Abstract
PURPOSE
Standard treatment for glioblastoma (GBM) includes surgery, radiation (RT), and temozolomide (TMZ), yielding a median overall survival (OS) of ≈14 months. Preclinical models suggest pharmacological ascorbate (P-AscH−) enhances RT/TMZ anti-tumor effect in GBM. We evaluated the safety of adding P-AscH− to standard RT/TMZ therapy.
EXPERIMENTAL DESIGN
This first-in-human trial was divided into an RT phase (concurrent RT/TMZ/ P-AscH−) and adjuvant (ADJ) phase (post RT/TMZ/ P-AscH− phase). Eight P-AscH− dose cohorts were evaluated in the RT phase until targeted plasma ascorbate levels were achieved (≥ 20 mM). In the ADJ phase, P-AscH− doses were escalated in each subject at each cycle until plasma concentrations were ≥ 20 mM. P-AscH− was infused 3 times weekly during the RT-phase and 2 times weekly during the ADJ-phase continuing for 6 cycles or until disease progression. Adverse events (AEs) were quantified by CTCAE(v4.03).
RESULTS
Eleven subjects were evaluable. No dose limiting toxicities occurred. Observed toxicities were consistent with historical controls. Adverse events related to study drug were dry mouth and chills. Targeted ascorbate plasma levels of 20 mM were achieved in the 87.5 g cohort; diminishing returns were realized in higher dose cohorts. Median progression free survival (PFS) was 9.4 months and median overall survival (OS) was 18 months. In subjects with undetectable MGMT promoter methylation (n=8), median PFS was 10 months and median OS was 23 months.
CONCLUSIONS
P-AscH−/RT/TMZ is safe with promising clinical outcomes warranting further investigation.
Keywords: Glioblastoma, GBM, pharmacological ascorbate, vitamin C
INTRODUCTION
Glioblastoma (GBM) is the most common primary brain malignancy in the United States (1). Standard treatment for GBM includes a combination of maximum safe surgical resection followed by radiation therapy (RT) and temozolomide (TMZ) followed by additional cycles of TMZ (2,3). Despite advances in GBM treatment, including the development of new biological agents, GBM patients continue to have a dismal prognosis with a median OS of 14 – 16 months, PFS of approximately 7 months, and 5-year survival of <10% (2,4,5).
Pharmacological ascorbate (P-AscH−), defined as a plasma ascorbate level ≥ 10 mM) has re-emerged as a possible cancer therapeutic being toxic to tumor cells yet relatively innocuous to normal cells (6–8). Recent phase 1 clinical trials demonstrate the safety of P-AscH− when administered with concurrent gemcitabine for stage IV pancreatic cancer (9), when combined with paclitaxel and carboplatin for ovarian cancer (10), as well when combined with radiation and gemcitabine for locally advanced pancreatic cancer (11). In vitro and orthotopic animal models demonstrate P-AscH− selectively increases cancer cell oxidative stress, thereby sensitizing cancer cells to radiation and chemotherapy (6,12,13).
Based on both the pre-clinical and clinical data, we designed and conducted a first-in-human phase 1 trial in newly diagnosed GBM subjects to estimate the maximum tolerated dose (MTD) of P-AscH− when added to the RT/TMZ regimen described by Stupp et al (2,4). The primary objective was to determine the safety and acute toxicity of P-AscH− when administered with standard therapy for GBM and to identify a recommended dose for a phase 2 clinical trial with P-AscH−. Secondary objectives were to estimate progression-free survival (PFS) and overall survival (OS) as well as characterize an initial safety profile. GBM was felt a promising site for P-AscH− application because of its poor prognosis as well as the known high concentration of ascorbate in the cerebrospinal fluid and central nervous system tissues (13–15).
METHODS
ETHICS & OVERSIGHT
This trial was designed by the principal investigator (Buatti) and supervising statisticians (TenNapel, B.J. Smith). The protocol was submitted to FDA under IND 105715 in 2012 (Cullen, sponsor) and registered on ClinicalTrials.gov 19 Dec 2012 under . Approval was obtained from The University of Iowa Institutional Review Board (Biomedical IRB01; IRB 201211713). Informed written consent was obtained from each subject prior to beginning the trial. The trial was conducted according to the Declaration of Helsinki, the Belmont Report, the United States Common Rule (45CFR§46), and the International Council on Harmonisation—Good Clinical Practice; all investigators were GCP trained. The University of Iowa Hospitals and Clinics data and safety monitoring committee (DSMC) reviewed data for compliance to protocol and participant safety. Safety and annual reports regarding this trial were submitted as required (21CFR§312.23, §312.32).
