Abstract
Background
Body mass index (BMI), waist circumference (WC) and waist-hip ratio (WHR) are all simple anthropometric tools used to categorize obesity status. This study aimed to determine associations between different anthropometric indices and the attainment of therapeutic lipid goals in patients with atherosclerotic cardiovascular disease (CVD) undergoing secondary prevention.
Methods
Between 2010 and 2014, this multi-center study enrolled 5718 patients undergoing secondary prevention for CVD. At study enrollment, total cholesterol, high-density lipoprotein protein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) were recorded. This cross-sectional study analyzed these three anthropometric obesity indices and correlations with achieving therapeutic lipid goals.
Results
Among the 5718 patients, multivariate analysis revealed that those with higher BMI or WC tended not to meet their HDL-C and TG therapeutic goals. However, neither BMI nor WC showed a relationship with achieving the LDL-C target. The patients with an elevated WHR (≥ 0.98 for males and ≥ 0.97 for females) were less likely to achieve all three lipid target values, including LDL-C (p = 0.05), HDL-C (p < 0.001) and TG (p < 0.001).
Conclusions
Among Taiwanese patients undergoing secondary prevention for CVD, the higher the WHR the lower the likelihood of achieving the lipid therapeutic goals.
Keywords: Body mass index, Lipid, Obesity
INTRODUCTION
Dyslipidemia is a common consequence of obesity and a strong risk factor for atherosclerosis-related cardiovascular disease (CVD). Excess body weight increases the risk of cardiovascular diseases, fatal coronary artery disease, insulin resistance, type 2 diabetes mellitus and sleep apnea.1-5 When assessing the degree of obesity, body mass index (BMI) is used to define the level of obesity, the waist-hip ratio (WHR) is used as an indicator of body fat distribution, and waist circumference (WC) serves as a marker of abdominal obesity.
Based on regional epidemiological studies, health authorities in Eastern and Western countries have proposed different obesity classifications.6,7 Men with low cardiorespiratory fitness have been associated with central obesity and high triglyceride (TG) to high density lipoprotein ratio.8 Furthermore, changes in body weight can lead to changes in lipid profile. For instance, life style changes can both reduce body weight in obese individuals and also lead to improvements in vascular inflammation, insulin resistance and help normalize plasma lipids and lipoprotein.9,10 Conversely, insufficient body weight reduction is associated with not achieving the TG treatment target.11,12 A lipid-lowering diet has been shown to lower the mortality rate in healthy middle-aged men with hyperlipidemia.13
Although most patients with CVD receive standard secondary prevention therapy, many subjects fail to reach their recommended lipid levels.14,15 An increasing BMI level has been reported to be an independent risk factor for the poor control of blood sugar, blood pressure, and low-density lipoprotein cholesterol (LDL-C) in diabetic patients.16,17 Additional evidence has highlighted the importance of intra-abdominal obesity or visceral obesity. A high WC has been shown to predict diabetes in high-risk patients with central obesity.18 Furthermore, the WHR has been shown to have a better association with metabolic risk factors and stronger correlation with CVD than BMI.19-22 This study aimed to determine the correlations between different anthropometric indices and the attainment of therapeutic lipid goals among patients undergoing secondary prevention for atherosclerotic CVD.
METHODS
Study population
T-SPARCLE, the Taiwanese Secondary Prevention for patients with AtheRosCLErotic disease (T-SPARCLE) Registry, was a large multi-center study approved by the Joint Institutional Review Board (ClinicalTrials.gov Identifier: NCT02223143). The design and methods of the T-SPARCLE Registry have been described in detail previously.23-25
This prospective observational study enrolled patients aged 18 years and older with stable symptomatic atherosclerotic CVD, including coronary artery disease (CAD), cerebrovascular disease and peripheral artery disease (PAD) receiving medical control, from 16 teaching hospitals in Taiwan. Participants with CAD were defined as those who had coronary artery stenosis greater than 50% by angiography, positive stress tests or a history of myocardial infarction. Participants with cerebrovascular disease were defined as those who had cerebral infarction, intra-cerebral hemorrhage, or a transient ischemic attack history. Participants with PAD were defined as those with an ankle-brachial index less than 0.90 or stenosis greater than 50% by angiography. Subjects with ≥ class III heart failure, malignancy, or on dialysis were excluded from this study. The lipid therapeutic targets were defined as LDL-C < 100 mg/dL, TG < 200 mg/dL, and high-density lipoprotein protein cholesterol (HDL-C) > 40 mg/dL in males and HDL-C > 50 mg/dL in females. We collected detailed baseline characteristics of the participants and evaluated the relationships between different obesity indices and attainment of therapeutic lipid levels in cross-sectional assessment. Informed consent was obtained from all of the participants.
Measurements
At the first clinical visit, vital signs, current medications, laboratory data and detailed history were recorded. At the time of enrollment, the complete lipid profiles including total cholesterol (TC), HDL-C, LDL-C and TG were evaluated, along with other blood biochemistry (including fasting blood sugar, liver function and renal function). Lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, and rosuvastatin were prescribed according to the physician’s judgment, and comparisons of statin potency were based on a previous study.21 This cross-sectional observational study analyzed three anthropometric indices: BMI, WHR and WC. We used the following BMI cut-off points as proposed in the Asia-Pacific guidelines on obesity and its treatment published in 2000: underweight (< 18.5 kg/m2), normal weight (18.5-22.9 kg/m2), overweight (23-24.9 kg/m2), obesity class I (25-29.9 kg/m2), and obesity class II (≥30 kg/m2).6 WHR and WC were analyzed as categorical variables by dividing them into four groups using quartiles.
