Abstract
Lysosomes are multi-functional, sub-cellular organelles with roles in plasma membrane repair, autophagy, pathogen degradation and nutrient sensing. Dysfunctional lysosomes underlie Alzheimers, Parkinsons and rare lysosomal storage diseases but their contributions to these pathophysiologies are unclear. Live imaging has revealed lysosome sub-populations with different physical characteristics including dynamics, morphology or cellular localization. Here we chemically resolve lysosome sub-populations using a DNA-based combination reporter that quantitatively images pH and chloride simultaneously in the same lysosome while retaining single lysosome information in live cells. We call this technology two-ion measurement or 2-IM. 2-IM of lysosomes in primary skin fibroblasts derived from normal individuals show two major lysosome populations, one of which is lost in primary cells derived from Niemann-Pick disease patients. When patient cells are treated with relevant therapeutic, the second population re-emerges. Chemically resolving lysosomes by 2-IM could enable decoding the mechanistic underpinnings of lysosomal diseases, monitoring disease progression or evaluating therapeutic efficacy.
Lysosomes are highly fusogenic organelles that regulate cellular processes such as innate immunity by fusion with the phagosome, cell membrane repair through fusion with the plasma membrane, autophagy by fusion with the autophagosome and nutrient sensing through the mTOR pathway1. Lysosome dysfunction is central to the pathology of common neurological disorders such as Alzheimer’s disease, Parkinson’s disease as well as ~60 rare, largely untreatable genetic diseases called lysosomal storage diseases2. It has been challenging to deconvolute how each of its multiple roles are affected in the diverse pathophysiologies associated with lysosome-related diseases.
Lysosomes are, by and large, regarded as a single population while assaying for a specific lysosomal function. However, recent promising studies have considered that sub-populations of lysosomes might perform sub-sets of tasks3. Indeed, many cell types have evolved specialized lysosomes that perform distinct functions. For instance in addition to lysosomes, skin cells have melanosomes4, neutrophils have azurophil granules5, cytotoxic T-cells have secretory lysosomes6 while every cell has autolysosomes7. Functional imaging based on physical parameters such as lysosome movement, morphology or spatial position within cells have revealed sub-populations that exhibit different behaviors and functions3,8. For example, autolysosomes and lysosomes adopt tubulovesicular and vesicular morphologies respectively9. Lysosomes have also been sorted into two populations based on how actively they move within the cell10. Spatial positioning of lysosomes is emerging as a correlate of lysosome function11,12. Nevertheless, the capacity to chemically discriminate between lysosome populations in live cells would significantly aid our understanding of lysosome biology by providing the ability to quantitatively correlate chemotypes with function. For example, in 1960s electron microscopy and bright field imaging could only distinguish up to three stages in melanosome maturation based on morphology and melanin content respectively13. However, when protein markers were used to chemotype melanosomes, it revealed four stages in melanosome maturation. Chemical resolution revealed the colorless, stage I melanosome that had eluded identification till then due to its high physical and morphological similarity with lysosomes14. However, there are still no methods to chemically resolve lysosome populations.
Specialized lysosomes have a different protein composition from normal lysosomes to enable the distinct biochemistries within their lumens. This lumenal biochemistry is facilitated by an optimal chemical milieu, of which key components are high concentrations of specific ions homeostatically maintained by the lysosome protein composition15,16. H+ and Cl− are two highly abundant ions in the lysosome that are critical to its function17. In fact, no other organelle has a greater concentration of either ion. Lysosomal pH is critical to lysosome maturation, cargo degradation and recycling of degraded material18. High lumenal Cl− in the lysosome is required for the activity of certain lysosome-resident hydrolases15,19. However, unlike other organelles, lumenal Cl− levels in the lysosome is independent of lumenal pH15,20. We therefore hypothesized that the levels of H+ and Cl− in the lysosome could form a basis to chemically discriminate lysosome populations in live cells.
Here we show that by measuring two ions - H+ and Cl− - simultaneously in the same lysosome and retaining this information with single lysosome addressability, one can resolve lysosomal sub-populations quantitatively in live cells. We call this technology two-ion measurement or 2-IM. A technology like 2-IM has proved elusive to realize thus far for several reasons. Cl−-sensitive small molecule probes offer the necessary chemical selectivity, molar brightness and long wavelength excitation, but not the required spatial addressability or organelle targetability21. Genetically encoded Cl− sensors offer stable spatial localization, but the response of these reporters to Cl− is pH sensitive22. This complicates analysis of most organelles as lumenal Cl− entry is coupled to their acidification. Fluorescent proteins label organelles with lower specificity than endocytic tracers and have lower dynamic range compared to DNA-based nanodevices22,23. DNA nanodevices comprise a range of biologically interfacable,24 quantitative imaging probes that unite the photophysical advantages of small molecules, the stable localization provided by proteins along with the precision of organelle targeting that is accessible to endocytic tracers16,25–28.