DESIGN
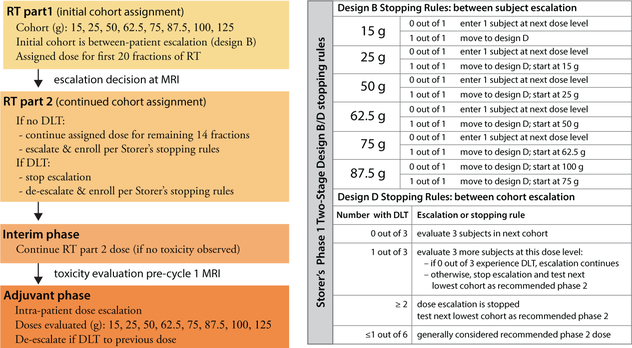
This open-label, single-center investigator-initiated clinical trial was divided into 2 phases: an RT-phase and adjuvant (ADJ) phase. To identify the recommended phase 2 dose of P-AscH− during the RT-phase, Storer’s two-phase design B/D was employed (16). Briefly, a single participant is assigned to a P-AscH− dose cohort. If no dose limiting toxicities (DLTs) occur, a second participant is assigned to the next dose cohort. After a DLT occurs, a traditional 3+3 dose evaluation is used for the remaining P-AscH− dose cohorts (16). With the 3+3 dose evaluation, a dose level is deemed too toxic if 2 out of 3 participants experience DLTs. Thus, the recommended phase 2 ascorbate dose for the RT-phase would be defined as either the highest dose tested where ≤ 1 out of 6 participants experienced a DLT or a dose that consistently sustained plasma ascorbate levels of ≥ 20 mM.
For the ADJ-phase, the study employed an intra-patient ascorbate dose escalation design until a DLT occured or a target plasma ascorbate level was achieved (≥ 20 mM). This design utilizes intra-patient dose escalation of 25 g to estimate the MTD or identify the dose required to achieve the target plasma ascorbate level of ≥ 20 mM. The ADJ-phase began one month after completing RT and after an MRI determined there was no associated normal tissue injury. The ADJ-phase starting ascorbate dose was the same dose the subject received during the RT-phase. The ascorbate intra-patient dose escalation design was key in determining the diminishing returns of higher doses of ascorbate (e.g., 100 g, 125 g). This design was selected because it would enable dose evaluation using the subject as their own control (if there was no toxicity for one cycle then the next cycle would permit an escalation of dose until plasma ascorbate levels ≥ 20 mM were reached). For the initial cohorts, it was known the ADJ ascorbate dose would be greater than the RT ascorbate dose.
DLTs were defined based on published literature (2,4) as well as the U.S. prescribing information for temozolomide (17) and include the following events regardless of attribution to ascorbate: infection (gr. 4), nausea or vomiting (gr. 3) despite maximal supportive care, and decreased neutrophil count (gr. 4) or platelet count (gr. 4) (Table 1). Additionally, a serious adverse event (as defined by FDA) with a reasonable attribution to ascorbate also met dose limiting toxicity criteria. The defined RT-phase window for evaluation was RT day 1 through the pre-adjuvant cycle 1 MRI whereas the ADJ-phase evaluation period was from cycle 1 day 1 of temozolomide through 30 days after the last ascorbate infusion. DLT criteria were consistent across these phases.
Table 1.
Adverse events occurring during RT-phase across all dose cohorts (n = 13) and ADJ-phase (n = 12)§
| Subjects experiencing an adverse event, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Radiation phase (RT-phase) | Adjuvant phase (ADJ-phase) | |||||||
| Grade† | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Hematologic | ||||||||
| Anemia | 5 (39) | 0 | 0 | 0 | 3 (25) | 0 | 0 | 0 |
| White blood cell decreased | 1 (8) | 4 (31) | 0 | 0 | 0 | 1 (8) | 1 (8) | 0 |
