Abstract
Background
It is unclear whether renal denervation (RDN) can safely result in blood pressure (BP) reductions in Asian hypertensive patients and whether such reductions would be sustainable. The study is to assess the safety and efficacy of RDN achieved by either main renal artery ablation using the Symplicity FlexTM catheter or main plus branch renal artery ablations using the Symplicity SpyralTM catheter in Taiwanese uncontrolled hypertensive patients enrolled in the Global SYMPLICITY Registry (GSR) with 3 years of follow-up.
Methods
The GSR is a prospective, open-label, and all-comer registry to evaluate the safety and effectiveness of RDN in patients with uncontrolled hypertension worldwide.
Results
Among 26 patients enrolled (mean age, 59.1 ± 13.8 years), 8 were treated with the Symplicity FlexTM catheter, and 18 were treated with the Symplicity SpyralTM catheter. Baseline office systolic BP was 168.2 ± 19.8 mmHg and diastolic BP was 89.0 ± 14.3 mmHg. Office BP reductions following RDN were sustained throughout the follow-up periods of up to 3 years in the Symplicity FlexTM group and 2 years in the Symplicity SpyralTM group. In the Symplicity FlexTM group, the office systolic BP reductions were 14.9 ± 14.7 mmHg and 29.7 ± 25.9 mmHg at 3 months and 3 years, respectively (both p < 0.05 from baseline). In the Symplicity SpyralTM group, the office systolic BP reductions were 21.2 ± 28.7 mmHg and 42.4 ± 10.7 mmHg at 3 months and 2 years, respectively (both p < 0.05 from baseline). There were no significant changes in heart rate or antihypertensive medication classes. Three protocol-defined adverse events occurred in 2 patients, including new-onset end-stage renal disease, stroke, and hospitalization for new-onset heart failure.
Conclusions
Given the susceptibility of Asian populations to hypertension, RDN, as a safe antihypertensive procedure with long-lasting BP-lowering effects, could reliably serve as an alternative or complementary BP-lowering strategy for patients with uncontrolled hypertension in Taiwan and other Asian countries.
Keywords: Blood pressure, Catheter ablation, Hypertension, Nerve, Renal artery
INTRODUCTION
Sympathetic overactivity plays an important role in the pathogenesis of hypertension and associated comorbidities. The role of sympathetic overactivity in driving sustained hypertension may be due to its effects on renal function, whereas sympathetic effects on systemic hemodynamics are mostly responsible for transient blood pressure (BP) elevation.1,2 Catheter-based renal denervation (RDN) is designed to disrupt renal afferent and efferent sympathetic nerves, which modulate central sympathetic outflow and renal physiology, in patients with uncontrolled hypertension.3
Despite the neutral efficacy results in the sham-controlled SYMPLICITY HTN-3 trial, in which main renal artery ablation was performed using the Symplicity FlexTM catheter,4 three carefully designed, randomized, sham-controlled RDN trials (SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE-HTN SOLO) have been published since 2017, with the SPYRAL HTN studies collecting plasma and urine samples to ensure medication adherence and employing next-generation catheters capable of four-quadrant energy delivery.5-7 All three feasibility trials showed similar and clinically meaningful BP reductions [approximately 10 mmHg in office systolic BP (SBP) and 6-9 mmHg in 24-hour ambulatory SBP] at 2 to 6 months in patients with mild to moderate or uncontrolled hypertension, irrespective of whether radiofrequency energy with dedicated branch artery ablation or ultrasound energy was applied. In the SYMPLICITY HTN-3 trial, which enrolled patients with resistant hypertension (uncontrolled hypertension while receiving maximally tolerated doses of ≥ 3 antihypertensive medications), the reductions in office SBP and ambulatory SBP (14.1 mmHg and 6.8 mmHg, respectively) at 6 months in patients in the RDN group were also comparable to the three recent RDN trials.4 Even though non-standardized medication adjustment may have caused marked BP reductions in the sham group and confounded the effectiveness assessment of RDN, a recent meta-analysis of radiofrequency ablation-based RDN trials with < 10% unplanned changes in antihypertensive medications during the follow-up periods showed consistent and statistically significant office and ambulatory BP reductions in the RDN group compared to the control group across all RDN trials, including SYMPLICITY HTN-Japan.8,9 Another recent meta-analysis of sham-controlled RDN trials also showed a BP-lowering benefit of RDN in first- and second-generation trials.10
According to the long-term follow-up results from the SYMPLICITY HTN-Japan and Korean data from the Global SYMPLICITY Registry (GSR), office BP reductions following RDN (25-30 mmHg for SBP and 10-15 mmHg for diastolic BP (DBP) at 12 to 36 months) were similar between patients with uncontrolled hypertension in these two Asian countries.11,12 The reductions in office BP at 12 months in the Korean uncontrolled hypertensive patients were more pronounced than those in Caucasian uncontrolled hypertensive patients in the GSR.11 However, only main renal artery ablations were conducted in both SYMPLICITY-Japan and GSR-Korea, and it is still not certain whether RDN achieved by both main and branch renal artery ablations can result in similar BP reductions in Asian hypertensive patients and whether those reductions would be sustainable over longer time periods.
Accumulating evidence suggests that RDN is associated with a < 1% rate of vascular access site complications and renal artery injury, as well as no excess in risks of renal dysfunction.13 However, data regarding the long-term safety, particularly with RDN achieved by main and branch renal artery ablations, from real-world registries in Asian countries are lacking. Therefore, the aim of this study was to assess the safety and efficacy of RDN, achieved by either main renal artery ablation or main plus branch renal artery ablations, in Taiwanese uncontrolled hypertensive patients enrolled in the GSR (GSR-Taiwan) with up to 3 years of follow-up.
METHODS
Study design
The Global SYMPLICITY Registry (GSR) is a prospective, open-label, single-arm, all-comer registry with approximately 3000 patients enrolled globally, including from Taiwan, to evaluate the safety and effectiveness of treatment with the Symplicity RDN system (Medtronic, Santa Rosa, CA, USA) in patients with uncontrolled hypertension and/or conditions associated with sympathetic nervous system activation in real-world clinical settings.14,15 National regulatory authorities and institutional review boards/ethics committees of the participating centers approved the registry, and informed consent was obtained from all patients. The GSR is registered at ClinicalTrials.gov (NCT01534299).
The GSR includes the Taiwan registry subgroup (GSR-Taiwan), which enrolls patients with resistant hypertension defined as a SBP persistently > 160 mmHg (or ≥ 150 mmHg if they have type 2 diabetes mellitus) while receiving ≥ 3 antihypertensive medications without changes for 2 weeks before enrollment. The exclusion criteria included prior renal artery intervention, main renal arteries < 4 mm in diameter or < 20 mm in length and hemodynamically or anatomically significant renal artery abnormalities, such as renal artery stenosis. The baseline estimated glomerular filtration rate (eGFR) was recommended to be > 45 ml/min/1.73 m2, as calculated by the Modification of Diet in Renal Disease formula. Patients with type 1 diabetes mellitus, stenotic valvular heart disease, myocardial infarction, unstable angina or cerebrovascular accident within 6 months of enrollment were also excluded, as were individuals with untreated secondary hypertension.
Before treatment and at every follow-up visit, investigators interviewed the patients and documented any changes to antihypertensive medications. The GSR, including GSR-Taiwan, recommends three BP and heart rate measurements at each visit, with the patient sitting quietly for at least 5 minutes prior to the first measurement and at least 1 minute between each measurement, according to standard practice guidelines.16,17 Ambulatory BP monitoring is not mandated in GSR-Taiwan.
Procedures
RDN was performed using the SymplicityTM generator and either the mono-electrode Symplicity FlexTM radiofrequency catheter or the Symplicity SpyralTM multielectrode catheter. When using the Symplicity FlexTM catheter, individual ablations were applied for 120 s each, with at least 4 to 6 ablations planned for each main renal artery in a helical pattern.4 When using the Symplicity SpyralTM catheter (Medtronic, Galway, Ireland), a standardized approach to target all accessible renal arterial vessels, including branch arteries and accessory arteries with a diameter greater than 3 mm and less than 8 mm, was conducted.5 The four electrodes on the Symplicity SpyralTM catheter were positioned to apply radiofrequency energy in all four quadrants of the main renal artery and branch/accessory vessels. Denervation time was defined as the interval between RDN catheter insertion and guide catheter removal. Procedure time was defined as the interval between arterial access site puncture to discharge from the catheterization laboratory.
Statistical analysis
Continuous data were reported as mean ± standard deviation, and between-group differences were tested using the Wilcoxon-Mann-Whitney test. Within-group differences in continuous variables from baseline to follow-up were tested using paired t-tests. Categorical data were presented as frequencies or percentages and compared using the χ2 or Fisher’s exact test, as appropriate. Changes in the number of medication class between baseline and 3 years were compared using McNemar’s test. Analyses were performed on the basis of the intention-to-treat principle. Missing data were not imputed. A two-tailed p value < 0.05 was considered to be statistically significant. All analyses were performed using the SAS statistical package (version 9.3, Cary, NC, USA).
RESULTS
Baseline characteristics and procedural data
At the time of this analysis, 26 patients had been enrolled in Taiwan. Of these patients, 8 were treated with the Symplicity FlexTM catheter, and 18 were treated with the Symplicity SpyralTM catheter. Overall, the baseline office SBP was 168.2 ± 19.8 mmHg and diastolic BP (DBP) was 89.0 ± 14.3 mmHg. The average body mass index at baseline was 30.3 ± 6.1 kg/m2, and the mean age was 59.1 ± 13.8 years. Eighteen (69.2%) of the 26 patients were male, 11.5% had a history of renal insufficiency (eGFR < 60 mL/min/1.73 m2), 19.2% had a smoking habit, and half had type 2 diabetes mellitus. During the RDN procedure, an average of 19.9 ± 7.7 total ablations were applied in the patients treated with the Symplicity FlexTM catheter (main renal artery ablations), compared to an average of 39.1 ± 18.2 ablations in the patients treated with the Symplicity SpyralTM catheter (main and branch renal artery ablations). In the Symplicity SpyralTM subgroup, we ablated an average of 3.5 branches of renal arteries per patient. The denervation time was significantly shorter in the patients treated with the Symplicity SpyralTM catheter, even though they received almost twice as many ablations. The average amount of contrast used was similar between both groups (Table 1).
Table 1. Baseline and procedural characteristics in the Global SYMPLICITY Registry-Taiwan.
| Characteristics | Symplicity FlexTM System (N = 8) | Symplicity SpyralTM System (N = 18) | p-value |
| Office systolic blood pressure, mmHg | 0.68 | ||
| Median (IQR) | 173.67 (152.50, 175.34) | 173.34 (163.67, 181.67) | |
| Office diastolic blood pressure, mmHg | 0.24 | ||
| Median (IQR) | 96.34 (83.17, 107.00) | 87.00 (75.00, 99.67) | |
| Heart rate (beats per minute) | 0.49 | ||
| Median (IQR) | 69.50 (65.34, 82.50) | 68.50 (63.00, 74.00) | |
| Age, years | 0.23 | ||
| Median (IQR) | 48.00 (43.50, 64.50) | 61.00 (54.00, 68.00) | |
| Male gender | 87.5% (7/8) | 61.1% (11/18) | 0.36 |
| Body mass index, kg/m2 | 0.17 | ||
| Median (IQR) | 32.20 (28.60, 34.90) | 28.70 (26.60, 31.00) | |
| Obesity (≥ 30 kg m-2) | 75.0% (6/8) | 33.3% (6/18) | 0.09 |
| Smoking/tobacco use | 25.0% (2/8) | 16.7% (3/18) | 0.63 |
| Diabetes mellitus type 2 | 50.0% (4/8) | 50.0% (9/18) | 1.00 |
| Renal insufficiency (eGFR < 60 ml/min/1.73 m2) | 12.5% (1/8) | 11.1% (2/18) | 1.00 |
| History of atrial fibrillation | 12.5% (1/8) | 5.6% (1/18) | 0.53 |
| Previous stroke | 0.0% (0/8) | 5.6% (1/18) | 1.00 |
| Previous myocardial infarction | 0.0% (0/8) | 5.6% (1/18) | 1.00 |
| Angina pectoris | 25.0% (2/8) | 5.6% (1/18) | 0.22 |
