Abstract
Early stages of chronic obstructive pulmonary disease (COPD) are characterized by the loss and narrowing of terminal bronchioles in the lung, resulting in “air-trapping,” often occurring before overt emphysema manifests. Individuals with an airway-predominant phenotype of COPD display extensive lung air-trapping and are at greater cardiovascular disease (CVD) risk than COPD patients with an emphysema-predominant phenotype. We hypothesized that the degree of computed tomography (CT)-quantified lung air-trapping would be associated with greater aortic and carotid artery stiffness and lower endothelial function, known biomarkers of CVD risk. Lung air-trapping was associated with greater aortic stiffness (carotid femoral pulse wave velocity, CFPWV) (r = 0.60, P = 0.007) and carotid β-stiffness (r = 0.75, P = 0.0001) among adults with (n = 10) and without (n = 9) a clinical diagnosis of COPD and remained significant after adjusting for blood pressure (BP) and smoking history (pack-years) (carotid β-stiffness: r = 0.68, P < 0.01; CFPWV r = 0.53, P = 0.03). The association between lung air-trapping and carotid β-stiffness remained significant after additionally adjusting for age and forced expiratory volume 1(FEV1) (r = 0.64, P = 0.01). In the COPD group only (n = 10), lung air-trapping remained associated with carotid β-stiffness (r = 0.82, P = 0.05) after adjustment for age, pack-years, and FEV1. In contrast, no association was observed between CFPWV and lung air-trapping after adjustment for BP, pack-years, age, and FEV1 (r = 0.12, P = 0.83). Lung air-trapping was not associated with endothelial function (brachial artery flow-mediated dilation) in the entire cohort (P = 0.80) or in patients with COPD only (P = 0.71). These data suggest that carotid artery stiffness may be a mechanism explaining the link between airway-predominant phenotypes of COPD and high CVD risk.
NEW & NOTEWORTHY Previous cross-sectional studies have demonstrated greater large elastic artery stiffness and lower endothelium-dependent dilation in chronic obstructive pulmonary disease (COPD) patients compared with controls. Furthermore, COPD patients with emphysema have greater aortic stiffness than non-COPD controls, and the degree of stiffness is associated with emphysema severity. The present study is the first to demonstrate that even before overt emphysema manifests, lung air-trapping is associated with carotid artery stiffness in COPD patients independent of blood pressure, age, or smoking history.
Keywords: chronic obstructive pulmonary disease, emphysema, flow-mediated dilation, pulse wave velocity, vascular function
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease of the lungs that is characterized by expiratory airflow limitation that is not fully reversible. However, although primarily a disease of the lungs, up to 50% of all deaths in individuals with COPD are attributed to cardiovascular disease (CVD) (7, 20, 35). Two mechanisms that are likely to contribute to this heightened CVD risk in individuals with COPD are elevated large elastic artery (i.e., carotid and aorta) stiffness and endothelial dysfunction. Large elastic artery stiffness is a strong predictor of CVD events and mortality in adults, independent of blood pressure (BP) and other known CVD risk factors. Specifically, carotid-femoral pulse wave velocity (CFPWV), the reference standard measurement of aortic stiffness, is a robust, independent predictor of CVD events (4, 27), and carotid artery stiffness is strongly associated with incidence of stroke (36). Both CFPWV and carotid β-stiffness are markedly greater in individuals with COPD compared with non-COPD controls (1, 25, 32), suggesting that large elastic artery stiffness may contribute to CVD risk in COPD.
In individuals with COPD, endothelial dysfunction is present in both the pulmonary and systemic vasculature and may represent a mechanistic link between airflow limitation and CVD risk (19, 31). Vascular endothelial dysfunction, indicated by impaired brachial artery flow-mediated dilation (FMD), is an independent predictor of CVD-related events in adults (34, 37, 38). Importantly, brachial artery FMD values are significantly lower in individuals with COPD than age- and sex-matched non-COPD controls and are negatively associated with COPD disease severity (3, 8). However, the extent to which vascular endothelial dysfunction and large elastic artery stiffness contribute to higher CVD risk among specific COPD phenotypes remains unclear.
COPD is a heterogeneous disorder that includes both emphysema- and airway-predominant phenotypes (21). Individuals with an airway-predominant phenotype display characteristic signs of small airway disease, such as increased airway wall thickness and higher concentrations of inflammatory cells and mucus exudates in the small conducting airways (internal diameter <2 mm) (18, 23). This structural remodeling results in the loss and narrowing of small airways and, consequently, becomes a major site of resistance to airflow in the lungs. In turn, the increase in airway resistance causes a greater amount of air to become trapped in the lungs at functional residual capacity (FRC) and residual volume (RV). Importantly, the development of lung air-trapping typically occurs in the earlier stages of COPD progression and precedes the development of emphysema (18, 23). Despite having only mild/moderate COPD, patients with an airway-predominant phenotype are at a greater CVD risk than emphysematous patients with more severe airflow limitation (6). However, whether increased large elastic artery stiffness and endothelial dysfunction explain, in part, the heightened CVD risk observed in COPD patients with an airway-predominant phenotype remains to be elucidated.