Statistical analysis
The participants were divided into five groups according to BMI (as defined above) and into four groups using quartiles in the analysis of WHR and WC. Male and female data were combined for the analyses. Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as percentages. Analysis of variance (ANOVA) was used to compare the means of continuous variables in different obesity groups, while the chi-square test was used to compare dichotomous variables. Univariate analysis was conducted to test possible determinant factors for therapeutic lipid target achievement. Multiple logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of various determinants for the recommended lipid targets.
Less than 10% of the study cohort had missing data on BMI, estimated glomerular filtration rate (eGFR), LDL-C, HDL-C, non-HDL-C, TG, TC, history of diabetes, and hypertension, and 24% had missing data on WC and hip circumference. Multiple imputation (PROC MI procedure in SAS) was used to handle missing values, and the predictor variables in the imputation model included BMI, eGFR, LDL-C, HDL-C, non-HDL-C, TG, TC, history of diabetes, hypertension, WC, and hip circumference, as well as important non-missing variables such as age and gender. The imputation step resulted in 20 complete data sets, each of which contained different estimates of the missing values for all of the patients. After imputation, we used PROC LOGISTIC in SAS to fit a binary logistic regression model for each dataset, and then PROC MIANALYZE to combine the results from the 20 binary logistic regression models.
Three multivariate analyses models were conducted according to the different obesity indices. We used multiple imputations to overcome the missing data in this cross-sectional study. The logistic models included all possible variables and were adjusted for each other. Data analyses were performed using the Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC), and the significance level was set at p < 0.05.
RESULTS
From January 2010 to August 2014, 5718 patients were enrolled in this study. Table 1 shows the demographics and clinical characteristics of the participants divided into the five BMI groups. Of the 5718 enrolled patients, 4273 (75%) were male. The patients with a larger BMI were younger, had higher body weight, WHR, WC and hip girth. With increasing BMI, the prevalence of hypertension and type 2 diabetes increased. However in the obese group, a history of ischemic stroke was less common. As part of the secondary prevention therapy for atherosclerotic CVD, more obese patients took statins or fibrate to achieve the lipid target based on the recommendations of the National Health Insurance program, and they still tended to have lower HDL-C and higher TG levels.
Table 1. Demographic data grouped by BMI (n = 5718).
| Variable | Underweight | Normal | Overweight | Obese I | Obese II | p-value |
| BMI < 18.50 | BMI 18.5-22.9 | BMI 23-24.9 | BMI 25-29.9 | BMI ≥ 30 | ||
| n = 62 | n = 944 | n = 1201 | n = 2678 | n = 833 | ||
| Age (year), mean ± SD | 73.8 ± 11.1 | 70.0 ± 11.3 | 67.3 ± 10.9 | 65.2 ± 11.2 | 61.4 ± 11.9 | < 0.01 |
| Male (%) | 53.2 | 69.2 | 77.8 | 77.0 | 70.8 | < 0.01 |
| Waist (cm), mean ± SD | 73.9 ± 7.7 | 83.4 ± 7.2 | 88.5 ± 6.6 | 94.6 ± 7.3 | 104.5 ± 8.8 | < 0.01 |
| Hip (cm), mean ± SD | 85.9 ± 6.1 | 92.4 ± 5.8 | 95.9 ± 5.7 | 100.7 ± 6.4 | 108.7 ± 8.6 | < 0.01 |
| WHR, mean ± SD | 0.80 ± 0.09 | 0.90 ± 0.08 | 0.92 ± 0.07 | 0.94 ± 0.07 | 0.97 ± 0.09 | < 0.01 |
| Weight (kg), mean ± SD | 44.3 ± 5.6 | 56.6 ± 6.4 | 64.1 ± 6.3 | 72.3 ± 8.0 | 86.2 ± 11.5 | < 0.01 |
| Height (cm), mean ± SD | 160.5 ± 7.4 | 161.9 ± 8.1 | 163.2 ± 7.8 | 163.2 ± 8.1 | 162.3 ± 8.8 | < 0.01 |
| History of HTN (%) | 62.9 | 68.2 | 68.3 | 74.1 | 82.0 | < 0.01 |
| History of DM (%) | 25.9 | 33.4 | 36.8 | 38.9 | 45.0 | < 0.01 |
| History of ischemic stroke (%) | 35.5 | 14.7 | 13.0 | 10.5 | 10.3 | < 0.01 |
| History of MI (%) | 58.1 | 77.4 | 75.9 | 76.2 | 76.6 | < 0.05 |
| Current smoker (%) | 9.7 | 13.9 | 16.0 | 16.5 | 17.5 | 0.14 |
| Statin use (%) | 54.8 | 65.6 | 67.5 | 68.7 | 69.5 | 0.05 |
| Fibrate use (%) | 0 | 2.7 | 3.5 | 6.6 | 8.6 | < 0.01 |
| Anti-platelets therapy use (%) | 82.3 | 86.4 | 87.1 | 86.8 | 84.9 | 0.49 |
| ARB or ACEI use (%) | 38.7 | 52.1 | 53.0 | 60.8 | 66.6 | < 0.01 |
| Beta-blockers use (%) | 30.7 | 45.9 | 51.0 | 56.6 | 58.6 | < 0.01 |
| CKD (eGFR < 30) (%) | 3.7 | 3.9 | 2.5 | 2.3 | 2.9 | 0.12 |
| TC (mg/dL), mean ± SD | 172.6 ± 39.3 | 171.9 ± 41.1 | 169.8 ± 38.3 | 169.0 ± 37.3 | 172.3 ± 39.4 | 0.14 |
| HDL-C (mg/dL), mean ± SD | 58.2 ± 17.4 | 49.4 ± 14.8 | 45.8 ± 13.0 | 43.5 ± 12.0 | 42.2 ± 12.0 | < 0.01 |
| LDL-C (mg/dL), mean ± SD | 92.2 ± 32.9 | 97.8 ± 35.5 | 97.8 ± 35.1 | 97.0 ± 33.0 | 99.1 ± 33.0 | 0.44 |
| TG (mg/dL), mean ± SD | 107.7 ± 77.8 | 115.9 ± 77.8 | 132.9 ± 97.1 | 144.1 ± 83.5 | 169.0 ± 118.4 | < 0.01 |
ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HBA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; SD, standard deviation; TC, total cholesterol; TG, triglyceride; WHR, waist-hip ratio.
