’allemand T, Thiry F, et al. Structured relaxation in the treatment of akathisia: case series. Neuropsychiatr Dis Treat. 2010;6:269–271. doi: 10.2147/NDT.S9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor DM, Barnes TRE, Young AH. The maudsley prescribing guidelines in psychiatry. 13. Oxford: Wiley Blackwell; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed.