Abstract
Background
Platelets with high hemostatic activity play an important role in the pathophysiology of acute coronary syndrome (ACS), and mean platelet volume (MPV) has been proposed to be an indicator of platelet reactivity. We evaluated the predictive value of MPV and the responsive value of MPV with different antiplatelet agents in association with the clinical outcomes of ACS patients.
Methods
A total of 1094 patients with ACS and 472 patients without ACS were included. Blood samples were taken at hospital admission, at routine follow-up within one year, and beyond one year. The patients were divided into a "high MPV group" (> 9.0 fl, n = 305), "medium MPV group" (7.9-9.0 fl, n = 517), and "low MPV group" (< 7.9 fl, n = 272). The average follow-up time was 2.4 years, and the endpoints were major adverse cardiovascular events (MACEs) including all-cause mortality, time to recurrent ACS, target vessel re-intervention and stroke.
Results
MPV was significantly higher in the patients with ACS than in those without ACS (8.6 ± 1.1 vs. 8.4 ± 1.0 fl, p = 0.007). MPV decreased in the following year (8.38 ± 1.02 fl, p < 0.001) and also beyond one year (8.38 ± 0.94 fl, p < 0.001) after ACS events. The changes in MPV were not significantly different between the patients receiving either clopidogrel or ticagrelor. The high MPV group had more cardiovascular risk factors and more MACEs than the low MPV group (p = 0.017).
Conclusions
A higher MPV in patients with ACS was associated with more cardiovascular risk factors and more cardiovascular events during clinical follow-up.
Keywords: Acute coronary syndrome, ACS, Mean platelet volume, MPV
INTRODUCTION
Acute coronary syndrome (ACS) is a cluster of symptoms that arise due to decreased blood flow in the coronary arteries, which causes the heart muscle to become unable to function properly or to die.1,2 In the pathogenesis of ACS, rupture of an atherosclerotic plaque in an artery supplying the heart muscle is the most common cause of ACS, in which platelets play a major role.
Mean platelet volume (MPV) is a machine-calculated measurement of the average volume of platelets found in circulating blood, and is typically included in manual and automated hematologic blood tests as part of the complete blood count. MPV test results can be used to make inferences about issues of platelet destruction and production in bone marrow, such as in myeloproliferative disease and aplastic anemia. Previous studies have demonstrated that platelet volume is associated with hemostatic potential3 and also with markers of inflammation, including interleukin-6 and tumor necrosis factor-α.4 It is generally accepted that larger platelets are more active metabolically and enzymatically than smaller ones.5
An increase in MPV in patients with cardiovascular risk factors has been reported in numerous studies. For example, elevated MPV in adult non-dipper hypertensives has been shown to be part of low-grade inflammation, with a correlation between MPV and C-reactive protein (CRP).6 In patients with diabetes, MPV has been found to be greater in those with microangiopathy (i.e., retinopathy, microalbuminuria), inflammation, nephropathy, atherosclerotic vascular disease and heart failure.7,8 Furthermore, in patients with chronic kidney disease (CKD), MPV has been shown to be significantly increased with CKD progression, and it might be a useful indicator of an increased risk of coronary artery disease (CAD).9 Patients with metabolic syndrome have also been reported to have an increased MPV.10 However, the correlation between smoking and MPV is controversial, and a study of young male subjects failed to reveal any association between smoking status and MPV.11 However, another study of 121 subjects exposed to long-term smoking suggested the positive effects of smoking cessation on platelet size.12
Several studies have also suggested that MPV may be associated with thrombosis formation. In patients with antiphospholipid syndrome, MPV has been shown to be a prognostic factor of thrombosis recurrence.13 In patients with atrial fibrillation, MPV has been associated with the presence of markers of left atrial stasis.14 In cancer patients, MPV has been shown to be significantly elevated in patients with deep vein thrombosis compared to the MPV level in patients without deep vein thrombosis.15
Antiplatelet therapy has also been shown to affect MPV level. Colkesen et al. reported that aspirin treatment did not affect MPV levels in patients with paroxysmal atrial fibrillation.16 However, a randomized controlled trial demonstrated that MPV was reduced in patients with acute ischemic stroke who used any antiplatelet medication.17 Aspirin resistance rates have been reported to be higher in patients with CAD and higher MPV values.18 Taken together, whether changes in MPV occur after the use of antiplatelet drugs remains uncertain. Furthermore, differences in the effects between conventional and newer adenosine diphosphate (ADP) receptor inhibitors such as clopidogrel and ticagrelor on MPV remain unknown. Therefore, in this study we analyzed data of a well-designed and detailed registration cohort comprising an ACS population with 2.4 ± 1.5 years of follow-up. The purpose of the present study was to analyze the predictive value of MPV and the responsive value of MPV with different antiplatelet agents in association with clinical outcomes. We hypothesized that inflammation may be associated with increased MPV during ACS, and that it may be associated with clinical outcomes.
