Abstract
OBJECTIVE
Preoperative embolization of brain arteriovenous malformations (AVMs) is performed to facilitate resection, although its impact on surgical performance has not been clearly defined. The authors tested for associations between embolization and surgical performance metrics.
METHODS
The authors analyzed AVM cases resected by one neurosurgeon from 2006 to 2017. They tested whether cases with and without embolization differed from one another with respect to patient and AVM characteristics using t-tests for continuous variables and Fisher’s exact tests for categorical variables. They used simple and multivariable regression models to test whether surgical outcomes (blood loss, resection time, surgical clip usage, and modified Rankin Scale [mRS] score) were associated with embolization. Additional regression analyses integrated the peak arterial afferent contrast normalized for the size of the region of interest (Cmax/ROI) into models as an additional predictor.
RESULTS
The authors included 319 patients, of whom 151 (47%) had preoperative embolization. Embolized AVMs tended to be larger (38% with diameter > 3 cm vs 19%, p = 0.001), less likely to have hemorrhaged (48% vs 63%, p = 0.013), or be diffuse (19% vs 29%, p = 0.045). Embolized AVMs were more likely to have both superficial and deep venous drainage and less likely to have exclusively deep drainage (32% vs 17% and 12% vs 23%, respectively; p = 0.002). In multivariable analysis, embolization was not a significant predictor of blood loss or mRS score changes, but did predict longer operating times (+29 minutes, 95% CI 2–56 minutes; p = 0.034) and increased clip usage (OR 2.61, 95% CI 1.45–4.71; p = 0.001). Cmax/ROI was not a significant predictor, although cases with large Cmax/ROI tended to have longer procedure times (+25 minutes per doubling of Cmax/ROI, 95% CI 0–50 minutes; p = 0.051).
CONCLUSIONS
In this series, preoperative embolization was associated with longer median resection times and had no association with intraoperative blood loss or mRS score changes.
Keywords: intracranial arteriovenous malformations, digital subtraction angiography, embolization, cerebrovascular procedures, vascular disorders
THE treatment of brain arteriovenous malformations (AVMs) remains a challenge. Among the modalities of AVM treatment, microsurgery has the highest rates of complete obliteration and lowest long-term risk of hemorrhage, but also the highest case fatality rate.27 Preoperative embolization of AVMs is often performed as an adjuvant to surgery, with the goal of making resection safer. Preoperative embolization may be used to reduce the AVM size, high-risk features, or nidal blood flow.13,16 Embolization has proved an effective way to modify the hemodynamics of an AVM nidus,22,24 although there is less evidence quantifying its effect on surgical performance. Surgical performance is often graded on the totality of AVM resection, operative time, and blood loss. However, in a review of microsurgical outcomes of patients with unruptured AVMs, blood loss was the only significant predictor of posttreatment neurological deficits.31 Evidence as to the clinical outcomes in cases with preoperative embolization, while generally encouraging, has shown mixed results.5,9, 10, 14, 15, 20, 25,28
One challenge in assessing the efficacy of embolization is the lack of a simple and quantitative method of measuring AVM flow characteristics. Previous studies have used subjective measurements of percent AVM nidus reduction from pre- and postembolization angiograms,13,16,28 MR angiography,1,26 and transcranial ultrasound with Doppler6,19 to quantify flow. However, these techniques are heterogeneous and difficult to standardize into practice. A potential alternative is the use of parametrically color-coded angiography12 (iFlow, Siemens Medical Inc.), a novel method to quantify time-density curves from 2D angiography, thus enabling derivation of time-to-peak and pseudo-flow rate measurements.3 There are a handful of AVM studies employing iFlow to detect differences between ruptured and unruptured AVMs, although its influence on operative performance is unknown.2,3,17
Another challenge is the heterogeneity of embolization materials and techniques. There may be a comparative advantage in using certain embolization materials over others.8,13, 16,30 A review of studies looking at either N-butyl 2-cyanoacrylate (NBCA) or ethylene vinyl alcohol copolymer (EVOH) found that EVOH had higher angiographic cure rates but worse neurological outcomes, although none of these studies examined the relative impact of embolization itself on operative performance variables.8
We performed a retrospective review of our microsurgical AVM cases with and without pre-microsurgical embolization. Our primary aim was to characterize the role of pre-microsurgical embolization on traditional surgical performance variables. Our outcomes were intraoperative blood loss, resection time, microclip usage, and change in modified Rankin Scale (mRS) score. Our hypothesis was that preoperative embolization positively affects microsurgical performance outcomes.
Our secondary aim was to assess the utility of parametric color-coded angiography in quantifying blood flow through the AVM nidus. The peak contrast density of the primary feeding artery normalized against the size of the region of interest (i.e., the diameter of that artery) (Cmax/ROI) is a measurement of pixel density, or blood density. We hypothesized that using the Cmax/ROI to identify “high-flow” and “low-flow” AVMs, we could predict patients with worse surgical outcomes.