| Lymphocyte count decreased | 0 | 3 (23) | 5 (39) | 2 (15) | 1 (8) | 4 (33) | 5 (42) | 0 |
| Neutrophil count decreased | 4 (31) | 1 (8) | 0 | 0 | 1 (8) | 0 | 1 (8) | 0 |
| Platelet count decreased | 5 (39) | 0 | 0 | 0 | 4 (33) | 1 (8) | 0 | 0 |
| Serum chemistry | ||||||||
| Hyperglycemia | 2 (15) | 1 (8) | 1 (8) | 0 | 2 (17) | 3 (25) | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 0 | 2 (17) | 3 (25) | 0 | 0 |
| Hypernatremia | 1 (7.7) | 0 | 1 (8) | 0 | 0 | 0 | 0 | 0 |
| Hyponatremia | 5 (39) | 0 | 0 | 0 | 1 (8) | 0 | 0 | 0 |
| Hypokalemia | 4 (31) | 0 | 1 (8) | 0 | 2 (17) | 0 | 0 | 0 |
| ALT increased | 1 (8) | 2 (15) | 1 (8) | 0 | 2 (17) | 0 | 0 | 0 |
| AST increased | 1 (8) | 1 (8) | 0 | 0 | 1 (8) | 0 | 0 | 0 |
| Constitutional | ||||||||
| Fatigue | 2 (15) | 3 (23) | 1 (8) | 0 | 2 (17) | 2 (17) | 0 | 0 |
| Headaches | 3 (23) | 2 (15) | 1 (8) | 0 | 2 (17) | 3 (25) | 0 | 0 |
| Nausea | 5 (39) | 5 (39) | 1 (8) | 0 | 5 (42) | 4 (33) | 0 | 0 |
| Vomiting | 5 (39) | 0 | 2 (15) | 0 | 5 (42) | 1 (8) | 0 | 0 |
| Dry Mouth | 8 (62) | 0 | 0 | 0 | 9 (75) | 1 (8) | 0 | 0 |
| Hypertension | 0 | 1 (8) | 0 | 0 | 0 | 0 | 1 (8) | 0 |
| Constipation | 4 (31) | 2 (15) | 0 | 0 | 6 (50) | 2 (17) | 0 | 0 |
| Fever | 3 (23) | 1 (8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Chills | 7 (54) | 0 | 0 | 0 | 6 (50) | 0 | 0 | 0 |
| Dose limiting toxicity | Description | Attribution |
|---|---|---|
| Infection, grade 4 | Life-threatening or urgent intervention required | Any |
| Nausea, grade 3 (despite maximal support) | Inadequate oral intake, or, tube feeding, TPN, or hospitalization are indicated | Any |
| Vomiting, grade 3 (despite maximal support) | ≥ 6 episodes in 24 h, or, tube feeding, TPN, or hospitalization are indicated | Any |
| Neutrophil count decreased, grade 4 | < 500 cells / mm3 | Any |
| Platelet count decreased grade 4 | < 25,000 cells / mm3 | Any |
| Serious adverse event | Death or a life-threatening event, or, causes or prolongs inpatient hospitalization, or, significant disruption of activities of daily life, or, congenital anomaly or birth defect |
possible, probable, or definite to P-AscH- |
Events are reported as most severe per subject with one incidence per subject per event. All adverse events regardless of attribution are reported.
Graded using the Common Terminology Criteria for Adverse Events version 4.03.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; P-AscH−, pharmacologic ascorbate
Adverse events (AEs) were quantified by National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) (Table 1).
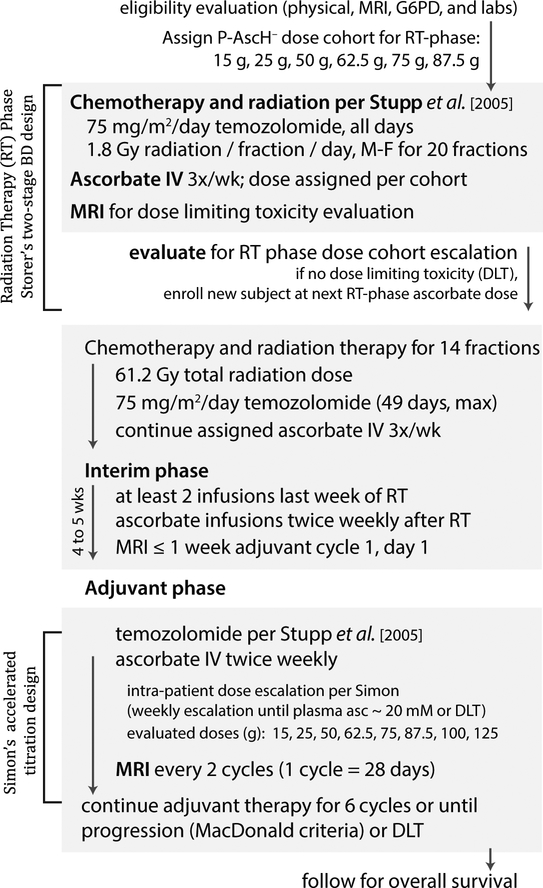
Day 1 of study was defined as RT fraction 1, serving as the first day of the RT-phase. Treatment continued until disease progression or completion of 6 adjuvant cycles. A schema for the study is provided (Figure 1).
Figure 1. Schematic diagram of phase 1 GBM clinical trial.
The primary objective was to determine the safety and tolerability of escalating pharmacological ascorbate doses combined with therapeutic radiation therapy and temozolomide (radiation phase) followed by continued treatment with pharmacological ascorbate combined with temozolomide (adjuvant phase) in GBM subjects.