METHODS
Study population
In this prospective hospital-based study, we enrolled a cohort of patients with ACS admitted to our hospital from September 15, 2012 to September 15, 2016. The study protocol was approved by the Institutional Ethical Committee (approval # A-ER-105-225). All patients diagnosed with unstable angina and myocardial infarction (MI) were included in the study. In addition to the ACS population, patients with non-obstructive coronary atherosclerosis confirmed by coronary angiography at our hospital were included as the non-ACS group. The exclusion criteria were either missing coronary angiography or MPV data.
Blood samples
Baseline blood samples were collected from all patients at the time of hospital admission or at the emergency room. Routine hematologic tests including hemoglobin, platelet count, MPV, and inflammation markers including white blood count (WBC) with differential and CRP were recorded. Other relative risk data including lipid profile, creatinine, and HbA1C were also recorded. A Coulter LH 750 Analyzer (Beckman-Coulter, Brea, CA, USA) was used to measures MPV.
Dual antiplatelet therapy for one year was recommended for patients with ACS, as previously described in the ACC/AHA guidelines.1 To compare the patients’ MPV values when receiving dual antiplatelet drugs or single antiplatelet drugs, MPV data were recorded during dual antiplatelet therapy (within one year) or single antiplatelet therapy (beyond one year) after ACS events.
Endpoints
To determine whether patients with a higher MPV were more prone to thrombus formation, TIMI flow data were recorded during percutaneous coronary interventions (PCIs). In addition, patients who received tirofiban for a large thrombus burden during PCI were also recorded.
During long-term follow-up, all ACS cases were divided into three groups according to MPV data. The "low MPV group" was defined as the patients with the lowest 25% of data, and the "high MPV group" was defined as those with the highest 25% of data. The other patients were classified into the "medium MPV group". The long-term primary endpoints were defined as major adverse cardiovascular events (MACEs), including all-cause mortality, time to recurrent ACS, target vessel re-intervention and stroke according to electrical medical records. All cases were followed until June 2017.
Statistical analysis
SPSS software version 21.0 (IBM, Armonk, NY, USA) was used for the statistical analysis. Continuous data were presented as mean ± standard deviation or as median (interquartile range), depending on the distribution. Dichotomous data were presented as numbers and percentages. Comparisons were conducted using the Student’s t-test or the Mann-Whitney U test for continuous variables which showed normal and non-parametric distribution, respectively. A chi-square test or Fisher’s exact test was used for categorical variables where appropriate. To compare the three MPV groups, ANOVA with linear trend was used for continuous variables, and linear-to-linear associations were used for categorical variables. A multivariate linear regression model was used to analyze correlations between continuous variables and MPV, and variables with p < 0.1 in bivariate linear regression were included. MACE-free curves were derived using the Kaplan-Meier method, and p values were calculated using the log-rank test. A Cox regression model was used to analyze relationships between variables and MACEs. Variables with p < 0.1 at baseline in the three groups were included in a simple regression model, and variables with p < 0.1 in simple regression were included in multiple regression analysis. A p value of < 0.05 was considered to be statistically significant.
