Abstract
Type 2 diabetes mellitus (T2DM) leads to exaggerated cardiovascular responses to exercise, in part due to an exaggerated exercise pressor reflex. Accumulating data suggest excessive oxidative stress contributes to an exaggerated exercise pressor reflex in cardiovascular-related diseases. Excessive oxidative stress is also a primary underlying mechanism for the development and progression ofT2DM. However, whether oxidative stress plays a role in mediating the exaggerated exercise pressor reflex in T2DM is not known. Therefore, this review explores the potential role of oxidative stress leading to increased activation of the afferent arm of the exercise pressor reflex. Several lines of evidence support direct and indirect effects of oxidative stress on the exercise pressor reflex. For example, intramuscular ROS may directly and indirectly (by attenuating contracting muscle blood flow) increase group III and IV afferent activity. Oxidative stress is a primary underlying mechanism for the development of neuropathic pain, which in turn is associated with increased group III and IV afferent activity. These are the same type of afferents that evoke muscle pain and the exercise pressor reflex. Furthermore, oxidative stress-induced release of inflammatory mediators may modulate afferent activity. Collectively, these alterations may result in a positive feedback loop that further amplifies the exercise pressor reflex. An exaggerated reflex increases the risk of adverse cardiovascular events. Thus, identifying the contribution of oxidative stress could provide a potential therapeutic target to reduce this risk in T2DM.
Keywords: Exercise pressor reflex, Neural cardiovascular control, Reactive oxygen species, Sympathetic activity, Blood pressure, Neuropathy
1. Introduction
Autonomic adjustments are pivotal in mediating appropriate cardiovascular responses to exercise. By decreasing parasympathetic activity to the heart while increasing sympathetic activity to the heart and peripheral vasculature, blood pressure increases, and blood flow is re-distributed away from inactive tissues. At the same time, sympathetically mediated vasoconstriction in the contracting skeletal muscle is blunted by local release of metabolites (i.e., functional sympatholysis) facilitating increases in muscle blood flow (Hansen et al., 2000; Remensnyder et al., 1962). Two primary mechanisms are known to increase sympathetic activity during exercise: Central command, a feed-forward mechanism originating in higher brain centers (Eldridge et al., 1985; Goodwin et al., 1972), and the exercise pressor reflex, a feedback mechanism with the afferent arm originating within the skeletal muscle (Alam and Smirk, 1937; McCloskey and Mitchell, 1972). Furthermore, arterial and cardiopulmonary baroreflexes modulate these adjustments (Fadel and Raven, 2012). The exercise pressor reflex is critical in evoking appropriate cardiovascular responses to exercise; however, an exaggerated reflex increases the risk of adverse cardiovascular events (Kurl et al., 2001; Laukkanen et al., 2001). Although several mechanisms are known to contribute to the autonomic adjustments of the circulation during exercise, this review will focus on the role of the exercise pressor reflex.
The afferent arm of the exercise pressor reflex is comprised of group III and IV afferents, whose peripheral endings are stimulated by mechanical and metabolic stimuli produced during muscle contraction (Adreani et al., 1997; Kaufman et al., 1983). Studies have shown that the exercise pressor reflex is exaggerated in several cardiovascular-related diseases, including hypertension (Leal et al., 2008), peripheral artery disease (PAD) (Baccelli et al., 1999; Stone et al., 2015b; Tsuchimochi et al., 2010), heart failure (Ives et al., 2016; Keller-Ross et al., 2014), and type 1 diabetes (T1DM) (Grotle et al., 2017), as well as kidney disease (Sprick et al., 2019). Furthermore, recent data have indicated that the exaggerated cardiovascular responses to exercise in type 2 diabetes mellitus (T2DM) patients (Holwerda et al., 2016a; Karavelioglu et al., 2013; Scott et al., 2008) are, in part, mediated by the exercise pressor reflex (Grotle et al., 2019a; Kim et al., 2019).
The prevalence of T2DM (US: ~30 million) and pre-diabetes (US: ~84 million) in younger, middle, and older adults are continuing to increase, posing a significant health concern as disease duration increases the risk of diabetic complications (Mayer-Davis et al., 2017; Centers for Disease Control and Prevention 2017, 2018). Individuals with diabetes are at a two to three-fold higher risk for cardiovascular disease (CVD), in which 84% of diabetics over 65 years die from a heart attack or stroke, contributing to a 10-year shorter life expectancy. Moreover, the high prevalence of comorbidities in diabetes, such as hypertension, obesity, and physical inactivity significantly increase mortality from CVD (Einarson et al., 2018). Although an exaggerated blood pressure response to exercise is a predictor for adverse cardiovascular events (Haffner et al., 1998; Mittleman and Siscovick, 1996), exercise training is recommended to combat cardiovascular risk factors in T2DM (Eriksson et al., 2001; Eriksson, 1999). However, in order to prescribe exercise safely and develop effective treatment strategies, it is essential to understand the mechanisms underlying cardiovascular responses to acute exercise.
In recent years, data have implicated oxidative stress as an underlying mechanism in evoking the exaggerated exercise pressor reflex in several cardiovascular-related diseases (Harms et al., 2017; Koba et al., 2009; Koba et al., 2013; Muller et al., 2012; Wang et al., 2009). In T2DM, oxidative stress is a primary mechanism for impaired vascular function, neuropathy, mitochondrial dysfunction, cardiomyopathies, and autonomic dysfunction (Giacco and Brownlee, 2010). Oxidative stress is a condition of cellular redox imbalance between the production and reduction (i.e., antioxidant defense) of free radicals, including reactive oxygen species (ROS: superoxide, hydrogen peroxide and hydroxyl radical) and reactive nitrogen species (RNS: peroxynitrite). In moderate amounts, free radicals are essential signaling molecules, whereas in higher amounts, they react with and can damage cellular structures such as DNA, lipids, and proteins (Valko et al., 2007). Accumulating evidence suggests that oxidative stress may be an efficacious stimulus, and unifying mechanism, for an exaggerated exercise pressor reflex. However, it is not known whether oxidative stress contributes to the exaggerated cardiovascular responses to exercise in T2DM.
Underlying mechanisms causing an exaggerated exercise pressor reflex in T2DM are incompletely understood. It is likely that these mechanisms involve increased production of metabolites and or changes to the group III and IV muscle afferents. Therefore, the purpose of this review is to discuss potential roles of peripheral oxidative stress in increasing the activity of the afferent arm of the exercise pressor reflex in T2DM.
2. The exercise pressor reflex
The exercise pressor reflex is essential in regulating cardiovascular and ventilatory responses to exercise. Mechanical and metabolic stimuli produced by contracting muscle stimulate receptors and channels on the peripheral endings of group III and IV skeletal muscle afferents (Alam and Smirk, 1937; Kaufman et al., 1983; McCloskey and Mitchell, 1972). Afferent signals from the muscle travel to the dorsal root ganglion (DRG) and then to the dorsal horn of the spinal cord before being transmitted centrally for integration in the rostral ventrolateral medulla (VLM) and nucleus tractus solitarius (NTS) in the medulla oblongata (Craig, 1995; Potts et al., 2002). Studies using electrically induced exercise (excluding central command) and pharmacological attenuation of afferent input from the lower limbs during voluntary exercise, support the importance of the exercise pressor reflex in regulating cardiovascular responses to exercise in humans (Amann et al., 2010; Amann et al., 2011).
The afferent arm of the reflex is comprised of thinly myelinated group III (AS fibers) and unmyelinated group IV (C-fibers) afferents (McCloskey and Mitchell, 1972). Group III afferents respond primarily to mechanical stimuli (i.e., mechanoreflex), such as light stroking or squeezing of the receptive field or stretching the Achilles tendon. These are fast-conducting afferents that discharge vigorously at the onset of muscle contraction in proportion to tension development and tend to decrease their discharge rate as the muscle fatigues (Hayes et al., 2009; Kaufman et al., 1983). In contrast, group IV afferents respond primarily to metabolic stimuli (i.e., metaboreflex), such as metabolites produced during muscle contraction. These are slower conducting afferents that increase their discharge as the muscle fatigues, in proportion to the production of metabolic by-products and with response latencies of 5–30 s (Kaufman et al., 1983; Kaufman et al., 1984). Although group III and IV muscle afferents primarily respond to mechanical or metabolic stimuli, respectively, both types of afferents exhibit polymodal activity in which some group III afferents respond to metabolic stimuli and some group IV afferents respond to mechanical stimuli (Kumazawa and Mizumura, 1977; Rotto and Kaufman, 1988; Rotto et al., 1990).