| Heart failure | 12.5% (1/8) | 11.1% (2/18) | 1.00 |
| Denervation procedure | |||
| Procedure time (min) | 0.13 | ||
| Median (IQR) | 144.50 (118.50, 161.50) | 107.50 (85.00, 158.00) | |
| Denervation time (min) | 0.02 | ||
| Median (IQR) | 103.50 (66.50, 113.00) | 58.00 (37.00, 76.00) | |
| Number of ablations | 0.01 | ||
| Median (IQR) | 19.50 (13.50, 25.50) | 40.50 (21.00, 54.00) | |
| Amount of contrast (ml) | 0.87 | ||
| Median (IQR) | 200.00 (160.00, 212.50) | 180.00 (100.00, 230.00) | |
| Hospital stay (day) | 0.2 | ||
| Median (IQR) | 2.50 (2.00, 3.50)0 | 2.00 (2.00, 2.00) |
eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Antihypertensive medications
An average of 4.6 ± 1.4 classes of antihypertensive medications were prescribed at baseline, which primarily included an angiotensin receptor blocker or angiotensin-converting enzyme inhibitor, a calcium channel blocker, a diuretic, and a beta-blocker (Table 2). There were no significant differences in the number of antihypertensive medication classes between baseline and 2 years (the FlexTM group) or 3 years (the SpyralTM group).
Table 2. Antihypertensive medications in patients enrolled in the Global SYMPLICITY Registry-Taiwan.
| Baseline | 6 Months | 12 Months | 24 Months | 36 Months | ||||||
| Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 18) | Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 17) | Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 16) | Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 9) | Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 0) | |
| Number of antihypertensive medication classes | 4.3 ± 1.3 | 4.8 ± 1.5 | 4.3 ± 1.5 | 4.3 ± 1.5 | 4.4 ± 1.4 | 4.7 ± 1.0 | 4.3 ± 1.5 | 5.0 ± 1.1 | 4.3 ± 1.5 | - |
| ACE inhibitors | 12.5% (1/8) | 5.6% (1/18) | 12.5% (1/8) | 0.0% (0/17) | 25.0% (2/8) | 6.3 % (1/16) | 25.0% (2/8) | 11.1% (1/9) | 25.0% (2/8) | - |
| Angiotensin receptor blockers | 75.0% (6/8) | 83.3% (15/18) | 62.5% (5/8) | 88.2% (15/17) | 75.0% (6/8) | 81.3% (13/16) | 62.5% (5/8) | 55.6% (5/9) | 62.5% (5/8) | - |
| Calcium channel blockers | 62.5% (5/8) | 83.3% (15/18) | 75.0% (6/8) | 76.5% (13/17) | 75.0% (6/8) | 93.8% (15/16) | 62.5% (5/8) | 88.9% (8/9) | 62.5% (5/8) | - |
| Diuretics | 62.5% (5/8) | 77.8% (14/18) | 62.5% (5/8) | 70.6% (12/17) | 62.5% (5/8) | 75.0% (12/16) | 62.5% (5/8) | 77.8% (7/9) | 62.5% (5/8) | - |
| Aldosterone antagonists | 37.5% (3/8) | 38.9% (7/18) | 37.5% (3/8) | 29.4% (5/17) | 37.5% (3/8) | 37.5% (6/16) | 37.5% (3/8) | 55.6% (5/9) | 37.5% (3/8) | - |
| Centrally acting sympatholytics | 0.0% (0/8) | 5.6% (1/18) | 12.5% (1/8) | 5.9% (1/17) | 0.0% (0/8) | 0.0% (0/16) | 0.0% (0/8) | 0.0% (0/9) | 0.0% (0/8) | - |
| Direct renin inhibitor | 0.0% (0/8) | 0.0% (0/18) | 0.0% (0/8) | 0.0% (0/17) | 0.0% (0/8) | 0.0% (0/16) | 0.0% (0/8) | 0.0% (0/9) | 0.0% (0/8) | - |
| β-blockers | 100.0% (8/8) | 72.2% (13/18) | 100.0% (8/8) | 64.7% (11/17) | 100.0% (8/8) | 81.3% (13/16) | 100.0% (8/8) | 88.9% (8/9) | 100.0% (8/8) | - |
| α-adrenergic blocker | 50.0% (4/8) | 77.8% (14/18) | 37.5% (3/8) | 58.8% (10/17) | 37.5% (3/8) | 56.3% (9/16) | 50.0% (4/8) | 66.7% (6/9) | 50.0% (4/8) | - |
| Direct-acting vasodilators | 25.0% (2/8) | 33.3% (6/18) | 25.0% (2/8) | 35.3% (6/17) | 25.0% (2/8) | 37.5% (6/16) | 25.0% (2/8) | 55.6% (5/9) | 25.0% (2/8) | - |
Values are presented as % or mean ± SD. ACE, angiotensin converting enzyme; SD, standard deviation.
