Abstract
Abstract
Multiple sclerosis (MS) is a chronic progressive disease and many patients transition from an initial relapsing–remitting course to a secondary progressive pattern. Accurate classification of disease status is critical to ensure that patients are treated appropriately and kept informed of their prognosis. Consensus terms defining the different forms of MS are available but were developed primarily for healthcare professionals (HCPs) and may be of limited value to patients. This article provides direct insights from four patients with MS, at different points in their disease trajectory, regarding their understanding of, and attitudes toward, MS progression. We also examine the utility of the current classification systems from the perspectives of patients and HCPs. Responses collected during in-depth, structured interviews and questionnaires portrayed the difficulties patients face accepting their MS diagnosis and treatment, revealed how understanding of the term “disease progression” varies considerably, and highlighted the challenges surrounding the period of transition to secondary progressive MS (SPMS). The terms describing different MS types were considered confusing and can make patients feel “compartmentalized” or “labeled”. Patients also struggled to relate these terms to their reality of living with MS, were reluctant to discuss progression with their HCPs, and feared being diagnosed with SPMS owing to concerns about treatment access. These insights highlight the need to develop patient-friendly language to describe MS progression; it may also be preferable for HCPs to describe MS as a disease spectrum in discussions with their patients.
Funding
Novartis Pharmaceuticals Corporation.
Plain Language Summary
Plain language summary available for this article.
Keywords: Classification, Multiple sclerosis, Patients, Perception, Progression, Terminology
Plain Language Summary
Multiple sclerosis (MS) usually worsens over time, with many patients transitioning from “relapsing–remitting” MS (RRMS) to “secondary progressive” MS (SPMS). Categorizing MS in this way may be useful for healthcare professionals (HCPs) because it can help to guide treatment decisions. However, these medical definitions of MS may be ambiguous to patients. Additionally, HCPs themselves do not always agree on the definition of SPMS.
Interviews with four MS patients and one physician who specializes in MS were conducted to understand the patients’ perspectives of how their disease worsens over time, and to assess how useful patients find the medical definitions used to describe different forms of MS.
From the interviews, it was clear that patients find it hard to come to terms with their diagnosis of MS, especially because they understand their disease will worsen over time. Patients may not fully understand the medical definitions used to describe different forms of MS and may not want to be identified in this way. They do not always think that these medical terms are relevant to them, as they do not reflect the reality of living with MS. In particular, some patients might not want to be identified as having SPMS, because this may limit their access to treatments. When speaking to their patients with MS, rather than using complex medical terms, doctors might want to describe MS as being part of a spectrum of disease.
Introduction
Multiple sclerosis (MS) can be a chronic progressive disease that over time places an increasing burden on patients. As such, accurate classification of disease status is critical to ensure that patients are treated and monitored appropriately and kept fully informed of their prognosis. Using clear and consistent terminology facilitates patient–physician communication and helps to define and compare MS clinical trial populations. Before 1995, definitions for the different forms of MS were inconsistent [1], so an international survey was conducted with MS experts to reach consensus on terminology describing the natural progression of MS [1]; these terms were revised in 2013 [2, 3].
In the following article, we provide an overview of how classification of MS disease progression has evolved over time. We present detailed insights from an MS healthcare professional (HCP) and from four patients with MS regarding their understanding of and attitudes toward MS disease progression and its impact. The four patient authors of this article were at different phases of the MS disease trajectory and were therefore able to provide insights based on their views from across the disease spectrum. At the time of the discussions, one patient had been newly diagnosed with relapsing–remitting MS (RRMS) as a young adult; a second was diagnosed as a teenager and is now an adult with RRMS. Another patient was diagnosed many years ago with RRMS and is relatively stable. Lastly, one patient had transitioned from RRMS to secondary progressive MS (SPMS). Insights were gathered from the patients via in-depth structured interviews and questionnaires, to collect qualitative data. The clinician perspective was provided by an MS specialist who also gave feedback on the patient responses to questions. Themes were identified, and literature searches were performed to look for corresponding data from the published literature. In-depth quantitative analysis methods were not used for this study owing to the sample size. In this article, we also explore the utility of MS disease classification terminology from the perspectives of the HCP and patient authors.
Much of the HCP-related research cited pertains to physicians, but we use the term HCP for both physicians and other professionals caring for patients with MS. The aim of this study was to assess the utility of the current classification systems from the HCP and patient perspectives, and to evaluate patient understanding of, and attitudes toward, MS progression from patients at different stages in their disease trajectory.
Methods
This article provides qualitative patient journey-based insights from the HCP and patient authors, all of whom are from the USA.
The HCP’s perspective was provided by the MS specialist Daniel Kantor, MD, FAAN, FANA, President Emeritus of the Florida Society of Neurology, President of the Medical Partnership 4 MS, Chief Medical Correspondent for MSWorld, and an active member of the Multiple Sclerosis Foundation’s Medical Advisory Board and the Multiple Sclerosis Association of America’s Healthcare Advisory Council.
Patients’ perspectives were provided by Jeri Burtchell, Kristen Fetty, Kit Minden, and Katelyn Miller. Jeri Burtchell (Florida, USA) is a patient activist who campaigns for better awareness in the patient community about clinical trials, an advocate for patients with MS, founder of Partners in Research, and Director of Patient Initiatives at HealthiVibe, LLC. In 1999, she was diagnosed with RRMS at 38 years of age. Kristen Fetty (West Virginia, USA) is a college student, studying accounting at Fairmont State University. Kristen was diagnosed with RRMS in 2016 at 20 years of age. Kit Minden (Virginia, USA) works full-time as a tutor and technical writer, and established the patient-support blog “Living for a Cure” in 2012 (wearelivingforacure.blogspot.co.uk). She was diagnosed with RRMS in 2011 at 55 years of age, but has since transitioned to SPMS. Katelyn Miller (Virginia, USA) was diagnosed with pediatric-onset MS in 2013 at the age of 13 years.
During manuscript development, a set of questions for use in patient interviews were drafted by Mark Rolfe PhD (Oxford PharmaGenesis, UK). These were reviewed by Brandon Brown (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), who has extensive experience as a clinical pharmacist in MS clinical centers, by Nina Jaitly (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), and by other Novartis medical representatives. Dr Kantor then provided his insights and perspectives directly on the questions. Patients’ insights and perspectives were gathered via a series of transcribed telephone interviews, conducted by Brandon Brown, Nina Jaitly, and Mark Rolfe, with the patient authors. The same questions were posed to each patient author. The patients were also sent written questions to review in advance of and after the phone interviews. Unedited and nonparaphrased quotes were taken directly from the transcriptions and used where appropriate throughout the manuscript. Separate MEDLINE searches identified scientific journal articles that supported patients’ and clinicians’ statements to contextualize the themes identified by the authors and to be representative of those of the wider MS population and other HCPs.
Compliance with Ethics Guidelines
This article provides personal perspectives and insights, and a review of the literature; it does not contain any new studies with human or animal subjects performed by any of the authors.
Disease Progression Classifications
A summary of the terms used to describe the different forms of MS, as defined by Lublin and colleagues in 1996 [1] and revised by them in 2013 [2, 3], is provided in Tables 1 and 2, respectively. Briefly, the 1996 classifications defined four main forms of MS: RRMS, primary progressive (PPMS), SPMS, and progressive relapsing MS (PRMS). When a patient is experiencing a flare up of SPMS symptoms, it is described as active SPMS; when the patient’s symptoms are stable, this is described as inactive SPMS. No consensus could be reached on a specific population corresponding to “relapsing progressive MS”, so the term was abandoned [1]. Although consensus was initially reached on the definition of “progressive relapsing MS” [1], this term was dropped in the 2013 revision and is now categorized as “primary progressive MS with activity” [3]. Use of “chronic progressive MS” was not recommended because the term was considered vague and could be replaced with one of the new definitions of progressive MS. Definitions for benign disease and malignant disease were also provided (Table 1) [1]. Not included in either Table 1 or 2 but nonetheless important to consider is “relapsing forms of MS” (RMS), which is a broad term that includes both RRMS and SPMS with superimposed relapses [4].