PARTICIPANTS
Adults with biopsy-proven glioblastoma were eligible if the recommended treatment was the standard RT/TMZ regimen described by Stupp et al (2). Descriptions of radiation volumes and doses to organs at risk may be found in Supplemental Tables 1 and 2. IDH1 testing for the R123H mutation was performed by PCR or next generation sequencing at the University of Iowa Diagnostic Laboratories. The R123H mutation is the most common IDH1 mutation (accounts for 90% of the known IDH1 mutations). MGMT promoter methylation status was determined by PCR analysis at ARUP laboratories, a national reference laboratory, as per University of Iowa Hospitals and Clinics clinical practice. Further detailed criteria are provided on ClinicalTrials.gov ().
TREATMENT
Standard therapy was congruent with Stupp et al (2). Briefly, RT was delivered to a total dose of 61.2 Gy in 34 fractions at 1 fraction per business day. Concomitant daily TMZ (75 mg/m2) was initiated with radiation and finished with RT completion or a maximum of 49 doses. Adjuvant TMZ was prescribed at 150 mg/m2 daily for 5 days of a 28-day cycle. A one-time TMZ dose escalation to 200 mg/m2 was allowed at cycle two as per prescribing information. All TMZ dose modifications were per FDA-approved TMZ prescribing information. Pneumocystis pneumonia prophylaxis and anti-nausea medication was administered as per institutional guidelines.
During the RT-phase, P-AscH− was infused three times weekly per the assigned dose cohort. During the ADJ-phase, P-AscH− was infused twice weekly and escalated weekly until a DLT occurred or the target plasma concentration was achieved (≥ 20 mM). Plasma ascorbate concentrations were evaluated at the end of each adjuvant cycle; if the concentration of ascorbate in plasma was lower than the target level, the next cycle’s dose was escalated.
ASSESSMENTS
For the purposes of adverse event collection and reporting, a subject was deemed evaluable if they received at least one dose of ascorbate. Thus, all participants have been included in AE reporting (Table 1). For dose evaluation and survival evaluation, subjects had to receive the entire radiation prescription, 90% of the prescribed temozolomide during radiation, and 90% of the prescribed ascorbate.
Adverse events were graded utilizing the CTCAE version 4.03. Adverse events solicited weekly included rash, nausea, vomiting, fatigue, dry mouth, headache, and diarrhea/constipation. During the RT-phase, a complete blood count and differential (CBC w/diff), electrolytes, and serum creatinine were obtained weekly with liver function tests obtained every other week during RT phase. During the ADJ-phase, a complete metabolic profile and CBC w/diff were obtained at each cycle day 1 with a follow-up CBC w/diff on day 22 for TMZ dose modifications.
Plasma ascorbate levels were assessed weekly during RT, weekly during intra-patient dose escalation, and then at the end of each adjuvant cycle once the target plasma concentration was achieved. Samples were drawn pre-infusion and immediately post-infusion (within 10 minutes). Tumor response was assessed using the MacDonald criteria (18). PFS was calculated as time elapsed from RT fraction one to day of progression. OS was calculated as time elapsed from RT fraction one to death from any cause or censored at last follow-up.
The systemic oxidative stress marker, 4-hydroxy-2-nonenal(4HNE) modified proteins, was assessed by dot-blot analysis in plasma samples collected at baseline (before radiation therapy, temozolomide therapy, or pharmacological ascorbate), week 3 of the RT-phase, week 5 of the RT-phase and week-7 of the RT phase as previously described.(19).
STATISTICAL ANALYSIS
The censor date for these data is October 8, 2018. The primary endpoint was frequency of DLT to estimate MTD as per Storer’s Design B/D and intrapatient dose escalation design (Figure 2) (16). Eleven subjects were evaluated for DLT analysis. All subjects who received at least one infusion of P-AscH− are included in safety analyses. Subjects deemed evaluable by DSMC (i.e., received adequate TMZ, RT, and P-AscH−) were included in the efficacy analysis (Figure 3). Descriptive statistics including medians, ranges, and standard deviations were calculated.
Figure 2. Graphical depiction of dose escalation rules applied to the clinical trial.
The clinical trial utilized two design methods: Storer’s phase 1 two-stage design B/D (interpatient dose escalation) followed by an intra-patient dose escalation to achieve an ascorbate plasma concentration of 20 mM. Dose limiting toxicities were assessed clinically but also at pre-defined radiographic checkpoints (fraction 20 of radiation and one month post-radiation). These checkpoints determined cohort entry as well as stopping rules.