RESULTS
A total of 1094 patients with ACS (average age 69 ± 13 years, males 71.1%) and 472 non-ACS patients (average age 62 ± 13 years, males 50.4%) were screened and enrolled (Figure 1). MPV was significantly higher in the MI group than in the non-ACS group (8.6 ± 1.1 vs. 8.4 ± 1.0 fl, p = 0.002) (Table 1). The subjects with a higher MPV were associated with a higher WBC (coefficient = 0.281, p < 0.001), higher HbA1C (coefficient = 0.144, p = 0.039), and lower platelet count (coefficient = -0.365, p < 0.001).
Figure 1.
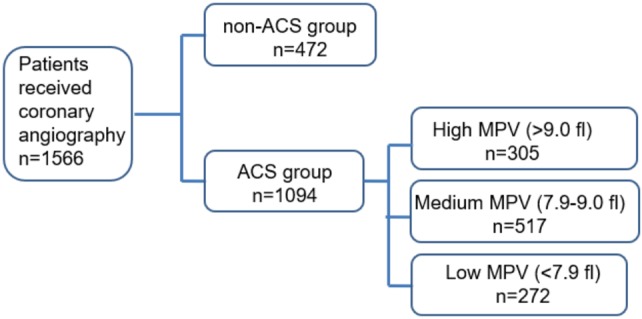
Consort flow diaphragm of the study.
Table 1. Clinical parameters of different study subgroups.
| Non-ACS (n = 472) | Whole ACS (N = 1094) | p value | MI (n = 784) | p value* | UA (n = 310) | p value# | |
| Gender (male) | 238 (50.4) | 778 (71.1) | < 0.001 | 567 (72.3) | < 0.001 | 211 (68.1) | < 0.001 |
| Age (y/o) | 62 ± 13 | 69 ± 13 | < 0.001 | 69 ± 14 | < 0.001 | 69 ± 12 | < 0.001 |
| BMI (Kg/m2) | N/A | 25 ± 4 | N/A | 25 ± 4 | N/A | 25 ± 4 | N/A |
| Smoking | N/A | 314 (28.7) | N/A | 252 (32.1) | N/A | 62 (20.0) | N/A |
| DM | 134 (28.4) | 562 (51.4) | < 0.001 | 395 (50.4) | < 0.001 | 167 (53.9) | < 0.001 |
| HTN | 248 (52.5) | 747 (68.3) | < 0.001 | 518 (66.1) | < 0.001 | 229 (73.9) | < 0.001 |
| DL | 162 (34.3) | 584 (53.4) | < 0.001 | 397 (50.6) | < 0.001 | 187 (60.3) | < 0.001 |
| CKD | 74 (15.7) | 458 (41.9) | < 0.001 | 349 (44.5) | < 0.001 | 109 (35.2) | < 0.001 |
| WBC (103/uL) | 7.5 ± 2.9 | 10.5 ± 4.8 | < 0.001 | 11.4 ± 5.1 | < 0.001 | 8.3 ± 3.1 | 0.001 |
| Hb (106/uL) | 13.2 ± 1.8 | 12.8 ± 2.5 | < 0.001 | 12.9 ± 2.6 | 0.005 | 12.5 ± 2.1 | < 0.001 |
| Plt (103/uL) | 219 ± 63 | 217 ± 75 | 0.502 | 218 ± 77 | 0.748 | 214 ± 69 | 0.265 |
| MPV (fL) | 8.4 ± 1.0 | 8.6 ± 1.1 | 0.007 | 8.6 ± 1.1 | 0.002 | 8.5 ± 1.1 | 0.283 |
| Cr (mg/dL) | 1.17 ± 1.33 | 2.13 ± 2.50 | < 0.001 | 2.20 ± 2.59 | < 0.001 | 1.97 ± 2.24 | < 0.001 |
| LDL (mg/dL) | 51 ± 17 | 112 ± 41 | < 0.001 | 113 ± 43 | < 0.001 | 109 ± 38 | < 0.001 |
| HbA1C (%) | 6.2 ± 1.3 | 6.7 ± 1.6 | < 0.001 | 6.7 ± 1.6 | 0.001 | 6.8 ± 1.6 | 0.001 |
Data are presented as mean ± SD or N (%). * Compare non-ACS group and MI group. # Compare non-ACS group and UA group.