Metabolic by-products and proteins comprising the metabolically sensitive component (i.e., metaboreflex) of the exercise pressor reflex include lactic acid, bradykinin, arachidonic acid, and its cyclooxygenase (COX) products, including ATP. These metabolites stimulate acid-sensing ion channel 3 (ASIC3), bradykinin B2 (B2) receptors, thromboxane A2 (TXA2) receptors, endoperoxide (EP) receptors, and purinergic 2× (P2X) receptors (Hanna and Kaufman, 2004; Kaufman et al., 1983; Rotto and Kaufman, 1988; Stone et al., 2015b), respectively. However, the exact role of these proteins in healthy and pathological conditions is still unclear (Stone and Kaufman, 2015) and difficult to differentiate due, in part, to redundancy (Stone et al., 2015b).
Less is known about proteins comprising the mechano-sensitive component (i.e., mechanoreflex) of the exercise pressor reflex. This lack of understanding is partially due to the scarcity of antagonists with specificity for mechano-sensitive proteins. Previous studies have used gadolinium, a non-selective antagonist that inhibits multiple classes of mechano-gated channels. Although gadolinium successfully blocks pressor responses to mechanical stimulation such as tendon stretch and muscle contraction (Hayes and Kaufman, 2001; Hayes et al., 2009), it cannot elucidate the contributions of individual channels. Conversely, the recent discovery of Piezo channels (found in two isoforms: Piezo1 and Piezo2) and their selective antagonist Grammostola spatulata mechanotoxin 4 (GsMTx-4) (Coste et al., 2010; Gnanasambandam et al., 2017), has led to studies that suggest that Piezo channels may be a significant mediator of the mechanical component of the exercise pressor reflex (Copp et al., 2016a). These studies have shown that injecting GsMTx-4 into the contracting muscle attenuates the exercise pressor reflex and mechanoreflex in healthy rats (Copp et al., 2016a; Sanderson et al., 2019), the exaggerated exercise pressor reflex and mechanoreflex in PAD rats (i.e. ligated femoral artery model) (Copp et al., 2016b) and the mechanoreflex in T1DM rats (Grotle et al., 2019b). Collectively, these findings highlight the importance of identifying signals and proteins involved in evoking the exercise pressor reflex and are crucial to developing effective therapeutic targets in disease.
3. Reflexive cardiovascular responses to exercise in T2DM
3.1. Human studies
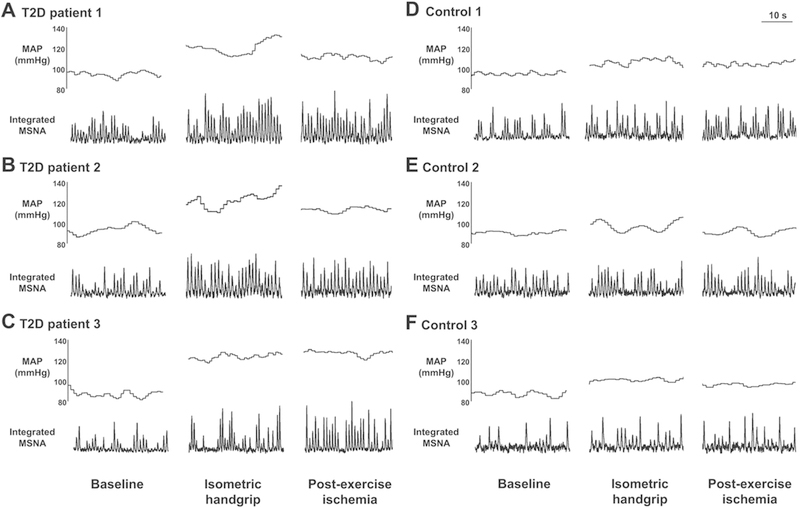
Individuals with T2DM have exaggerated blood pressure responses, as well as reduced tolerance, to exercise. Even independently, both of these conditions increase the risk of cardiovascular disease and mortality (Laukkanen et al., 2001; Poitras et al., 2018). Several studies have shown that blood pressure responses to both maximal and moderate intensity dynamic exercise are exaggerated in adolescents (Pinto et al., 2014), middle-aged adults (Regensteiner et al., 2009; Scott et al., 2008), and older adults with T2DM (O’Connor et al., 2015). Although these responses are common, surprisingly few studies have tried to elucidate the underlying mechanisms. A recent study by Holwerda et al. provided some critical insight into the reflexive control of circulation in T2DM (Holwerda et al., 2016a). They found that individuals with T2DM, independent of resting hypertension, had exaggerated pressor and sympathetic responses to isometric handgrip exercise, an effect which remained during post-exercise circulatory occlusion (i.e., metaboreflex), compared to healthy age-matched controls (Fig. 1). Interestingly, these responses were significantly correlated with disease severity (Holwerda et al., 2016a). Whether T2DM leads to an impaired arterial baroreflex control of sympathetic outflow is unclear (de Moura-Tonello et al., 2016; Holwerda et al., 2016b; Ruiz et al., 2005). However, one study suggested that any impairment in baroreflex control in T2DM may be due to obesity and not diabetes per se (Holwerda et al., 2016b). Thus, further investigation is warranted. Collectively, these findings indicate that the reflexive control of the circulation is altered in T2DM.
Fig. 1.
Original recordings of muscle sympathetic nerve activity (MSNA) and mean arterial pressure (MAP) in 3 type 2 diabetes patients (T2D; A-C) and 3 control subjects (D-F) at baseline, during 30% maximal voluntary contraction (MVC) isometric handgrip, and during post-exercise ischemia (PEI).
3.2. Animal studies
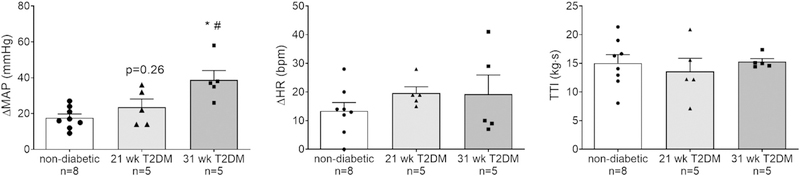
Recently, studies using an unanesthetized, decerebrate rat preparation in which the exercise pressor reflex was isolated by electrically stimulating hindlimb muscle contractions have provided important insight into the effect of T2DM on the exercise pressor reflex. Specifically, these studies have shown that the exercise pressor reflex is exaggerated in two rat models of T2DM. For example, Kim et al. demonstrated that sympathetic and pressor responses to electrically-induced static muscle contraction were exaggerated in a high-fat diet and low-dose streptozotocin-induced T2DM rat model (Kim et al., 2019). However, it was not determined whether these responses changed with severity and progression of the disease. We began investigating whether the progression of T2DM changes the expression of the exercise pressor reflex. Unlike the study by Kim et al., we used the UC Davis-Type 2 Diabetes Mellitus rat model (UCD-T2DM) that develops diabetes naturally (un-treated) over time with similar pathophysiology as that seen in humans (Cummings et al., 2008). This model has allowed us to follow the progression of the disease through the development and progression of T2DM. Indeed, we found that as rats progressed with T2DM, the pressor response to static contraction incrementally increased until it was significantly greater than that in healthy rats (Fig. 2) and only after plasma glucose and HbA1c levels were high and sustained for an extended time (Grotle et al., 2019a). These findings support the association with disease severity found in the study by Holwerda et al. (2016a). Furthermore, these rats exhibited an exaggerated mechanoreflex, which suggests that enhanced mechanical sensitivity of group III and IV afferents may partially mediate the exaggerated pressor reflex in T2DM. Collectively, these studies strongly support the idea that T2DM leads to an exaggerated exercise pressor reflex.
Fig. 2.
Means ± SE and individual data showing that statically contracting the hindlimb muscles evoked an exaggerated peak pressor response in the 31-wk compared with responses in 21-wk T2DM and non-diabetic rats (A). Peak changes in heart rate were not different among the groups (B). Developed tensions (tension-time index (TTI)) were similar among groups (C). *p < 0.05 (1-way ANOVA) indicates statistically greater response compared with non-diabetic rats. #p < 0.05 (1-way ANOVA) indicates statistically greater pressor response compared with 21-wk T2DM rats.