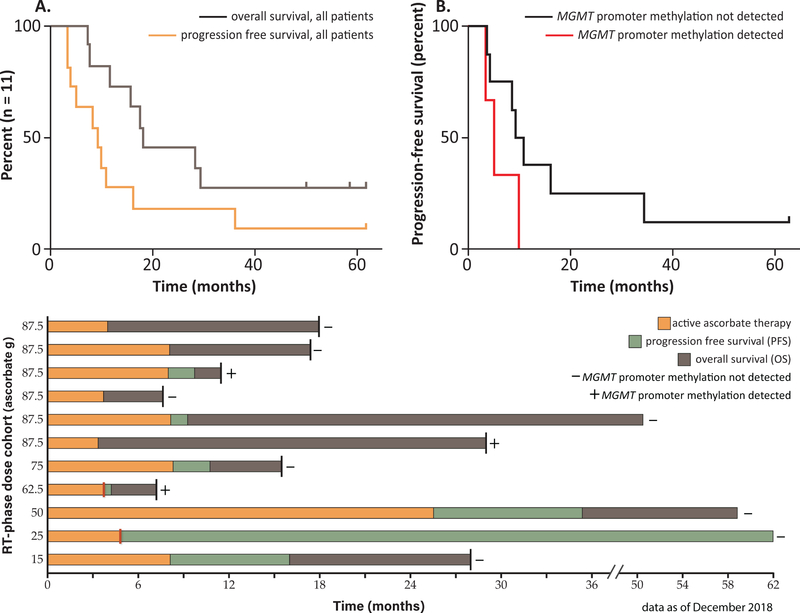
Figure 3. Overall and progression free survival of phase 1 GBM clinical trial subjects.
Historical median progression free survival for GBM patients treated with radiation and temozolomide therapy is approximately 7 months and median overall survival is 14 – 16 months. (A) Kaplan-Meier progression free survival curve (orange line) and overall survival curve (gray line) of all subjects treated with pharmacological ascorbate in combination with therapeutic radiation and temozolomide. (B) Kaplan-Meier progression free survival curve of subjects without detection of MGMT promoter methylation (black line) and with MGMT promoter methylation (red line). Subjects were treated with pharmacological ascorbate in combination with therapeutic radiation and temozolomide. (C) Swimmers plot showing outcomes of the 11 evaluable subjects. Active ascorbate therapy is indicated by orange bars, progression free survival is indicated by green bars, and overall survival is indicated by grey bars. The radiation phase ascorbate dose cohort of each subject is indicated along the y-axis. Red bars indicate subjects who chose to terminate the trial prematurely for personal reasons. Death is indicated by a black line at the end of the bar. Subjects with undetectable MGMT promoter methylation are indicated with a minus sign while subjects with detectable MGMT promoter methylation are indicated with a plus sign. The 62.5 g dose cohort subject and 87.5 g dose cohort subject who remains alive had isocitrate dehydrogenase (IDH) mutations.
RESULTS
Thirteen subjects consented to study participation; baseline demographics are summarized in Table 2. Median age at enrollment was 53 years (range of 25 – 71 years). Seven males and six females were included in the study. Ten patients had maximum safe resection and three had biopsies of tumor. Median time from biopsy or surgery to RT fraction 1 was 3.1 weeks (range of 1.3 to 4.6 weeks). Of the 11 subjects included in the survival analysis, 8 subjects had no MGMT promoter methylation detected. Two subjects had identified IDH1 mutations.
Table 2.
Baseline characteristics of study patients at enrollment
| Characteristic | n = 13†(accrued) | n = 11†(evaluable) |
|---|---|---|
| Age, years, median (range) | 53 (25–71) | 53 (25–68) |
| Gender, male, n (%) | 7 (54) | 6 (45) |
| Race, white, n (%) | 13 (100) | 11 (100) |
| Ethnicity, non-Hispanic, n (%) | 13 (100) | 11 (100) |
| Karnofsky Performance Status, n (%) | ||
| 70 | 4 (31) | 2 (18) |
| 80 | 3 (23) | 3 (27) |
| 90 | 5 (38) | 5 (45) |
| 100 | 1 (8) | 1 (9) |
| Preoperative lesion location, n (%) | ||
| Frontal | 5 (38) | 5 (45) |
| Temporal | 5 (38) | 3 (27) |
| Parietal | 2 (15) | 2 (18) |
| Occipital | 1 (8) | 1 (9) |
| Extent of resection, n (%) | ||
| Gross | 8 (62) | 6 (55) |
| Subtotal | 2 (15) | 2 (18) |
| Biopsy only | 3 (23) | 3 (27) |
| IDH mutation status, n (%) | ||
| Mutant | 1 (15) | 1 (9) |
| Wildtype | 12 (85) | 10 (91) |
| MGMT promoter methylation, n (%) | ||
| Detected | 3 (23) | 3 (27) |
| Not detected | 5 (38) | 5 (45) |
| Indeterminant (methylation levels < 1%) | 3 (23) | 3 (27) |
| Not tested | 2 (15) | 0 (0) |
Due to rounding, percentages may not total to exactly 100
Two subjects were withdrawn from study. The first 15 g subject developed a pulmonary embolism three weeks into the RT-phase necessitating a treatment break. The pulmonary embolism was determined to be attributable to a previous medical history of deep venous thrombosis. The DSMC concurred and authorized a second subject at 15 g. The first 50 g subject was withdrawn due poor tolerance of TMZ due to prior medical history of diverticulitis, which was aggravated by the TMZ. The DSMC determined this was unrelated ot study drug another subject was was enrolled to the 50 g cohort. Thus, there are 11 evaluable subjects for primary (safety) and secondary (survival) analysis.