ACS, acute coronary syndrome; BMI, body mass index; CKD, chronic kidney disease; Cr, creatinine; DL, dyslipidemia; DM, diabetes mellitus; Hb, hemoglobin; HTN, hypertension; LDL, low-density lipoprotein; MI, myocardial infarction; MPV, mean platelet volume; Plt, platelet; SD, standard deviation; UA, unstable angina; WBC, white blood cell.
To determine whether dual antiplatelet therapy within the first 12 months of treatment after an ACS event affected the MPV level, we analyzed 500 patients who received MPV follow-up based on the follow-up period, and found that the MPV decreased within one year (8.38 ± 1.02 fl, n = 376, p < 0.001) and also beyond one year (8.38 ± 0.94 fl, n = 237, p < 0.001) after an ACS event (Figure 2). Of these 500 patients, 435 received clopidogrel and 56 received ticagrelor. The changes in MPV were not significantly different between these patients.
Figure 2.
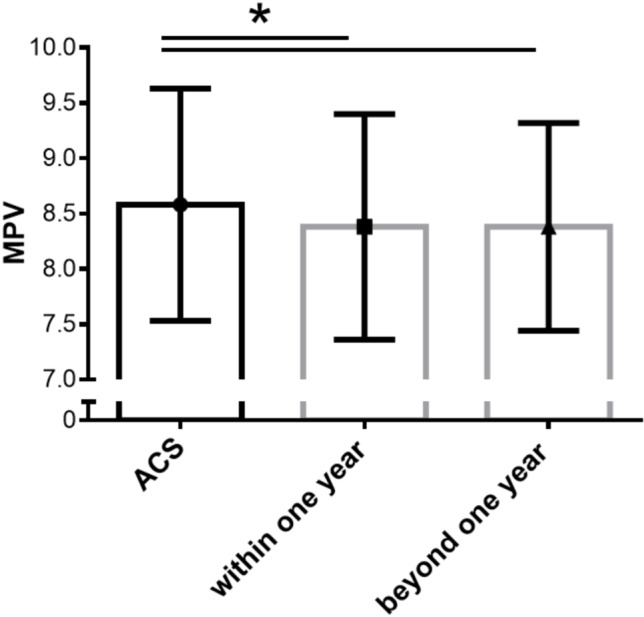
The changes in MPV after ACS event. MPV decrease after ACS event (8.58 ± 1.05 fl) within one year (8.38 ± 1.02 fl, p < 0.001, n = 376) and beyond one year (8.38 ± 0.94 fl, p < 0.001, n = 237). ACS, acute coronary syndrome; MPV, mean platelet volume.
However, in association analysis, the patients with TIMI 0 coronary flow did not have a higher MPV value (8.5 ± 1.1 vs. 8.6 ± 1.0 fl, p = 0.111). The patients who received tirofiban probably due to a larger thrombus burden during PCI also did not have a higher MPV value (8.7 ± 1.3 vs. 8.6 ± 1.1 fl, p = 0.63).
To support our hypothesis that the value of MPV plays a pathological role in cardiovascular outcomes, we further divided the patients into a high MPV group (highest 25%, MPV > 9.0 fl; n = 305), medium MPV group (MPV: 7.9-9.0 fl; n = 517), and low MPV group (lowest 25%, MPV < 7.9 fl; n = 272) (Figure 1). In baseline characteristics, the high MPV group had more cardiovascular risk factors, including age, diabetes mellitus, hypertension, old stroke, and also CKD than the low MPV group (Table 2).