Table 1.
Consensus terminology for MS disease course and severity, as defined by Lublin et al. in 1996 [1]
| Disease course definitions | |
|---|---|
| RRMS |
Clearly defined disease relapses with full recovery, or with sequelae and residual deficit upon recovery Periods between disease relapses characterized by a lack of disease progression |
| PPMS | Disease progression from onset, with occasional plateaus and temporary minor improvements allowed |
| SPMS | Initial relapsing–remitting disease course, followed by progression with or without occasional relapses, minor remissions, and plateaus |
| PRMSa |
Progressive disease from onset with clear acute relapses, with or without full recovery Periods between relapses characterized by continuing progression |
| Disease severity definitions | |
|---|---|
| Benign MS | Disease in which the patient remains fully functional in all neurologic systems 15 years after disease onset |
| Malignant MS | Disease with a rapid progressive course, leading to significant disability in multiple neurologic systems or death in a relatively short time after disease onset |
MS multiple sclerosis, PPMS primary progressive MS, PRMS progressive relapsing MS, RRMS relapsing–remitting MS, SPMS secondary progressive MS
aIdentified as a rare disease form only
Table 2.
Descriptions of the classifications of relapsing–remitting and progressive MS, as defined by Lublin et al. in 2013 [2, 3]
| Relapsing–remitting disease | ||
|---|---|---|
| CIS |
A clear-cut syndrome such as optic neuritis, brain stem/cerebellar dysfunction, or partial myelitis Characteristics of inflammatory demyelination that could be MS are present, but McDonald 2010 criteria [7] of dissemination in time are yet to be fulfilled |
Active |
| Not active | ||
| RRMS | MRI evidence of dissemination in space, as well as gadolinium-enhancing and non-enhancing T2 lesions on a single MRI scan and/or a subsequent event | Active |
| Not active | ||
| Progressive disease | ||
|---|---|---|
| PPMS | Progressive accumulation of disability from onset | Active and with progression |
| Active, but without progression | ||
| SPMS | Progressive accumulation of disability after initial relapsing course | Not active, but with progression |
| Not active and without progression | ||
| Definitions | ||
|---|---|---|
| Active | Clinical relapses and/or MRI activity (gadolinium-enhancing MRI lesions or new/enlarging T2 lesions) assessed at least annually | |
| Not active | Absence of MRI activity (if activity assessments are not available, disease activity is “indeterminate”) | |
| Progression | Measured by clinical evaluation at least annually | |
| Worsening disease | A documented increase in neurologic dysfunction/disability as a result of relapses or progressive disease or both | |
| Disease progression | Reserved solely for patients in a progressive phase of MS | |
| Confirmed progression | An increase in neurologic dysfunction confirmed throughout a defined time interval (e.g. 3, 6, or 12 months) | |
CIS clinically isolated syndrome, MRI magnetic resonance imaging, MS multiple sclerosis, PPMS primary progressive MS, PRMS progressive relapsing MS, RRMS relapsing–remitting MS, SPMS secondary progressive MS
At the time, the classifications shown in Table 1 were considered to represent the range of all MS clinical forms. They were incorporated rapidly into routine clinical practice and used to define inclusion criteria for many subsequent clinical trials; however, it was acknowledged that future updates may be needed to reflect advances in disease understanding [2, 3]. Indeed, the classifications were revised in 2013 (published in 2014), the main changes being recognition of clinically isolated syndrome (CIS) and omission of the term PRMS. In addition, MS subclassifications of disease status were introduced to capture whether patients had active disease or not, and whether their disease was progressing or stable [2, 3]. Definitions of worsening disease, disease progression, and confirmed progression were also provided, but the terms “benign disease” and “malignant disease” were recommended to be used only with caution (Table 2) [2, 3]. The new subclassifications allowed more selective inclusion criteria to be used in MS clinical trials than previously, and new study outcomes to be measured. They also helped shape the design of new MS clinical trials and biomarker studies, and facilitated the evaluation of new and existing treatments [2, 3].
Alongside the development of these classification systems, new criteria for diagnosing MS were introduced in 2001 (the “McDonald criteria”) [5], with updates and revisions published in 2005 [6], 2010 [7], and 2017 (published in 2018) [8]. Notably, the 2010 revisions allowed MS to be diagnosed on the basis of a single magnetic resonance imaging (MRI) scan that indicates dissemination of lesion activity in both time and space, so in terms of the MS disease course, very few patients remain categorized as CIS [7]. The 2010 revisions also stated that these diagnostic criteria can be applied to the vast majority of pediatric-onset cases [7]. Disease presentation is most common in young adults, but up to 5% of cases appear in childhood or adolescence [9–11]; pediatric-onset MS almost always follows a relapsing–remitting disease course [7].
Recognizing, Diagnosing, and Understanding Disease Progression
HCP’s Perspective
Owing to the heterogeneous nature of RMS, providing an accurate prognosis remains a challenge. For example, it is difficult to identify which patients with RRMS will transition to SPMS and how long this will take. However, the literature consistently reports that approximately 50–80% of untreated patients with RRMS will develop SPMS [12, 13] within a median time of approximately 20 years after onset [13–15].
Timely and accurate recognition of progression ensures disease management optimization, and is most likely achieved via regular follow-up and assessment. However, even with regular assessment, identifying the point at which transition occurs remains challenging: there is no universally accepted definition of SPMS, and there are no definitive tests that can identify when the progressive phase has been entered [16, 17]. Indeed, among patients with relapsing disease, a diagnosis of SPMS is typically retrospective, following a period of gradually accumulating disability that occurs independently of relapses [2, 3, 16, 17]. Diagnosis is further complicated by the fact that normal age-related changes, such as reduced muscle strength, fatigue, cognitive disturbances, and continence issues [18], overlap with symptoms of MS progression.
Uncertainty surrounding the transition period may contribute to delayed diagnosis. Indeed, HCPs (N = 11) participating in a UK-based study reported that the lack of an objective test for SPMS (and the associated uncertainty) meant that recognizing transition took time, with repeated assessments often required before the diagnosis was confirmed [19]. Similarly, in a US retrospective study of 123 patients with RRMS, a 3-year period of “diagnostic uncertainty” was noted for 14 of the 20 patients who transitioned to SPMS [20].
A further issue is the current lack of widely used effective treatments for SPMS [21]. Although HCPs recognize the value of communicating openly with patients about progression, initiating such conversations can be challenging [19]. Taking all this into account, it is perhaps unsurprising that HCPs may be cautious in diagnosing their patients with SPMS [20].
Recent advances suggest that identifying SPMS may become easier in the future. For example, when 576 candidate definitions of SPMS were evaluated systematically, the best-performing (in terms of reproducibility, accuracy, and timeliness) was an objective definition of SPMS based on the patient’s Expanded Disability Status Scale (EDSS) score and past relapses [16]. This suggests that a unified definition of SPMS may soon be achievable. A screening tool to support HCPs in identifying patients with SPMS is also being developed [22], and a UK pilot study of a web-based questionnaire allowing patients to capture disease-progression outcomes showed good agreement between patient- and HCP-measured disability scores, suggesting potential utility for monitoring disease progression in the long term [23]. Combining different disability outcome assessments, especially patient- and HCP-reported measures, increases the likelihood of detecting progression [24], and nuclear magnetic resonance spectroscopic analysis has, for the first time, revealed serum metabolite biomarkers that differentiate between patients with RMS and those with SPMS, predicting the patient’s disease classification with high sensitivity and specificity [25]. Finally, various studies have identified factors that predict shorter time to, younger age at, or increased risk of transitioning to SPMS. These risk factors include male sex [14, 15], older age at MS onset [15, 26, 27], previous relapses [15, 28], and specific clinical features at disease onset [14, 26, 27].