Methods
A total of 319 consecutive patients who underwent microsurgical resection of a brain AVM by a single neurosurgeon from January 2006 to January 2017 were reviewed. All AVM patients had postoperative angiography that demonstrated no residual AVM. Patients who had stereotactic radiosurgery or prior resection were excluded. The neurosurgeon selected cases for preoperative embolization. Of the 319 patients, 151 (47%) underwent preoperative embolization. Ethics approval was received from the institutional review board, and informed consent was waived. Records were retrospectively reviewed for all patients, and clinical and angioarchitectural data were recorded. Variables included patient age at presentation, AVM size, eloquence of adjacent brain regions, diffuseness of the nidus, complexity of venous drainage, and rupture prior to surgery.11,23 All angioarchitectural measurements were recorded by a neurointerventional radiologist for the express purpose of the AVM research. Determination of eloquence (yes/no), diffuseness (sharp/diffuse), complexity of venous drainage (superficial/deep/both), and rupture prior to surgery (yes/no) were the subjective assessments of the neurointerventional radiologist.
Embolizations were performed by 6 neurointerventional radiologists, and details of the procedure were the purview of the neurointerventional radiologist. The number of embolization sessions, materials used, number of feeding arteries targeted, and high-risk features targeted were recorded from the embolization procedure records.
Outcomes included intraoperative blood loss, resection time, microclip usage, and changes in mRS score.28 In addition to the traditional measures of operative performance (blood loss and resection time), microclip usage was included as a surgical outcome because our surgeon noted that in his practice, clips are representative of larger vessel bleeding and more complex cases. Intraoperative blood loss was retrospectively recorded and defined as the estimated blood loss from the anesthesia case record. Resection time was defined as the total procedure time documented in the anesthesia record, as microscope time was heterogeneously recorded in the records. Microclip usage was retrospectively recorded from the surgical records. Pretreatment mRS scores were assigned by members of the research team based on patient records and were reviewed by the clinical team. Posttreatment (follow-up) mRS scores were based on phone interviews, mailings, record review, or in-person visits conducted by the research team at regular postoperative time intervals. The posttreatment mRS score was based on the last patient follow-up time. The mean follow-up was 1.46 years for the embolization group and 1.42 years for the nonembolization group; no significant difference was detected between the 2 groups (p = 0.85). Team members were aware of the patient’s care, including embolization.
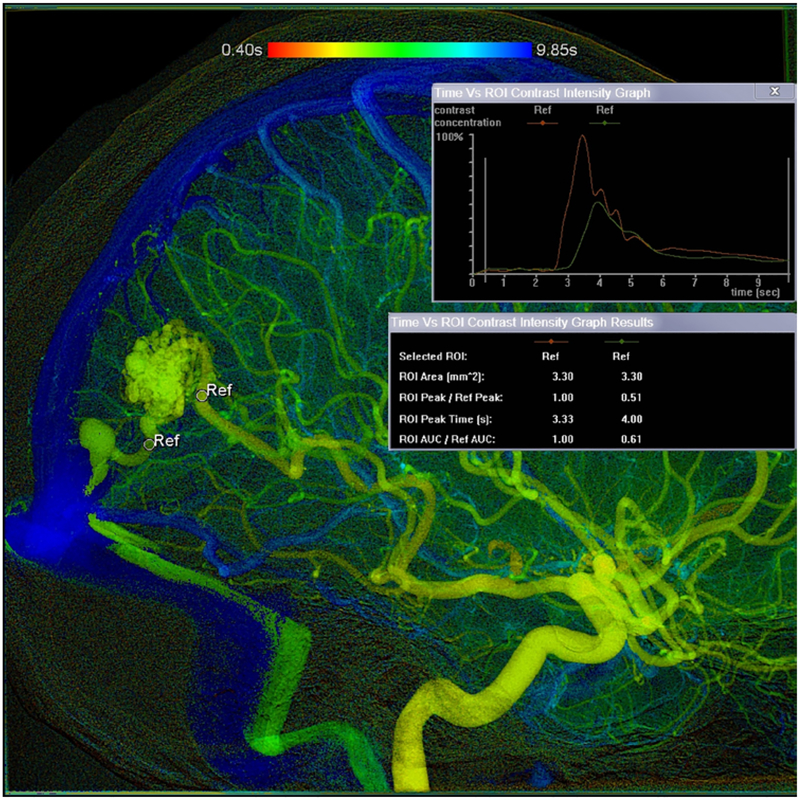
From the 319 patients, we identified 172 cases with iFlow-compatible imaging (those with imaging after 2010) and measured the Cmax/ROI. The Cmax/ROI was defined as the peak contrast of the primary feeding artery to the AVM normalized for the diameter of that feeding artery. This value is essentially a measurement of pixel density (blood), and, when plotted as function of time, a proxy for flow. We derived the Cmax of the primary feeding artery to the AVMs from the pretreatment angiograms. For AVMs with multiple feeding arteries, the dominant arterial afferent was selected for study. Dominance was defined as the composite of vessel diameter (objective) and contribution of nidal flow (subjective). In instances in which multiple vessels demonstrated equivalent contributions to AVM flow, the vessel most clearly identified was selected for iFlow measure. The primary feeding artery and draining vein were marked with a vessel-specific region of interest (ROI) as anatomically close to the nidus as possible in order to minimize artifact from en-passage flow (arterial) and nonopacified flow (venous). The diameter of the ROI was adjusted to the size of the smallest artery or vein in each patient. The Cmax, or peak contrast density, was then obtained from iFlow software as a measure of pixel density and standardized against the size of the ROI (Fig. 1). All iFlow measurements were obtained by a single neurointerventional radiologist.
FIG. 1.
Image created in iFlow from the pretreatment 2D angiogram of a surgical AVM patient. The primary feeding artery and draining vein were marked with a vessel-specific ROI, and the diameter of the ROI was adjusted in each patient. The time density curve was created by iFlow.