Table 2 shows the demographics and clinical characteristics of the participants divided into quartiles according to the WHR. The participants with a higher WHR had a larger WC, BMI, and body weight. With increasing WHR, the prevalence of hypertension and type 2 diabetes also increased. More obese participants grouped by WHR took statins and tended to have lower HDL-C, higher TG but similar LDL-C level.
Table 2. Demographic data grouped by waist-hip ratio (n = 4547).
| Variable | WHR < 0.91 for male, WHR < 0.85 for female | WHR 0.91-0.93 for male, WHR 0.85-0.88 for female | WHR 0.94-0.97 for male, WHR 0.89-0.96 for female | WHR ≥ 0.98 for male, WHR ≥ 0.97 for female | p-value |
| n = 1085 | n = 956 | n = 1373 | n = 1133 | ||
| Age (year), mean ± SD | 65.55 ± 11.65 | 65.19 ± 11.67 | 66.07 ± 11.28 | 66.83 ± 11.21 | < 0.01 |
| Male (%) | 74.93 | 70.92 | 67.73 | 81.29 | < 0.01 |
| Waist (cm), mean ± SD | 84.88 ± 8.02 | 90.44 ± 7.70 | 94.21 ± 7.54 | 100.68 ± 9.4 | < 0.01 |
| Hip (cm), mean ± SD | 99.16 ± 8.02 | 100.00 ± 8.09 | 99.88 ± 7.75 | 98.67 ± 9.23 | < 0.01 |
| Weight (kg), mean ± SD | 65.81 ± 10.47 | 68.57 ± 10.98 | 70.12 ± 11.55 | 74.53 ± 13.83 | < 0.01 |
| Height (cm), mean ± SD | 163.56 ± 7.84 | 162.72 ± 8.28 | 162.07 ± 8.41 | 163.33 ± 8.01 | < 0.01 |
| BMI (kg/m2), mean ± SD | 24.60 ± 3.38 | 25.84 ± 3.30 | 26.64 ± 3.49 | 27.86 ± 4.28 | < 0.01 |
| History of HTN (%) | 69.93 | 71.34 | 73.76 | 77.85 | < 0.01 |
| History of DM (%) | 25.61 | 31.80 | 38.29 | 44.84 | < 0.01 |
| History of ischemic stroke (%) | 12.90 | 10.98 | 11.58 | 11.65 | 0.56 |
| History of MI (%) | 75.85 | 77.72 | 79.61 | 79.96 | 0.07 |
| Current smoker (%) | 13.27 | 14.54 | 16.24 | 19.42 | < 0.01 |
| Statin use (%) | 62.03 | 66.95 | 67.30 | 69.90 | < 0.01 |
| Fibrate use (%) | 3.0 | 6.2 | 6.0 | 6.5 | < 0.01 |
| Anti-platelets therapy use (%) | 81.66 | 84.83 | 84.78 | 87.91 | < 0.01 |
| ARB or ACEI use (%) | 55.58 | 58.26 | 61.84 | 63.20 | < 0.01 |
| Beta-blockers use (%) | 45.99 | 51.05 | 49.38 | 48.37 | 0.04 |
| CKD (eGFR < 30) (%) | 2.09 | 2.00 | 1.90 | 3.63 | 0.03 |
| TC (mg/dL), mean ± SD | 171.97 ± 39.69 | 171.74 ± 38.78 | 171.92 ± 38.23 | 171.52 ± 39.69 | 0.99 |
| HDL-C (mg/dL), mean ± SD | 48.84 ± 15.43 | 45.59 ± 12.92 | 44.89 ± 13.11 | 43.38 ± 12.19 | < 0.01 |
| LDL-C (mg/dL), mean ± SD | 98.81 ± 35.43 | 98.41 ± 34.81 | 98.36 ± 33.66 | 97.77 ± 33.27 | 0.92 |
| TG (mg/dL), mean ± SD | 124.25 ± 81.25 | 136.23 ± 78.41 | 144.77 ± 97.53 | 153.92 ± 95.08 | < 0.01 |
Abbreviation as Table 1.
Table 3 shows the participants divided into quartiles of WC. The patients with a higher WC had a larger hip circumference, weight, WHR and BMI. With increasing WC, the prevalence of hypertension and type 2 diabetes also increased. The participants tended to have lower HDL-C and higher TG values as the WC increased.
Table 3. Demographic data group by waist circumference (n = 4589).