Six P-AscH− dose cohorts were evaluated in the RT-phase and interim phase including 15 g, 25 g, 50 g, 62.5 g, 75 g, and 87.5 g ascorbate infusions. DLTs (Table 1) were not realized during the RT-phase or interim phase between completing RT and beginning the adjuvant phase (1 month) (Supplementary Table 3).
Eight P-AscH− cohorts were evaluated during the ADJ-phase including the RT-phase cohorts in addition to 100 g and 125 ascorbate infusions. During the ADJ-phase, all subject ascorbate doses were escalated until target plasma ascorbate levels were achieved (≥ 20 mM) (13). The 87.5 g dose consistently achieved plasma ascorbate concentrations of ≥ 20 mM in both the RT and ADJ phases. The 100 g and 125 g doses did not significantly increase plasma ascorbate concentrations above 20 mM. The increase in infusion time (+30 min per 25 g) was also considered. Thus, 87.5 g was identified as the recommended phase 2 ascorbate dose during both the RT-phase and ADJ-phase.
Of the 11 evaluable subjects, 2 withdrew during the ADJ-phase: one to enter into another clinical trial and the other to return to family out-of-state (subjects with red lines in Figure 3). Three subjects were withdrawn due to disease progression during adjuvant therapy phase. Six subjects completed all prescribed adjuvant cycles. On average, subjects received 188 days of ascorbate out of a maximum 245 days. The most common reason for withdrawal was disease progression (Figure 3) (Supplementary Table 4).
Overall, there were 85 adverse events (CTCAE grades 1, 2, 3, and 4) experienced during the RT-phase; due to the exhaustive listing of grade 1 and 2 constitutional events, not all are listed in Table 1. In review, subjects (n = 6) assigned to the recommended phase 2 dose (R2PD) cohort of 87.5 g experienced similar frequency of adverse events compared to subjects (n=7) assigned to lower doses. Specifically, the R2PD cohort had 41 grade 1, 21 grade 2, 7 grade 3, and 1 grade 4 adverse event. The other lower dose subjects totaled 44, 22, 9, and 2, adverse events respectively.
Adverse events directly attributable to the ascorbate infusions were dry mouth and chills (Table 1). Dry mouth occurred in 75% of the subjects and was thought to be related to the osmotic shift caused by the salt content of the infusion. Subjects reported the symptom resolved about an hour post-infusion. Chills were reported in about 54% of the subjects; it is suspected the temperature (4◦C) of the P-AscH− infusion fluid was the cause. Hypokalemia grade 3 (2.9 mEq/L; lower limit of normal (LLN) = 3.5 mEq/L) not requiring inpatient admission was reported in 1 subject, occurring during the RT-phase and was not associated with background therapy or the subject’s medical history and was therefore associated to P-AscH−.
The most frequent, severe hematologic AEs occurring during the RT-phase were decreased lymphocyte counts (Table 1). In addition to hypokalemia, the most frequent, severe chemistry AEs were single instances of hyperglycemia (gr. 3), hypernatremia (gr. 3), and elevated AST (gr. 3). None of these events were believed to be related to P-AscH−. The most frequent, severe constitutional AE was vomiting (Table 1) and this was considered most likely related to standard TMZ chemotherapy.
The observed hematologic events during the ADJ-phase were similar with only a single grade 3 absolute neutrophil count decrease. There was also an absence of ≥ grade 3 fatigue, nausea, vomiting, and pain during the adjuvant phase (Table 1).
A total of 4 serious adverse events (SAE) occurred during the clinical trial. Of these, 3 were not attributed to P-AscH− but instead were consistent with prior medical history (pulmonary embolism, diverticulitis) or anticipated with GBM (headache requiring inpatient pain management). The remaining SAE was a seizure that occurred immediately after the screening test dose of 15 g. Upon admission, the subject was found to be febrile (grade 1) and have a pseudomeningocele. While it was believed the seizure etiology was most likely fever or the primary GBM post-surgery, P-AscH− was attributed as possibly contributing due the contemporaneousness of the event. The subject asked to undergo another 15 g test dose and tolerated the second infusion. The subject completed all therapy without further issues.