Patients’ Perspectives
The terminology defined by Lublin et al. in 1996 [1] and 2013 [2, 3] was developed primarily with HCPs and researchers in mind. However, as healthcare becomes increasingly patient-centric, it is important to consider whether this terminology is understood by patients, and whether they consider it relevant or useful. Indeed, at the point of diagnosis, some patients may not even have heard of MS, as Jeri and Kristen explained.
“I didn’t even know what MS was—I had it confused with muscular dystrophy.” (Jeri)
“Before diagnosis, I had never even heard of MS. I thought the lesions were signs of cancer.” (Kristen)
Regarding the current terminology HCPs use to describe MS progression, Jeri and Kit felt that the definitions were confusing and unhelpful, making patients feel they are being put into categories that are not applicable to themselves or to other patients.
“The terms can make patients feel compartmentalized. All of the patients with MS who I have met, regardless of what form of MS they had, seem so different from each other. So part of me thinks that there is something different going on with each of us, but that it’s similar enough to be called MS.” (Jeri)
“I find it confusing, to be honest. We have the classifications to group us, but it can be so different. No one is the same—everyone is affected differently. I don’t think that the classifications of MS necessarily fit everyone who has MS. They don’t help us live with the reality of having MS. Our lives are based around what’s happening now, what’s happening to us today. The classifications don’t help us decide what treatments to take. They just tell us that doctors want to put us into a group.” (Kit)
Other patients, especially those diagnosed only recently, may be relatively unaware of the different classifications; this was the case for Kristen.
“I’m not really familiar with the different types of MS. I didn’t even know that there were different types of MS.” (Kristen)
There are many reasons why it may be beneficial for patients to have a good knowledge and understanding of MS. For example, findings from a systematic review showed that patients who were uncertain about their MS or who lacked a “coherent understanding” of it scored more highly on measures of psychological distress than patients who had a clear understanding of their disease [29].
However, patients are often expected to find this information themselves. In a small qualitative study of UK patients with MS (N = 15), respondents reported receiving little support or information about their prognosis from the HCP when they were diagnosed [30]. This lack of information, combined with an expectation that patients would be able to source and interpret information themselves, was something Jeri, Kit, and Kristen all experienced.
“I researched MS online, but the Internet was so new that finding good sources of information was not easy. I mostly learned through anecdotes and conversations with other patients in online forums.” (Jeri)
“They sent me home with a stack of literature about nine different types of medicine and told me to read and decide which one I wanted to take… After we set up a patient support group (Living for a Cure), I started researching things to help other people. I would then apply what I learned to my own situation.” (Kit)
“Googling your symptoms is the worst thing you can do when you have MS. I’m a millennial, so of course I went straight to the Internet when I was diagnosed. Bad idea.” (Kristen)
The impact of being diagnosed with a progressive disease can have a profound psychological effect on patients, as Jeri, Kristen, and Katelyn recalled.
“I remember being terrified and also in deep denial. When I think back, I can still remember how it felt like the end. When I got home, I felt deflated. We now knew that I really did have MS, but we did not know what was coming next.” (Jeri)
“I was 20 years old when I was diagnosed, and I was terrified.” (Kristen)
“I was diagnosed at 13… It was a very confusing and difficult time; I was told I was making it up, which made me feel worse and depressed… I felt overwhelmed and was very angry. It was very hard for my parents too.” (Katelyn)
Katelyn experienced relief when she finally received her diagnosis, a process that was complicated by the fact that her elder brother had experienced symptoms similar to hers when he was very young.
“My brother, who’s 5 years older than me, came down with symptoms related to MS in his senior year of high school; he had numbness and tingling like me, and the doctors told him he had MS but he never received treatment. His symptoms went away, and he hasn’t had any symptoms since then. A year later it happened to me. I didn’t understand it as a kid, I thought my disease would go the same way as his.” (Katelyn)
Similarly, Kit was relieved to have MS confirmed, following years of uncertainty and misdiagnoses.
“I was looking forward to any diagnosis that would lead to me being treated. When I got my MS diagnosis, it clicked with me—it was suddenly very real. I didn’t really have a problem getting the diagnosis, but then they couldn’t do much for me.” (Kit)
Even though Kit is now very well informed about MS, she would like more information about the different MS classifications.
“I feel like there have to be more forms of MS than the ones that are commonly talked about. I read some years ago that there were six different types of MS, so I would like more information on the different types, and why someone like me never had RRMS.” (Kit)
Jeri and Kit also highlighted a need for better-quality patient materials, although these seem to have improved in recent years.
“Until I joined a clinical study, most of my information came from MS groups, pamphlets (which weren’t particularly patient-friendly), and online information. The literature wasn’t nearly as patient-friendly as it is now.” (Jeri)
“The MS books are glossy. Multiple Sclerosis for Dummies tells us a lot about MS, but not about how to live with MS. It’s frustrating; we need something more, some better information.” (Kit)
Clearly, some patients do not feel that their informational needs on the prognosis and management of MS are being met. Since well-informed patients are able to take an active role in making decisions about their own treatment, access to accurate medical information is vital. The Internet allows such information to be disseminated easily, and HCPs could guide patients toward unbiased peer-reviewed or nonprofit-endorsed sources of online information, such as the National Multiple Sclerosis Society (nationalmssociety.org), the Multiple Sclerosis Association of America (mymsaa.org), and the Multiple Sclerosis Foundation (msfocus.org)—trusted advocacy groups with a strong online presence. With the rise of the Internet and social media, patients are also able to connect with each other more than ever before. Social media platforms allow patients with MS to share information from their personal experience, to give support and advice to each other, and to pass on their knowledge of the disease and its treatment [31, 32]. Nevertheless, despite the wealth of MS-related information and support available online, patients may remain unaware of, or not fully understand, the medical terminology used to describe different forms of MS. It should also be noted that some sources of patient information may be biased, factually incorrect, or even harmful [32].
One final consideration is that for many patients, their primary concern may not be about the term used to classify their disease, but whether that classification affects their ability to access treatments or participate in studies. As one patient commented in one of Jeri’s online articles, “When all is said and done, I don’t care what you call me, just don’t call me anything that will take away my ability to try any medical therapy or clinical trial out there” [33].
Transitioning to SPMS
HCP’s Perspective
Supporting patients during the transition to SPMS can generate significant challenges for HCPs [19]. In order to provide appropriate support, it is essential that HCPs understand patients’ potential concerns and needs around this time.
In a UK study exploring the experiences of seven specialist MS HCPs and nine patients who were transitioning to SPMS, common themes identified by all participants included the importance of providing adequate information and support during the transition period, both to patients and to HCPs [34]. Participants also felt that timing and delivery of patient information should be carefully considered, and that sensitive communication was paramount; providing appropriate training and support for HCPs on this topic may be beneficial [34].
In a separate UK study of HCPs (N = 11) working with patients with MS, including MS specialists and nonspecialists, numerous challenges associated with providing support during the transition period were identified [19]. Managing “invisible” symptoms (e.g., changes in mood, memory, and personality) and providing appropriate psychological support were considered particularly problematic, because HCPs felt that they lacked the necessary skills and resources to manage these issues effectively [19]. Although HCPs recognized the value of communicating openly with patients about SPMS, they found it difficult to know when to initiate such conversations [19]. Discussing SPMS shortly after receiving a diagnosis of MS was considered too soon (a sentiment echoed by UK patients in a separate study [30]), but waiting until transition was imminent was considered too late [19]. Because their patients rarely raised the subject of transition themselves, HCPs in the study found it difficult to know when to tackle the issue [19]. Communicating prognostic information as part of a more general and continuous “joined-up care” approach may be more successful than relying on a single one-off conversation [30].
Although guidelines, such as those in the UK, emphasize the need for open and honest communication and information provision for patients with MS [35], no specific recommendations are provided regarding how information is communicated at an individual level, especially in relation to potential prognosis [29].