| Variable | Waist < 88 for male, Waist < 83 for female | Waist 88-93.14 for male, Waist 83-88.99 for female | Waist 93.15-99.99 for male, Waist 89-95.99 for female | Waist ≥ 100 for male, Waist ≥ 96 for female | p-value |
| n = 1138 | n = 1126 | n = 1128 | n = 1191 | ||
| Age (year), mean ± SD | 66.27 ± 11.20 | 65.78 ± 11.16 | 66.46 ± 11.23 | 65.51 ± 12.15 | 0.04 |
| Male (%) | 73.46 | 75.49 | 71.10 | 74.31 | 0.11 |
| Waist (cm), mean ± SD | 81.06 ± 5.07 | 89.35 ± 2.64 | 95.00 ± 2.74 | 105.27 ± 6.38 | < 0.01 |
| Hip (cm), mean ± SD | 92.21 ± 5.58 | 97.00 ± 5.19 | 100.79 ± 5.61 | 107.35 ± 7.75 | < 0.01 |
| WHR, mean ± SD | 0.88 ± 0.060 | 0.92 ± 0.057 | 0.95 ± 0.061 | 0.98 ± 0.081 | < 0.01 |
| Weight (kg), mean ± SD | 60.44 ± 8.11 | 66.51 ± 8.25 | 70.95 ± 9.25 | 81.05 ± 12.06 | < 0.01 |
| Height (cm), mean ± SD | 161.77 ± 7.64 | 162.44 ± 7.99 | 163.02 ± 8.26 | 164.28 ± 8.55 | < 0.01 |
| BMI (kg/m2), mean ± SD | 23.08 ± 2.45 | 25.17 ± 2.30 | 26.70 ± 2.65 | 30.03 ± 3.71 | < 0.01 |
| History of HTN (%) | 69.04 | 71.29 | 73.32 | 79.60 | < 0.01 |
| History of DM (%) | 29.61 | 35.00 | 37.76 | 39.53 | < 0.01 |
| History of ischemic stroke (%) | 12.92 | 14.65 | 10.37 | 9.57 | < 0.01 |
| History of MI (%) | 77.59 | 76.38 | 79.88 | 79.43 | 0.15 |
| Current smoker (%) | 15.64 | 15.99 | 15.60 | 16.88 | 0.82 |
| Statin use (%) | 67.22 | 66.52 | 67.64 | 64.99 | 0.54 |
| Fibrate use (%) | 2.9 | 5.5 | 4.9 | 8.2 | < 0.01 |
| Anti-platelets therapy use (%) | 85.50 | 85.17 | 85.46 | 83.38 | 0.42 |
| ARB or ACEI use (%) | 54.31 | 56.57 | 63.21 | 65.49 | < 0.01 |
| Beta-blockers use (%) | 45.78 | 47.87 | 52.13 | 52.64 | < 0.01 |
| CKD (eGFR < 30) | 2.34 | 2.46 | 2.17 | 2.64 | 0.92 |
| TC (mg/dL), mean ± SD | 174.16 ± 41.76 | 172.24 ± 38.53 | 170.03 ± 37.57 | 170.80 ± 37.82 | 0.07 |
| HDL-C (mg/dL), mean ± SD | 49.10 ± 15.31 | 45.96 ± 13.80 | 44.78 ± 12.19 | 42.86 ± 12.15 | < 0.01 |
| LDL-C (mg/dL), mean ± SD | 99.88 ± 37.37 | 98.66 ± 33.71 | 96.90 ± 32.61 | 97.99 ± 32.77 | 0.25 |
| TG (mg/dL), mean ± SD | 124.35 ± 95.98 | 138.29 ± 81.91 | 143.35 ± 84.47 | 154.68 ± 94.28 | < 0.01 |
Abbreviation as Table 1.
In univariate analysis, all three obesity indices were negatively correlated with achieving HDL-C and TG therapeutic targets, however there was no correlation with LDL-C target attainment (Table 4). A history of diabetes mellitus and beta-blocker use were positively correlated with LCL-C and negatively correlated with HDL-C and TG therapeutic goal attainment. A history of myocardial infarction, statin and antiplatelet therapy were correlated with LDL-C therapeutic goal attainment, and a history of ischemic stroke was negatively correlated with LDL-C therapeutic goal attainment. Current smokers, fibrate use, ACEI/ARB use, low physical activity, and CKD (eGFR < 30 ml/min) were negatively correlated with HDL-C and TG therapeutic goal attainment.
Table 4. Univariate analysis for different lipid target attainment (A) LDL-C, (B) HDL-C, (C) TG.
| (A) | ||||
| Variable | LDL < 100 | LDL ≥ 100 | OR, 95% Cl | p-value |
| Age (y), mean ± SD | 66.8 ± 11.4 | 65.0 ± 11.7 | 1.01 (1.01-1.02) | < 0.001 |
| Male (%) | 76.1 | 72.9 | 1.18 (1.05-1.34) | < 0.01 |
| Waist (cm), mean ± SD | 93.0 ± 9.95 | 92.9 ± 9.89 | 1.00 (1.00-1.01) | 0.75 |
| Hip (cm), mean ± SD | 99.7 ± 8.1 | 99.5 ± 8.2 | 1.00 (1.00-1.01) | 0.57 |