Using a DNA nanodevice that can ratiometrically image pH and [Cl−] simultaneously with single lysosome addressability, we could discriminate lysosome chemotypes on a two-dimensional map that correlates lumenal pH with lumenal [Cl−]. Lysosome profiles of cells obtained from healthy individuals revealed a high chloride, high acidity population that was absent in cells derived from patients afflicted with Niemann Pick A, B or C diseases. Interestingly, treating these cultured patient cells with the known therapeutic for these diseases led to a reemergence of the high chloride high acidity population.
Design and in vitro characterization of ChloropHore
We describe the design, creation and characterization of a DNA-based, ratiometric, fluorescent combination reporter of pH and chloride called ChloropHore. ChloropHore is a 61-base pair DNA duplex comprising three strands C1, C2 and P (Supplementary Table S1) and bears three distinct domains (Fig. 1a). Two of these are fluorescent, ratiometric reporter domains that are previously reported, namely a Cl− reporter domain, Clensor16,29 and a pH-reporter domain called the I-switch15,25,30–32 Each reporter domain is fused to either end of an “integration domain”, which comprises a 27-mer duplex, that serves to integrate the pH and the Cl− reporter domains into a single DNA assembly. This 27-mer duplex also helps in targeting, because its anionic nature aids recognition and trafficking by scavenger receptors in a DNA sequence independent manner. To match the pH sensing regime of ChloropHore to the low pH regimes encountered in mammalian lysosomes we also made ChloropHoreLy, a variant that used modified 5’-bromocytosines in the C-rich region (Supplementary Table S1)30,33. The formation and specificity of ChloropHore and ChloropHoreLy were confirmed by a gel shift assay, circular dichroism spectroscopy and UV melting studies (Supplementary Fig. S1–S3).
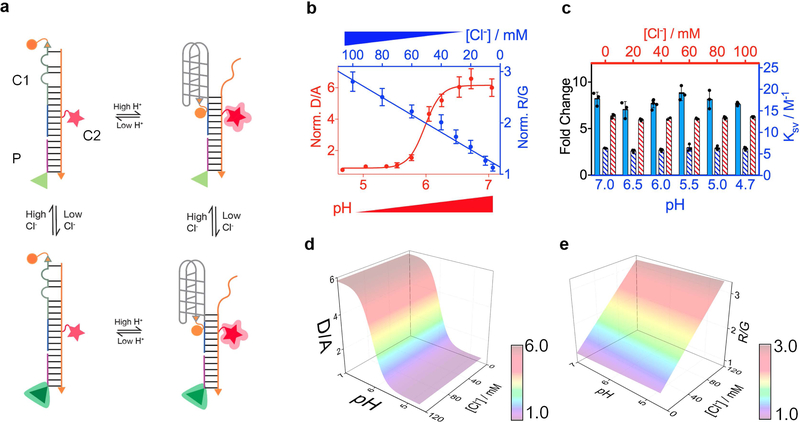
Figure 1. Design and characterization of ChloropHore.
(a) Schematic of the working principle: A pH-induced change in FRET between Alexa546 (donor, orange sphere) and Alexa647 (acceptor, red star) reports pH ratiometrically. A Cl− sensitive fluorophore (BAC, green triangle) and Alexa647 report Cl− ratiometrically. (b) pH and Cl− response profiles of ChloropHore: Normalized fluorescence intensity ratio (D/A) of donor (D) and acceptor (A) upon donor excitation in vitro as a function of pH and 50 mM Cl− (red). Normalized fluorescence intensity ratio (R/G) of Alexa 647 (R) and BAC (G) as a function of Cl− concentration at pH 7 (blue). Values were normalized 5 mM Cl− for R/G or pH 4 for D/A. (c) Performance of the pH sensing module (red) at different [Cl−] and Cl− sensing module at different pH (blue). Fold changes in D/A (red hatched bars) or R/G (blue hatched bars) for the pH and Cl− sensing modules are shown. Stern Volmers constant (Ksv, solid blue bars) for Cl− sensing at each pH. Calibration surface plot of the fluorescence intensity ratios of (d) D/A and (e) R/G of ChloropHore as a function of Cl− and pH. Error bars indicate the mean ± s.e.m. of three independent measurements.