Statistical Analysis
We tested whether cases with and without embolization differed from each other with respect to patient and AVM characteristics using Fisher’s exact tests for categorical variables and t-tests for continuous variables. Characteristics analyzed included nidus size, eloquence, venous drainage, age, diffuse nidus, and hemorrhage prior to treatment, as these variables have a known association with surgical outcomes and are incorporated in the Spetzler-Martin and supplemental Lawton-Young AVM grading systems.11,23
We tested for associations with surgical outcomes using regression analyses. The outcomes analyzed were 1) intraoperative blood loss in milliliters, 2) resection time in minutes, 3) surgical clip usage (yes/no), and 4) worsening of mRS score after resection compared with pretreatment status (yes/no). To examine if the embolization material was associated with surgical outcomes, we ran an analysis restricted to embolization cases. In this analysis, we tested whether outcomes were associated with embolization material, represented as indicator variables, using linear and logistic regression models. To test for associations between preoperative embolization (yes/no) and surgical outcomes, we used simple and multivariable linear and logistic regression models. Multivariable models included embolization, AVM size, eloquence, venous drainage, age, rupture status prior to treatment, and diffuseness as predictors.11,23
To assess the utility of quantifying AVM flow with iFlow in predicting surgical outcomes, multivariable regression models were rerun on the sample subset with iFlow data and included Cmax/ROI as an additional predictor. Since the standardized Cmax/ROI was right-skewed and subject to outliers, we log-transformed (base 2) values prior to analysis. To test the utility of iFlow as a screening measure to identify patients who would benefit from embolization, we ran another set of multivariable models including the interaction term of embolization and Cmax/ROI.
Intraoperative blood loss was log-transformed to accommodate for outliers; consequently, coefficients from linear regression analyses with intraoperative blood loss as the outcome were exponentiated in order to interpret results as proportional increases (PIs); for example, a PI of 2 indicates a doubling, and a PI of less than 1 indicates a decrease. Logistic regression results from the surgical clip and mRS analyses are presented as odds ratios. Follow-up time for mRS scores varied by patient, so models with mRS score as an outcome further adjusted for log-transformed (base 2) time postsurgery, up to a maximum of 1 year. All regression results are presented with 95% confidence intervals. Varying degrees of missing outcome and covariate information led to the exclusion of some observations during particular analyses. The level of significance was set at p < 0.05. Data analysis was performed using Stata (version 15.1, StataCorp).
Results
Clinical and Angioarchitectural Characteristics
Of 319 patients who underwent microsurgical resection of a brain AVM, 151 (47%) underwent preoperative embolization. Seven patients (2%) had previously undergone embolization at an outside facility. Table 1 describes the patient and AVM characteristics. The average patient age was 36 years (range 5–90 years), and there was no difference found between the embolization and nonembolization groups with respect to Lawton-Young supplemental scale age categories (p = 0.74).
TABLE 1.
Patient and AVM characteristics
| Characteristic | Embolization | No Embolization | p Value |
|---|---|---|---|
| No. of patients | 151 | 168 | |
| Age, yrs | 0.739 | ||
| <20 | 35 (23) | 42 (25) | |
| 20–40 | 52 (34) | 51 (30) | |
| >40 | 64 (42) | 75 (45) | |
| Female sex | 83 (55) | 81 (48) | 0.262 |
| Nidus size, cm | 0.001 | ||
| <3 | 91/146 (62) | 124/154 (81) | |
| 3–6 | 52/146 (36) | 26/154 (17) | |
| >6 | 3/146 (2) | 4/154 (3) | |
| Eloquent area | 65/145 (45) | 80/154 (52) | 0.247 |
| Venous drainage | 0.002 | ||
| Deep & superficial | 46/146 (32) | 25/151 (17) | |
| Deep only | 18/146 (12) | 35/151 (23) | |
| Superficial only | 82/146 (56) | 91/151 (60) | |
| Spetzler-Martin grade | 0.026 | ||
| I | 25/145 (17) | 43/152 (28) | |
| II | 64/145 (44) | 51/152 (34) | |
| III | 41/145 (28) | 46/152 (30) | |
| IV | 15/145 (10) | 9/152 (6) | |
| V | 0/145 (0) | 3/152 (2) | |
| Ruptured prior to treatment | 73 (48) | 105 (63) | 0.013 |
| Diffuse nidus | 28/145 (19) | 45/153 (29) | 0.045 |
| Pre-resection mRS score | 0.190 | ||
| 0 | 25 (17) | 26/167 (16) | |
| 1 | 53 (35) | 43/167 (26) | |
| 2 | 30 (30) | 35/167 (21) | |
| 3 | 21 (14) | 20/167 (12) | |
| 4 | 10 (7) | 19/167 (11) | |
| 5 | 12 (8) | 24/167 (14) | |
| mRS score at last follow-up | 0.707 | ||
| 0 | 38/140 (27) | 35/160 (22) | |
| 1 | 62/140 (44) | 67/160 (42) | |
| 2 | 25/140 (18) | 35/160 (22) | |
| 3 | 7/140 (4) | 7/160 (4) | |
| 4 | 6/140 (4) | 9/160 (6) | |
| 5 | 1/140 (1) | 3/160 (2) | |
| 6 | 1/140 (1) | 4/160 (3) |
Values are number (%) or number/total not missing (%).