Table 2. Baseline characteristics of different groups based on mean platelet volume values.
| High MPV (> 9.0 fl) (n = 305) | Medium MPV (7.9-9.0 fl) (n = 517) | Low MPV (< 7.9 fl) (n = 272) | p value | |
| Age (y/o) | 70 ± 14 | 69 ± 13 | 68 ± 13 | 0.025 |
| Sex (male) | 212 (69.5) | 370 (71.6) | 196 (72.1) | 0.493 |
| Smoking | 89 (29.2) | 145 (28.0) | 80 (29.4) | 0.997 |
| DM | 182 (59.7) | 263 (50.9) | 117 (43) | < 0.001 |
| HTN | 224 (73.4) | 353 (68.3) | 170 (62.5) | 0.005 |
| Old stroke | 39 (12.8) | 67 (13.0) | 16 (5.9) | 0.011 |
| DL | 158 (51.8) | 278 (53.8) | 148 (54.4) | 0.518 |
| CKD | 150 (49.2) | 220 (42.6) | 88 (32.4) | < 0.001 |
| Cr (mg/dL) | 2.18 ± 2.35 | 2.17 ± 2.57 | 2.02 ± 2.51 | 0.447 |
| HbA1C (%) | 7.0 ± 1.7 | 6.7 ± 1.7 | 6.4 ± 1.3 | < 0.001 |
| LDL (mg/dL) | 111 ± 43 | 111 ± 42 | 114 ± 38 | 0.389 |
| CRP (mg/L) | 68.5 ± 68.9 | 58.9 ± 71.7 | 46.2 ± 53.2 | 0.062 |
| ACS | 0.421 | |||
| STEMI | 83 (27.2) | 136 (26.3) | 78 (28.7) | |
| NSTEMI | 143 (46.9) | 238 (46.0) | 106 (39) | |
| UA | 79 (25.9) | 143 (27.7) | 88 (32.4) | |
| P2Y12 inhibitor | 0.409 | |||
| Clopidogrel | 265 (86.9) | 405 (78.3) | 226 (83.1) | |
| Ticagrelor | 29 (9.5) | 41 (7.9) | 36 (13.2) | |
| ACEi/ARB use | 182 (59.7) | 259 (50.1) | 181 (66.5) | 0.135 |
| Beta blocker use | 174 (57.0) | 266 (51.5) | 164 (60.3) | 0.491 |
| Statin use | 186 (61.0) | 299 (57.8) | 188 (69.1) | 0.056 |
Data are presented as mean ± SD or N (%).
ACEi/ARB, angiotensin-converting-enzyme inhibitor/angiotensin II receptor blockers; ACS, acute coronary syndrome; CKD, chronic kidney disease; Cr, creatinine; CRP, C-reactive protein; DL, dyslipidemia; DM, diabetes mellitus; HTN, hypertension; LDL, low-density lipoprotein; MPV, mean platelet volume; NSTEMI, non-ST elevation myocardial infarction; SD, standard deviation; STEMI, ST elevation myocardial infarction; UA, unstable angina.
The follow-up time was 2.4 ± 1.5 years. MACEs occurred in 36.7% (112/305) of the patients in the high MPV group, 31.7% (164/517) of the patients in the medium MPV group, and 27.9% (76/272) of the patients in the low MPV group (p = 0.024) (Table 3). In outcome Kaplan-Meier curves, the high MPV group had a significantly higher risk of MACEs than the low MPV group (p = 0.017) (Figure 3). After adjusting for variables, multivariate regression analysis indicated that the independent factors for MACEs were MPV > 9.0 (fl), creatinine, age, and low-density lipoprotein (Table 4).
Table 3. Major adverse cardiovascular events in different mean platelet volume groups.
| High MPV (> 9.0 fl) (n = 305) | Medium MPV (7.9-9.0 fl) (n = 517) | Low MPV (< 7.9 fl) (n = 272) | p value* | |
| Total MACE | 112 (36.7) | 164 (31.7) | 76 (27.9) | 0.024 |
| All-cause mortality | 54 (17.7) | 56 (10.8) | 33 (12.1) | 0.039 |
| Target vessel revascularization | 33 (10.8) | 61 (11.8) | 22 (8.1) | 0.31 |
| Recurrent ACS | 58 (19.0) | 107 (20.7) | 43 (15.8) | 0.354 |
| Stroke | 5 (1.6) | 1 (0.2) | 4 (1.5) | 0.76 |
Data are presented as N (%).