Patients’ Perspectives
To date, understanding of the experiences and needs of patients during the transition period is somewhat fragmentary, but a group of Italian MS experts is attempting to address this issue via the ManTra (Managing the Transition to SPMS) initiative [36]. This project aims to gather in-depth insights into the experiences and needs of patients who have recently transitioned to SPMS, from which a user-led resource will be developed, designed to empower and improve the quality of life of these patients [36].
Although there is only limited scientific literature on this topic, excerpts from patient blogs reveal just how difficult a time the transition period can be, with one blogger describing the transition as feeling like she was “losing my grip on life” [37]. Another blogger described, after a period of time when her symptoms were getting worse, the initial sense of relief she experienced when she relapsed. Not relapsing had always been a goal of disease management but could be a sign of transitioning to SPMS [38].
In a UK study exploring the experiences of patients during the transition to SPMS, progression marked a major turning point for many patients and was accompanied by a range of cognitive, emotional, and behavioral responses [34]. Themes identified in these responses suggested that patients underwent a four-stage “transition journey”, starting out by questioning whether the transition was actually happening, then accepting that the transition had occurred, followed by dealing with the day-to-day issues of living with SPMS before finally “brushing oneself off and moving on” [34]. Similarly, in a separate UK study of patients with relapsing or progressive forms of MS (N = 15), a coping strategy adopted by many patients was to try and remain focused on the present as much as possible [30].
When Kit transitioned to SPMS, she was initially confined to a wheelchair. After receiving intensive physical therapy, she was able to walk short distances with a walker, but she still faced a range of daily challenges. She also tries to focus on living in the here and how, concentrating on day-to-day activities that require her immediate attention.
“Progression has meant becoming more and more restricted… I can have trouble holding pens, and I can struggle to dress myself. I don’t cook because I can’t reach things in the kitchen. I would also forget to turn the stove off. It takes 2 h to get ready to go out, and I don’t shop as I just don’t have the energy. When the fatigue is bad I can fall asleep in the middle of things. Also, when tutoring, there are some days when I just can’t do the math problems. I’m doing okay, but it’s hard. I prefer not to think about it.” (Kit)
“My best bet is to think, what do I have to do today so I can get on with my life and my job? What bills do I have to pay?” (Kit)
Owing to the difficulties in objectively identifying progressive disease, the onus of recognizing the transition to SPMS often falls on patients. In a qualitative study of 20 UK patients with MS who were close to transitioning or who had recently transitioned to SPMS, it was the patients’ own gradual realization that they were entering the secondary progressive phase that prompted subsequent discussions with their HCP, with a confirmatory diagnosis made later [39]. Although some patients were happy reaching this realization themselves, others were frustrated that their neurologist had not initiated discussions [39]. Of concern, some patients discovered that they had SPMS only by chance, after overhearing a conversation or receiving a copy of the letter written to their general (i.e., primary care) practitioner (GP) [39].
Kit also commented on the fact that HCPs may not have time to discuss patients’ concerns at length.
“Doctors don’t have to deal with the reality of having MS and often don’t want to explore that very much with us. Most of them don’t have time, I think. It’s not a criticism; it’s just a fact of life. Even MS specialists don’t really deal with the nitty-gritty of our lives. This focus would be critical to help sharing ideas.” (Kit)
For patients who are still in the relapsing–remitting phase of the disease, coming to terms with the possibility of transitioning to SPMS in the future can also be upsetting, as Jeri explained.
“I knew MS was progressive and degenerative, but I don’t really remember how I found out about the implications. The woman who set up my MS group had only ever had one MS attack, so I guess she may have CIS, but there were people who had progressive MS and were in wheelchairs, and I knew one lady who died from complications. So I knew there was a wide array of what MS could look like and it terrified me. I couldn’t go to the support group for a while because I saw so many people who were depressed and in wheelchairs—I was frightened for my future.” (Jeri)
Even though Jeri has had RRMS for 18 years, the prospect of progressing still frightens her, and she is reluctant to raise the topic with her HCP.
“I’m afraid to discuss progression with my doctor—I don’t want to discuss it. I don’t want to find out that I am progressing. The thought of transitioning to SPMS from my current diagnosis, which I’ve known for years, frightens me. It’s an unknown, even though I’ve been involved with the MS community for so long.” (Jeri)
For other patients, such as Kristen and Katelyn, progression may be less of an immediate concern, especially if their MS is being managed well.
“The lesions I have already are well controlled with my current medication. The way I understand it is that as long as the lesions don’t continue to form, it shouldn’t cause me too many problems.” (Kristen)
“Time will tell, I guess. I know that the therapies also slow down progression. I’ve only been on my current treatment for just over a year, and I realize that there is the possibility of another relapse, but I feel that now I’m getting the right treatment, I’ll be able to tackle it effectively—now that we know what’s going on.” (Kristen)
“I’ve not had any conversations about future progression… I’m aware it may happen. I’m okay with it; I accepted that a long time ago.” (Katelyn)
“I’m ready to handle whatever I need to handle. I’m a fighter—and I’ll do whatever I need to do.” (Katelyn)
Living With and Managing MS Progression
HCP’s Perspective
A detailed discussion of the range of pharmacological and nonpharmacological treatments for progressive MS is beyond the scope of this article. However, it should be acknowledged that although approximately 15 drugs are approved by the US Food and Drug Administration (FDA) for use in RRMS [40], there are currently no licensed treatments approved for nonrelapsing SPMS [21, 40, 41], although the EXPAND study of siponimod in SPMS did show treatment effect in patients with nonrelapsing SPMS [42]. Indeed, nearly all immunomodulatory and immunosuppressant agents tested in SPMS have failed to demonstrate consistent or meaningful effects on disability [17, 43], and an international registry-based study suggested that MS disease-modifying therapies (DMTs) offered no benefit in delaying nonrelapse-related disability progression in patients with SPMS [44]. However, recently, siponimod and cladribine have been approved in the USA for CIS, RRMS, and active SPMS [45, 46].
Long-term treatment with first-generation DMTs can delay progression from RRMS to SPMS [47], but the optimal duration for which patients should receive DMTs remains controversial [48]. Translating efficacy data from clinical trials into routine clinical practice may also be complicated by the 2010 revisions to the MS diagnostic criteria [7] and the 2013 updates to MS classifications [2, 3]. Thus, before recommending any treatment strategy, HCPs must assimilate a vast array of information, and weigh up potential risks and benefits [41].
Until recently no widely available DMTs with an acceptable benefit–risk ratio were available for SPMS. Symptom control therefore was the mainstay of care for the majority of patients with SPMS; however, hope remains for the future [49]. A recent clinical trial in patients with SPMS demonstrated a reduced risk of disability progression associated with a novel DMT relative to placebo [42], although real-world evidence is still lacking. Progress is being made in other areas, including an increased understanding of the pathophysiological mechanisms underlying progressive disease [50, 51], the identification of novel approaches that may help increase the success of future SPMS trials [52], and the optimization of rehabilitative strategies aimed specifically at improving the quality of life of patients with progressive MS [43].
Treatment guidelines have also been drafted recently by the American Academy of Neurology (AAN) [41] and the Congress of the European Committee for Treatment and Research in Multiple Sclerosis and the European Academy of Neurology (ECTRIMS/EAN) [40, 53]. Although the ECTRIMS/EAN guidelines do not seem to make any specific treatment recommendations for patients with SPMS, the AAN guidelines recommend that HCPs should advise discontinuation of DMTs only in patients with SPMS who do not have ongoing relapses (or gadolinium-enhancing lesions on MRI), and in addition who have not been ambulatory (EDSS score ≥ 7) for at least 2 years, although this is only a level C recommendation [41].
This heightened attention from the wider MS community, combined with increased global collaborative research efforts to overcome the challenges of progressive MS [54, 55], suggests that significant advances may be made in the years to come [49].