Embolized AVMs tended to fall into larger AVM categories than did nonembolized AVMs (38% with diameter > 3 cm vs 19%, p = 0.001). Venous drainage was associated with embolization (p = 0.002), as embolized AVMs were less likely to have exclusively deep venous drainage (12% vs 23%) and more likely to have both superficial and deep drainage (32% vs 17%). Embolized AVMs were less likely to have diffuse nidi (19% vs 29%, p = 0.045) and were less likely to be ruptured prior to treatment (48% vs 63%, p = 0.013).
Embolization
For 134 embolization cases where the embolization material was known, NBCA was the most common material used (n = 71, 53%), followed by a combination of materials (n = 23, 17%) and PVA (polyvinyl alcohol, n = 15, 11%). Other materials included EVOH, coils, and embospheres. Of 128 patients for whom the details of embolization were recorded, 66 (52%) underwent 2 or more embolizations. The most common high-risk feature targeted for embolization was a feeding artery aneurysm (12%). Other features targeted included intranidal aneurysms and fistulas.
In regression analysis, embolization material was associated with resection time (p = 0.003); patients who underwent embolization with coils had the longest average resection times, while those with ethanol and embospheres had the shortest. However, sample sizes for these materials were small (Table 2). Embolization material was not associated with blood loss (p = 0.688), clip usage (p = 0.256), or mRS score changes (p = 0.269).
TABLE 2.
Characteristics of embolization (n = 134)
| No. of Patients (%) | |
|---|---|
| Material used | |
| NBCA | 71 (53) |
| PVA | 15 (11) |
| EVOH | 12 (9) |
| Coils | 8 (6) |
| Ethanol | 3 (2) |
| Embospheres | 2 (1) |
| Combination | 23 (17) |
| Staged embolization | 66 (52) |
| Multiple pedicles | 59 (45) |
| High-risk features targeted | |
| Intranidal aneurysm | 11 (9) |
| Feeding artery aneurysm | 18 (14) |
| Intranidal fistula | 11 (9) |
Surgical and Clinical Outcomes
Blood loss information was collected for 270 patients. The median blood loss was 300 mL (range 25–8700 mL). Embolization was not associated with a statistically significant increase in blood loss in simple or multivariable linear regression (PI 1.21, 95% CI 0.99–1.49 [p = 0.061] and PI 1.05, 95% CI 0.85–1.30 [p = 0.631], respectively) (Table 3). Both age and AVM size were associated with blood loss (likelihood ratio test, p < 0.001 for both variables), as pediatric patients and smaller AVMs had less bleeding.
TABLE 3.
Multipredictor model of intraoperative blood loss (n = 270)
| Predictor | PI | 95% CI | p Value |
|---|---|---|---|
| Embolization | 1.05 | 0.85–1.30 | 0.631 |
| Age, yrs | |||
| <20 | Ref | ||
| 20–40 | 1.80 | 1.36–2.39 | <0.001 |
| >40 | 1.59 | 1.21–2.10 | 0.001 |
| Nidus size, cm | |||
| <3 | Ref | ||
| 3–6 | 1.77 | 1.37–2.30 | <0.001 |
| >6 | 2.04 | 1.05–3.95 | 0.035 |
| Eloquent area | 0.98 | 0.79–1.21 | 0.864 |
| Venous drainage | |||
| Superficial only | Ref | ||
| Deep only | 1.20 | 0.90–1.60 | 0.217 |
| Deep & superficial | 1.03 | 0.80–1.32 | 0.833 |
| Diffuse nidus | 0.94 | 0.73–1.21 | 0.634 |
| Ruptured | 0.96 | 0.77–1.19 | 0.698 |
Resection time was available for 290 patients; the average time was 329 minutes (range 106–910 minutes). In simple and multivariable linear regression, embolization cases took longer (+49 minutes, 95% CI 22–77 minutes [p = 0.001]; and +29 minutes, 95% CI 2–56 minutes [p = 0.034], respectively) (Table 4). In multivariable analysis, larger AVMs were also associated with longer resection time relative to small AVMs (+225 minutes, 95% CI 139–311 minutes [p < 0.001] for AVMs > 6 cm; +107 minutes, 95% CI 74–140 minutes [p < 0.001] for 3- to 6-cm AVMs). Other variables associated with longer resection time included eloquent brain location (+35 minutes, 95% CI 8 to 62 minutes; p = 0.011), deep venous drainage (+40 minutes, 95% CI 3–76 minutes; p = 0.032), and both deep and superficial venous drainage (+52 minutes, 95% CI 20–83 minutes; p = 0.002) when compared with the superficial drainage reference group.
TABLE 4.