Median PFS (n = 11) was 9.4 months (95% CI: 5.1, NA; range: 3.4, 63 months; the upper 95% confidence limits for the medians are not estimable (NA) because the upper confidence limits of the Kaplan-Meier curves do not drop below 50% survival.). Median OS (n = 11) was 18 months (95% CI: 16, NA; range: 7.3, 63 months). Currently, three subjects remain alive. Of these, one subject has no evidence of disease progression with last prescribed therapy in March of 2014 (Figure 3). The remaining two experienced disease progression and are receiving salvage therapy (Figure 3). Historical data demonstrate patients without MGMT promoter methylation have a worse prognostic outcome relative to those with a methylated MGMT promoter (4). In our study, subjects without MGMT promoter methylation had a median PFS of 10 months (95% CI: 8.5, NA; range 3.7, 63 months) and median OS of 23 months (95% CI: 18, NA; range 7.8, 63). Furthermore, the three subjects who remain alive all had undetectable MGMT promoter methylation (Figure 3) and two were IDH wild type. Overall response rate did not vary according to sex or age.
The systemic oxidative stress marker, 4-hydroxy-2-nonenal (4HNE) modified proteins, were assessed in select subject plasma samples prior to and during the RT-phase. In these subjects, 4HNE modified proteins decreased over the course of treatment supporting the hypothesis that pharmacological ascorbate acts as an anti-oxidant systemically and may protect against radiation and chemotherapy induced toxicity (Supplementary Figure 1).(20)
DISCUSSION
This study reports the first-in-human combination of pharmacological ascorbate, radiation therapy, and temozolomide therapy. Data suggest P-AscH− is safe when combined with RT and TMZ, with minimal toxicity relative to standard RT/TMZ therapy. While not powered to detect changes in effect size, data suggest P-AscH− may enhance RT/TMZ effectiveness in GBM patients, improving both PFS and OS, especially in subjects with undetectable MGMT promoter methylation and IDH wild type status. Results are sufficiently promising to merit a phase II investigation.
Several additional early phase clinical trials have found P-AscH− to be safe when combined with chemotherapy and may reduce cancer therapy associated normal tissue injury (9–11,13). In stage III or IV ovarian cancer, pharmacological ascorbate combined with conventional carboplatin and paclitaxel demonstrated reduced incidence of gastrointestinal and hematopoietic toxicity while trending toward improved overall survival and time to disease progression (10). Similarly, patients with stage IV pancreas cancer treated with pharmacological ascorbate and gemcitabine demonstrated the safety and tolerability of ascorbate combined with cancer therapy with a suggestion of improved progression free survival and overall survival (21). In locally advanced pancreatic cancer, pharmacological ascorbate combined with radiation therapy and gemcitabine showed an increase in progression free survival and a trend towards an increase in overall survival (11).
To our knowledge, this clinical trial was the first to combine P-AscH− with RT and TMZ for a primary brain tumor. Compared to RT/TMZ alone for GBM therapy (2,4), P-AscH− did not appear to increase hematologic toxicity but instead may provide protective effect. For example, grade 3 or 4 thrombocytopenia was not observed in this small study but had an 11% incidence in temozolomide alone treatment as reported by Stupp et al. (2) and 5% reported incidence in the TEMODAR prescribing information (3). The other hematologic toxicities reported were all consistent with the effects of temozolomide. Data submitted to the FDA identify a common ≥ grade 3 reaction as fatigue (13%), yet in contrast, the single grade 3 fatigue experienced in this study was related to diverticulitis (with the subject having a prior medical history). Protection against neurocognitive decline was not assessed as part of the phase 1 study but will be considered in subsequent clinical trials. These initial data suggest P-AscH− may increase RT and TMZ therapeutic tolerability, increasing a subject’s quality of life and decreasing therapeutic toxicity (22).
Median PFS (n = 11) was 9.4 months (95% CI: 5.1, NA; range: 3.4, 63 months; the upper 95% confidence limits for the medians are not estimable (NA) because the upper confidence limits of the Kaplan-Meier curves do not drop below 50% survival.). Median OS (n = 11) was 18 months (95% CI: 16, NA; range: 7.3, 63 months). Currently, three subjects remain alive. Of these, one subject has no evidence of disease progression with last prescribed therapy in March of 2014 (Figure 3). The remaining two experienced disease progression and are receiving salvage therapy (Figure 3). Historical data demonstrate patients without MGMT promoter methylation have a worse prognostic outcome relative to those with a methylated MGMT promoter (4). In our study, subjects without MGMT promoter methylation had a median PFS of 10 months (95% CI: 8.5, NA; range 3.7, 63 months) and median OS of 23 months (95% CI: 18, NA; range 7.8, 63). Furthermore, the three subjects who remain alive all had undetectable MGMT promoter methylation (Figure 3) and two were IDH wild type. Overall response rate did not vary according to sex or age.