Blood pressures and heart rate
The effectiveness of renal denervation on office SBP reductions were comparable in both groups. Office SBP reductions were sustained for up to 3 years in the Symplicity FlexTM group and up to 2 years in the Symplicity SpyralTM group (Figure 1). In the Symplicity FlexTM group, office SBP decreased by 14.9 ± 14.7 mmHg (n = 7, p = 0.037), 24.6 ± 12.2 mmHg (n = 6, p = 0.004), 28.4 ± 25.6 mmHg (n = 8, p = 0.017), 19.7 ± 28.8 mmHg (n = 6, p = 0.155) and 29.7 ± 25.9 mmHg (n = 8, p = 0.014) at 3 months, 6 months, 1 year, 2 years, and 3 years, respectively (Figure 1A). In the Symplicity SpyralTM group, office SBP decreased by 21.2 ± 28.7 mmHg (n = 15, p = 0.013), 31.2 ± 24.6 mmHg (n = 14, p < 0.001), 25.6 ± 28.5 mmHg (n = 15, p = 0.004) and 42.4 ± 10.7 mmHg (n = 6, p < 0.001) at 3 months, 6 months, 1 year, and 2 years, respectively (Figure 1A). Office DBP reductions were generally consistent with SBP reductions (Figure 1B). DBP reductions were sustained until the end of follow-up by 16.8 ± 17.6 mmHg (n = 8, p = 0.030) in the Symplicity FlexTM group at 3 years, and by 22.7 ± 16.1 mmHg (n = 6, p = 0.018) in the Symplicity SpyralTM group at 2 years. There were no significant changes in heart rate in both groups (Figure 1C).
Figure 1.
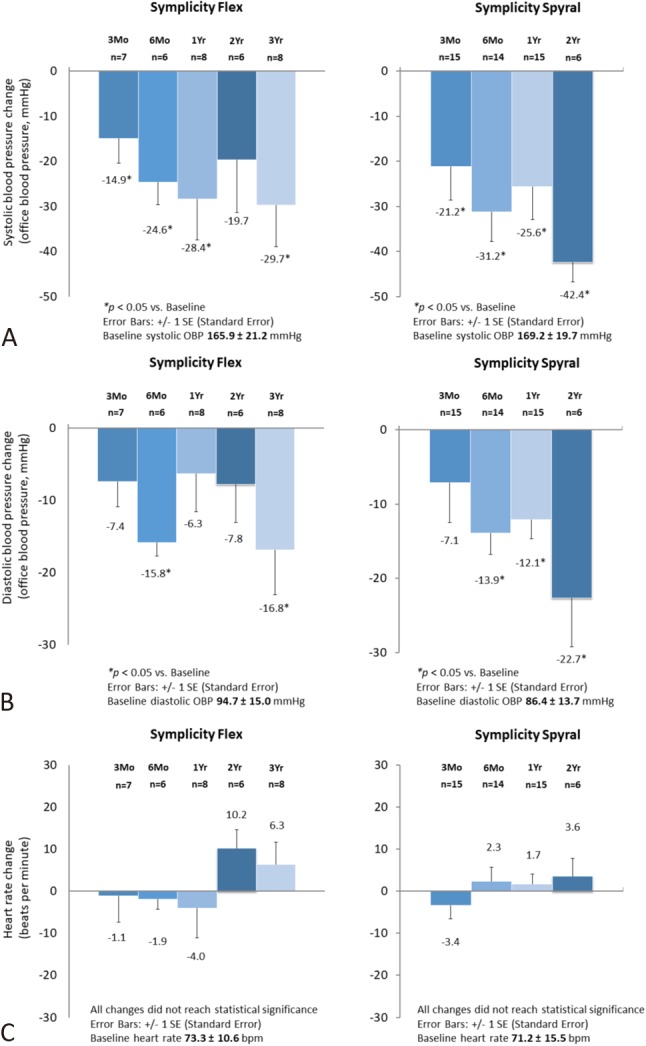
Changes in office systolic blood pressure (A), office diastolic blood pressure (B), and heart rate (C) in patients treated with the Symplicity FlexTM catheter and those with the Symplicity SpyralTM catheter.