Patients’ Perspectives
Patients with progressive MS often (and justifiably) express frustration at the lack of available treatments for them [49]. For example, one patient from the USA who had progressive MS noted in their blog that hearing they had a progressive disease for which no treatments were available felt like being told to “go home and make the best of it” [56].
For those at an early stage of disease, adhering to treatment can be a challenge. Katelyn was a teenager when she started injectable therapy; although initially enthusiastic, Katelyn found adherence challenging because of the side effects of injectable treatment.
“Once I was diagnosed, I was ready to get on treatment! I wanted to be a normal kid.” (Katelyn)
“I’ve been on several injections and had side effects—they made me feel like I was sick, and I ached from bruising. I refused to take them at one point; my parents thought I was, but I wasn’t. This made my MS worse, until I was forced to accept that I had to get on treatment. I used to pray for a pill—it would have been a lot easier, I would have taken that.” (Katelyn)
As well as coming to terms with treatment regimens and side effects, there may be practical challenges to overcome relating to school, work, or family commitments. Indeed,
“I was 13 when diagnosed, right in the middle of the school year. I had to pull out of school because I felt so bad. I missed several months and had to finish the year at home—but I hated it; I wanted to go to school.” (Katelyn)
“I had a close group of friends; it wasn’t hard to tell them [about the diagnosis] and they were so supportive and very nice.” (Katelyn)
“I never really had a life plan or goal until I was diagnosed with MS, then everything changed. I was a printer and graphic artist. I had had a print shop for 10 years, but when I was diagnosed, I had to close it up.” (Jeri)
“I had to change jobs. I worked in a retail store where I was on my feet the whole time and it became really difficult.” (Kristen)
“It was right around finals time when I was diagnosed. Juggling doctors’ appointments with my class schedule was not fun, and walking around campus was even worse.” (Kristen)
“When I was first diagnosed, we struggled a lot; it was terrible. I struggled to work as a tutor because of the cognitive fog—I couldn’t speak and write at the same time. I found the cognitive fog very frustrating. I am the center of my family. It’s my job to make sure that everyone else is okay and that everything runs properly. Yet I can’t do that.” (Kit)
Other people’s perceptions of MS can also have a significant impact on patients’ lives, as Kristen explained.
“I have been amazed at some of the things people have had the audacity to say to me. My boss told me I would end up in a wheelchair within 10 years. I’m 20 years old and didn’t know what was happening to me, so I was very shaken up by that.” (Kristen)
In an Italian study of patients with progressive MS (N = 22), their caregivers (N = 30), and HCPs (N = 18), patients had difficulties expressing their precise needs and requirements, but maintaining personal hygiene and receiving coordinated care and psychosocial support emerged as major themes [57]. Many patients reported being lonely and expressed a need for supportive social networks. Being able to maintain their roles in their families and the local community was also important for many patients [57].
For all patients, regular checkups and reviews with their HCPs are critical to ensuring that any disease changes are detected as early as possible, so that management plans can be adjusted accordingly. Kristen and Katelyn explained how this approach underpins their disease management.
“We go to my clinic to get my MRI done every 6 months and discuss any problems we have. They do the 25-Foot Walk Test and the 9-Hole Peg Test. I also have cognitive and vision tests. I also see my regular physician every 6 months.” (Kristen)
“I take my treatment, and I see my neurologist every 6 months; we discuss my symptoms and I have an MRI.” (Katelyn)
Both Jeri and Katelyn noted that adjusting to their symptoms is important in managing the condition.
“I try to get plenty of rest and pace myself and listen to my body and recognize when I’m overdoing it. If I go shopping, I’ll use a cart, even if I’m only getting one item. I live in Florida, so I try not to go out in the day too much, because it will just take all my energy.” (Jeri)
“I’m a single mother with a preschool age son, and I’m trying to manage my MS and my family on my own—this is one of the biggest challenges of living with my MS.” (Katelyn)
“I’m proactive and positive; I exercise right, I eat right. I always appreciate advice and guidance. My faith is also important to me, which helps.” (Katelyn)
Jeri and Kit highlighted how maintaining a good patient–HCP relationship was fundamental to receiving appropriate treatment and support and taking control of their disease.
“When I got the definite diagnosis, my doctor thought interferons were too risky so started me on Copaxone. I stayed on it for 7 or 8 years, but realized it wasn’t working for me. However, my doctor said it was, and that I would be so much worse without it. He wouldn’t switch me, even though I was having so many relapses. I wasn’t living for those 7 or 8 years. I floundered. It was exhausting, physically and mentally. I got depressed. I felt hopelessness and despair. There was a pivotal moment where I thought about killing myself, before I decided to do something. My GP put me in contact with [a new specialist], who happened to be the Principal Investigator for a clinical study. That was the key moment for me taking back control.” (Jeri)
“My previous neurologist, who wasn’t an MS specialist, was wonderful. He has since retired and I found another neurologist, but he was an MS specialist and very research focused, and he did not support me well. I’m now looking to attend a new MS center to get better support.” (Kit)
In the absence of widespread approved pharmacological treatments specifically for SPMS, various studies have examined the impact of nonpharmacological approaches on outcomes such as gait, mobility, fatigue, quality of life, and daily functioning [58–70]. Most studies focused on rehabilitative strategies, with successful interventions including total-body recumbent stepper training [58]; robot-assisted gait training [59]; functional electrical stimulation [60–62]; physiotherapy [59]; supported treadmill training [58, 63]; minimal acupuncture [64]; autologous hematopoietic cell transplantation [65]; energy conservation training [66]; transdermal histamine [67]; outpatient rehabilitation [68]; a program combining a modified Paleolithic diet with exercise, electrical stimulation, and stress management [69]; and hyperbaric oxygen therapy [70].
The patient authors of this article have limited experiences of these nonpharmacological approaches, but Kit found that exercise was beneficial in managing her SPMS, and all three patient authors reported that dietary changes had helped them manage their disease (although Kit mentioned that this may not help everyone). Although there is some evidence that gluten may be a trigger for autoimmune disease, and that changes in diet can improve symptoms of MS [69, 71], no particular diet has been demonstrated to have any significant benefit in MS [72, 73].
“I try to eat right. Food plays an important role in how I feel. On days when I eat fewer sweet things, I have less spasticity at night. When I was first diagnosed, I used to get horrible gastrointestinal issues, but these stopped when I gave up dairy.” (Jeri)
“The diet is hard, but I really do try to manage that.” (Kristen)
“I received intensive physical therapy and worked with an occupational therapist, who gave me a lot of exercises to do. I stopped eating gluten and cut down on dairy. By following an anti-inflammatory diet, I have managed to stop or slow a lot of the progression. However, I don’t understand why for, say, 60% of people, diet is really significant [in managing their MS], but for the other 40% it doesn’t make any difference at all in terms of how it feels, or how their disease progresses.” (Kit)
Redefining MS Progression as a Spectrum Disease
HCP’s Perspective
The idea that MS could be a spectrum disorder has been suggested previously [12, 74, 75], with some going as far as to propose that the spectrum of pathology actually extends to all healthy individuals, with clinical MS arising only once a certain threshold has been crossed [76]. Given that the current classification system underpins routine clinical practice and clinical research, redefining MS as a disease spectrum may not always be appropriate from an HCP perspective. However, the way that HCPs talk about MS progression to their patients could be reviewed, to help patients fully understand their condition, prognosis, and how this relates to their lives.
Patients’ Perspectives
As outlined earlier, patients do not seem to find the current MS classification system useful, with a major reason being the difficulty in relating these terms to the realities of living with MS, as Kit explained.
“The classifications can be frustrating. Some people in my [online support] group have a hard time believing their diagnosis, because the classifications don’t relate to the realities of their lives.” (Kit)
Compartmentalizing patients in this way can also have negative psychological and practical implications, as Jeri pointed out.