4. Oxidative stress and the exercise pressor reflex
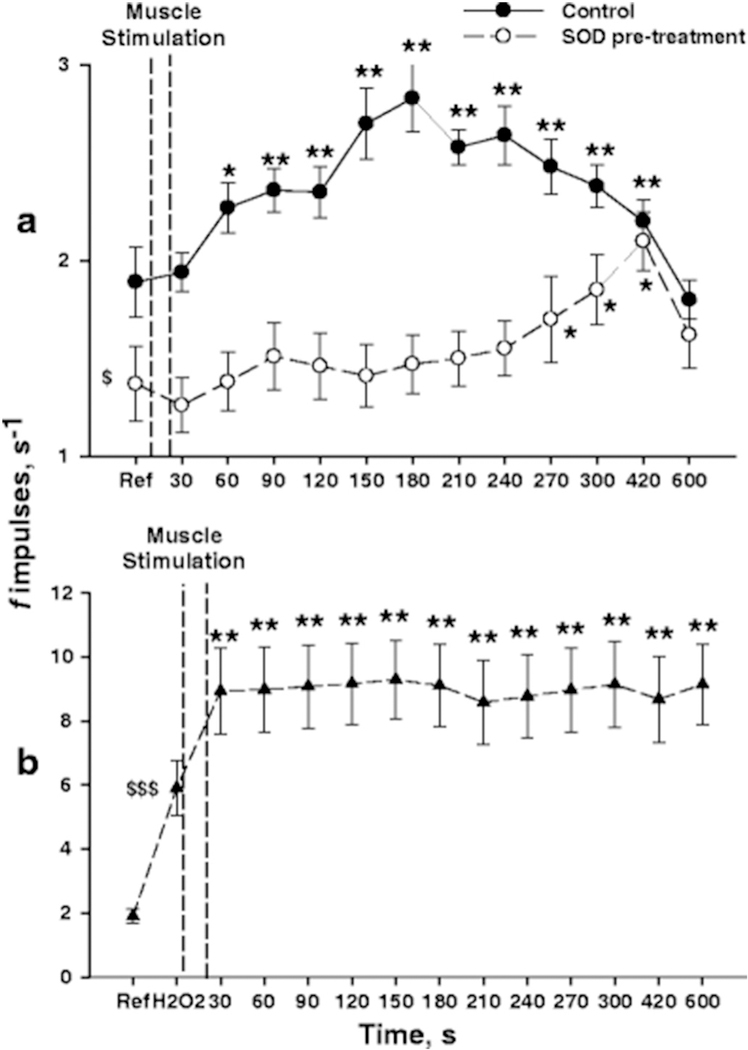
Several studies have demonstrated that free radicals produced during muscle contraction play a role in evoking the exercise pressor reflex. It is well known that muscle contraction generates two to three-fold increases in free radicals, primarily superoxide and nitric oxide (NO). Additionally, superoxide can react with NO to form the highly reactive peroxynitrite (Davies et al., 1982; Powers and Hogan, 2016). Although these free radicals have essential signaling functions in normal conditions, insufficient neutralization in pathological conditions may cause them to accumulate and react with surrounding structures such as sensory afferent endings located in the muscle (Alessio et al., 2000; Davies et al., 1982; Steinberg et al., 2007). Wang et al. were the first to investigate the role of oxidative stress on the exercise pressor reflex and found that infusing a superoxide dismutase (SOD) mimetic inhibitor that increases superoxide production augmented the exercise pressor reflex in healthy rats (Wang et al., 2009). Delliaux et al. were the first to provide electrophysiological evidence of an effect of ROS on afferent activity when they showed that infusing a ROS donor (H2O2) increased impulse activity of group IV afferents (i.e., metaboreceptors) in healthy Sprague Dawley rats. Furthermore, infusing SOD before infusing the ROS donor abolished the responses. More importantly, they showed that infusing SOD before rhythmically contracting the hindlimb muscles significantly attenuated the activity of group IV afferents (Fig. 3) (Delliaux et al., 2009a). Thus, these findings provide compelling evidence that free radicals produced in the contracting muscle can stimulate afferent endings in the muscle and thereby evoke the exercise pressor reflex.
Fig. 3.
Response of group IV afferents to muscle stimulation in healthy Sprague Dawley rats. A) The changes in spontaneous activity of group IV afferents elicited by muscle stimulation are studied in control condition (closed circles: n = 37 units) and after intramuscular injection of SOD (open circles: n = 32 units). B) H2O2 injection before muscle stimulation markedly increased the baseline discharge rate but the response to muscle stimulation was not accentuated compared to control condition (n = 33 units). Dashed vertical bars indicate the 1-min muscle stimulation. Asterisk denote significant changes in averaged discharge rate (f impulses) measured prior to beginning muscle stimulation (*p < 0.05; **p < 0.01). Symbol $ indicates that the averaged resting discharge rate was significantly lower in rats pre-treated with SOD ($p < 0.05) and markedly higher after H2O2 injection ($ $p < 0.01).
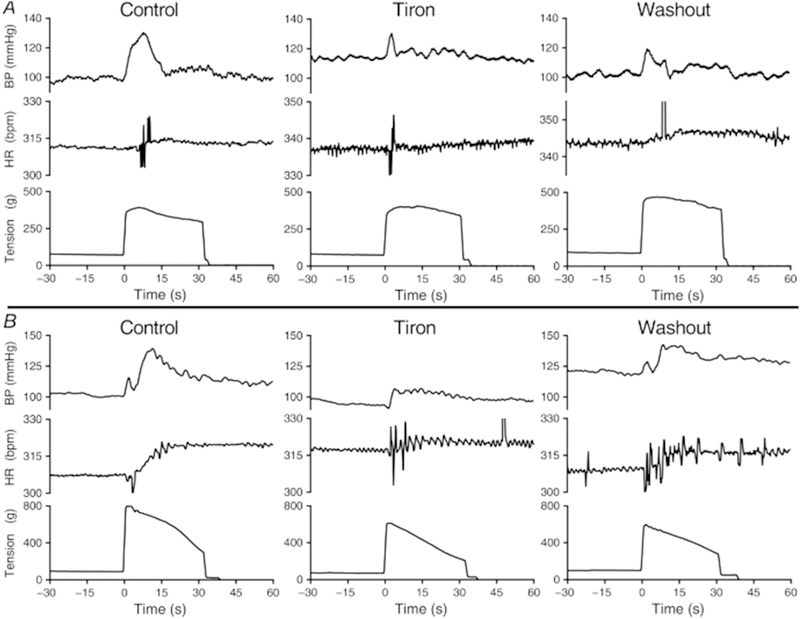
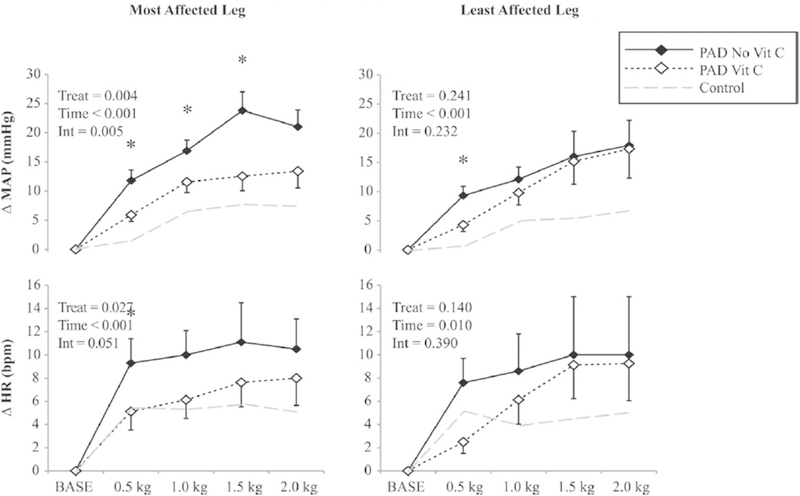
In recent years, studies have demonstrated that reducing intramuscular ROS effectively attenuates the exaggerated exercise pressor reflex in several cardiovascular-related diseases (Harms et al., 2017; Koba et al., 2009; Koba et al., 2013; Muller et al., 2012). For example, locally infusing tiron (superoxide scavenger) into the contracting muscle attenuated the exaggerated exercise pressor reflex in PAD rats (i.e. ligated femoral artery model; Fig. 4) (Harms et al., 2017). Moreover, infusing tempol (SOD mimetic) also reduces the exaggerated pressor reflex in rat models of hypertension (Koba et al., 2013), and heart failure (Koba et al., 2009). Likewise, Muller et al. found that acute intravenous infusion of a non-specific antioxidant (ascorbic acid) attenuated the exaggerated pressor response to low-intensity, one-leg rhythmic plantar flexion in PAD patients (Fig. 5). Electrically contracting the same muscle resulted in similar responses, thus suggesting these responses were independent of central command (Muller et al., 2012). Other studies have demonstrated that local infusion of tempol or tiron during intermittent hindlimb contractions (low metabolite production) attenuated the peak pressor and the synchronization of the renal sympathetic response with tension development (Koba et al., 2009; Koba et al., 2013). These findings are consistent with an augmented mechanoreflex in the same diseases (Copp et al., 2016b; Ives et al., 2016; Leal et al., 2008). Collectively, these data indicate that intramuscular superoxide is an efficacious stimulus for the exercise pressor reflex and may partially mediate its effect by stimulating or sensitizing mechanosensitive afferents.