Safety
Safety outcomes are shown in Table 3. One patient in the Symplicity FlexTM subgroup was hospitalized for new-onset heart failure, which occurred in the third year following RDN. One patient in the Symplicity SpyralTM group developed end-stage renal disease at 6 months, which was deemed not to be related to the procedure by the investigator, and subsequently suffered a stroke event in the second year following RDN. There were no deaths, strokes, myocardial infarctions, renal events, or procedure-related vascular complications in the remaining 24 patients.
Table 3. Safety outcomes of patients enrolled in the Global SYMPLICITY Registry-Taiwan.
| Symplicity FlexTM (N = 8) | Symplicity SpyralTM (N = 18) | |
| Death | 0 | 0 |
| Cardiovascular events | ||
| Cardiovascular death | 0 | 0 |
| Stroke | 0 | 1* |
| Hospitalization for new onset heart failure | 1 | 0 |
| Hospitalization for hypertensive crisis/emergency | 0 | 0 |
| Myocardial infarction | 0 | 0 |
| Renal events | ||
| New onset end stage renal disease | 0 | 1* |
| Procedure-related vascular complications | 0 | 0 |
* Events occurring in the same patient.
DISCUSSION
In the present analysis of GSR-Taiwan, the first real-world registry of RDN for patients with uncontrolled hypertension in Taiwan, we demonstrated that 1) office BP reductions were sustained throughout the follow-up periods (up to 3 years), 2) the office BP reductions were comparable between patients undergoing main renal artery ablations and those undergoing main and branch renal artery ablations, 3) the magnitude of office SBP reductions at 12 months was similar to that observed in GSR-Korea, and was numerically greater than that in Caucasians in the GSR,11 and 4) the short- and long-term safety profiles were consistent regardless of the device used or the number of individual lesions applied. Although the sample size is relatively small, the encouraging findings of GSR-Taiwan lend support to the clinical application of RDN, a legitimate antihypertensive strategy, for uncontrolled hypertension in Taiwan and other Asian countries.
The early BP-lowering effect following RDN observed in this study is noteworthy. In fact, the SYMPLICITY HTN-2 trial demonstrated significant BP reductions at 1 month following main renal artery ablations,18 despite the SYMPLICITY HTN-3 trial showing similar BP reductions in sham and treatment groups.4 With advances in RDN strategy, including branch and accessory renal artery ablations, the SPYRAL HTN-OFF MED study demonstrated significant BP reductions at 3 months.5 Likewise, in the present GSR-Taiwan analysis, the patients treated with the Symplicity SpyralTM catheter who underwent main and branch renal artery ablations had numerically greater office SBP reductions at 3 months compared to the patients treated with the Symplicity FlexTM catheter. Whether a more comprehensive renal artery ablation strategy can result in earlier BP reductions awaits further verification.
Another main finding of this study is that RDN achieved substantial reductions in office BP throughout the 2 to 3 years of follow-up in GSR-Taiwan. In addition, these reductions in SBP and DBP are greater than those observed in sham-controlled trials and similar to findings in the SYMPLICITY HTN-1,19 SYMPLICITY HTN-2, SYMPLICITY-Japan, and the GSR, despite differences in baseline characteristics among these trials. In addition to sustained BP reductions, it is of interest that SBP continued to decline throughout the follow-up period. This long-term decreasing trend in SBP following RDN has also been observed in Korean patients in the GSR and Japanese patients in the SYMPLICITY-Japan.11,12 However, for Caucasians in the GSR, the reductions in SBP remained stable between 6 months (-20.9 mmHg) and 12 months (-20.1 mmHg). In contrast, continuous declines in SBP after 1 year following RDN were documented in the SYMPLICITY HTN-1 and SYMPLICITY HTN-2 trials,19,20 which enrolled mainly Caucasian populations. Further investigations are needed to elucidate whether there are ethnic differences in the long-term BP-lowering response to RDN.