The fluorescence response characteristics of ChloropHore and ChloropHoreLY were investigated as function of pH and [Cl−] in order to determine their pH and [Cl−] sensitive regimes. The gradual increase of D/A ratio of ChloropHore and ChloropHoreLy revealed their pH reporting capabilities between pH 5.5 and 6.5 (Fig. 1b) and pH 4.5 and 6.5 respectively (Supplementary Fig. S4b), the latter being well suited to measure the pH of highly acidic human lysosomes. Both ChloropHore and ChloropHoreLy show a sigmoidal increase in D/A as a function of pH in the sensitive regime, and fold changes in D/A ratios of 5.5 and 3 respectively. Notably, the fold change in D/A ratio remained invariant for both ChloropHore and ChloropHoreLy over [Cl−] ranging from 5 mM to 120 mM (Fig. 1c and Supplementary Fig. S4c). Thus, the pH sensing characteristics of ChloropHore and ChloropHoreLy are insensitive to changes in physiological [Cl−]. This is illustrated by a 3D surface plot of D/A as a function of pH performed at different fixed values of [Cl−] is shown in Fig 1d (Supplementary Figure S4d).
In parallel, the R/G ratio shows a linear dependence with increasing [Cl−], showing ~2.5 fold change upon increasing [Cl−] from 5 mM to 120 mM (Fig. 1b,c and Supplementary Fig. S4b,c). Again, for both ChloropHore and ChloropHoreLy the Stern Volmer constant (Ksv) and the fold change in R/G stayed constant as a function of pH from pH 4.5–7.0 (Fig. 1c and Supplementary Fig. S4c). This is illustrated by a 3D surface plot of R/G as a function of Cl− performed at different fixed values of pH as shown in Fig 1e (Supplementary Figure S4e). Thus, the [Cl−] sensing characteristics of ChloropHore and ChloropHoreLy are insensitive to changes in physiological pH. This indicates that in ChloropHore and ChloropHoreLy, the sensing characteristics of the H+ sensing module is unaffected by integration to the Cl− sensing module and vice versa thus enabling potential detection of these two ions in parallel.
Two Ion measurement with single endosome addressability
To simultaneously measure lysosomal pH and [Cl−] in live cells, we targeted ChloropHore to the lysosomes of human dermal fibroblasts (HDF). Human dermal fibroblasts (HDF) express scavenger receptors (SR) that uptake anionic ligands34. Therefore DNA nanodevices can be trafficked to organelles on the endolysosomal pathway in diverse living systems (Fig. 2a)24,32,35–37. Upon incubating ChloropHore with HDF cells for 1 h (referred to as 1 h “pulse”), we found excellent uptake into punctate endosomes (Fig. 2b,d,f). The uptake was effectively competed out by 10 equivalents of maleylated BSA revealing that in HDF cells, ChloropHore is internalized via the SR pathway (Fig. 2b–c) 34.
Figure 2. Trafficking pathway of ChloropHore in human dermal fibroblasts.
(a) Trafficking of ChloropHore along the scavenger receptor-mediated endocytic pathway. (b-c) ChloropHore uptake in primary skin fibroblasts (HDF cells) is competed out by excess maleylated BSA (mBSA, 10 μM). Cells are imaged in Alexa647 channel. AF: autofluorescence. (d-g) ChloropHore labels lysosomes in HDF cells. (d) Representative images of co-localization between lysosomes of HDF cells labeled with FITC Dextran (green) and LAMP-1 RFP (red) with the corresponding Pearson’s correlation coefficient (e). (f) Representative images of lysosomes of HDF cells labelled with TMR dextran (TMR; green) and ChloropHore (Alexa647, red). (g) Pearson’s correlation coefficient of colocalization between ChloropHore and lysosomes as a function of ChloropHore chase times. Experiments were performed in triplicate. Error bars indicate the mean of three independent experiments ± s.e.m. (n = 20 cells).
We then estimated the time required for lysosomal localization of ChloropHore, by its time-dependent colocalization with a lysosomal marker. Pulsing HDF cells with 10 KDa fluorescent dextrans (0.25 mg/mL) for 1 h followed by a 16 h chase effectively marked lysosomes, as revealed by colocalization with LAMP1-RFP (Fig. 2d–e). Next, we pulsed ChloropHore in HDF cells where lysosomes were pre-labeled with TMR-Dextran as above, washed and imaged the cells at various chase times. ChloropHore and TMR-Dextran showed maximal co-localization at 9 h (Fig. 2f–g, Supplementary Figure S5). Stability measurements revealed that ChloropHore and ChloropHoreLy had a half-life of 20 hours and were stable for at least 10 h in HDF lysosomes (Supplementary Fig. S5)38.