Median OS (n = 11) was 18 months and median PFS was 9.4 months. These survival data compare favorably to historical GBM patients treated with radiation and temozolomide therapy alone with a median OS of 14.6 months and PFS of 7 months (2). Furthermore, historical GBM patients with an un-methylated MGMT promoter treated with radiation and temozolomide therapy have a median OS of only 12.7 months (23). In the 8 subjects enrolled in this clinical trial with un-detectable MGMT promoter methylation (“-“ sign in Figure 3), median OS was 23 months and median PFS was 10 months. Currently, of the 3 subjects who remain alive, 2 have an unfavorable un-methylated MGMT promoter and wild type IDH1 mutation (Figure 3).
We found P-AscH− to be safe when combined with RT/TMZ in the treatment of newly diagnosed GBM. The most frequent adverse events were transient dry mouth and chills, which were mild and associated acutely with infusion. Based on the encouraging nature of these data, a phase 2 clinical was trial initiated to assess the efficacy of P-AscH− in GBM subjects treated with RT/TMZ ().
Supplementary Material
Statement of Translational Research Relevance.
Pharmacological ascorbate (intravenous infusions of high dose vitamin C) has been shown to be efficacious in pre-clinical studies, both in vitro and in vivo, for treatment of glioblastoma multiforme (GBM). This study translates the pre-clinical findings into a phase 1, first-in-human clinical trial combining pharmacological ascorbate with radiation and temozolomide in the treatment of GBM. The primary objective was to determine the safety of this combination and explore the impact, if any, on treatment outcome. Data support pharmacological ascorbate is safe and well-tolerated when combined with radiation and temozolomide and may improve therapeutic outcome. Clinical trials combining pharmacological ascorbate, temozolomide, and radiation continue.
ACKNOWLEDGEMENTS
The authors sincerely thank the clinical trial participants, their families, and the caregivers for making this trial possible. The authors also thank Dr. Luke Szweda for providing the 4-HNE antibody. Funding for this trial and support for the investigators was provided by the University of Iowa Department of Radiation Oncology, the Burke Family Foundation, the Holden Comprehensive Cancer Center, and P01CA217797 (BGA, KLB, JJC, DRS, JMB).
Footnotes
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359(5):492–507 doi 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–96 doi 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN). 2018 19 November. NCCN treatment guidelines: Central nervous system cancers. <https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf>. Accessed 2018 19 November.
- 4.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology 2009;10(5):459–66 doi 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370(8):699–708 doi 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 2008;105(32):11105–9 doi 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta 2012;1826(2):443–57 doi 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, et al. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med 2011;50(11):1610–9 doi 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol 2013;71(3):765–75 doi 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 2014;6(222):222ra18 doi 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 11.Alexander MS, Wilkes JG, Schroeder SR, Buettner GR, Wagner BA, Du J, et al. Pharmacological ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res 2018. doi 10.1158/0008-5472.CAN-18–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, Cieslak JA 3rd, Welsh JL, Sibenaller ZA, Allen BG, Wagner BA, et al. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res 2015;75(16):3314–26 doi 10.1158/0008-5472.CAN-14-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2(−) and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 2017;32(2):268 doi 10.1016/j.ccell.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 14.May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem 2012;56:85–103 doi 10.1007/978-94-007-2199-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice ME. Ascorbate compartmentalization in the CNS. Neurotox Res 1999;1(2):81–90. [DOI] [PubMed] [Google Scholar]
- 16.Storer BE. Design and analysis of phase I clinical trials. Biometrics 1989;45(3):925–37. [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration (FDA). 2016 15 June. TEMODAR(R) prescribing information. <https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021029s031lbl.pdf>. Accessed 2019 15 June.
- 18.Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8(7):1277–80. [DOI] [PubMed] [Google Scholar]
- 19.Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clinical Cancer Research 2013;19(14):3905–13 doi 10.1158/1078-0432.CCR-12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfeld JD, Alexander MS, Waldron TJ, Sibenaller ZA, Spitz DR, Buettner GR, et al. Pharmacological ascorbate as a means of sensitizing cancer cells to radio-chemotherapy while protecting normal tissue. Semin Radiat Oncol 2019;29(1):25–32 doi 10.1016/j.semradonc.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol 2013;71(3):765–75 doi 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration (FDA). 2018 22 June. FDA public workshop: 2018 clinical outcome assessments in cancer clinical trials. <https://www.fda.gov/NewsEvents/MeetingsConferencesWorkshops/ucm602540.htm>. Accessed 2018 22 June. [Google Scholar]
- 23.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352(10):997–1003 doi 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.