* p value for trend was analyzed by linear-by-linear association.
ACS, acute coronary syndrome; MACE, major adverse cardiovascular events; MPV, mean platelet volume.
Figure 3.
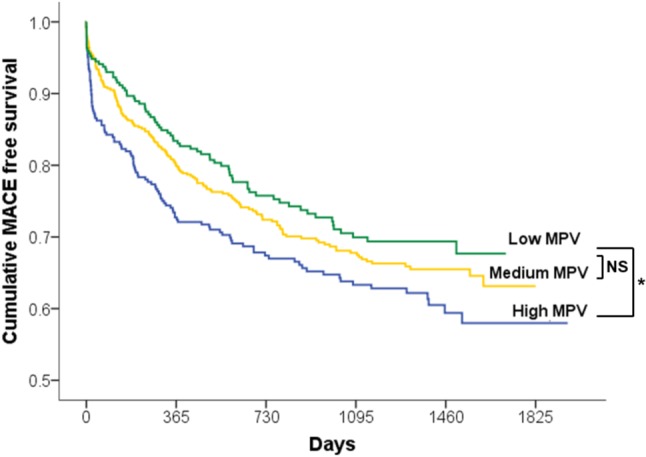
Kaplan-Meier curve of cumulative MACE-free survival. Compare with “low MPV group”, “high MPV group” had higher risk of MACE with 2.4 ± 1.5 year follow up (* p = 0.017); however, medium MPV group was not statistically higher (p = 0.288).
Table 4. Multivariable predictors of major adverse cardiovascular events.
| Hazard ratio (95% CI) | p value | |
| MPV > 9.0 (fl) | 1.31 (1.03-1.68) | 0.030 |
| Cr (mg/dL) | 1.15 (1.11-1.19) | < 0.001 |
| Age | 1.01 (1.00-1.02) | 0.005 |
| LDL (mg/dL) | 0.99 (0.99-1.00) | < 0.001 |
| DM | 1.18 (0.93-1.50) | 0.177 |
| Smoking | 0.92 (0.69-1.23) | 0.567 |
| Sex (male) | 1.00 (0.76-1.30) | 0.982 |
Note: Whole ACS patients were included to analysis (n = 1094). Cr, age, and LDL were continuous variables. Others were categorical variables.
Cr, creatinine; CI, confidence interval; DM, diabetes mellitus; LDL, low-density lipoprotein; MPV, mean platelet volume.
DISCUSSION
The results of the present study suggest that MPV may indicate an unstable and activated platelet status. In the analysis of data from this cohort study, differences in MPV were found between the non-ACS and ACS groups, and patients with MI had the highest MPV. This fit our initial hypothesis that MPV may be associated with the stability of coronary plaque, and especially with plaque rupture as in ACS events. MPV probably acts as a marker of platelet status, and can be influenced by common cardiovascular risk factors.
In our previous study, we showed that inflammatory marker secretory type II phospholipase A2 responded rapidly to inflammatory activity in atherosclerotic arteries and plaque rupture.19 Gasparyan et al. suggested that MPV is a reflection of both pro-inflammatory and pro-thrombotic conditions, where thrombopoietin and numerous inflammatory cytokines including interlukin-1, interlukin-6, and tumor necrosis factor-α, regulate thrombopoiesis.20 In the current study, we showed that higher MPV was associated with higher WBC, indicating an enhanced response of inflammatory status. We hypothesize that a high MPV may reflect high inflammatory status, and may be associated with the functional change of platelets or the replication rate of platelet production. Further studies should be conducted to investigate other inflammatory factors and their associations with platelet volume. Another recent study also supported our findings and reported that patients with MI with systemic inflammatory response syndrome were associated with an increased risk of one-year all-cause mortality.21
In the present study, data were driven mainly from the treatment-associated MPV values, regardless of the treatment or timing of the ACS events. From a pathophysiologic point of view, a higher MPV may cause more thrombus formation, but not the occurrence or initiation of ACS events. A previous study suggested that MPV is a strong independent predictor of impaired angiographic reperfusion.22 To understand the impact of treatment strategy, we analyzed the baseline TIMI 0 flow andtirofibanuse during PCI which also indicated a larger thrombus burden, but no significant difference in MPV was noted. The pathology of impaired angiographic reperfusion is believed to be multifactorial, which may be the reason for the insignificant result.