Next, we investigated the in-cell pH and [Cl−] sensing characteristics of ChloropHore. We clamped lumenal pH and [Cl−] in ChloropHore- labeled HDF cells by incubation in clamping buffers of fixed pH and [Cl−] containing nigericin, monensin and tributyltin chloride at high [K+]15,16. Figure 3a shows representative fluorescence images of a cell clamped at the indicated pH and [Cl−] imaged in the D, A, R and G channels along with the corresponding D/A and R/G maps. Histograms of D/A and R/G values of 150 endosomes clamped at different pH and [Cl−] are shown in Supplementary Fig. S6. ChloropHore showed a ~5.5 fold-change in D/A value from pH 4.0 to pH 7.0 across all tested values of [Cl−], with the in vitro 3D surface plot being quantitatively recapitulated in cells (Fig. 3b, Supplementary Fig. S7). Similarly, ChloropHore response in terms of R/G as a function of [Cl−] was performed at different fixed values of physiological pH (Fig. 3c). Both the Ksv and fold-change in R/G from 5 mM to 120 mM [Cl−] was constant from pH 4.0–7.0, with the in vitro 3D surface plot of R/G being quantitatively recapitulated in cells (Fig. 3c, Supplementary Fig. S7). This revealed that ChloropHore and ChloropHoreLy can simultaneously report pH and [Cl−] with performance characteristics in cells that closely match their in vitro pH and [Cl−] sensing properties.
Figure 3. Intracellular calibration of ChloropHore and ChloropHoreLy.
(a) Fluorescence images of primary HDF cells labeled with ChloropHore, clamped at the indicated pH and [Cl−], imaged in the donor (D), acceptor (A), reference (R), BAC (G) channel and the corresponding pseudocolour D/A (pH) and R/G (Cl−) maps. (b-c) In cell calibration surface corresponding to the pH and Cl− response profiles of the sensing modules in ChloropHore at various [Cl−] and pH values respectively. (d) Representative scatter plot of D/A versus the R/G values of endosomes in primary HDF cells from a normal individual clamped at the indicated pH and [Cl−]. Each data point corresponds to a single endosome. (e) The scatter plot in (d) represented as a density plot in pseudocolour where red and blue correspond to populations with higher and lower frequencies of occurrence, i.e., a 2-IM profile. (f-h) 2-IM profiles of HDF cells clamped in indicated varying pH and fixed [Cl−]. (i-k) 2-IM profiles of HDF cells at fixed pH and increasing [Cl−]. a-k, experiments were performed in duplicate (n = 15 cells, n = 150 endosomes). (l) Single endosome clamping in HDF cells. ChloropHore labeled cells clamped at indicated pH (i) and Cl− (ii) were clamped to a different indicated pH and same Cl− (iv) (m) 2D-scatter plots and their projected histograms on a single axis of each clamping step of the same endosomes are shown. Gray arrow represents the direction of change in D/A (pH) values for each endosome. (n) ChlorophoreLY labeled HDF clamped at indicated pH (i) and Cl− (ii) were clamped to the same pH (iii) but different [Cl−] (iv) (o) 2D”scatter plots and their projected histograms on a single axis for each clamping step. Gray arrow indicates direction of change in R/G (Cl−) values for each endosome. Scale bars, 10 μm. l-o, experiments were performed in duplicate (n = 30 endosomes).
Next, we sought to simultaneously map both ions in endosomes while retaining this information with single endosome addressability in live cells. Therefore, the D/A value-reflecting lumenal pH-in a given endosome is plotted against the R/G value in the same endosome-reflecting lumenal [Cl−]-for 150 endosomes, which is represented as a scatter plot with each data point corresponding to a single endosome (Fig. 3d). For clearer visualization, this data is represented as a density plot color coded according to their frequencies of occurrence (Fig. 3e). We refer to this method of simultaneous quantitative imaging of two ions in a single endosome, while retaining concentration information with single endosome addressability as two-ion measurement (2-IM) and the corresponding density plot as a 2-IM profile. Figures 3f–h shows the 2-IM profile of 150 endosomes clamped at the same [Cl−] (100 mM) but different pH, while Figure 3i–k shows the 2-IM profile of 150 endosomes clamped at the same pH (pH 6.5), but different [Cl−].