Multipredictor model of procedure time in minutes (n = 290)
| Predictor | Coefficient | 95% CI | p Value |
|---|---|---|---|
| Embolization | 29 | 2 to 56 | 0.034 |
| Age, yrs | |||
| <20 | Ref | ||
| 20–40 | 2 | −33 to 37 | 0.907 |
| >40 | −4 | −39 to 30 | 0.807 |
| Nidus size, cm | |||
| <3 | Ref | ||
| 3–6 | 107 | 74 to 140 | <0.001 |
| >6 | 225 | 139 to 311 | <0.001 |
| Eloquent area | 35 | 8 to 62 | 0.011 |
| Venous drainage | |||
| Superficial only | Ref | ||
| Deep only | 40 | 3 to 76 | 0.032 |
| Deep & superficial | 52 | 20 to 83 | 0.002 |
| Diffuse nidus | −9 | −41 to 22 | 0.556 |
| Ruptured | −19 | −46 to 8 | 0.162 |
Surgical clip information was collected for 287 patients. One hundred eighteen (37%) patients required use of surgical clips during resection. Preoperative embolization was associated with increased odds of surgical clip use in simple and multivariable logistic regression analyses (OR 2.70, 95% CI 1.69–4.32 [p < 0.001] and OR 2.61, 95% CI 1.45–4.71 [p = 0.001]; respectively) (Table 5). Other factors that increased odds of clip usage included large nidus size (likelihood ratio test, p < 0.001) and eloquent brain location (OR 2.73, 95% CI 1.50–4.99; p = 0.001). AVMs that hemorrhaged prior to treatment had decreased odds of requiring surgical clips (OR 0.24, 95% CI 0.13–0.45; p < 0.001).
TABLE 5.
Multipredictor model of clip usage (n = 287)
| Predictor | OR | 95% CI | p Value |
|---|---|---|---|
| Embolization | 2.61 | 1.45–4.71 | 0.001 |
| Age, yrs | |||
| <20 | Ref | ||
| 20–40 | 1.30 | 0.60–2.78 | 0.507 |
| >40 | 1.19 | 0.55–2.56 | 0.658 |
| Nidus size, cm | |||
| <3 | Ref | ||
| 3–6 | 3.62 | 1.86–7.04 | <0.001 |
| >6 | 5.00 | 0.79–31.72 | 0.088 |
| Eloquent area | 2.73 | 1.50–4.99 | 0.001 |
| Venous drainage | |||
| Superficial only | Ref | ||
| Deep only | 1.41 | 0.63–3.18 | 0.407 |
| Deep & superficial | 1.95 | 1.00–3.80 | 0.049 |
| Diffuse nidus | 1.69 | 0.84–3.40 | 0.141 |
| Ruptured | 0.24 | 0.13–0.45 | <0.001 |
Of 264 cases with clinical outcome data, 58 (19%) had a worse mRS score during a postsurgical follow-up assessment (median time of 1.1 years) when compared with pretreatment status. Embolized cases were not significantly more likely to have the same or improved mRS score in multivariable analysis (OR 0.74, 95% CI 0.36–1.52; p = 0.409) (Table 6). Factors strongly predictive of mRS score changes were eloquent brain location (OR 2.75, 95% CI 1.35–5.39; p = 0.005) and hemorrhage prior to resection (OR 0.10, 95% CI 0.04–0.24; p < 0.001).
TABLE 6.
Multipredictor model of worsening mRS score (n = 264)
| Predictor | OR | 95% CI | p Value |
|---|---|---|---|
| Embolization | 0.74 | 0.36–1.52 | 0.409 |
| Age, yrs | |||
| <20 | Ref | ||
| 20–40 | 1.30 | 0.45–3.73 | 0.629 |
| >40 | 2.52 | 0.92–6.93 | 0.073 |
| Nidus size, cm | |||
| <3 | Ref | ||
| 3–6 | 1.14 | 0.47–2.76 | 0.770 |
| >6 | 2.10 | 0.19–23.52 | 0.548 |
| Eloquent area | 2.75 | 1.35–5.59 | 0.005 |
| Venous drainage | |||
| Superficial only | Ref | ||
| Deep only | 2.31 | 0.83–6.42 | 0.110 |
| Deep & superficial | 1.77 | 0.76–4.11 | 0.186 |
| Diffuse nidus | 1.30 | 0.53–3.15 | 0.566 |
| Ruptured | 0.10 | 0.04–0.24 | <0.001 |
| Log2 recovery time, yrs* | 0.73 | 0.61–0.89 | 0.001 |
Recovery time is from surgery to last follow-up assessment (set to a 1-year maximum).
iFlow Characteristics
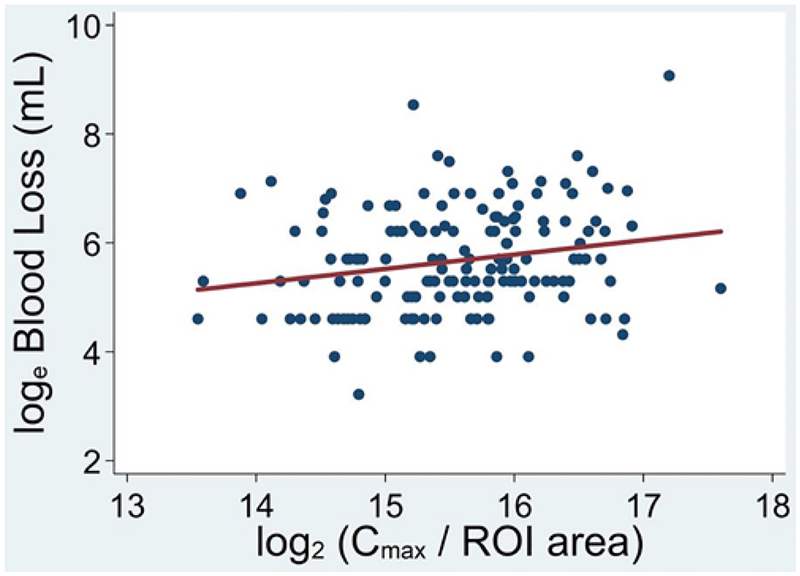
Data from iFlow were available for 172 patients. The average log-transformed Cmax/ROI, a proxy for flow, was 15.6 ± 0.8 (range 11.0–17.6). The interquartile range of the log-transformed standardized Cmax/ROI was approximately 1 unit (i.e., a doubling). We found that each doubling of Cmax/ROI was associated with increased blood loss in simple regression analysis (Fig. 2), but not in multivariable analysis (PI 1.30, 95% CI 1.07–1.59 [p = 0.009] and PI 1.16, 95% CI 0.94–1.43 [p = 0.157], respectively). This iFlow parameter was associated with longer procedure times in a simple linear regression, but not in multivariable analysis (+37 minutes, 95% CI 11–63 minutes [p = 0.005] and +25 minutes, 95% CI 0–50 minutes [p = 0.051], respectively). We were unable to detect an association between Cmax/ROI and clip usage in simple and multivariable regression analyses (OR 1.54, 95% CI 0.99–2.38 [p = 0.053] and OR 1.47, 95% CI 0.82–2.64 [p = 0.196], respectively). We were unable to detect an association between Cmax/ROI and the mRS change outcome when either adjusting just for follow-up time or in the full multivariable analysis (OR 1.35, 95% CI 0.79–2.32 [p = 0.272] and OR 1.19, 95% CI 0.61–2.31 [p = 0.606]). In analyses that included an interactions term, in no instance was an outcome associated with the interaction of embolization with Cmax/ROI (p > 0.2 for all), indicating a lack of evidence that embolization differentially benefits or disadvantages AVMs with high iFlow values undergoing resection.