In the study design of GSR, the antihypertensive medications were adjusted at the discretion of the physicians. Although substantial BP reductions were achieved throughout the follow-up period, the BP achieved may just have reached the targets recommended by the hypertension management guidelines (130/80 mmHg or 140/90 mmHg) given the relatively high SBP (160 to 170 mmHg) at baseline.17,21 This may explain why there was no significant change in the number of antihypertensive medication classes. While no changes in the number of medications occurred from baseline to 2-3 years of follow-up, medication adherence was not measured, so the impact of medication adherence on these data cannot be determined. Based on the National Reimbursement Claims Database in Taiwan from 2001 to 2007, the medication adherence rate, defined as having medications refilled for ≥ 80% of days in the year after the initiation of antihypertensive treatment, was only 18.6%.22 In the DENERHTN trial,23 the prevalence of non-adherence to antihypertensive medications at 6 months was approximately 50%. Medication nonadherence might obscure the antihypertensive effect of any BP-lowering strategy, including RDN.
In this study, only one case developed end-stage renal disease within 12 months following RDN, and this case was judged by the investigator to be not related to the procedure. While there were no reported cases of new-onset renal artery stenosis in the 18 patients who underwent comprehensive branch renal artery ablations during the follow-up period of up to 2 years, this may have been underestimated since computed tomographic or magnetic resonance renal angiography was not required during follow-up in this study. Because of the limited sample size, some rare adverse events may have been overlooked. The 2-year safety profile of the patients undergoing main and branch renal artery ablations with the Symplicity SpyralTM catheter is reassuring.
Limitations
Some limitations should be addressed. First, as commonly seen in registries, not all patients enrolled in the GSR-Taiwan completed 3 years of follow-up. In addition, the sample size is relatively small, especially in the Symplicity SpyralTM subgroup, in which the 2-year outcomes mainly came from 6 patients. Despite the modest sample size, our findings mirror those of other subgroup publications as well as the overall GSR study findings. Second, the study did not include a control arm and thus could not evaluate potential placebo or Hawthorne effects on the outcomes.24 Third, some confounding factors, especially medication adherence and doses of anti-hypertensive medications, which may have influenced the therapeutic effects, were not evaluated. Fourth, ambulatory BP monitoring was not routinely required in the follow-up period.25,26 We thus could not assess the 24-hour BP-lowering efficacy of RDN. However, office BP and heart rate were strictly measured, and the extent of office BP reductions was consistent with that in observations from other Asian populations. Finally, serum creatinine levels were not routinely collected. Thus, it was hard to identify subclinical renal function changes.
CONCLUSIONS
This GSR-Taiwan 3-year follow-up study is the first to show that RDN achieved statistically significant and sustained office BP reductions throughout the study period in patients with uncontrolled hypertension in Taiwan. The reductions in office BP following RDN were maintained for 2-3 years after the procedure. Office BP reductions were comparable irrespective of branch artery ablations. Even though the patients undergoing main and branch artery ablations had numerically greater office SBP reductions at 3 and 6 months, the modest sample size and non-randomized nature of the study did not allow for comparisons of BP reductions for patients with ablations applied only in the main renal artery to those with ablations also in the renal artery branch vessels. Finally, the safety profile was favorable with RDN, regardless of whether branch artery ablation was performed. Given the susceptibility of Asian populations to hypertension,27 RDN, as a safe antihypertensive procedure with long-lasting BP-lowering effects, could reliably serve as an alternative or complementary BP-lowering strategy for patients with uncontrolled hypertension in Taiwan and other Asian countries.