To illustrate the capability of 2-IM to address single endosomes, we subjected ChloropHoreLy-labeled HDF cells to different clamped states of pH and [Cl−] in series due to its optimal pH responsivity in lysosomes (Supplementary Fig. S8). ChloropHoreLy labeled endosomes were clamped at the indicated pH and Cl− and imaged in D, A, R and G channels (Figure 3l,n). Figure 3l(i-ii) shows the D/A and corresponding R/G maps of cells clamped at pH 4.5 and 5 mM Cl−. The D/A and corresponding R/G maps of these same cells subsequently clamped at the same value of [Cl−], but at pH 6.5 are shown in Figure 3l(iii-iv) (Supplementary Fig. S9). Figure 3m shows a plot of individual endosomes in each clamped state, with black lines connecting the same endosome in either clamped state. It is clear that when [Cl−] was constant and pH changed, all the endosomes show increased D/A and negligible variation of R/G, moving parallel to the Y-axis. Similarly, Figure 3n(i-iv) shows the D/A and R/G maps of cells clamped at 100 mM Cl− and pH 6.5 that have subsequently been clamped at the same value of pH, but at 5 mM [Cl−]. Again, when pH was constant and [Cl−] was changed, individual endosomes moved from right to left, parallel to the X-axis (Fig. 3o). Thus, in addition to population measurements, 2-IM provides information with single endosome resolution.
Two Ion measurement chemically resolves lysosome populations
The 2-IM profile of lysosomes in fibroblasts of healthy individuals reveals populations with two distinct chemotypes. ChloropHoreLy labeled lysosomes in HDF cells were imaged to obtain D/A and R/G maps (Fig. 4a(i)–(ii)). The corresponding 2-IM profile revealed a major population of ~68% lysosomes that contained relatively low chloride, with R/G <1.3 (Fig. 4b(i)). However, there is a minor population (~23%) with R/G > 1.5 and D/A < 1.25 with higher lumenal Cl− and proton content (Fig. 4b(i)). This was consistent over experimental replicates on samples derived from the same individual as well as across multiple normal individuals (Fig. 4b(i)–(vi)). 2-IM in lysosomes of diverse cell types such as BHK-21 cells, murine macrophages and T-47D cells also showed lysosomes chemotypes with these characteristics (Supplementary Fig. S10–12). The high Cl−, high acidity population was lost upon pharmacologically inhibiting either V-ATPase or chloride channels with bafilomycin A1 or 5-nitro-2-(3-phenylpropylamino) benzoic acid, that selectively block lysosomal proton or Cl− accumulation respectively (Fig. 4c–d).
Figure 4. 2-IM chemically resolves lysosome populations.
(a) Respective pseudocolour D/A and R/G map of HDF cells derived from normal individuals 1, NP-A patient 1 and NP-C patient 1 labeled with ChlorophoreLY. 2-IM profiles and the histograms of D/A, R/G ratios of (b,i) normal individual (N.I.) and in presence of (c) bafilomycin A1 or (d) NPPB. 2-IM profiles of lysosomes of primary HDF cells from the same normal individual showing three replicates (b,ii-vi), two different normal individuals 2–3, (e) NP-A, NP-B, NPC patients. (f) Scatter plots of lysosome sizes versus their R/G or D/A values in a normal individual and an NP-A patient. 2-IM profile and corresponding histograms of D/A, R/G ratios of (g) HDF cells treated with 65 μM amitriptyline (AH) or 20 μM U18666A (h) 2-IM profiles of NP-A, NP-B patient fibroblasts in the presence of 5 μg of acid sphingomyelinase (ASM) and NP-C patient fibroblasts in the presence of 50 μM of ß-CD. Experiments were performed in duplicate (n = 550 lysosome, = 55 cells). Scale bars 10 μm.
In order to understand how these lysosomal populations are affected upon pathological lysosomal storage, we subjected fibroblasts of patients with lysosomal storage disorders to 2-IM profiling. Lysosomal storage disorders arise due to genetic defects in proteins that affect the lysosomal degradation of specific biomolecules. Further, dysfunctional lysosomes in a range of lysosomal storage disorders show reduced lumenal Cl− and/or H+ as a result of flawed lysosomal integrity15. We applied 2-IM to study three related lysosomal storage disorders, i.e., the Niemann Pick disease variants, due to their similarity of presentation, the fact that mutations lie in only one of three identified genes, all three gene products are lysosome-resident, and importantly, therapeutics are available for these diseases39,40.