| WHR, mean ± SD | 0.933 ± 0.079 | 0.935 ± 0.085 | 0.80 (0.38-1.70) | 0.56 |
| Weight (kg), mean ± SD | 69.8 ± 12.3 | 69.9 ± 12.2 | 1.00 (1.00-1.00) | 0.75 |
| Height (cm), mean ± SD | 163.0 ± 8.1 | 162.7 ± 8.2 | 1.01 (1.00-1.01) | 0.14 |
| BMI (kg/m2), mean ± SD | 26.2 ± 3.7 | 26.4 ± 3.8 | 0.99 (0.98-1.00) | 0.17 |
| History of HTN % | 73.6 | 71.8 | 1.09 (0.97-1.23) | 0.15 |
| History of DM % | 42.2 | 33.5 | 1.45 (1.29-1.63) | < 0.001 |
| History of ischemic stroke % | 9.3 | 13.1 | 0.68 (0.57-0.80) | < 0.001 |
| History of MI % | 78.5 | 72.6 | 1.38 (1.22-1.56) | < 0.001 |
| Current smoker % | 16.0 | 16.8 | 0.94 (0.82-1.09) | 0.44 |
| Alcohol consumption % | 15.1 | 14.7 | 1.03 (0.89-1.20) | 0.73 |
| Statin use | 74.1 | 63.6 | 1.64 (1.46-1.84) | < 0.001 |
| Fibrate use | 5.3 | 6.1 | 0.85 (0.68-1.07) | 0.19 |
| Anti-platelets therapy use % | 88.7 | 84.1 | 1.47 (1.26-1.72) | < 0.001 |
| ARB or ACEI use % | 59.7 | 57.0 | 1.12 (1.00-1.25) | 0.05 |
| Beta-blockers use % | 57.3 | 52.5 | 1.22 (1.09-1.36) | < 0.001 |
| Physical activity < 3 times per week % | 73.1 | 73.3 | 0.99 (0.88-1.12) | 0.88 |
| TC, mg/dL, mean ± SD | 148.5 ± 24.4 | 199.8 ± 34.2 | 0.92 (0.92-0.93) | < 0.001 |
| HDL-C (mg/dL), mean ± SD | 44.5 ± 13.2 | 45.5 ± 12.7 | 0.99 (0.99-1.00) | < 0.005 |
| TG (mg/dL), mean ± SD | 137.5 ± 97.6 | 142.7 ± 84.1 | 1.00 (1.00-1.00) | 0.04 |
| CKD (eGFR < 30) | 2.6 | 2.2 | 1.22 (0.84-1.77) | 0.34 |
| (B) | ||||
| Variable | HDL > 40 for male and HDL > 50 for female | HDL ≤ 40 for male and HDL ≤ 50 for female | OR, 95% Cl | p-value |
| Age (y), mean ± SD | 67.0 ± 11.4 | 65.3 ± 11.7 | 1.01 (1.01-1.02) | < 0.001 |
| Male (%) | 77.9 | 71.5 | 1.41 (1.25-1.60) | < 0.001 |
| Waist (cm), mean ± SD | 92.0 ± 9.8 | 94.2 ± 9.9 | 0.98 (0.97-0.98) | < 0.001 |
| Hip (cm), mean ± SD | 99.1 ± 8.0 | 100.4 ± 8.3 | 0.98 (0.97-0.99) | < 0.001 |
| WHR, mean ± SD | 0.929 ± 0.079 | 0.940 ± 0.084 | 0.18 (0.08-0.39) | < 0.001 |
| Weight (kg), mean ± SD | 68.7 ± 11.7 | 71.2 ± 12.7 | 0.98 (0.98-0.99) | < 0.001 |
| Height (cm), mean ± SD | 163.1 ± 7.8 | 162.7 ± 8.4 | 1.01 (1.00-1.01) | 0.08 |
| BMI (kg/m2), mean ± SD | 25.8 ± 3.7 | 26.9 ± 3.8 | 0.93 (0.91-0.94) | < 0.001 |
| History of HTN, % | 72.2 | 73.7 | 0.93 (0.82-1.04) | 0.22 |
| History of DM, % | 32.1 | 45.4 | 0.57 (0.51-0.64) | < 0.001 |
| History of ischemic stroke, % | 10.7 | 11.1 | 0.97 (0.82-1.15) | 0.73 |
| History of MI, % | 74.6 | 77.1 | 0.87 (0.77-0.99) | 0.04 |
| Current smoker, % | 14.8 | 17.7 | 0.80 (0.70-0.93) | < 0.05 |
| Alcohol consumption, % | 16.6 | 13.2 | 1.31 (1.13-1.53) | < 0.01 |
| Statin use | 70 | 68.1 | 1.09 (0.98-1.23) | 0.13 |
| Fibrate use | 3.7 | 7.9 | 0.45 (0.35-0.57) | < 0.001 |
| Anti-platelets therapy use, % | 86.4 | 87 | 0.96 (0.82-1.12) | 0.59 |
| ARB or ACEI use, % | 56.9 | 59.7 | 0.89 (0.80-0.99) | 0.03 |
| Beta-blockers use, % | 50.7 | 60.6 | 0.67 (0.60-0.74) | < .001 |
| Physical activity < 3 times per week % | 71.3 | 75.8 | 0.79 (0.70-0.90) | < 0.005 |
| TC, mg/dL, mean ± SD | 176.3 ± 38.4 | 162.1 ± 36.8 | 1.01 (1.01-1.01) | < 0.001 |
| LDL-C (mg/dL), mean ± SD | 99.8 ± 33.5 | 94.7 ± 34.0 | 1.00 (1.00-1.00) | < 0.001 |
| TG (mg/dL), mean ± SD | 115.3 ± 67.2 | 165.1 ± 105.2 | 0.99 (0.99-0.99) | < 0.001 |
| CKD (eGFR < 30) | 1.7 | 3.2 | 0.54 (0.37-0.78) | < 0.005 |
| (C) | ||||