Fig. 4.
Original data traces showing the pressor (BP) and cardioaccelerator (HR) responses to static contraction for two PAD rats (i.e. ligated femoral artery model; A and B) during consecutive infusions of saline (control, left), tiron (center) and saline (washout, right) into the superficial epigastric artery. Tiron reduced the peak pressor and cardioaccelerator responses to contraction, as well as the duration of the pressor responses, compared with responses during saline infusion controls. Reinfusion of saline following tiron contraction (washout) partially restored the pressor and cardioaccelerator responses to contraction.
Fig. 5.
Effect of intravenous ascorbic acid (Vit C) on the change in mean arterial pressure (MAP) and heart rate (HR) in patients with peripheral arterial disease (PAD) during bouts of one-leg, low intensity rhythmic plantar flexion exercise. The left panel show responses to contractions in the most affected leg, while the right panel show responses to muscle contraction in the least affected leg. Control data in healthy individuals (grey dashed line) from protocol 1 is included for reference. Mean data from the first 20 s of each exercise stage are presented. Data are mean ± SEM, n = 8,*p < 0.05 between treatments at a specific time point. INT = interaction.
NADPH oxidase contributes importantly to skeletal muscle superoxide production during exercise (Powers and Hogan, 2016; Sakellariou et al., 2014) and appears to be the primary source for sensitizing the exercise pressor reflex (Harms et al., 2017; Wang et al., 2009). This is probably due to the assembly process of the active complex on the muscle plasma membrane causing the catalytic unit (Nox2) to be oriented in such a way that it can release superoxide into the interstitial space where the group III and IV afferent endings are located (Ferreira and Laitano, 2016; Sakellariou et al., 2014). This role is supported by the finding that inhibiting NADPH assembly (gp91ds-tat) attenuates the pressor response to muscle contraction in rats with ligated femoral artery (i.e. PAD model). Moreover, higher indices of intramuscular NADPH oxidase (Nox2 and p67phox) found in the skeletal muscle of PAD rats compared to healthy controls further supports this role and suggest that pathological conditions increase the activity of NADPH oxidase (Harms et al., 2017). Collectively, these findings provide strong evidence that NADPH oxidase-mediated production of superoxide plays a significant role in evoking an exaggerated exercise pressor reflex in cardiovascular-related diseases.
5. Oxidative stress and T2DM
Oxidative stress is a unifying mechanism underlying diabetic complications, such as vascular dysfunction and neuropathy (Giacco and Brownlee, 2010; Robson et al., 2018; Ziegler et al., 2015). Hyperglycemia and hyperinsulinemia both contribute to oxidative stress in T2DM and may independently have sympathoexcitatory effects (Joyner and Limberg, 2013; Marfella et al., 2000; Marfella et al., 2001; Marfella et al., 1995). Whether glucose and insulin have similar effects during exercise is not known; however, we do know that acute and chronic events of hyperglycemia stimulate superoxide production in a variety of tissues (Greene et al., 1999; Russell et al., 2002; Sedeek et al., 2010). In addition, increased superoxide production stimulates classical pathways such as polyol, hexosamine, protein kinase C (PKC), and formation of advanced glycation end products (AGE), which further increase tissue damage. Endothelial cells and peripheral nerves are especially vulnerable to the deleterious effects of superoxide as their glucose uptake is dependent on external glucose concentration (Giacco and Brownlee, 2010). T2DM impairs insulin-stimulated glucose disposal in skeletal muscle; however, exercise facilitates glucose disposal by an insulin-independent mechanism (Goodpaster et al., 2014; Oguri et al., 2009). Moreover, ROS produced by muscle contraction is thought to play a role in mediating exercise-stimulated glucose uptake (Kellogg 3rd et al., 2017; Sandstrom et al., 2006). Thus, the combination of hyperglycemia and muscle contraction likely exacerbates oxidative stress within the contracting muscle.
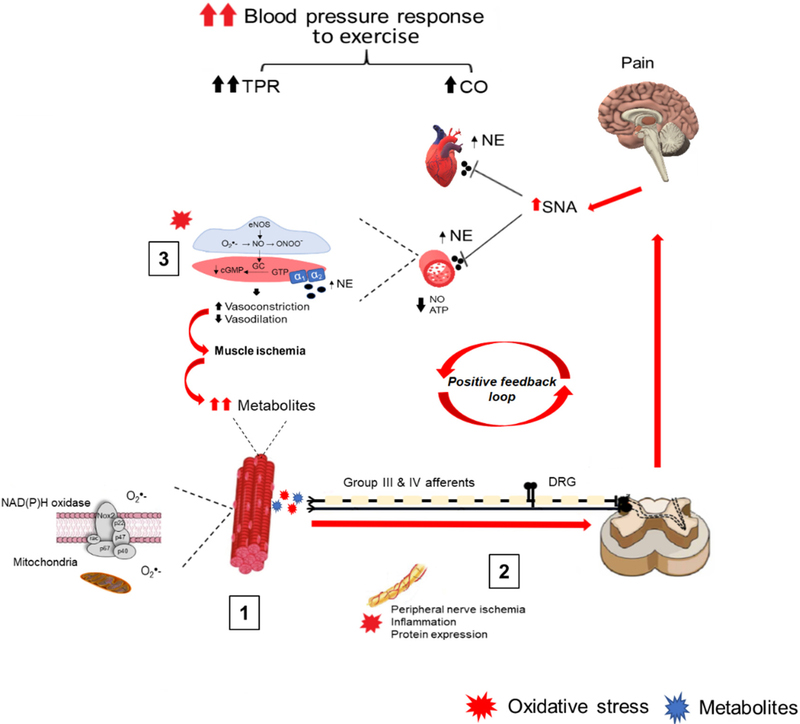
The mechanisms by which oxidative stress mediates its effects are complex, multifactorial, and incompletely understood. However, accumulating evidence supports a direct effect of oxidative stress within the contracting muscle in modulating the exercise pressor reflex in diseases associated with excessive oxidative stress (Harms et al., 2017; Koba et al., 2009; Koba et al., 2013; Muller et al., 2012). T2DM also lead to excessive oxidative stress. Thus, it is possible that oxidative stress also modulates the exercise pressor reflex in T2DM by similar mechanisms. Furthermore, oxidative stress may modulate group III and IV afferent activity by other mechanisms. These include acute and chronic vascular impairments affecting contracting muscle blood flow, increased group III and IV afferent activity, and upregulation of inflammatory mediators that can modulate sensory afferent activity (Fig. 6).
Fig. 6.
Theoretical model depicting the potential direct and indirect roles of peripheral oxidative stress in causing an exaggerated blood pressure response to exercise in type 2 diabetes mellitus (T2DM). In response to muscle contraction, NADPH oxidase release superoxide into the interstitial space, which increases the activity of group III and IV afferents (1). Increased oxidative stress along the afferent nerves and dorsal root ganglion (DRG) may further potentiate the afferent feedback (2). The increased afferent activity is relayed back to the brain stem increasing sympathetic outflow and may also be further relayed to the cerebral cortex causing increased pain sensation. On the vascular side, oxidative stress attenuates contracting muscle blood flow by reducing nitric oxide (NO) bioavailability and impairing functional sympatholysis. The accelerated muscle ischemia leads to accumulation of metabolites and increases the stimulation of group III and IV afferents (3). Collectively, these impairments result in a powerful positive feedback loop causing an over-stimulation and potentiation of group III and IV afferent activity ultimately causing an exaggerated reflexive increase in blood pressure during exercise.