A previous study reported that aspirin treatment did not affect MPV levels in patients with paroxysmal atrial fibrillation.16 We compared differences between patients who received conventional or newer ADP receptor inhibitors after ACS events, and our data demonstrated a non-significant difference in MPV changes between groups. We also compared the patients’ MPVs when they received dual antiplatelet drug within one year or single antiplatelet drug use beyond one year according to the current guidelines, and found that MPV was not affected by the number of antiplatelet drugs. In fact, our results indicated that MPV was not affected by antiplatelet drugs. This was probably due to the limited number of cases who used newer antiplatelet agents. Further studies are needed to verify the differences using more real-world data.
Interestingly, we found that MPV decreased after an ACS event either within one year or beyond one year (Figure 2). MPV levels in the MI group were also higher than in the non-ACS group. Poulter et al. reported that actin nodules are podosome-like structures in platelets during early spreading.23 Additionally, Klages et al. reported that G12/G13-mediated Rho/Rho-kinase dependent regulation of myosin light chain phosphorylation participated in changes in platelet shape.24 Further research is needed to determine whether or not the podosome-like structural change of platelets or rho kinase inhibitors change MPV.
Martin et al. showed that MPV was an independent risk factor for further ischemic events, including recurrent MI.25 A recent study also showed that increased MPV was associated with heart failure-related hospitalization in patients with a reduced ejection fraction.26 In agreement with that study, our data demonstrated that the high MPV group had more MACEs than the low MPV group. The high MPV group also had more cardiovascular risk factors, especially diabetes mellitus and CKD. This fit our initial hypothesis that MPV may be a link between cardiovascular risk factors and cardiovascular outcomes.
This study has several limitations. First, the blood samples were collected from patients in the emergency room at variable time points after ACS. MPV might show different changes with time, and these changes may have influenced our study results. Second, based on the results of this cohort study, some selection bias may have occurred because all of the patients received coronary angiography. We supposed that patients who needed coronary angiography would have had more cardiovascular risk factors. Therefore, differences between the ACS and non-ACS groups would be reduced correspondingly. Third, patients without MPV tests during follow-up may have led to some bias. Fourth, this was a single-center study using one type of automated analyzer. Further studies should be designed to determine the relationship between MPV and resistance to newer ADP receptor inhibitors, which were not analyzed in the present study.
CONCLUSIONS
We found that MPV was increased in patients presenting with ACS, regardless of the antiplatelet therapy strategy. A higher MPV was associated with more cardiovascular risk factors and more cardiovascular events. MPV may be a link between cardiovascular risk factors and cardiovascular outcomes.