Niemann Pick A (NP-A) and Niemann Pick B (NP-B) diseases arise due to defects in the enzyme acid sphingomyelinase (ASM)39, for which enzyme replacement therapy is available41. Niemann Pick C (NP-C) arises due to defective cholesterol transport due to mutations in any one of two key proteins, NPC1 or NPC240, and for which clinical trials using cyclodextrin derivatives are under way42. Three patient samples corresponding to NP-A and NP-C disease and two for NP-B were studied based on sample availability, common mutations and characterization by enzyme activity (Supplementary Table S2).
After verifying that ChloropHoreLy could label the lysosomes in every patient sample (Supplementary Fig. S13g–l), we subjected each to 2-IM (Fig. 4e). Typical D/A and R/G maps of ChloropHoreLy labeled lysosomes in fibroblasts derived from NP-A and NP-C patients are shown in Fig 4a(iii)–(vi). The 2-IM profiles of fibroblasts derived from skin biopsies of all the patient samples showed that the high chloride, high acidity lysosome population was absent (Fig 4e(i)–(viii)). Particularly, the 2-IM profile of NP-C patient samples showed a high degree of monodispersity compared to healthy samples (Fig. 4e(vi)–(viii)).
Although lysosomal pH correlates with spatial position in certain cell types, no such dependence is available yet for lysosomal Cl-43. Peripheral lysosomes show lower acidity in C2C12 murine myoblasts, human adipose microvascular endothelial cells and HeLa cells, but not primary human dendritic cells or CHO cells43. Interestingly, 2-IM profiles in primary HDF cells showed that peripheral lysosomes had higher pH than perinuclear lysosomes (Fig. 4a(i), arrowhead). Further, in NP-A and NP-C cells the spatial heterogeneity in lysosomal pH is lost, with lysosomes becoming uniformly hypoacidic (Fig. 4a(iii)&(v)). In contrast to pH, no such spatial correlation could be observed for lumenal Cl− in HDF cells from normal individuals (Fig. 4a(ii)). However, in NP-A and NP-C patient samples, the lumenal Cl− of the perinuclear lysosomes was more affected and lower than those of peripheral lysosomes (Fig. 4a(iv)&(vi)). Taken together, this suggests that the milieu of the perinuclear lysosomes is more compromised than the rest, and that the high chloride high acidity population could correspond to these lysosomes. We also explored whether there was any correlation between lumenal ions and lysosome size (Fig. 4f (i)–(iv)). These studies revealed that the high chloride lysosomes were smaller in size, and their numbers were depleted in patient cells. No such clear correlation emerged for lysosomal pH.
ChloropHoreLy enables evaluation of therapeutic efficacy
In order to understand how the high chloride high acidity population was affected upon inducing pathological lysosomal storage, we created cell culture models of NP-A/B and NP-C diseases. In order to mimic the sphingomyelin and cholesterol accumulation characterizing these disorders, we treated HDF cells from a healthy individual with amitriptyline hydrochloride (AH)44 and U18666A45 that inhibit ASM and NPC1 respectively. We performed 2-IM after verifying that ChloropHoreLy effectively labels lysosomes in these cells (Supplementary Fig. S13c–f). The 2-IM profiles showed a monodisperse lysosome population in both cell culture models, and a depletion of the high chloride, high acidity lysosome population (Fig. 4g(i)&(ii)).
Next, we investigated whether this high chloride, high acidity lysosome population could be recovered upon complementing patient cells with the corresponding therapeutic. Recombinant human acid sphingomyelinase (rhASM) is used to treat NP-A/B patients by enzyme replacement therapy41. We therefore incubated fibroblasts from NP-A and NP-B patient samples with rhASM as outlined in literature46. These cells are expected to internalize rhASM from the extracellular milieu, and traffic it to lysosomes, where it degrades sphingomyelin and mitigates storage46. The 2-IM profile of patient cells treated with rhASM showed the reemergence of the high chloride high acidity lysosome population (Fig. 4h(i)&(ii)).
NP-C does not arise from the deficiency of a degradative enzyme, but rather due to a transport defect, for which there is still no FDA approved therapeutic. However, o-2-hydroxypropyl-β-cyclodextrin (βCD) treatment for 24 h has been shown to improve cholesterol transport from the lysosome to the endoplasmic reticulum thereby delivering cholesterol to the ER for esterification and reducing storage in the lysosome47. Interestingly, treating NP-C patient samples mutated either in NPC1 (Fig. 4h(iii)&(iv)) or NPC2 (Fig. 4h(v)) with βCD showed a loss of monodispersity in the 2-IM profile and the appearance of lysosomes with higher lumenal Cl− and H+ content.