FIG. 2.
We found that each doubling of Cmax/ROI was associated with increased blood loss in simple regression analysis, but not in multivariable analysis.
Discussion
In the largest study to date of preoperative embolization compared with surgery alone, we show that embolization is associated with longer median resection times and surgical clip usage and not with decreased blood loss. Embolization is often an adjuvant therapy for AVMs, although there are limited data to support its superiority to resection alone. The literature mainly represents case series9,22,24,29 and retrospective studies.5,10,14,15,20 Notably, Jafar et al. conducted a retrospective study of operative time and blood loss metrics for 41 patients with preoperative embolization compared with surgery alone.10 Pasqualin,20 Morgan,14,15 and DeMeritt5 and their colleagues also each conducted small, retrospective studies of posttreatment morbidity and mortality for preoperative embolization compared with surgery alone. The literature demonstrates that embolization may improve outcomes for some AVMs, although without clear consensus. Our study is the largest describing quantitative operative metrics for preoperative embolization with surgery-only controls and reveals an uncertain role for embolization as a positive surgical modifier.
Preoperative embolization is proposed to be most helpful in the treatment of higher-grade AVMs,18,24,27 and, as such, embolized AVMs are characteristically different from those that are not. In our analysis, AVMs selected for embolization were not only larger on average than those that were not embolized, but also more likely to have both superficial and deep venous drainage. Similarly, Jafar et al. found that cases selected for embolization were of higher overall Spetzler-Martin grade.10 Pasqualin et al. likewise found that AVMs selected for embolization tended to be larger and located in eloquent brain regions.20 The benefit of embolization may in part be that patients with higher-grade lesions were able to undergo curative microsurgery, as opposed to other treatment modalities with hypothetical risks of future bleeding from incompletely treated AVMs. For example, Ding et al. and Patibandla et al. noted 10-year radiosurgical obliteration rates of Spetzler-Martin grade III and IV/V lesions of 78% and 37%, respectively, both carrying a 4% risk of permanent neurological injury.7,21 Compared with such radiosurgical efficacy, the higher and more immediate rates of cure of embolization-enabled microsurgical resection may represent a longer-term benefit.
The heterogeneity of embolization also confounds interpretation of the procedure’s impact on surgical performance. The aim of embolization is not unilateral; some operators focus on AVM flow reduction rather than penetrating the nidus. Embolization material also affects success of embolization as determined by angiography. Loh and Duckwiler noted that EVOH yielded a higher percent of nidal embolization than NBCA.13 Many surgeons primarily employ EVOH when attempting such nidal reduction. Our series reflects a practice largely focused on flow reduction, as evidenced by a higher percentage of cases using NBCA. This is not to say that NBCA cannot penetrate and reduce nidal volume, but that our series tilts away from this as embolization’s major function. EVOH may have a greater impact on operative performance with its ability for more complete nidal obliteration, though with greater risk of periprocedural complications. Of patients in the embolization group, 2 (1%) had a complication associated with the procedure. Both were perforations related to microwave manipulation, and neither patient suffered permanent injury related to the complication. In other studies, some of which used EVOH more commonly, complication rates associated with embolization ranged from 2% to 5%.18,23,29
Previous studies have found that multimodal treatment with embolization is associated with decreased intraoperative blood loss.13,20,22 Pasqualin et al. reported decreased rates of intraoperative complications in patients with embolization compared with size-matched patients who had surgery alone,19 while Jafar et al. found that there was no significant difference in intraoperative blood loss or resection time for AVMs with preoperative embolization.10 In their multivariable analysis, Jafar et al. found that AVM size and Spetzler-Martin grade were not predictive of blood loss for embolization cases, arguing that embolization could potentially offset such higher-risk features. Our study corroborates that there is no statistical difference in intraoperative blood loss between the embolized and nonembolized groups. At best, the effect of embolization adjusted for covariates was only a 15% reduction in blood loss (from PI 1.05, 95% CI 0.85–1.30; p = 0.631). For operating time, Jafar et al. found that after embolization larger AVMs still took longer to resect, but embolization decreased the resection time compared with AVMs of similar Spetzler-Martin grade without embolization. Our results differed in that we found that embolization was still predictive of increased operating time even in a multivariable model.