In this study, we aimed to determine whether the magnitude of lung air-trapping was associated with large elastic artery stiffness and endothelial dysfunction in individuals with COPD with little or no emphysema. Given the high CVD risk observed in COPD patients with an airway-predominant phenotype, we hypothesized that the degree of computed tomography (CT)-measured lung air-trapping would be associated with greater aortic and carotid artery stiffness and lower endothelial function among adults with COPD.
METHODS
Subjects.
Individuals with COPD and non-COPD controls were recruited from previous pulmonary research cohorts, including the COPDGene and SPIROMICS studies at The University of Iowa. A total of 10 individuals with COPD [age 66 ± 8 yr; 5F/5M; Global Initiative for COPD, GOLD (Global Initiative for Obstructive Lung Disease) stages 1–3; four current smokers], with little or no emphysema, and nine controls without COPD (age 59 ± 13 yr, five women and four men; two current smokers), who had had a recent (within the past 2 yr) research lung CT scan from the Iowa Comprehensive Lung Imaging Center, were enrolled in the study. COPD disease severity was classified using the GOLD staging system that uses spirometry to define the presence and severity of airflow limitation from mild (stage 1) to very severe (stage 4) (29). We completed comprehensive noninvasive assessments of vascular function, including aortic stiffness (CFPWV), carotid β-stiffness (carotid ultrasound and applanation tonometry), endothelial function (brachial artery FMD), spirometry, and blood gases in the Institute for Clinical and Translational Science (ICTS) Clinical Research Unit. Previous lung CT scans were reanalyzed at the time of the present study to quantify air-trapping and emphysema in the Advanced Pulmonary Physiomic Imaging Laboratory (APPIL). Exclusion criteria for both the COPD and control groups included concomitant respiratory disorder other than asthma, use of antibiotics or steroids for COPD exacerbation within the past month, use of 24-h supplemental oxygen, pregnancy, uncontrolled cancer within the past 5 yr, lung cancer, lung surgery, pulmonary hypertension, insulin-dependent diabetes, prior neurological condition (e.g., epilepsy), recent (3 mo) myocardial infarction, stroke, recent eye surgery (6 wk), renal failure, heart failure, substance use disorder (not tobacco), cystic fibrosis, or chest radiation therapy.
Measurements.
Participants reported to the laboratory after an 8-h overnight fast, refrained from antihypertensive medications for 12 h or PDE5 inhibitors for 72 h, and did not smoke or use bronchodilators for at least 3 h before the visit. Medical history, current medication use, and symptom severity were collected by self-report at the time of the present study. All procedures were approved by the University of Iowa Institutional Review Board (IRB), and all participants read and signed an IRB-approved informed consent document before participating. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Iowa (15). REDCap is a secure, Web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry, 2) audit trails for tracking data manipulation and export procedures, 3) automated export procedures for seamless data downloads to common statistical packages, and 4) procedures for importing data from external sources.
Carotid artery stiffness.
Participants underwent carotid ultrasound imaging with a 7–12-MHz linear transducer (LOGIQ 7; GE Healthcare, New York, NY) used to calculate carotid artery β-stiffness index from 30-s ultrasound images and the carotid artery pressure waveform using applanation tonometry, as previously described in our laboratory (11). Briefly, maximal systolic and minimum diastolic diameters were analyzed ~2 cm from the carotid bulb by a single researcher (Vascular Analysis Tools Analyzer 5.5; Medical Imaging Applications, Coralville, IA). Carotid systolic and diastolic BP were derived from the carotid site and calibrated to the mean and diastolic pressure obtained from a brachial BP cuff and tonometry-acquired brachial BP waveform [noninvasive hemodynamics (NIHem) workstation; Cardiovascular Engineering, Norwood, MA]. To determine the stiffness of the vessel wall without the influence of BP, β-stiffness index was calculated as β = ln (carotid systolic BP/carotid diastolic BP/Δd) × (d), where Δd represents the change in diameter (end-systolic diameter minus end-diastolic diameter) and d represents the end-diastolic diameter (17).
Aortic stiffness.