5.1. Muscle blood flow
Oxidative stress plays a significant role in causing endothelial dysfunction, which in turn contributes to attenuating blood flow in contracting muscle in T2DM (Frisbee et al., 2018; Kingwell et al., 2003; McVeigh et al., 1992). Moreover, the magnitude of attenuation likely depends on the severity of endothelial dysfunction (Womack et al., 2009). The interaction between the local release of vasoactive substances and sympathetically mediated vasoconstriction allows for precise regulation of muscle blood flow during exercise. However, T2DM leads to exaggerated sympathetic activity (Holwerda et al., 2016a; Kim et al., 2019) and attenuated blood flow responses to exercise (Groen et al., 2019; Kingwell et al., 2003; Lalande et al., 2008), which accelerates muscle ischemia. Muscle ischemia, both acute and chronic, accelerate the onset of muscle fatigue and pain (Frisbee et al., 2019; Senefeld et al., 2019). More importantly, muscle ischemia is a efficacious stimulus for metabolically sensitive muscle afferents and mediates an augmented reflexive increase in blood pressure (Kuczmarski et al., 2018; Spranger et al., 2015; Tsuchimochi et al., 2010). Thus, accelerated muscle ischemia is consistent with an exaggerated metaboreflex in T2DM (Holwerda et al., 2016a). In healthy, cardiac output is the primary contributor to the metaboreflex-induced increases in blood pressure. However, T2DM leads to an enhanced contribution of peripheral vascular resistance (Roberto et al., 2019). Collectively, these data suggest that oxidative stress indirectly increase the stimulation of metabolically sensitive group III and IV afferents by accelerating muscle ischemia and metabolite accumulation within the contracting muscle.
5.1.1. Vasoconstriction and vasodilation
Studies have shown that oxidative stress increases the production of vasoconstrictors such as endothelin-1 and thromboxane A2, as well as augments reactivity to sympathetic stimulation, which could contribute to reducing muscle blood flow in T2DM (Frisbee et al., 2018; Oguri et al., 2009; Reynolds et al., 2017; Stepp and Frisbee, 2002). For example, oxidative stress and its by-products (8-isoprostane) promote the synthesis of endothelin-1 and thromboxane A2 and can stimulate atherogenesis while inhibiting pro-angiogenic pathways (Kahler et al., 2001; Kahler et al., 2000; Kolluru et al., 2012). Thus, oxidative stress promotes both structural and functional changes to the vasculature. Other lines of evidence suggest that oxidative stress plays an acute role in impairing vasodilation. For example, one study showed that acutely attenuating oxidative stress (tempol) improve blood flow, oxygen uptake, and performance of skeletal muscle in T2DM rats (Frisbee et al., 2018). Moreover, inhibiting NADPH oxidase-mediated production of superoxide (apocynin) was found to restore endothelial function in T2DM rats with NO dysfunction (Fukatsu et al., 2007; Hayashi et al., 2005). In older humans, infusion of ascorbic acid acutely increased blood flow primarily by improving endothelial NO bioavailability (Crecelius et al., 2010). Indeed, decreased signaling of NO appear to be the critical mediator of attenuated blood flow in T2DM (Frisbee et al., 2018); Kingwell et al., 2003). Thus, these data provide strong evidence for a role of oxidative stress in attenuating muscle blood flow in T2DM.
Superoxide can reduce nitric oxide bioavailability by its reaction with NO forming peroxynitrite, a highly efficacious free radical. Peroxynitrite promotes uncoupling of endothelial and neuronal NO synthases (eNOS, nNOS), which synthesizes NO from L-Arginine. This uncoupling, in turn, can lead to further production of superoxide while also reducing endogenous SOD. Ultimately these alterations reduce the antioxidant capacity of the cell and promote cellular damage in the vasculature (Giacco and Brownlee, 2010; Gliemann et al., 2014; Schiffrin, 2008; Zou et al., 2002). In addition to its role in mediating endothelial-dependent vasodilation, NO plays a significant role in attenuating sympathetic vasoconstriction during exercise (i.e., functional sympatholysis). Intraluminal ATP is another mediator of endothelial- dependent vasodilation by stimulating NO production. ATP also attenuates sympathetic vasoconstriction (Mortensen et al., 2009; Rosenmeier et al., 2004). Interestingly, studies have shown that plasma concentrations and signaling of ATP are lower in T2DM and correspond to an attenuated leg blood flow response to ATP infusion and exercise (Groen et al., 2019; Thaning et al., 2010). Thus, these findings suggest that reduced NO bioavailability and endothelium function impair the ability to vasodilate and override sympathetic vasoconstriction during exercise.
5.1.2. Functional sympatholysis
One study found that oxidative stress can impair functional sympatholysis and promote muscle ischemia during exercise in both rats and humans (Fadel et al., 2012). However, whether T2DM leads to impaired functional sympatholysis capacity is currently unclear. For example, Thaning et al. found preserved functional sympatholysis in T2DM individuals with an intact response to acetylcholine (Thaning et al., 2011). On the other hand, preliminary data from Bock et al., showed attenuated functional sympatholysis in T2DM individuals with a reduced response to acetylcholine (Bock et al., 2019). Thus, these findings suggest that any impairment in functional sympatholysis is dependent on endothelium function. Nonetheless, data do support a significant role of oxidative stress in attenuating blood flow in contracting skeletal muscle. Collectively, these findings provide compelling evidence that oxidative stress indirectly contributes to an exaggerated exercise pressor reflex by reducing blood flow delivery to the working muscles.
5.2. Group III and IV afferent activity
Oxidative stress is a likely mediator of an exaggerated exercise pressor reflex in T2DM due to its ability to increase afferent activity (Delliaux et al., 2009b). A longitudinal study found that superoxide was the strongest predictor for impairments in sensory and autonomic nerves and was associated with increased mortality among T2DM patients (Ziegler et al., 2015). Although, the specific mechanisms by which oxidative stress mediates these effects are still unclear, we do know that oxidative stress plays a significant role in the development and progression of neuropathic pain (Giacco and Brownlee, 2010), which is also mediated by increased group III and IV afferent activity (Janig, 2011; Khan et al., 2002; Orstavik et al., 2006; Pitcher and Henry, 2000). Moreover, group III and IV muscle afferents play a dual role in evoking muscle pain and the exercise pressor reflex (Franz and Mense, 1975; Graven-Nielsen and Mense, 2001; Kaufman et al., 1983). Considering the high prevalence of neuropathic pain (> 50%) in T2DM and that it initially affects distal limb nerves, it is likely that oxidative stress also contributes to an exaggerated exercise pressor reflex by modulating group III and IV afferent activity.
Increased sensitization or damage of group III and group IV afferents results in mechanical allodynia, which is characterized as a painful response to a normally non-painful mechanical stimulus (Janig, 2011; Khan et al., 2002; Orstavik et al., 2006; Pitcher and Henry, 2000; Xu et al., 2015). Indeed, it appears that the sensitivity to mechanical deformation of the muscle is heightened in diabetic rats as they have an exaggerated pressor response to tendon stretch (Grotle et al., 2019a; Grotle et al., 2019b). Studies on mechanical allodynia have suggested that oxidative stress can enhance the activity and increase the expression of mechanically sensitive ion channels leading to neuronal hyperexcitability (Bogeski and Niemeyer, 2014; Hsieh, 2008; Liu and Gutterman, 2002; Lolignier et al., 2015). Moreover, free radicals may lower the activation threshold or prolong the inactivation phase of excitatory ion channels (Hsieh, 2008; Liu and Gutterman, 2002; Schluter and Leffler, 2016). Interestingly, some of the channels associated with mechanical allodynia are also known to play a role in evoking the exercise pressor reflex. For example, the mechanically activated cation channel Piezo plays a role in evoking the mechanical component of the exercise pressor reflex (i.e., mechanoreflex) and mechanical allodynia in rat models of PAD (i.e. ligated femoral artery model) (Copp et al., 2016b), T1DM (Grotle et al., 2019b), and neuropathy (Eijkelkamp et al., 2013; Park et al., 2008). Similarly, oxidative stress may modulate exci tatory voltage-gated Nav1.7 channels, which play a role in the spinal transmission of the exercise pressor reflex (Stone et al., 2015a) and neuropathic pain (Dib-Hajj et al., 2010; Dib-Hajj et al., 2013). Moreover, other studies have shown that oxidative stress can modulate Nav1.7 channel function by reducing the threshold for activation as well as slowing the inactivation of the channel (Schluter and Leffler, 2016). Although speculative, these findings suggest that oxidative stress may directly modulate ion channels and receptors that play a role in evoking the exercise pressor reflex.