Acknowledgments
The study was granted by NCKUH-10603060 from National Cheng Kung University Hospital, Tainan, Taiwan.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Damman P, van’t Hof AW, Ten Berg JM, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: comments from the Dutch ACS working group. Neth Heart J. 2017;25:181–185. doi: 10.1007/s12471-016-0939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson CB, Jakubowski JA. The pathophysiology and clinical relevance of platelet heterogeneity. Blood. 1988;72:1–8. [PubMed] [Google Scholar]
- 4.Kaushansky K. The molecular mechanisms that control throm-bopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbmayer WM, Haushofer A, Radek J, et al. Platelet size, fibrinogen and lipoprotein(a) in coronary heart disease. Coron Artery Dis. 1995;6:397–402. doi: 10.1097/00019501-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209:278–282. doi: 10.1016/j.atherosclerosis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Bavbek N, Kargili A, Kaftan O, et al. Elevated concentrations of soluble adhesion molecules and large platelets in diabetic patients: are they markers of vascular disease and diabetic nephropathy? Clin Appl Thromb Hemost. 2007;13:391–397. doi: 10.1177/1076029607303615. [DOI] [PubMed] [Google Scholar]
- 8.Tavil Y, Sen N, Yazici H, et al. Coronary heart disease is associated with mean platelet volume in type 2 diabetic patients. Platelets. 2010;21:368–372. doi: 10.3109/09537101003628421. [DOI] [PubMed] [Google Scholar]
- 9.Ju HY, Kim JK, Hur SM, et al. Could mean platelet volume be a promising biomarker of progression of chronic kidney disease? Platelets. 2015;26:143–147. doi: 10.3109/09537104.2014.890179. [DOI] [PubMed] [Google Scholar]
- 10.Nechita A, Delcea C, Enache V, et al. Metabolic syndrome and mean platelet volume variation in patients with chest pain and negative cardiac enzymes. J Med Life. 2013;6:156–160. [PMC free article] [PubMed] [Google Scholar]
- 11.Arslan E, Yakar T, Yavasoglu I. The effect of smoking on mean platelet volume and lipid profile in young male subjects. Anadolu Kardiyol Derg. 2008;8:422–425. [PubMed] [Google Scholar]
- 12.Terres W, Becker P, Rosenberg A. Changes in cardiovascular risk profile during the cessation of smoking. Am J Med. 1994;97:242–249. doi: 10.1016/0002-9343(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Rupa-Matysek J, Gil L, Wojtasinska E, et al. The relationship between mean platelet volume and thrombosis recurrence in patients diagnosed with antiphospholipid syndrome. Rheumatol Int. 2014;34:1599–1605. doi: 10.1007/s00296-014-2996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Providencia R, Faustino A, Paiva L, et al. Mean platelet volume is associated with the presence of left atrial stasis in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. 2013;13:40. doi: 10.1186/1471-2261-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilir C, Engin H, Bilir F. Increased mean platelet volume in deep vein thrombosis patients with cancer. J Hematol. 2013;2(2):64–68. [Google Scholar]
- 16.Colkesen Y, Coskun I, Muderrisoglu H. The effect of aspirin on mean platelet volume in patients with paroxysmal atrial fibrillation. Platelets. 2013;24:263–266. doi: 10.3109/09537104.2012.682106. [DOI] [PubMed] [Google Scholar]
- 17.Haungsaithong R, Udommongkol C, Nidhinandana S, et al. The changes in mean platelet volume after using of antiplatelet drugs in acute ischemic stroke: a randomized controlled trial. J Med Assoc Thai. 2015;98:852–857. [PubMed] [Google Scholar]
- 18.Koh YY, Kim HH, Choi DH, et al. Relation between the change in mean platelet volume and clopidogrel resistance in patients undergoing percutaneous coronary intervention. Curr Vasc Pharmacol. 2015;13:687–693. doi: 10.2174/1570161112666141017121118. [DOI] [PubMed] [Google Scholar]
- 19.Liu PY, Li YH, Tsai WC, et al. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J. 2003;24:1824–1832. doi: 10.1016/j.ehj.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 21.Huang WC, Chou RH, Chang CC, et al. Systemic inflammatory response syndrome is an independent predictor of one-year mortality in patients with acute myocardial infarction. Acta Cardiol Sin. 2017;33:477–485. doi: 10.6515/ACS20170603A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 23.Poulter NS, Pollitt AY, Davies A, et al. Platelet actin nodules are podosome-like structures dependent on Wiskott-Aldrich syndrome protein and ARP2/3 complex. Nat Commun. 2015;6:7254. doi: 10.1038/ncomms8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klages B, Brandt U, Simon MI, et al. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 26.Kaya H, Kutay Yildirimli M, Kurt R, et al. Mean platelet volume as a predictor of heart failure-related hospitalizations in stable heart failure outpatients with sinus rhythm. Acta Cardiol Sin. 2017;33:292–300. doi: 10.6515/ACS20160930A. [DOI] [PMC free article] [PubMed] [Google Scholar]