An auscultatory BP was recorded by an investigator using a built-in cuff microphone, allowing for accurate identification of 1st and 5th Korotkoff sounds (NIHem workstation; Cardiovascular Engineering, Norwood, MA). Arterial tonometry of the brachial, radial, femoral, and carotid artery was performed with a custom transducer, and waveforms were signal-averaged and gated to the ECG R-wave. Average systolic and diastolic BPs were used to calibrate peak and trough of the signal-averaged BP waveform. BP measurements were overseen by a single investigator. The pressure waveform was gated to the R-wave of the ECG to determine the change in time difference between the foot of the carotid and femoral diastolic pressure waveforms. The distance between the carotid and femoral pulse sites was measured as the distance between the suprasternal notch (SSN) and the carotid waveform minus the distance from the SSN to the femoral pulse site to account for parallel transmission. Corrected distances were divided by the carotid and femoral foot-to-foot delay to calculate CFPWV (26).
Brachial artery endothelial function.
Brachial artery FMD was determined using ultrasonography (LOGIQ 7; GE Healthcare) with a linear array transducer, as previously described (30). Briefly, after baseline measurements of brachial artery diameter were performed, a cuff on the upper forearm was inflated to a suprasystolic pressure (250 mmHg) for 5 min. Following the 5 min of occlusion, the cuff was rapidly deflated, and measurements continued for an additional 2 min. Baseline and deflation images were sent in real-time to an offline vascular imager (Vascular Analysis Tools Analyzer 5.5; Medical Imaging Applications, LLC, Coralville, IA) that used automated edge detection to determine changes in brachial artery diameter in response to reactive hyperemia. Responses are expressed as absolute (mm) and percent change (%∆) from baseline diameter.
Spirometry.
Pre- and post-albuterol spirometry testing was performed using the Vmax spirometer (Vyaire) and in accordance with American Thoracic Society guidelines (9). After three baseline forced vital capacity (FVC) tests were completed, four puffs of albuterol (90 μg per actuation) were administered from a metered dose inhaler with a spacer. After 15 min, the participants were prompted to perform three additional FVC maneuvers. Postalbuterol values were used to determine the presence or absence of COPD using a fixed cut-off of forced expiratory volume in 1 s (FEV1)/FVC less than 0.70. Post-albuterol FEV1 percent predicted values were used to identify the GOLD stage in participants that had COPD.
Lung imaging.
Participants previously underwent (within 2 yr; average 6 mo) volumetric chest CT scans after β2-agonist bronchodilation at full inspiration (i.e., total lung capacity, TLC) and at the end of full expiration (i.e., RV) (16, 33). The rate of progression of CT-quantifiable lung disease over several months is very minimal, and individuals with COPD are classified as a “rapid progressor” if they have a one-year increase in emphysema index greater than 1%. Therefore, it is not expected that there would be significant differences in the extent of emphysema or air-trapping between the time of the previous lung CT scan and the present study (10). At the time of the present study, quantitative lung imaging software (APOLLO, VIDA Diagnostics) was used to import, segment, and analyze the CT lung images. Emphysema was defined as the % of lung voxels on inspiratory scan with mean attenuation <−950 Hounsfield units (HU) at TLC, as previously described (16). Lung air-trapping was defined two ways: the % of lung voxels with mean attenuation <−856 HU on expiratory scan at RV (33), or expiratory/inspiratory ratio (Exp/Insp) of mean lung attenuation on expiratory and inspiratory scans (16).
Statistical analyses.
All data are reported as means ± SD. Demographic and clinical characteristics of the groups were compared using independent t-tests for continuous variables or χ2 for group variables. Unadjusted Pearson correlations and partial correlations (adjusted for age, mean arterial pressure, pack-years, and FEV1) between CT-quantified air-trapping and carotid-femoral PWV and carotid β-stiffness were performed.
RESULTS
As expected, individuals with COPD had increased airflow limitation (P < 0.001), increased smoking history (pack-years) (P < 0.001), and increased measures of lung air-trapping expressed as the % of lung voxels less than −865 HU at RV (P < 0.01) and expiration/inspiration mean attenuation ratio (P = 0.03) (Tables 1 and 2) compared individuals with and without COPD. There were no differences in age, body mass index, % oxygen saturation (, %), arterial partial pressure of oxygen (Po2), arterial partial pressure of carbon dioxide (Pco2), brachial BP, or % emphysema (all P > 0.05) between COPD patients and controls (Tables 1 and 2). Individuals with COPD had significantly higher CFPWV compared with controls (Table 2), but no differences in carotid β-stiffness (P = 0.26) or brachial artery FMD (P = 0.32) were observed between individuals with COPD and controls (Table 2).
Table 1.