“It is about how other people perceive me.” (Jeri)
“SPMS scares me. To put a label on what I’m going through would do me more harm than good. I’m scared about how transitioning might affect my options in terms of treatment. Would I still be able to access treatments? Will my insurance still cover the cost of treatments that are only approved for RRMS?” (Jeri)
Indeed, in a recent survey carried out in the USA, 50% of patients with SPMS reported no DMT use, compared with 27% of patients with RRMS (p < 0.001) [77]. These figures may reflect that patients with SPMS have limited access to DMTs in the USA. The situation may be similar in Europe, where only those patients with SPMS who are also experiencing relapsing activity qualify for interferon therapy [78, 79]. Patients with SPMS without relapses, who account for the majority of patients with SPMS, are not eligible for treatment with DMTs according to the EU licensed indications, so these patients would have limited treatment options. UK guidelines recommend that HCPs consider stopping treatment when SPMS develops [80], and in the USA, drugs approved only for RMS may not be reimbursed for SPMS. However, times are changing and the FDA has now approved DMTs for relapsing forms of MS, including CIS, RRMS, and active SPMS [45, 46]. Given the potential treatment access implications of being diagnosed with SPMS, it is therefore understandable why patients do not want to be identified in this way and why HCPs may be cautious in applying such a description to their patients. Patients with PPMS may face similar difficulties in accessing treatments. For example, despite the fact that clinical trials have shown that ocrelizumab can slow disability worsening in patients with PPMS, the National Institute for Health and Care Excellence (NICE) in the UK recently took the decision to deny funding of ocrelizumab in this setting, citing unacceptable cost-effectiveness estimates [81]. Indeed, although there is clear evidence that DMTs can provide benefits for patients with PPMS, there are currently no such agents approved for this patient population.
Rather than identifying a patient as having a certain MS subtype, it may be more helpful for patients if MS is described in terms of a spectrum of disease when HCPs discuss the natural disease course with their patients. This concept resonated well with Jeri and Katelyn.
“Transitioning is not definitive; it’s more gradual. I like the idea of MS being described as a spectrum. Everybody is on it, just at different points. We’re all part of the same journey, just at different stages at a given moment. If doctors explained it like this to the patients, we might feel less compartmentalized. It might help us feel connected.” (Jeri)
“I like it—I think it accurately represents my MS course.” (Katelyn)
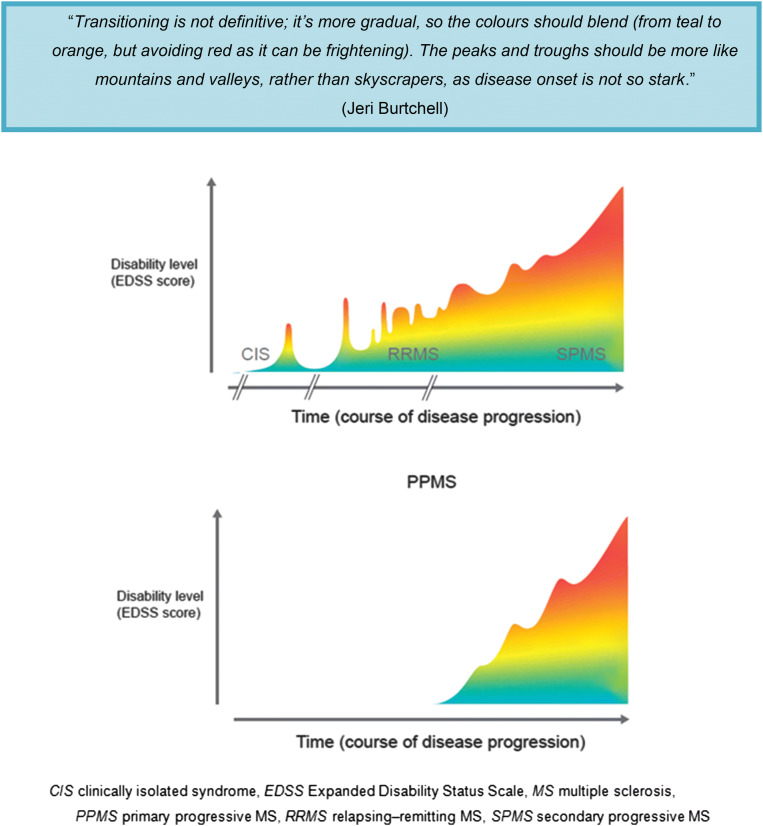
Figure 1 provides an example of how this spectrum could be depicted graphically. Patients may find it easier to relate the changes they are experiencing to a graphical representation of the MS disease course than to a list of definitions, as is used currently. Table 3 summarizes the most important issues relating to MS disease progression from the perspectives of HCPs and patients.
Fig. 1.
Patients’ perspectives on MS progression and subsequent schematic illustrating MS as a disease spectrum, rather than as distinct clinical forms. The figure depicts a representative course of MS disease progression from RRMS to SPMS (top) and for PPMS (bottom). Note that progression does not necessarily correlate with time; a patient can remain stable for a long period of time without their disease worsening. Importantly, disease progression takes a different course (i.e., the figure would look different) for each patient with MS
Table 3.
Summary of the most important issues relating to MS disease progression from the perspective of HCPs and patients
| Theme | Issues from the HCP’s perspective | Issues from patients’ perspective |
|---|---|---|
| Recognizing, diagnosing, and understanding disease progression | No universally accepted definition of SPMS | Confusing and unhelpful definitions of MS classifications |
| Diagnostic uncertainty during transition | Lack of awareness or information about MS classifications | |
| Difficulty identifying the point of transition to SPMS | ||
| Lack of reliable tests for SPMS | Lack of (good quality) information about prognosis | |
| Difficulty discriminating from age-related changes | Relief when receiving a diagnosis | |
| Subclinical symptoms complicate diagnosis | Concern about lack of access to treatments or to clinical studies for SPMS | |
| Transitioning to SPMS | Need for adequate information and skills to handle communications during transition period, including invisible symptoms and psychological support | Onus on patient to recognize transition to SPMS |
| Need for adequate information and support during transition period | ||
| Difficulty knowing the right point in time to discuss progression with a patient—not too soon postdiagnosis and not too late | Need for communications to be time- and content-sensitive | |
| Conflicting feelings about not relapsing | ||
| Emotional impact of transitioning; many patients cope by focusing on the present | ||
| Limited time with HCPs to discuss concerns | ||
| Concerns may be minimized if MS is being managed well | ||
| Living with and managing MS progression | Limited therapies for SPMS | Frustration at limited availability of DMTs for SPMS |
| Uncertainty about optimal duration of DMT treatment to delay progression from RRMS to SPMS | Negative impact on quality of life and ability to work as MS progresses | |
| Lack of clear treatment guidelines for patients with SPMS | Regular checkups with HCPs help detect changes in disease progression at an early stage | |
| Mixed benefits of nonpharmacological approaches | ||
| Redefining MS progression as a spectrum disease | Discussing MS progression as a spectrum may help patients understand their condition better | Feeling frustrated and compartmentalized by MS classifications |
| Redefining MS as a spectrum may not be relevant to clinical research | Limited access to treatments once diagnosed with SPMS | |
| Description of MS as a spectrum of disease may be more readily understood versus describing by subtype |
DMT disease-modifying therapy, HCP healthcare professional, MS multiple sclerosis, RRMS relapsing–remitting MS, SPMS secondary progressive MS
Conclusions
The usefulness of current MS classification systems to HCPs and patients appears to be discrepant, at least according to the patients surveyed here. Patients with MS may not understand the term “disease progression” and its related categories, and may struggle to relate the terms used to the impact that MS has on their lives. Being identified as having SPMS is of particular concern to patients because of the potential impact on access to MS treatments and clinical trials. Better information is needed for patients regarding the natural MS disease course and how this translates into the daily reality of living with the condition. There is also an apparent need to improve the HCP–patient dialog. HCPs are advised to take the time to understand each patient’s unique situation and how MS impacts their lives. It is also important for HCPs to take into account the patient’s level of understanding of MS during discussions about their disease. In particular, it may be preferable to some patients if HCPs described MS progression as a disease spectrum when discussing prognosis with their patients.