REFERENCES
- 1.Mann SJ. Neurogenic hypertension: pathophysiology, diagnosis and management. Clin Auton Res. 2018;28:363–374. doi: 10.1007/s10286-018-0541-z. [DOI] [PubMed] [Google Scholar]
- 2.Li YD, Lin TK, Tu YR, et al. Blood pressure reactivity and recovery to anger recall in hypertensive patients with type D personality. Acta Cardiol Sin. 2018;34:417–423. doi: 10.6515/ACS.201809_34(5).20180330A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm M, Linz D, Urban D, et al. Renal sympathetic denervation: applications in hypertension and beyond. Nat Rev Cardiol. 2013;10:465–476. doi: 10.1038/nrcardio.2013.89. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 5.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 6.Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 7.Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 8.Liu YM, Lin PL, Liao FC, et al. Effect of radiofrequency-based renal denervation: the impact of unplanned medication change from a systematic review and meta-analysis. Acta Cardiol Sin. 2019;35:144–152. doi: 10.6515/ACS.201903_35(2).20181231A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kario K, Ogawa H, Okumura K, et al. SYMPLICITY HTN-Japan - First Randomized Controlled Trial of catheter-based renal denervation in Asian patients. Circ J. 2015;79:1222–1229. doi: 10.1253/circj.CJ-15-0150. [DOI] [PubMed] [Google Scholar]
- 10.Sardar P, Bhatt DL, Kirtane AJ, et al. Sham-controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol. 2019;73:1633–1642. doi: 10.1016/j.jacc.2018.12.082. [DOI] [PubMed] [Google Scholar]
- 11.Kim BK, Bohm M, Mahfoud F, et al. Renal denervation for treatment of uncontrolled hypertension in an Asian population: results from the Global SYMPLICITY Registry in South Korea (GSR Korea). J Hum Hypertens. 2016;30:315–321. doi: 10.1038/jhh.2015.77. [DOI] [PubMed] [Google Scholar]
- 12.Kario K, Yamamoto E, Tomita H, et al. Sufficient and persistent blood pressure reduction in the final long-term results from SYMPLICITY HTN-Japan - safety and efficacy of renal denervation at 3 years. Circ J. 2019;83:622–629. doi: 10.1253/circj.CJ-18-1018. [DOI] [PubMed] [Google Scholar]
- 13.Wang TD, Lee YH, Chang SS, et al. 2019 Consensus Statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on renal denervation for the management of arterial hypertension. Acta Cardiol Sin. 2019;35:199–230. doi: 10.6515/ACS.201905_35(3).20190415A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohm M, Mahfoud F, Ukena C, et al. Rationale and design of a large registry on renal denervation: the Global SYMPLICITY registry. EuroIntervention. 2013;9:484–492. doi: 10.4244/EIJV9I4A78. [DOI] [PubMed] [Google Scholar]
- 15.Bohm M, Mahfoud F, Ukena C, et al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015;65:766–774. doi: 10.1161/HYPERTENSIONAHA.114.05010. [DOI] [PubMed] [Google Scholar]
- 16.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Chiang CE, Wang TD, Lin TH, et al. The 2017 Focused Update of the Guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213–225. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 19.Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 20.Esler MD, Bohm M, Sievert H, et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–1759. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.Wang TD, Chen YH, Huang CH, et al. Bidirectional adherence changes and associated factors in patients switched from free combinations to equivalent single-pill combinations of antihypertensive drugs. Hypertension. 2014;63:958–967. doi: 10.1161/HYPERTENSIONAHA.113.02455. [DOI] [PubMed] [Google Scholar]
- 23.Azizi M, Pereira H, Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) trial. Circulation. 2016;134:847–857. doi: 10.1161/CIRCULATIONAHA.116.022922. [DOI] [PubMed] [Google Scholar]
- 24.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attar A, Sadeghi AA, Amirmoezi F, Aghasadeghi K. Low dose spironolactone monotherapy in the management of stage I essential hypertension: a pilot randomized, double-blind, placebo-controlled trial. Acta Cardiol Sin. 2018;34:59–65. doi: 10.6515/ACS.201801_34(1).20170903B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. doi: 10.1097/00004872-200304000-00013. [DOI] [PubMed] [Google Scholar]