Conclusion
In summary, we describe a new technology to chemically resolve lysosome sub-populations in living cells based on the rationale that different sub-populations of lysosomes contain a different ionic microenvironment due to their distinct roles. This is a two-dimensional method where quantification of two different ions in the lysosome, i.e., lumenal pH and Cl−, can be correlated with single lysosome addressability. We exploit the principle that populations that are challenging to discriminate based on a single parameter, can be resolved in two dimension (2D) based on a second independent parameter. This is seen in the case of 2D nuclear magnetic resonance spectroscopy and 2D gel electrophoresis48,49. However, in order to achieve this for organelles such as the lysosome, it is essential to be able to measure both parameters i.e., pH and Cl− in parallel in a given lysosome, while retaining single lysosome addressability.
The present combination-reporter succeeds because it uses the 1:1 stoichiometry of DNA hybridization to integrate four functions with stoichiometric precision onto a single probe: (i) a pH sensing function (ii) a Cl− sensing function (iii) an internal standard for simultaneous ratiometric quantitation of both Cl− & pH and (iv) a lysosome targeting function for addressability50. 2-IM is a highly sensitive method that chemically resolved a high-chloride, high acidity lysosome population in human fibroblasts isolated from skin biopsies of normal, healthy humans. The significance of this high-chloride, high-acidity population was revealed upon 2-IM investigation of fibroblasts derived from skin biopsies of patients afflicted with three variants of Niemann Pick disease, where this population was lost, resulting in highly monodisperse 2-IM profiles. Replenishing cells with the relevant therapeutic, i.e., the defective enzyme, recovered the high-chloride, high-acidity population. However, treatment with a molecule documented to have a limited therapeutic efficacy showed marginal recovery of this population.
Our studies indicate that 2-IM profiling of lysosomes is a promising method to screen for potential lead compounds for Niemann Pick diseases in an unbiased way, potentially identify suitable patient cohorts for clinical trials, monitor therapeutic efficacy or track disease progression. Although we currently cannot ascribe specific functions to the high chloride high acidity population, the ability to resolve lysosome chemotypes in live cells may allow one to more quantitatively investigate the contribution of diverse lysosomal functions in health and disease.
Methods
Methods, including statements of data availability and any associated accession codes and references, are available in the online version of the paper.
Supplementary Material
Acknowledgements
This work was supported by the University of Chicago Women’s Board, Pilot and Feasibility award from an NIDDK center grant P30DK42086 to the University of Chicago Digestive Diseases Research Core Center, MRSEC grant no. DMR-1420709, Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust, C-084 and University of Chicago start-up funds to Y.K. Y.K. is a Brain Research Foundation Fellow.
Footnotes
Additional information
Supplementary information is available in the online version of the paper. Reprints and permission information is available online at www.nature.com/reprints.
Competing financial interests
The authors declare no competing financial interests.
Data availability. The data that support the plots within this paper and the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Settembre C, Fraldi A, Medina DL & Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14, 283–296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraldi A, Klein AD, Medina DL & Settembre C. Brain disorders due to lysosomal dysfunction. Annu Rev Neurosci 39, 277–295 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Pu J, Guardia CM, Keren-Kaplan T. & Bonifacino JS Mechanisms and functions of lysosome positioning. J Cell Sci 129, 4329–4339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasmeier C, Hume AN, Bolasco G. & Seabra MC Melanosomes at a glance. J Cell Sci 121, 3995–3999 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Faurschou M. & Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5, 1317–1327 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Blott EJ & Griffiths GM Secretory lysosomes. Nat Rev Mol Cell Biol 3, 122–131 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N, Yoshimori T. & Levine B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertoft H, Wärmegård B. & Höök M. Heterogeneity of lysosomes originating from rat liver parenchymal cells. Metabolic relationship of subpopulations separated by density-gradient centrifugation. Biochem J 174, 309–317 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X. et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 18, 404–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matteoni R. & Kreis TE Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol 105, 1253–1265 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu J. et al. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell 33, 176–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornak U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Seiji M, Shimao K, Birbeck MSC & Fitzpatrick TB Subcellular localization of melanin biosynthests. Ann N Y Acad Sci 100, 497–533 (2006). [PubMed] [Google Scholar]
- 14.Raposo G, Tenza D, Murphy DM, Berson JF & Marks MS Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol 152, 809–824 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty K, Leung K. & Krishnan Y High lumenal chloride in the lysosome is critical for lysosome function. elife 6, e28862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha S, Prakash V, Halder S, Chakraborty K. & Krishnan Y A pH-independent DNA nanodevice for quantifying chloride transport in organelles of living cells. Nat Nanotechnol 10, 645–651 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Van Dyke RW Proton pump-generated electrochemical gradients in rat liver multivesicular bodies. Quantitation and effects of chloride. J Biol Chem 263, 2603–2611 (1988). [PubMed] [Google Scholar]