Acknowledgements
The authors thank all the participants involved in this research.
Funding
The study and the article processing charges were funded by Novartis Pharmaceuticals Corporation.
Medical Writing and/or Editorial Assistance
Editorial assistance was provided by Oxford PharmaGenesis Ltd (Oxford, UK) and was funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA). The authors acknowledge Brandon Brown and Nina Jaitly, of Novartis Pharmaceuticals Corporation and Mark Rolfe, of Oxford PharmaGenesis Ltd, who helped to facilitate the patient interviews.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors had full access to the information presented in this study and take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosures
Daniel Kantor has received research support from Novartis and speaking/consulting honoraria, not related to this publication. Jeri Burtchell is the founder of Partners in Research and Director of HealthiVibe, LLC. As an employee of HealthiVibe, Jeri has worked on projects with many pharmaceutical companies, including Novartis. She also serves on the patient advisory board of CureClick. Kristen Fetty, Katelyn Miller, and Kit Minden have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8223368.
References
- 1.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/WNL.46.4.907. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-multiple-sclerosis_en-0.pdf.
- 5.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 9.Chitnis T, Glanz B, Jaffin S, Healy B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler. 2009;15:627–631. doi: 10.1177/1352458508101933. [DOI] [PubMed] [Google Scholar]
- 10.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59:1006–1010. doi: 10.1212/WNL.59.7.1006. [DOI] [PubMed] [Google Scholar]
- 11.Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3:43–46. doi: 10.1177/135245859700300105. [DOI] [PubMed] [Google Scholar]
- 12.Antel J, Antel S, Caramanos Z, Arnold DL, Kuhlmann T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 2012;123:627–638. doi: 10.1007/s00401-012-0953-0. [DOI] [PubMed] [Google Scholar]
- 13.Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129:584–594. doi: 10.1093/brain/awh721. [DOI] [PubMed] [Google Scholar]
- 14.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:1039–1043. doi: 10.1136/jnnp.2010.208173. [DOI] [PubMed] [Google Scholar]
- 15.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:67–75. doi: 10.1136/jnnp-2012-304333. [DOI] [PubMed] [Google Scholar]
- 16.Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139:2395–2405. doi: 10.1093/brain/aww173. [DOI] [PubMed] [Google Scholar]
- 17.Plantone D, De Angelis F, Doshi A, Chataway J. Secondary progressive multiple sclerosis: definition and measurement. CNS Drugs. 2016;30:517–526. doi: 10.1007/s40263-016-0340-9. [DOI] [PubMed] [Google Scholar]
- 18.Multiple Sclerosis International Federation. MS in focus. Ageing with MS. 2015. https://www.msif.org/wp-content/uploads/2015/01/Ageing-with-MS-FINAL-web.pdf. Accessed 1 Dec 2018.
- 19.Davies F, Wood F, Brain KE, et al. The transition to secondary progressive multiple sclerosis: an exploratory qualitative study of health professionals’ experiences. Int J MS Care. 2016;18:257–264. doi: 10.7224/1537-2073.2015-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz Sand I, Krieger S, Farrell C, Miller AE. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler. 2014;20:1654–1657. doi: 10.1177/1352458514521517. [DOI] [PubMed] [Google Scholar]
- 21.Nandoskar A, Raffel J, Scalfari AS, Friede T, Nicholas RS. Pharmacological approaches to the management of secondary progressive multiple sclerosis. Drugs. 2017;77:885–910. doi: 10.1007/s40265-017-0726-0. [DOI] [PubMed] [Google Scholar]
- 22.Ziemssen T, Simsek D, Lahoz R, di Cantogno VE. Development of a screening tool to support identification of patients with secondary progressive multiple sclerosis (SPMS) Value Health. 2015;18:A763. doi: 10.1016/j.jval.2015.09.2497. [DOI] [Google Scholar]
- 23.Leddy S, Hadavi S, McCarren A, Giovannoni G, Dobson R. Validating a novel web-based method to capture disease progression outcomes in multiple sclerosis. J Neurol. 2013;260:2505–2510. doi: 10.1007/s00415-013-7004-1. [DOI] [PubMed] [Google Scholar]
- 24.Kragt JJ, Nielsen JM, van der Linden FA, Polman CH, Uitdehaag BM. Disease progression in multiple sclerosis: combining physicians’ and patients’ perspectives? Mult Scler. 2011;17:234–240. doi: 10.1177/1352458510385505. [DOI] [PubMed] [Google Scholar]
- 25.Dickens AM, Larkin JR, Griffin JL, et al. A type 2 biomarker separates relapsing-remitting from secondary progressive multiple sclerosis. Neurology. 2014;83:1492–1499. doi: 10.1212/WNL.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tedeholm H, Skoog B, Lisovskaja V, Runmarker B, Nerman O, Andersen O. The outcome spectrum of multiple sclerosis: disability, mortality, and a cluster of predictors from onset. J Neurol. 2015;262:1148–1163. doi: 10.1007/s00415-015-7674-y. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar S, Alroughani R, Ahmed SF, Al-Hashel JY. Prognostic indicators of secondary progression in a paediatric-onset multiple sclerosis cohort in Kuwait. Mult Scler. 2016;22:1086–1093. doi: 10.1177/1352458515608960. [DOI] [PubMed] [Google Scholar]
- 28.Skoog B, Tedeholm H, Runmarker B, Oden A, Andersen O. Continuous prediction of secondary progression in the individual course of multiple sclerosis. Mult Scler Relat Disord. 2014;3:584–592. doi: 10.1016/j.msard.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29:141–153. doi: 10.1016/j.cpr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Dennison L, McCloy Smith E, Bradbury K, Galea I. How do people with multiple sclerosis experience prognostic uncertainty and prognosis communication? A qualitative study. PLoS One. 2016;11:e0158982. doi: 10.1371/journal.pone.0158982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantor D, Bright JR, Burtchell J. Perspectives from the patient and the healthcare professional in multiple sclerosis: social media and participatory medicine. Neurol Ther. 2018;7:37–49. doi: 10.1007/s40120-017-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantor D, Bright JR, Burtchell J. Perspectives from the patient and the healthcare professional in multiple sclerosis: social media and patient education. Neurol Ther. 2018;7:23–36. doi: 10.1007/s40120-017-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burtchell J. New MS disease categories: how will they affect you? 2014. https://www.healthline.com/health-news/new-ms-disease-categories-defined-061714#1. Accessed 1 Dec 2018.
- 34.O’Loughlin E, Hourihan S, Chataway J, Playford ED, Riazi A. The experience of transitioning from relapsing remitting to secondary progressive multiple sclerosis: views of patients and health professionals. Disabil Rehabil. 2017;39:1821–1828. doi: 10.1080/09638288.2016.1211760. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Clinical guideline [CG186]. 2014. https://www.nice.org.uk/guidance/cg186. Accessed 1 Dec 2018. [PubMed]
- 36.Giovannetti AM, Giordano A, Pietrolongo E, et al. Managing the transition (ManTra): a resource for persons with secondary progressive multiple sclerosis and their health professionals: protocol for a mixed-methods study in Italy. BMJ Open. 2017;7:e017254. doi: 10.1136/bmjopen-2017-017254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemelle N. Here’s my transition to secondary-progressive multiple sclerosis. 2013. https://multiplesclerosis.net/living-with-ms/heres-my-transition-to-secondary-progressive-multiple-sclerosis/. Accessed 1 Dec 2018.
- 38.Emrich L. The transition from relapsing to secondary progressive MS. 2013. https://multiplesclerosis.net/living-with-ms/the-transition-from-relapsing-to-secondary-progressive-ms/. Accessed 1 Dec 2018.