5.3. Inflammatory mediators
Oxidative stress is associated with an increased release of inflammatory mediators that may also play a role in modulating group III and IV afferent activity (Cheng et al., 2009; Franz and Mense, 1975; Mense, 2009). Additionally, oxidative stress contributes to peripheral nerve ischemia, which further disrupts peripheral nerve function resulting in the release of neurotrophic factors (Okamoto et al., 2001). Although neurotrophic factors have regenerative functions, T2DM disrupts their regenerative capacity and promotes chronic inflammation (Mirza et al., 2015; Singh et al., 2016). Likely candidates that contribute to an exaggerated exercise pressor reflex in T2DM include bradykinin (Mense, 1977), arachidonic acid and its cyclooxygenase products (Rotto and Kaufman, 1988; Rotto et al., 1990), ATP (Hanna and Kaufman, 2003; Stone et al., 2014), substance P (Kaufman et al., 1988), nerve growth factor (NGF) (Cheng et al., 2009; Lu et al., 2012), and pro-inflammatory cytokines (Al-Mazidi et al., 2018; Copp, 2015; Miller et al., 2009). Substantial literature suggests bradykinin, which is released from contracting muscle (Stebbins et al., 1990), can stimulate group III and IV muscle afferents (Franz and Mense, 1975; Kaufman et al., 1982; Pan et al., 1993). Moreover, bradykinin is known to play a role in evoking an exaggerated mechanoreflex in PAD rats (i.e. ligated femoral artery model) (Lu et al., 2013), as well as modulating muscle and neuropathic pain (Franz and Mense, 1975). Bradykinin has also been found to cause an 8-fold increase in mechanically activated Piezo2 currents (Dubin et al., 2012). However, whether inflammatory mediators, such as bradykinin, or mechanically gated Piezo channels play a role in evoking the exaggerated exercise pressor reflex in T2DM is not known. Nonetheless, these data indicate that oxidative stress contributes to increasing group III and IV afferent activity either by a direct effect on ion channel activity and/or by stimulating the release of inflammatory mediators that modulate afferent activity.
6. Future directions and conclusion
There are many unanswered questions in terms of the reflexive control of the circulation in T2DM. In this review, we provide supporting evidence for direct and indirect effects of oxidative stress in contributing to an exaggerated exercise pressor reflex. Future studies should focus on determining specific mechanisms behind superoxide and other ROS in evoking the exercise pressor reflex. These mechanisms may act through direct stimulation or changes in the expression of receptors and channels that mediate the exercise pressor reflex. In order to provide a comprehensive understanding of the role of oxidative stress on the reflexive control of circulation, we believe that these additional studies should be conducted in both animal models and humans.
In conclusion, individuals with T2DM have exaggerated cardiovascular responses to exercise due, in part, to an exaggerated exercise pressor reflex. There is accumulating evidence supporting oxidative stress as a unifying mechanism in contributing to an exaggerated exercise pressor reflex. Specifically, oxidative stress may directly or indirectly increase the activity of group III and IV afferents, evoking neuropathic pain and an exaggerated exercise pressor reflex. Ultimately, the pathological alterations associated with oxidative stress may result in a powerful positive feedback loop that further amplifies the stimulation of group III and IV afferents, and thus, the cardiovascular responses to exercise. Understanding the role of oxidative stress in neurovascular regulation is essential to identifying novel therapies that reduce the risk of adverse cardiovascular events in individuals with T2DM.
Acknowledgments
The authors acknowledge funding support from National Institutes of Health Grant R01-HL144723.
Footnotes
Declaration of Competing Interest
None.
References
- Adreani CM, Hill JM, Kaufman MP, 1997. Responses of group III and IV muscle afferents to dynamic exercise. J.Appl.Physiol 82, 1811–1817. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH, 1937. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J.Physiol 89, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL, 2000. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med. Sci. Sports Exerc 32, 1576–1581. [DOI] [PubMed] [Google Scholar]
- Al-Mazidi S, Alotaibi M, Nedjadi T, Chaudhary A, Alzoghaibi M, Djouhri L, 2018. Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy. Eur. J. Pain 22, 810–821. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA, 2010. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS, 2011. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J.Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M, 1999. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50, 361–374. [DOI] [PubMed] [Google Scholar]
- Bock J, Hughes W, Ueda K, Feider A, Hanada S, Kruse N, Iwamoto E, Casey D, 2019. Greater al- and a2-adrenergic mediated vasoconstriction in contracting skeletal muscle of type 2 diabetic humans. FASEB J. 33, 696.619. [DOI] [PubMed] [Google Scholar]
- Bogeski I, Niemeyer BA, 2014. Redox regulation of ion channels. Antioxid. Redox Signal 21, 859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2017, 2018. Diabetes Report Card. In: Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services. [Google Scholar]
- Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL, 2009. Nerve growthfactor mediates mechanical allodynia in a mouse model of type 2 diabetes. J. Neuropathol. Exp. Neurol 68, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, 2015. Role Played by Interleukin-6 in Evoking the Exercise Pressor Reflex in Decerebrate Rats: Effect of Femoral Artery Ligation. vol. 309 pp. H166–H173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP, 2016a. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J. Physiol 594, 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP, 2016b. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am. J. Physiol. Heart Circ. Physiol 310, H1233–H1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A, 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 1995. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J. Comp. Neurol 361, 225–248. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA, 2010. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am. J. Physiol. Heart Circ. Physiol 299, H1633–H1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ, 2008. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am. J. Phys. Regul. Integr. Comp. Phys 295, R1782–R1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L, 1982. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun 107, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y, 2009a. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch. 459, 143–150. [DOI] [PubMed] [Google Scholar]
- Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y, 2009b. Reactive oxygen species and inflammatory mediators enhance muscle spindles mechanosensitivity in rats. Pflugers Arch. 457, 877–884. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG, 2010. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci 33, 325–347. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG, 2013. The Na(V)1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci 14, 49–62. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A, 2012. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN, 2013. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun 4, 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson TR, Acs A, Ludwig C, Panton UH, 2018. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol 17, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG, 1985. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir. Physiol 59, 313–337. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Lindstrom J, Tuomilehto J, 2001. Potential for the prevention of type 2 diabetes. Br. Med. Bull 60, 183–199. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, 1999. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med. 27, 381–391. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Raven PB, 2012. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp. Physiol 97, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Farias Iii M, Gallagher KM, Wang Z, Thomas GD, 2012. Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate-tolerant rats and humans. J. Physiol 590, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Laitano O, 2016. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med 98, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M, Mense S, 1975. Muscle receptors with group IV afferent fibers responding to applications of bradykinin. Brain Res. 92, 369–383. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Lewis MT, Kasper JD, Chantler PD, Wiseman RW, 2018. Type II diabetes mellitus in the Goto-Kakizaki rat impairs microvascular function and contributes to premature skeletal muscle fatigue. J. Appl. Physiol 126, 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Lewis MT, Kasper JD, Chantler PD, Wiseman RW, 2019. Type 2 diabetes mellitus in the Goto-Kakizaki rat impairs microvascular function and contributes to premature skeletal muscle fatigue. J. Appl. Physiol 126, 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu A, Hayashi T, Miyazaki-Akita A, Matsui-Hirai H, Furutate Y, Ishitsuka A, Hattori Y, Iguchi A, 2007. Possible usefulness of apocynin, an NADPH oxidase inhibitor, for nitrate tolerance: prevention of NO donor-induced endothelial cell abnormalities. Am. J. Physiol. Heart Circ. Physiol 293, H790–H797. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M, 2010. Oxidative stress and diabetic complications. Circ. Res 107, 1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann L, Nyberg M, Hellsten Y, 2014. Nitric oxide and reactive oxygen species in limb vascular function: what is the effect of physical activity? Free Radic. Res 48, 71–83. [DOI] [PubMed] [Google Scholar]
- Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI, Suchyna TM, 2017. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys. J 112, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Bertoldo A, Ng JM, Azuma K, Pencek RR, Kelley C, Price JC, Cobelli C, Kelley DE, 2014. Interactions among glucose delivery, transport, and phosphorylation that underlie skeletal muscle insulin resistance in obesity and type 2 diabetes: studies with dynamic PET imaging. Diabetes 63, 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH, 1972. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J.Physiol.(Lond.) 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven-Nielsen T, Mense S, 2001. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin. J. Pain 17, 2–10. [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ, Obrosova I, Feldman EL, 1999. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur. J. Pharmacol 375, 217–223. [DOI] [PubMed] [Google Scholar]
- Groen MB, Knudsen TA, Finsen SH, Pedersen BK, Hellsten Y, Mortensen SP, 2019. Reduced skeletal-muscle perfusion and impaired ATP release during hypoxia and exercise in individuals with type 2 diabetes. Diabetologia 62, 485–493. [DOI] [PubMed] [Google Scholar]
- Grotle AK, Garcia EA, Huo Y, Stone AJ, 2017. Temporal changes in the exercise pressor reflex in type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol 313, H708–H714 (ajpheart 00399 02017). [DOI] [PubMed] [Google Scholar]
- Grotle AK, Crawford CK, Huo Y, Ybarbo KM, Harrison ML, Graham JL, Stanhope KL, Havel PJ, Fadel PJ, Stone AJ, 2019a. Exaggerated cardiovascular responses to muscle contraction and tendon stretch in UCD Type-2 diabetes mellitus rats. Am. J. Physiol. Heart Circ. Physiol 317, H479–H486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotle AK, Garcia EA, Harrison ML, Huo Y, Crawford CK, Ybarbo KM, Stone AJ, 2019b. Exaggerated mechanoreflex in early stage type 1 diabetic rats: role of piezo channels. Am. J. Phys. Regul. Integr. Comp. Phys 316, R417–R426. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M, 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med 339, 229–234. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP, 2003. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J. Appl. Physiol 94, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP, 2004. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J.Appl.Physiol 96, 1166–1169. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Thomas GD, 2000. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol. Scand 168, 489–503. [DOI] [PubMed] [Google Scholar]
- Harms JE, Kuczmarski JM, Kim JS, Thomas GD, Kaufman MP, 2017. The role played by oxidative stress in evoking the exercise pressor reflex in health and simulated peripheral artery disease. J. Physiol 595, 4365–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Juliet PA, Kano-Hayashi H, Tsunekawa T, Dingqunfang D, Sumi D, Matsui-Hirai H, Fukatsu A, Iguchi A, 2005. NADPH oxidase inhibitor, apocynin, restores the impaired endothelial-dependent and -independent responses and scavenges superoxide anion in rats with type 2 diabetes complicated by NO dysfunction. Diabetes Obes. Metab 7, 334–343. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP, 2001. Gadolinium attenuates exercise pressor reflex in cats. Am. J. Physiol. Heart Circ. Physiol 280, H2153–H2161. [DOI] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Koba S, Kaufman MP, 2009. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J. Physiol 587, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ, 2016a. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am. J. Physiol. Heart Circ. Physiol 310, H300–H309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ, 2016b. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol 311, H1170–H1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CP, 2008. Redox modulation of A-type K+ currents in pain-sensing dorsal root ganglion neurons. Biochem. Biophys. Res. Commun 370, 445–449. [DOI] [PubMed] [Google Scholar]
- Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS, 2016. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med. Sci. Sports Exerc 48, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janig W, 2011. Mechanical allodynia generated by stimulation of unmyelinated afferent nerve fibres. J. Physiol. 589, 4407–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Limberg JK, 2013. Insulin and sympathoexcitation: it is not all in your head. Diabetes 62, 2654–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler J, Mendel S, Weckmuller J, Orzechowski HD, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T, 2000. Oxidative stress increases synthesis of big endothelin-1 by activation of the endothelin-1 promoter. J. Mol. Cell. Cardiol 32, 1429–1437. [DOI] [PubMed] [Google Scholar]
- Kahler J, Ewert A, Weckmuller J, Stobbe S, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T, 2001. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J. Cardiovasc. Pharmacol 38, 49–57. [DOI] [PubMed] [Google Scholar]
- Karavelioglu Y, Karapinar H, Gul I, Kucukdurmaz Z, Yilmaz A, Akpek M, Kaya MG, 2013. Blood pressure response to exercise is exaggerated in normotensive diabetic patients. Blood Press. 22, 21–26. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH, 1982. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ. Res 50, 133–139. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH, 1983. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA, 1984. Effect of ischemia on responses of group III and IV afferents to contraction. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 57, 644–650. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rotto DM, Rybicki KJ, 1988. Pressor reflex response to static muscular contraction: its afferent arm and possible neurotransmitters. Am. J. Cardiol 62, 58E–62E. [DOI] [PubMed] [Google Scholar]
- Keller-Ross ML, Johnson BD, Joyner MJ, Olson TP, 2014. Influence of the metaboreflex on arterial blood pressure in heart failure patients. Am. Heart J 167, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL 3rd, McCammon KM, Hinchee-Rodriguez KS, Adamo ML, Roman LJ, 2017. Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic. Biol. Med 110, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan GM, Chen SR, Pan HL, 2002. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 114, 291–299. [DOI] [PubMed] [Google Scholar]
- Kim HK, Hotta N, Ishizawa R, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA, Mizuno M, 2019. Exaggerated pressor and sympathetic responses to stimulation of the mesencephalic locomotor region and exercise pressor reflex in type II diabetic rats. Am. J. Phys. Regul. Integr. Comp. Phys 317, R270–R279. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK, 2003. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26, 899–904. [DOI] [PubMed] [Google Scholar]
- Koba S, Gao Z, Sinoway LI, 2009. Oxidative stress and the muscle reflex in heart failure. J. Physiol 587, 5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Watanabe R, Kano N, Watanabe T, 2013. Oxidative stress exaggerates skeletal muscle contraction-evoked reflex sympathoexcitation in rats with hypertension induced by angiotensin II. Am. J. Physiol. Heart Circ. Physiol 304, H142–H153. [DOI] [PubMed] [Google Scholar]
- Kolluru GK, Bir SC, Kevil CG, 2012. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med 2012, 918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski JM, Unrath K, Thomas GD, 2018. Exaggerated cardiovascular responses to treadmill running in rats with peripheral arterial insufficiency. Am. J. Physiol. Heart Circ. Physiol 314, H114–H121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, 1977. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J. Physiol 273, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT, 2001. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke 32, 2036–2041. [DOI] [PubMed] [Google Scholar]
- Lalande S, Gusso S, Hofman PL, Baldi JC, 2008. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med. Sci. Sports Exerc 40, 612–617. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Kurl S, Lakka TA, Tuomainen TP, Rauramaa R, Salonen R, Eranen J, Salonen JT, 2001. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J. Am. Coll. Cardiol 38, 72–79. [DOI] [PubMed] [Google Scholar]
- Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA, 2008. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol 295, H1429–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD, 2002. Oxidative stress and potassium channel function. Clin. Exp. Pharmacol. Physiol 29, 305–311. [DOI] [PubMed] [Google Scholar]
- Lolignier S, Eijkelkamp N, Wood JN, 2015. Mechanical allodynia. Pflugers Arch. 467, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xing J, Li J, 2012. Role for NGF in augmented sympathetic nerve response to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J. Appl. Physiol 113, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xing J, Li J, 2013. Bradykinin B2 receptor contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. Am. J. Physiol. Heart Circ. Physiol 304, H1166–H1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfella R, Verrazzo G, Acampora R, La Marca C, Giunta R, Lucarelli C, Paolisso G, Ceriello A, Giugliano D, 1995. Glutathione reverses systemic hemodynamic changes induced by acute hyperglycemia in healthy subjects. Am. J. Phys 268, E1167–E1173. [DOI] [PubMed] [Google Scholar]
- Marfella R, Nappo F, De Angelis L, Paolisso G, Tagliamonte MR, Giugliano D, 2000. Hemodynamic effects of acute hyperglycemia in type 2 diabetic patients. Diabetes Care 23, 658–663. [DOI] [PubMed] [Google Scholar]
- Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D, 2001. Acute hyperglycemia induces an oxidative stress in healthy subjects. J. Clin. Invest 108, 635–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L, 2017. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med 376, 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH, 1972. Reflex cardiovascular and respiratory responses originating in exercising muscle. J. Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR, 1992. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35, 771–776. [DOI] [PubMed] [Google Scholar]