| Variable | TG < 200 | TG ≥ 200 | OR, 95% Cl | p-value |
| Age (y), mean ± SD | 67.0 ± 11.3 | 62.1 ± 12.0 | 1.14 (0.98-1.34) | < 0.001 |
| Male (%) | 74.8 | 72.2 | 1.04 (1.03-1.04) | 0.10 |
| Waist (cm), mean ± SD | 92.5 ± 9.9 | 95.0 ± 10.3 | 0.98 (0.97-0.98) | < 0.001 |
| Hip (cm), mean ± SD | 99.3 ± 8.2 | 100.5 ± 8.7 | 0.98 (0.97-0.99) | < 0.001 |
| WHR, mean ± SD | 0.931 ± 0.083 | 0.947 ± 0.075 | 0.12 (0.05-0.31) | < 0.001 |
| Weight (kg), mean ± SD | 69.2 ± 11.9 | 72.9 ± 13.4 | 0.98 (0.97-0.98) | < 0.001 |
| Height (cm), mean ± SD | 162.8 ± 8.1 | 162.9 ± 8.4 | 1.00 (0.99-1.01) | 0.76 |
| BMI (kg/m2), mean ± SD | 26.1 ± 3.7 | 27.3 ± 3.8 | 0.92 (0.90-0.94) | < 0.001 |
| History of HTN, % | 72.5 | 74.7 | 0.89 (0.76-1.05) | 0.17 |
| History of DM, % | 37.1 | 46.7 | 0.67 (0.59-0.78) | < 0.001 |
| History of ischemic stroke, % | 11.4 | 10.4 | 1.12 (0.89-1.40) | 0.37 |
| History of MI, % | 76.2 | 76.4 | 0.99 (0.84-1.17) | 0.96 |
| Current smoker, % | 14.4 | 24.1 | 0.53 (0.45-0.63) | < 0.001 |
| Alcohol consumption, % | 14.0 | 19.4 | 0.68 (0.57-0.81) | < 0.001 |
| Statin use | 69.1 | 65.5 | 1.18 (1.02-1.37) | 0.03 |
| Fibrate use | 3.6 | 17.7 | 0.17 (0.14-0.22) | < 0.001 |
| Anti-platelets therapy use, % | 86.5 | 87.2 | 0.94 (0.76-1.16) | 0.61 |
| ARB or ACEI use, % | 57.4 | 62.0 | 0.82 (0.71-0.95) | < 0.01 |
| Beta-blockers use, % | 53.8 | 59.5 | 0.79 (0.69-0.92) | < 0.005 |
| Physical activity < 3 times per week, % | 71.2 | 79.0 | 0.66 (0.55-0.78) | < 0.001 |
| TC, mg/dL, mean ± SD | 166.3 ± 36.7 | 189.4 ± 40.4 | 0.99 (0.98-0.99) | < 0.001 |
| HDL-C (mg/dL), mean ± SD | 46.3 ± 13.0 | 37.7 ± 10.7 | 1.07 (1.06-1.08) | < 0.001 |
| LDL-C (mg/dL), mean ± SD | 97.1 ± 96.2 | 98.9 ± 96.1 | 1.00 (1.00-1.00) | 0.16 |
| CKD (eGFR < 30) | 2.3 | 4.3 | 0.53 (0.36-0.78) | < 0.005 |
Abbreviation as Table 1.
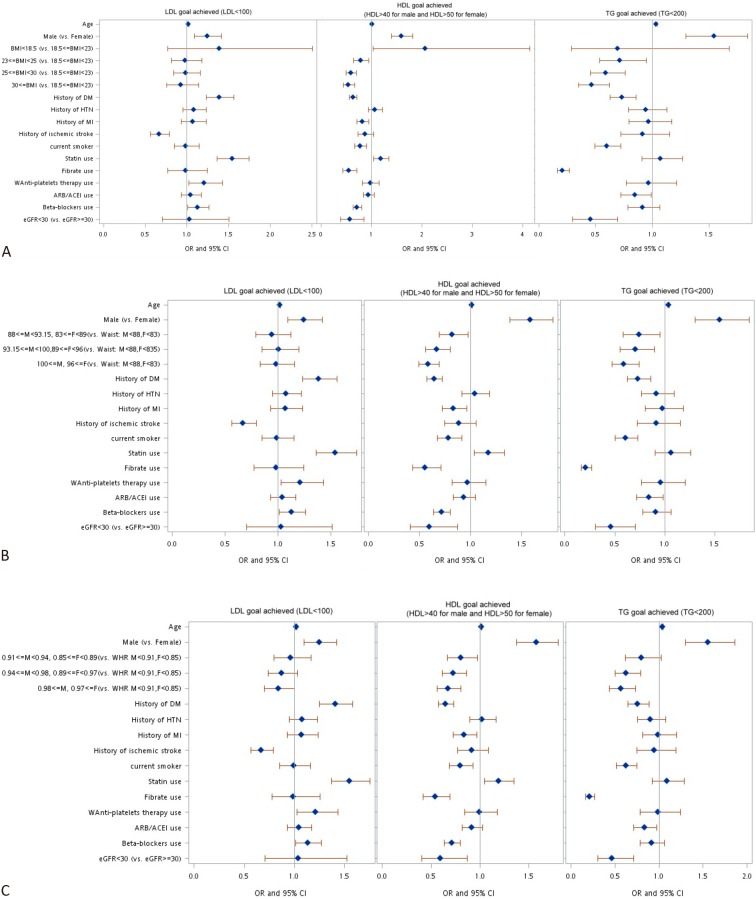
Three multiple logistic regression models were used to investigate the associations among the three anthropometric indices (BMI, WHR and WC) and the likelihood of attaining therapeutic lipid profile goals. Although the p-value of LDL-C attainment in different obesity groups lacked significance in the univariate analysis, we included the three different obesity indices into multivariate analysis, the results of which are shown in Figure 1. These multivariate analyses with different anthropometric indices showed some consistent results. For example, the participants who were older or male were more likely to reach the therapeutic lipid goals. Although diabetic patients tended to achieve the LDL-C target, they were less likely to achieve their HDL-C and TG target values. In contrast, the patients with a history of ischemic stroke were less likely to reach their LDL-C target. The subjects who smoked and those who took fibrates were less likely to achieve the HDL-C and TG targets. In addition, the patients with CKD (eGFR < 30 ml/min) were less likely to achieve the HDL-C and TG goals.
Figure 1.
Multivariate analysis for lipid therapeutic target achievement in different anthropometric model (A) Body mass index model. (B) Waist circumference model. (C) Waist-hip ratio model. ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL-c, high-density lipoprotein; HTN, hypertension; LDL-C, low-density lipoprotein; MI, myocardial infarction; WHR, wiast hip ratio.