Previous studies have found that patients who undergo embolization followed by microsurgical resection have better neurological outcomes than patients treated with surgery alone, despite having larger and higher-grade AVMs.5,25,29 Embolization does not, however, appear to improve neurological outcomes for all patients. Those with an unruptured presentation are more likely to experience posttreatment neurological deficits.9 Patients with Spetzler-Martin grade I and II AVMs may also be less likely to benefit from embolization, as the procedure exposes them to additional risk.4,9,14,18,27 Morgan et al. found that embolization with EVOH did not improve postsurgical clinical outcomes or decrease the risk of hemorrhage compared with historical controls.14 In a separate study, Morgan et al. also found increased rates of major morbidity and mortality for embolization compared with surgery alone.15
Despite such evidence, others have found that while preoperative embolization was associated with increased transient deficits or no difference in the immediate postoperative period, it was associated with better long-term clinical outcomes compared with surgery.5,20 DeMeritt et al. hypothesized that as embolized AVMs were larger, patients could show more improvement in clinical status following the resolution of transient deficits related to postoperative edema or brain retraction.5 Pasqualin et al. found that patients with AVMs located in eloquent brain regions who underwent embolization were significantly less likely to have long-term deficits.20 In our multivariable model, we found no significant relationship between embolization and mRS score.
AVM Flow and Patient Selection
One of the critical issues related to AVM embolization is our lack of a standardized manner to assess AVM flow. There is evidence noting the use of MRI to quantify AVM flow,1,26 with Alaraj et al. noting that nidal flow measured by quantitative MR angiography was decreased in AVMs that had undergone embolization of multiple arterial pedicles or a direct arteriovenous shunt.1 MRI is instructive but has limited practical application as decisions in endovascular practice are most often made using catheter angiography. Parametric color-coding, a quantitative, pseudo-flow technique, is powerful in that it can consistently and immediately be generated using DSA, permitting real-time decision-making during procedures. Our result that Cmax/ROI was a predictor of blood loss and operative time in simple regression analysis suggests its potential in quantitative flow dynamics and in clinical decision-making. Although the incorporation of this variable into our models does not yet suggest which patients might benefit from embolization, its accessibility permits more widespread adoption by the neurointerventional community.
Previous studies have correlated iFlow variables with clinical characteristics of AVM patients, specifically ruptured presentation.2,3,17 Variables significantly associated with hemorrhage in other studies include time-to-peak contrast in the feeding artery,17 the ratio of time-to-peak contrast in the draining vein to feeding artery,3 and mean transit time.2,3,26 In a retrospective comparison of ruptured and unruptured AVMs, Burkhardt et al. found significantly longer venous drainage times in the ruptured AVMs,2 while Chen et al. found that mean contrast transit time was associated with silent microhemorrhage in unruptured AVMs.3 It is possible that further analysis will reveal additional variables predictive of surgical outcomes or otherwise identify AVM patients at risk for worse outcomes. Furthermore, other iFlow variables may show utility as screening measures to determine which patients could benefit from embolization.
Limitations
The study was retrospective and therefore is subject to bias. Embolization cases were performed by 6 neurointerventional radiologists, which may be an additional confounding factor in assessing the efficacy of embolization. One neurosurgeon performed the resection in all cases, which lends internal validity to our study but could affect the generalizability of the results.
In this retrospective analysis, we did not control for craniotomy approach or level of trainee involvement. However, given that multivariable models controlled for AVM size and eloquent location, as well as other Spetzler-Martin variables, surgical variability associated with craniotomy approach may be captured. In future prospective studies related to the topic, it will be important to control for level of trainee involvement.
All iFlow measurements were performed by a single neurointerventional radiologist. Performance characteristics of this technique, including intra- and interobserver reliability, were not assessed as part of this study, as this use of iFlow was exploratory. Subsequent work in iFlow needs to be done to assess performance characteristics.
Conclusions
Embolized AVMs have higher Spetzler-Martin grades and are associated with a number of specific variables independently predictive of worse outcomes, including larger AVM size and more complex venous drainage. We found that preoperative embolization did not have a clearly beneficial effect on operative performance measures. In multivariable analysis, preoperative embolization was not associated with intraoperative bleeding and was associated with longer resection time and increased use of surgical clips. We found that the iFlow variable, arterial Cmax/ROI, was associated with increased blood loss and operating time in simple regression analysis, regardless of embolization status, suggesting its possible utility in identifying patients at greater treatment risk. Parametric color-coded angiography is a simple, quantitative measurement that may identify patients at risk for adverse surgical outcomes, or have utility as a screening measure for patients who would benefit from embolization.
ABBREVIATIONS
- AVM
arteriovenous malformation
- Cmax
peak contrast density of the primary feeding artery
- Cmax/ROI
Cmax normalized for the size of the ROI (i.e., the diameter of the artery)
- EVOH
ethylene vinyl alcohol copolymer
- mRS
modified Rankin Scale
- NBCA
N-butyl 2-cyanoacrylate
- PI
proportional increase
- PVA
polyvinyl alcohol
- ROI
region of interest
Footnotes
Disclosures
Dr. Hetts reports being a consultant for Cerenovus and Micro-Vention Terumo; ownership of Stryker; direct ownership of stock in ThrombX; and a research contract with Siemens.