- Mense S, 1977. Nervous outflow from skeletal muscle following chemical noxious stimulation. J. Physiol 267, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, 2009. Algesic agents exciting muscle nociceptors. Exp. Brain Res 196, 89–100. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA, 2009. Cytokine and chemokine regulation of sensory neuron function. Handb. Exp. Pharmacol 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, Koh TJ, 2015. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J. Pathol 236, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman MA, Siscovick DS, 1996. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol. Clin 14, 263–270. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y, 2009. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am. J. Phys. Regul. Integr. Comp. Phys 296, R1140–R1148. [DOI] [PubMed] [Google Scholar]
- de Moura-Tonello SC, Porta A, Marchi A, de Almeida Fagundes A, Francisco Cde O, Rehder-Santos P, Milan-Mattos JC, Simoes RP, Gois Mde O, Catai AM, 2016. Cardiovascular variability analysis and baroreflex estimation in patients with type 2 diabetes in absence of any manifest neuropathy. PLoS One 11, e0148903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI, 2012. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J. Physiol 590, 6237–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor E, Green S, Kiely C, O’Shea D, Egana M, 2015. Differential effects of age and type 2 diabetes on dynamic vs. peak response of pulmonary oxygen uptake during exercise. J. Appl. Physiol 118, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Oguri M, Adachi H, Ohno T, Oshima S, Kurabayashi M, 2009. Effect of a single bout of moderate exercise on glucose uptake in type 2 diabetes mellitus. J. Cardiol 53, 8–14. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA, 2001. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol 169, 386–391. [DOI] [PubMed] [Google Scholar]
- Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jorum E, Torebjork HE, 2006. Abnormal function of C-fibers in patients with diabetic neuropathy. J. Neurosci 26, 11287–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL, Longhurst JC, 1993. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J. Appl. Physiol 75, 2061–2068. [DOI] [PubMed] [Google Scholar]
- Park SP, Kim BM, Koo JY, Cho H, Lee CH, Kim M, Na HS, Oh U, 2008. A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. Pain 137, 208–217. [DOI] [PubMed] [Google Scholar]
- Pinto TE, Gusso S, Hofman PL, Derraik JG, Hornung TS, Cutfield WS, Baldi JC, 2014. Systolic and diastolic abnormalities reduce the cardiac response to exercise in adolescents with type 2 diabetes. Diabetes Care 37, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Henry JL, 2000. Cellular mechanisms of hyperalgesia and spontaneous pain in a spinalized rat model of peripheral neuropathy: changes in myelinated afferent inputs implicated. Eur. J. Neurosci 12, 2006–2020. [DOI] [PubMed] [Google Scholar]
- Poitras VJ, Hudson RW, Tschakovsky ME, 2018. Exercise intolerance in type 2 diabetes: is there a cardiovascular contribution? J. Appl. Physiol 124, 1117–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Lee SM, Anguelov PI, 2002. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Auton. Neurosci 98, 64–69. [DOI] [PubMed] [Google Scholar]
- Powers SK, Hogan MC, 2016. Exercise and oxidative stress. J. Physiol 594, 5079–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, Miller TM, Groves BM, Wolfel EE, 2009. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med. Sci. Sports Exerc 41, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ, 1962. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ. Res 11, 370–380. [DOI] [PubMed] [Google Scholar]
- Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP, 2017. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J. Appl. Physiol 122, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto S, Milia R, Doneddu A, Pinna V, Palazzolo G, Serra S, Orru A, Hosseini Kakhak SA, Ghiani G, Mulliri G, Pagliaro P, Crisafulli A, 2019. Hemodynamic abnormalities during muscle metaboreflex activation in patients with type 2 diabetes mellitus. J. Appl. Physiol 126, 444–453. [DOI] [PubMed] [Google Scholar]
- Robson R, Kundur AR, Singh I, 2018. Oxidative stress biomarkers in type 2 diabetes mellitus for assessment of cardiovascular disease risk. Diabetes Metab. Syndr 12, 455–462. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J, 2004. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J. Physiol 558, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP, 1988. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J. Appl. Physiol 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP, 1990. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J. Appl. Physiol 68, 861–867. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Monbaron D, Parati G, Perret S, Haesler E, Danzeisen C, Hayoz D, 2005. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 46, 162–167. [DOI] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL, 2002. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 16, 1738–1748. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Jackson MJ, Vasilaki A, 2014. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res 48, 12–29. [DOI] [PubMed] [Google Scholar]
- Sanderson BC, Rollins KS, Hopkins TD, Butenas AL, Felice KP, Ade CJ, Copp SW, 2019. GsMTx4 reduces the reflex pressor response during dynamic hindlimb skeletal muscle stretch in decerebrate rats. Phys. Rep 7, e13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A, 2006. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol 575, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL, 2008. Oxidative stress, nitric oxide synthase, and superoxide dismutase: a matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension 51, 31–32. [DOI] [PubMed] [Google Scholar]
- Schluter F, Leffler A, 2016. Oxidation differentially modulates the recombinant voltage-gated Na(+) channel alpha-subunits Nav1.7 and Nav1.8. Brain Res. 1648, 127–135. [DOI] [PubMed] [Google Scholar]
- Scott JA, Coombes JS, Prins JB, Leano RL, Marwick TH, Sharman JE, 2008. Patients with type 2 diabetes have exaggerated brachial and central exercise blood pressure: relation to left ventricular relative wall thickness. Am. J. Hypertens 21, 715–721. [DOI] [PubMed] [Google Scholar]
- Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL, 2010. Critical role of Nox4- based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol 299, F1348–F1358. [DOI] [PubMed] [Google Scholar]
- Senefeld JW, Limberg JK, Lukaszewicz KM, Hunter SK, 2019. Exercise-induced hyperemia is associated with knee extensor fatigability in adults with type 2 diabetes. J. Appl. Physiol 126, 658–667. [DOI] [PubMed] [Google Scholar]
- Singh K, Agrawal NK, Gupta SK, Sinha P, Singh K, 2016. Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J. Diabetes Complicat 30, 99–108. [DOI] [PubMed] [Google Scholar]
- Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA, 2015. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am. J. Physiol. Heart Circ. Physiol 309, H1440–H1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprick JD, Morison DL, Fonkoue IT, Li Y, DaCosta D, Rapista D, Choi H, Park J, 2019. Metabolic acidosis augments exercise pressor responses in chronic kidney disease. Am. J. Phys. Regul. Integr. Comp. Phys 317, R312–R318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC, 1990. Bradykinin release from contracting skeletal muscle of the cat. J. Appl. Physiol 69, 1225–1230. [DOI] [PubMed] [Google Scholar]
- Steinberg JG, Ba A, Bregeon F, Delliaux S, Jammes Y, 2007. Cytokine and oxidative responses to maximal cycling exercise in sedentary subjects. Med. Sci. Sports Exerc 39, 964–968. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Frisbee JC, 2002. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol 282, H816–H820. [DOI] [PubMed] [Google Scholar]
- Stone AJ, Kaufman MP, 2015. The exercise pressor reflex and peripheral artery disease. Auton. Neurosci 188, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Yamauchi K, Kaufman MP, 2014. Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am. J. Physiol. Heart Circ. Physiol 306, H396–H404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, Kaufman MP, 2015a. Role played by NaV 1.7 channels on thin-fiber muscle afferents in transmitting the exercise pressor reflex. Am. J. Phys. Regul. Integr. Comp. Phys 309, R1301–R1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, Kim JS, Kaufman MP, 2015b. Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. J. Appl. Physiol 119, 1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaning P, Bune LT, Hellsten Y, Pilegaard H, Saltin B, Rosenmeier JB, 2010. Attenuated purinergic receptor function in patients with type 2 diabetes. Diabetes 59, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB, 2011. Functional sympatholysis during exercise in patients with type 2 diabetes with intact response to acetylcholine. Diabetes Care 34, 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP, 2010. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am. J. Physiol. Heart Circ. Physiol 299, H106–H113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J, 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol 39, 44–84. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W, 2009. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J. Appl. Physiol 107, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR, 2009. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J. Am. Coll. Cardiol 53, 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR, 2015. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med 21, 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M, 2015. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 52, 65–72. [DOI] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA, 2002. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest 109, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]