The subjects with a BMI ≥ 23 kg/m2 were less likely to achieve the HDL-C and TG goals, although no effect was noted on LDL-C target achievement. Similarly, the patients with a larger WC were less likely to achieve the HDL-C and TG goals, but this finding had no impact on achieving the LDL-C target. In contrast, the patients with a higher WHR (≥ 0.98 for males and ≥ 0.97 for females) were significantly less likely to achieve all three lipid target values, including LDL-C (OR 0.84, CI 0.70-1.00, p = 0.0535), HDL-C (OR 0.67, CI 0.56-0.80, p < 0.0001) and TG (OR 0.57, CI 0.44-0.73, p < 0.0001). These three models showed that WHR had a better correlation with all three lipid therapeutic targets than BMI or WC.
DISCUSSION
Overweight and obesity are increasingly prevalent in many countries and are related to multiple cardiovascular risk factors. For example, obesity has a strong association with dyslipidemia,26-28 and dyslipidemia is an important risk factor of atherosclerotic disease. However, few studies have focused on the association between anthropometric indices and dyslipidemic control in patients with atherosclerotic CVD, especially in Asian populations.11,12,16,17 To the best of our knowledge, this is the first study to show a significant correlation between obese Taiwanese grouped by WHR and the achievement of lipid control, including HDL-C, LDL-C and TG, during secondary prevention for atherosclerotic CVD.
In a study of 750 Iranian adults without any significant past medical history, WC and WHR showed stronger correlations with TC, TG, LDL-C, TC/HDL-C levels than BMI.27 Of the specific lipid parameters, TG showed the closest correlation with WHR (r = 0.309, p < 0.001) and WC (r = 0.308, p < 0.001), whereas HDL-C level was weakly correlated with WC (r = -0.088, p < 0.05) but not with WHR. Our study showed that all three anthropometric indices (i.e., BMI, WHR and WC) were significantly negatively associated with the likelihood of achieving HDL-C and TG target levels in patients under secondary prevention for atherosclerotic CVD in cross-sectional assessments. However, neither BMI nor WC was significantly correlated with achieving target LDL-C levels. This finding may be partially explained by the fact that lipid lowering guidelines emphasize LDL-C control over HDL-C or TG control; therefore, it is possible that more obese patients took statins to reach the guideline-recommended target LDL-C level.29 This important finding may encourage clinicians to be even more aggressive in their efforts to control TG and HDL-C levels when treating obese patients with dyslipidemia.
In addition, according to the multivariate analyses, the patients with a higher WHR were significantly less likely to achieve their LDL-C goal. Because BMI does not take into account fat distribution of the body, obesity defined by WC has a higher sensitivity and specificity for identifying the presence of CVD.30,31 Waist circumference is the preferred measure of abdominal obesity, however people with the same WC may have a different body shape or BMI due to racial differences. In contrast, the WHR, calculated as WC divided by hip circumference, can lower the misinterpretation of single measurements. Waist and hip girths show opposing relationships to body fat, lipid composition and insulin concentration, with a larger waist girth indicating an increased health risk, but a larger hip girth indicating a protective effect on CVD risk.32 This has been shown in many previous studies. For example, BMI has repeatedly failed to accurately predict mortality risk or prognosis in patients with acute myocardial infarction.33-35 It is possible that these previous studies used an inappropriate anthropometric index for their analyses. In a case-control study involving 27098 patients from 52 countries, Yusuf et al. found that WHR had the strongest association with the incidence of myocardial infarction worldwide.36 Rexrode et al. reported that WHR was a risk factor for coronary heart disease in an 8-year follow-up observation study in a cohort of women.37 Taken together, WHR may be a more suitable anthropometric index to predict the risk of mortality or disease prognosis, and it may also be strongly associated with the achievement of therapeutic target lipid levels during interventions. Therefore, in clinical practice, physicians should pay more attention to treating dyslipidemia in patients with a high WHR.
Limitations
In this cross-sectional observational study, we used the lipid profile at enrollment for analysis. We assumed that all of the patients would receive lipid-lowering treatment according to clinical guidelines, and this is a quality limitation of this study. We also used multiple imputation to replace missing data and to overcome the lack in the multivariate analyses. In addition, the impact of switching statins before this study would be a limitation since this is a cross-sectional study.
CONCLUSIONS
In conclusion, obese Taiwanese patients undergoing secondary prevention for atherosclerotic CVD showed a significant negative correlation with achieving lipid control, including LDL-C, HDL-C, and TG. WHR had better correlation than BMI or WC with achieving therapeutic lipid targets in these Taiwan atherosclerotic CVD patients undergoing secondary prevention for atherosclerotic CVD.
FUNDING
The Taiwan Consortium of Lipid and Atherosclerosis is sponsored by the Taiwan Society of Lipids & Atherosclerosis since 2009 and Taiwan Ministry of Science and Technology since 2012 (Project code: NRPB-TR11: 100-2325-B-002-075).