References
- 1.Alaraj A, Amin-Hanjani S, Shakur SF, Aletich VA, Ivanov A, Carlson AP, et al. : Quantitative assessment of changes in cerebral arteriovenous malformation hemodynamics after embolization. Stroke 46:942–947, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Burkhardt JK, Chen X, Winkler EA, Cooke DL, Kim H, Lawton MT: Delayed venous drainage in ruptured arteriovenous malformations based on quantitative color-coded digital subtraction angiography. World Neurosurg 104:619–627, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Cooke DL, Saloner D, Nelson J, Su H, Lawton MT, et al. : Higher flow is present in unruptured arteriovenous malformations with silent intralesional microhemorrhages. Stroke 48:2881–2884, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conger A, Kulwin C, Lawton MT, Cohen-Gadol AA: Diagnosis and evaluation of intracranial arteriovenous malformations. Surg Neurol Int 6:76, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMeritt JS, Pile-Spellman J, Mast H, Moohan N, Lu DC, Young WL, et al. : Outcome analysis of preoperative embolization with N-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol 16:1801–1807, 1995 [PMC free article] [PubMed] [Google Scholar]
- 6.Diehl RR, Henkes H, Nahser HC, Kühne D, Berlit P: Blood flow velocity and vasomotor reactivity in patients with arteriovenous malformations. A transcranial Doppler study. Stroke 25:1574–1580, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Ding D, Starke RM, Kano H, Lee JYK, Mathieu D, Pierce J, et al. : Stereotactic radiosurgery for Spetzler-Martin Grade III arteriovenous malformations: an international multicenter study. J Neurosurg 126:859–871, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Elsenousi A, Aletich VA, Alaraj A: Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: a meta-analysis. J Neurointerv Surg 8:265–272, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Hartmann A, Mast H, Mohr JP, Pile-Spellman J, Connolly ES, Sciacca RR, et al. : Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke 36:2431–2435, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ: The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg 78:60–69, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL: A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 66:702–713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KW, Tsai FY, Chen WL, Liu CK, Kuo CL: Intracranial venous hemodynamics and rupture of cerebral aneurysm. Neuroradiol J 27:703–709, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh Y, Duckwiler GR: A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Clinical article. J Neurosurg 113:733–741, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Morgan MK, Davidson AS, Koustais S, Simons M, Ritson EA: The failure of preoperative ethylene-vinyl alcohol copolymer embolization to improve outcomes in arteriovenous malformation management: case series. J Neurosurg 118:969–977, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Morgan MK, Zurin AAR, Harrington T, Little N: Changing role for preoperative embolisation in the management of arteriovenous malformations of the brain. J Clin Neurosci 7:527–530, 2000 [DOI] [PubMed] [Google Scholar]
- 16.n-BCA Trial Investigators: N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 23:748–755, 2002 [PMC free article] [PubMed] [Google Scholar]
- 17.Norris JS, Valiante TA, Wallace MC, Willinsky RA, Montanera WJ, terBrugge KG, et al. : A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: a prospective, blinded analysis of 31 cases. J Neurosurg 90:673–679, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Ogilvy CS, Stieg PE, Awad I, Brown RD Jr, Kondziolka D, Rosenwasser R, et al. : AHA Scientific statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 32:1458–1471, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Pasqualin A, Barone G, Cioffi F, Rosta L, Scienza R, Da Pian R: The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 28:370–379, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Pasqualin A, Scienza R, Cioffi F, Barone G, Benati A, Beltramello A, et al. : Treatment of cerebral arteriovenous malformations with a combination of preoperative embolization and surgery. Neurosurgery 29:358–368, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Patibandla MR, Ding D, Kano H, Xu Z, Lee JYK, Mathieu D, et al. : Stereotactic radiosurgery for Spetzler-Martin Grade IV and V arteriovenous malformations: an international multicenter study. J Neurosurg 129:498–507, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Purdy PD, Samson D, Batjer HH, Risser RC: Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol 11:501–510, 1990 [PMC free article] [PubMed] [Google Scholar]
- 23.Spetzler RF, Martin NA: A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E: Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg 67:17–28, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Starke RM, Komotar RJ, Otten ML, Hahn DK, Fischer LE, Hwang BY, et al. : Adjuvant embolization with N-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke 40:2783–2790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todaka T, Hamada J, Kai Y, Morioka M, Ushio Y: Analysis of mean transit time of contrast medium in ruptured and unruptured arteriovenous malformations: a digital subtraction angiographic study. Stroke 34:2410–2414, 2003 [DOI] [PubMed] [Google Scholar]
- 27.van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJE, et al. : Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 306:2011–2019, 2011 [DOI] [PubMed] [Google Scholar]
- 28.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J: Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Viñuela F, Dion JE, Duckwiler G, Martin NA, Lylyk P, Fox A, et al. : Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg 75:856–864, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Weber W, Kis B, Siekmann R, Jans P, Laumer R, Kühne D: Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery 61:244–254, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wong J, Slomovic A, Ibrahim G, Radovanovic I, Tymianski M: Microsurgery for ARUBA Trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformation)–eligible unruptured brain arteriovenous malformations. Stroke 48:136–144, 2017 [DOI] [PubMed] [Google Scholar]