- 39.Davies F, Edwards A, Brain K, et al. ‘You are just left to get on with it’: qualitative study of patient and carer experiences of the transition to secondary progressive multiple sclerosis. BMJ Open. 2015;5:e007674. doi: 10.1136/bmjopen-2015-007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medscape. European and American new draft guidelines for MS. 2016. http://www.medscape.org/viewarticle/870690. Accessed 1 Dec 2018.
- 41.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 42.Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- 43.Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 2015;14:194–207. doi: 10.1016/S1474-4422(14)70231-5. [DOI] [PubMed] [Google Scholar]
- 44.Lorscheider J, Jokubaitis VG, Spelman T, et al. Anti-inflammatory disease-modifying treatment and short-term disability progression in SPMS. Neurology. 2017;89:1050–1059. doi: 10.1212/WNL.0000000000004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novartis Pharmaceutical Corporation. Prescribing information. Mayzent® 2019. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mayzent.pdf. Accessed May 1, 2019.
- 46.EMD Serono. Prescribing information. Mavenclad®. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf. Accessed 1 May 2019.
- 47.Tedeholm H, Lycke J, Skoog B, et al. Time to secondary progression in patients with multiple sclerosis who were treated with first generation immunomodulating drugs. Mult Scler. 2013;19:765–774. doi: 10.1177/1352458512463764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Amico E, Ziemssen T, Cottone S. To stop or not to stop disease modifying therapies in secondary progressive multiple sclerosis, that is the question. Expert Rev Neurother. 2017;17:847–849. doi: 10.1080/14737175.2017.1340831. [DOI] [PubMed] [Google Scholar]
- 49.Coetzee T, Zaratin P, Gleason TL. Overcoming barriers in progressive multiple sclerosis research. Lancet Neurol. 2015;14:132–133. doi: 10.1016/S1474-4422(14)70323-0. [DOI] [PubMed] [Google Scholar]
- 50.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 51.Zeller D, Classen J. Plasticity of the motor system in multiple sclerosis. Neuroscience. 2014;283:222–230. doi: 10.1016/j.neuroscience.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 52.Ontaneda D, Fox RJ, Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 2015;14:208–223. doi: 10.1016/S1474-4422(14)70264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 54.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler. 2012;18:1534–1540. doi: 10.1177/1352458512458169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.International Progressive MS Alliance. Progressive MS. Challenges. http://www.progressivemsalliance.org/progressive-ms/challenges/. Accessed 1 Dec 2018.
- 56.Jackson K. My silver lining. MS Connection Blog. http://www.msconnection.org/Blog/January-2014/My-Silver-Lining. Accessed 1 Dec 2018.
- 57.Borreani C, Bianchi E, Pietrolongo E, et al. Unmet needs of people with severe multiple sclerosis and their carers: qualitative findings for a home-based intervention. PLoS One. 2014;9:e109679. doi: 10.1371/journal.pone.0109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18:221–229. doi: 10.7224/1537-2073.2015-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straudi S, Fanciullacci C, Martinuzzi C, et al. The effects of robot-assisted gait training in progressive multiple sclerosis: a randomized controlled trial. Mult Scler. 2016;22:373–384. doi: 10.1177/1352458515620933. [DOI] [PubMed] [Google Scholar]
- 60.Taylor P, Barrett C, Mann G, Wareham W, Swain I. A feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis. Neuromodulation. 2014;17:75–84. doi: 10.1111/ner.12048. [DOI] [PubMed] [Google Scholar]
- 61.Esnouf JE, Taylor PN, Mann GE, Barrett CL. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Mult Scler. 2010;16:1141–1147. doi: 10.1177/1352458510366013. [DOI] [PubMed] [Google Scholar]
- 62.Ratchford JN, Shore W, Hammond ER, et al. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010;27:121–128. doi: 10.3233/NRE-2010-0588. [DOI] [PubMed] [Google Scholar]
- 63.Pilutti LA, Lelli DA, Paulseth JE, et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011;92:31–36. doi: 10.1016/j.apmr.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 64.Donnellan CP, Shanley J. Comparison of the effect of two types of acupuncture on quality of life in secondary progressive multiple sclerosis: a preliminary single-blind randomized controlled trial. Clin Rehabil. 2008;22:195–205. doi: 10.1177/0269215507082738. [DOI] [PubMed] [Google Scholar]
- 65.Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105:2601–2607. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 66.Vanage SM, Gilbertson KK, Mathiowetz V. Effects of an energy conservation course on fatigue impact for persons with progressive multiple sclerosis. Am J Occup Ther. 2003;57:315–323. doi: 10.5014/ajot.57.3.315. [DOI] [PubMed] [Google Scholar]
- 67.Gillson G, Wright JV, DeLack E, Ballasiotes G. Transdermal histamine in multiple sclerosis: part one—clinical experience. Altern Med Rev. 1999;4:424–428. [PubMed] [Google Scholar]
- 68.Di Fabio RP, Choi T, Soderberg J, Hansen CR. Health-related quality of life for patients with progressive multiple sclerosis: influence of rehabilitation. Phys Ther. 1997;77:1704–1716. doi: 10.1093/ptj/77.12.1704. [DOI] [PubMed] [Google Scholar]
- 69.Lee JE, Bisht B, Hall MJ, et al. A multimodal, nonpharmacologic intervention improves mood and cognitive function in people with multiple sclerosis. J Am Coll Nutr. 2017;36:150–168. doi: 10.1080/07315724.2016.1255160. [DOI] [PubMed] [Google Scholar]
- 70.Meneghetti G, Sparta S, Rusca F, et al. Hyperbaric oxygen therapy in the treatment of multiple sclerosis. A clinical and electrophysiological study in a 2 year follow-up. Riv Neurol. 1990;60:67–71. [PubMed] [Google Scholar]
- 71.Russell RD, Lucas RM, Brennan V, et al. Reported changes in dietary behavior following a first clinical diagnosis of central nervous system demyelination. Front Neurol. 2018;9:161. doi: 10.3389/fneur.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mische LJ, Mowry EM. The evidence for dietary interventions and nutritional supplements as treatment options in multiple sclerosis: a review. Curr Treat Options Neurol. 2018;20(4):8. doi: 10.1007/s11940-018-0494-5. [DOI] [PubMed] [Google Scholar]
- 73.Farinotti M, Vacchi L, Simi S, et al. Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev. 2012;12:CD004192. doi: 10.1002/14651858.cd004192.pub3. [DOI] [PubMed] [Google Scholar]
- 74.Heard RN. The spectrum of multiple sclerosis. Curr Allergy Asthma Rep. 2007;7:280–284. doi: 10.1007/s11882-007-0042-y. [DOI] [PubMed] [Google Scholar]
- 75.Siva A. The spectrum of multiple sclerosis and treatment decisions. Clin Neurol Neurosurg. 2006;108:333–338. doi: 10.1016/j.clineuro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Haegert DG. Clinical multiple sclerosis occurs at one end of a spectrum of CNS pathology: a modified threshold liability model leads to new ways of thinking about the cause of clinical multiple sclerosis. Med Hypotheses. 2005;65:232–237. doi: 10.1016/j.mehy.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat. 2017;13:1349–1357. doi: 10.2147/NDT.S132079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bayer. Betaferon (recombinant interferon beta-1b) Summary of product characteristics. Last updated July 2018. https://www.medicines.org.uk/emc/product/1121/smpc. Accessed 9 Aug 2018.
- 79.Novartis. Extavia (recombinant interferon beta-1b) summary of product characteristics. Last updated July 2018. https://www.medicines.org.uk/emc/product/6529. Accessed 9 Aug 2018.
- 80.Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15:273–279. doi: 10.1136/practneurol-2015-001139. [DOI] [PubMed] [Google Scholar]
- 81.National Institute for Health and Care Excellence (NICE). Appraisal consultation document. Ocrelizumab for treating primary progressive multiple sclerosis. 2018. https://www.nice.org.uk/guidance/ta585/documents/appraisal-consultation-document.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.