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Kawada T, Otsuka T, Inagaki H, et al. Insulin resistance, as expressed by HOMA-R, is strongly determined by waist circumference or body mass index among Japanese working men. Obes Res Clin Pract. 2010;4:e1–e82. doi: 10.1016/j.orcp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Logue J, Murray HM, Welsh P, et al. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011;97:564–568. doi: 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 3.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajani UA, Lotufo PA, Gaziano JM, et al. Body mass index and mortality among US male physicians. Ann Epidemiol. 2004;14:731–739. doi: 10.1016/j.annepidem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Tsai WC, Wu KY, Lin GM, et al. Clinical characteristics of patients less than forty years old with coronary artery disease in Taiwan: a cross-sectional study. Acta Cardiol Sin. 2017;33:233–240. doi: 10.6515/ACS20161026A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1-253. [PubMed] [Google Scholar]
- 7.The Asia-Pacific perspective: redefining obesity and its treatment. International Diabetes Institute. 2000 [Google Scholar]
- 8.Vega GL, Grundy SM, Barlow CE, et al. Association of triglyceride-to-high density lipoprotein cholesterol ratio to cardiorespiratory fitness in men. J Clin Lipidol. 2016;10:1414–1422 e1411. doi: 10.1016/j.jacl.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 10.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 11.Papademetriou V, Piller LB, Ford CE, et al. Characteristics and lipid distribution of a large, high-risk, hypertensive population: the lipid-lowering component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2003;5:377–384. doi: 10.1111/j.1524-6175.2003.03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto X, Valdivielso P, Perez de Juan JM, et al. Predictive factors of achieving therapeutic goals of hypertriglyceridemia. Curr Med Res Opin. 2014;30:19–26. doi: 10.1185/03007995.2013.850069. [DOI] [PubMed] [Google Scholar]
- 13.Hjerkinn EM, Sandvik L, Hjermann I, Arnesen H. Effect of diet intervention on long-term mortality in healthy middle-aged men with combined hyperlipidaemia. J Intern Med. 2004;255:68–73. doi: 10.1046/j.0954-6820.2003.01248.x. [DOI] [PubMed] [Google Scholar]
- 14.Vedin O, Hagstrom E, Stewart R, et al. Secondary prevention and risk factor target achievement in a global, high-risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol. 2013;20:678–685. doi: 10.1177/2047487312444995. [DOI] [PubMed] [Google Scholar]
- 15.Wang KF, Wu CH, Chang CC, et al. Determinants of treatment modification in hypercholesterolemic patients. Acta Cardiol Sin. 2017;33:156–164. doi: 10.6515/ACS20161215A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertoni AG, Clark JM, Feeney P, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complications. 2008;22:1–9. doi: 10.1016/j.jdiacomp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Holecki M, Handzlik-Orlik G, Almgren-Rachtan A, et al. The decreased achievement of therapeutic goal in lipid lowering therapy in obese and diabetic patients in Poland. Pharmacol Rep. 2017;69:6–12. doi: 10.1016/j.pharep.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Janiszewski PM, Janssen I, Ross R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care. 2007;30:3105–3109. doi: 10.2337/dc07-0945. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Turin TC, Kita Y, et al. Associations of obesity measures with metabolic risk factors in a community-based population in Japan. Circ J. 2007;71:776–781. doi: 10.1253/circj.71.776. [DOI] [PubMed] [Google Scholar]
- 20.Wildman RP, Gu D, Reynolds K, et al. Are waist circumference and body mass index independently associated with cardiovascular disease risk in Chinese adults? Am J Clin Nutr. 2005;82:1195–1202. doi: 10.1093/ajcn/82.6.1195. [DOI] [PubMed] [Google Scholar]
- 21.Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254:555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin WY, Lee LT, Chen CY, et al. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. Int J Obes Relat Metab Disord. 2002;26:1232–1238. doi: 10.1038/sj.ijo.0802040. [DOI] [PubMed] [Google Scholar]
- 23.Ho LT, Yin WH, Chuang SY, et al. Determinants for achieving the LDL-C target of lipid control for secondary prevention of cardiovascular events in Taiwan. PloS One. 2015;10:e0116513. doi: 10.1371/journal.pone.0116513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeng JS, Yin WH, Huang CC, et al. Guideline-adherent therapy in patients with cardiovascular diseases in Taiwan. J Formos Med Assoc. 2015;114:1000–1007. doi: 10.1016/j.jfma.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Lin TH, Chuang SY, Chu CY, et al. The impact of chronic kidney disease on lipid management and goal attainment in patients with atherosclerosis diseases in Taiwan. Int J Med Sci. 2014;11:381–388. doi: 10.7150/ijms.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bays HE, Chapman RH, Grandy S, Group SI. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61:737–747. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chehrei A, Sadrnia S, Keshteli AH, et al. Correlation of dyslipidemia with waist to height ratio, waist circumference, and body mass index in Iranian adults. Asia Pac J Clin Nutr. 2007;16:248–253. [PubMed] [Google Scholar]
- 28.Mamabolo RL, Sparks M, Moss SJ, Monyeki MA. The association between dyslipidemia and anthropometric indicators in black and white adolescents residing in Tlokwe Municipality, North-West Province, South Africa: the PAHL study. Afr Health Sci. 2014;14:929–938. doi: 10.4314/ahs.v14i4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YH, Chao TH, Liu PY, et al. Lipid lowering therapy for acute coronary syndrome and coronary artery disease: highlights of the 2017 Taiwan Lipid Guidelines for high risk patients. Acta Cardiol Sin. 2018;34:371–378. doi: 10.6515/ACS.201809_34(5).20180629A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Heymsfield SB, Toyoshima H, et al. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81:409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 31.Wessel TR, Arant CB, Olson MB, et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292:1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 32.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann J, Gersh BJ, Goldfinger JZ, et al. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol. 2014;114:9–16. doi: 10.1016/j.amjcard.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Jiang D, Zhang B, et al. Impact of obesity on the outcome of Chinese patients with ST-segment myocardial infarction undergoing urgent percutaneous coronary intervention. Acta Cardiol. 2012;67:541–548. doi: 10.1080/ac.67.5.2174128. [DOI] [PubMed] [Google Scholar]
- 35.Widlansky ME, Sesso HD, Rexrode KM, et al. Body mass index and total and cardiovascular mortality in men with a history of cardiovascular disease. Arch Intern Med. 2004;164:2326–2332. doi: 10.1001/archinte.164.21.2326. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 37.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]