- 18.Luzio JP, Pryor PR & Bright NA Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8, 622–632 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Weinert S. et al. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl-accumulation. Science 328, 1401–1403 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Guzman RE, Grieschat M, Fahlke C. & Alekov AK ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem Neurosci 4, 994–1003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biwersi J, Tulk B. & Verkman AS Long-wavelength chloride-sensitive fluorescent indicators. Anal Biochem 219, 139–143 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Arosio D. et al. Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat Methods 7, 516–518 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Kuner T. & Augustine GJ A Genetically Encoded Ratiometric Indicator for Chloride. Neuron 27, 447–459 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Surana S, Shenoy AR & Krishnan Y. Designing DNA nanodevices for compatibility with the immune system of higher organisms. Nat Nanotechnol 10, 741–747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modi S, Nizak C, Surana S, Halder S. & Krishnan Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. Nat Nanotechnol 8, 459–467 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Chu TC et al. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res 66, 5989–5992 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro AV, Han D, Shih WM & Yan H. Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol 6, 763–772 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia D. et al. Quantum dot-loaded monofunctionalized DNA icosahedra for single-particle tracking of endocytic pathways. Nat Nanotechnol 11, 1112–1119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prakash V, Saha S, Chakraborty K. & Krishnan Y. Rational design of a quantitative, pH-insensitive, nucleic acid based fluorescent chloride reporter. Chem Sci 7, 1946–1953 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi S, Halder S, Nizak C. & Krishnan Y. Recombinant antibody mediated delivery of organelle-specific DNA pH sensors along endocytic pathways. Nanoscale 6, 1144–1152 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Modi S. et al. A DNA nanomachine that maps spatial and temporal pH changes inside living cells. Nat Nanotechnol 4, 325–330 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Surana S, Bhat JM, Koushika SP & Krishnan Y. An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism. Nat Commun 2, 340 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Halder S. & Krishnan Y. Design of ultrasensitive DNA-based fluorescent pH sensitive nanodevices. Nanoscale 7, 10008–10012 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Gough PJ & Gordon S. The role of scavenger receptors in the innate immune system. Microbes Infect 2, 305–311 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Bhatia D, Surana S, Chakraborty S, Koushika SP & Krishnan Y. A synthetic icosahedral DNA-based host-cargo complex for functional in vivo imaging. Nat Commun 2, 339 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Krishnan Y. & Simmel FC Nucleic acid based molecular devices. Angew Chem Int Ed Engl 50, 3124–3156 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Veetil AT et al. Cell-targetable DNA nanocapsules for spatiotemporal release of caged bioactive small molecules. Nat Nanotechnol 12, 1183–1189 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Surana S, Bhatia D. & Krishnan Y. A method to study in vivo stability of DNA nanostructures. Methods 64, 94–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuchman EH & Desnick RJ Types A and B Niemann-Pick disease. Mol GenetMetab 120, 27–33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanier MT & Millat G. Niemann-Pick disease type C. Clin Genet 64, 269–281 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Desnick RJ & Schuchman EH Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet 3, 954–966 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Ory DS et al. Intrathecal 2-hydroxypropyl-P-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. The Lancet 390, 1758–1768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson DE, Ostrowski P, Jaumouillé V. & Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212, 677–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornhuber J. et al. Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J Med Chem 51, 219–237 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Cenedella RJ Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids 44, 477–487 (2009). [DOI] [PubMed] [Google Scholar]
- 46.He X. et al. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 1432, 251–264 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Devany J, Chakraborty K. & Krishnan Y. Subcellular nanorheology reveals lysosomal viscosity as a reporter for lysosomal storage diseases. Nano Lett 18, 1351–1359 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Aue WP, Bartholdi E. & Ernst RR Two-dimensional spectroscopy. Application to nuclear magnetic resonance. J Chem Phys 64, 2229–2246 (1976). [Google Scholar]
- 49.Gygi SP, Corthals GL, Zhang Y, Rochon Y. & Aebersold R. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc Natl Acad Sci U SA 97, 9390–9395 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakraborty K, Veetil AT, Jaffrey SR & Krishnan Y. Nucleic Acid-Based Nanodevices in Biological Imaging. Annu Rev Biochem 85, 349–373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.