Participant characteristics
| Controls (n = 9) | COPD (GOLD 1–3) (n = 10) | P Value | |
|---|---|---|---|
| Age, yr | 59 ± 13.0 | 66 ± 8.0 | 0.20 |
| Sex, n (% female) | 5 (56%) | 5 (50%) | 0.81 |
| Body mass index, kg/m2 | 29.4 ± 5.4 | 29.0 ± 6.6 | 0.88 |
| Smoker >10 pack year, n (%) | 2 (22%) | 9 (90%)** | 0.003 |
| Smoking history (pack-years) | 6.4 ± 12.9 | 45.9 ± 21.0*** | <0.001 |
| Current smokers | 2 (22%) | 4 (40%) | 0.41 |
| Former smokers | 0 (0%) | 5 (56%) | 0.01 |
| Medications, n (%) | |||
| Antihypertensive | 4 (44%) | 3 (30%) | 0.52 |
| Inhaled corticosteroids | 0 | 1 (10%) | 0.33 |
| Short-acting β2 agonist | 0 | 2 (20%) | 0.16 |
| Long-acting muscarinic antagonist | 0 | 2 (10%) | 0.16 |
| Inhaled corticosteroid/long acting β2 agonist combination | 0 | 1 (10%) | 0.33 |
| Brachial systolic blood pressure, mmHg | 123 ± 14.2 | 133 ± 8.9 | 0.10 |
| Brachial diastolic blood pressure, mmHg | 65 ± 10.0 | 70 ± 6.0 | 0.20 |
| Brachial pulse pressure, mmHg | 60 ± 16.9 | 64 ± 10.2 | 0.48 |
| Carotid systolic blood pressure, mmHg | 116 ± 12.9 | 129 ± 9.2* | 0.02 |
| Carotid diastolic blood pressure, mmHg | 65 ± 9.4 | 70 ± 6.3 | 0.15 |
| Carotid pulse pressure, mmHg | 52 ± 17.1 | 60 ± 9.4 | 0.22 |
| Carotid augmentation index, % | 6.7 ± 12.8 | 9.8 ± 11.6 | 0.58 |
| Heart rate, beats/min | 72 ± 11.6 | 72 ± 11.4 | 0.94 |
| C-reactive protein, mg/l | 1.9 ± 1.4 | 3.2 ± 2.1 | 0.40 |
Data are expressed as means ± SD. COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Obstructive Lung Disease.
P < 0.05,
P < 0.01,
P < 0.001 vs. Controls.
Table 2.
Spirometry, lung computed tomography, and arterial data
| Controls (n = 9) | COPD (GOLD 1–3) (n = 10) | P Value | |
|---|---|---|---|
| Postbronchodilator FEV1% predicted | 110 ± 15.0 | 64 ± 15.8*** | <0.001 |
| Postbronchodilator FEV1/FVC, % | 79 ± 4.3 | 55 ± 8.5*** | <0.001 |
| % Air-trapping on lung CT (% of lung voxels −865 HU at RV) | 4.1 ± 3.9 | 17.7 ± 13.1** | <0.01 |
| Air-trapping on lung CT (Exp/Insp mean attenuation ratio) | 0.78 ± 0.1 | 0.85 ± 0.1* | 0.03 |
| % Emphysema on lung CT (% of lung voxels <−950 HU at TLC) | 1.3 ± 1.2 | 2.9 ± 2.8 | 0.14 |
| Radiologist qualitative rating of emphysema on lung CT, n (%) | 2 (22%) | 6 (60%) | 0.10 |
| Arterial Po2, mmHg | 84.6 ± 11.2 | 74.4 ± 10.3 | 0.06 |
| Arterial Pco2, mmHg | 38.3 ± 3.2 | 40.4 ± 5.6 | 0.35 |
| O2 saturation from pulse oximetry, % | 96.8 ± 1.5 | 95.9 ± 2.7 | 0.09 |
| 6MWT-total distance walked, ft | 1902.8 ± 271 | 1603.8 ± 400 | 0.08 |
| Carotid artery β-stiffness index, U | 10.6 ± 4.7 | 13.3 ± 5.1 | 0.26 |
| Carotid-femoral pulse wave velocity, cm/s | 760 ± 147.2 | 999 ± 292.7* | 0.04 |
| Carotid artery intimal-medial thickness, mm | 0.62 ± 0.1 | 0.68 ± 0.1 | 0.32 |
| Brachial artery FMD, %Δ | 6.8 ± 5.1 | 5.0 ± 2.1 | 0.32 |
| Brachial artery FMD absolute change, mm | 0.28 ± 0.2 | 0.21 ± 0.1 | 0.31 |
Data are expressed as means ± SD. CT, computed tomography; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume; FMD, flow-mediated dilation; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease; HU, Hounsfield units; Pco2, partial pressure of carbon dioxide; Po2, partial pressure of oxygen; RV, residual volume; TLC, total lung capacity; 6MWT, 6-min walk test.
P < 0.05,
P < 0.01,
P < 0.001 vs. Controls.
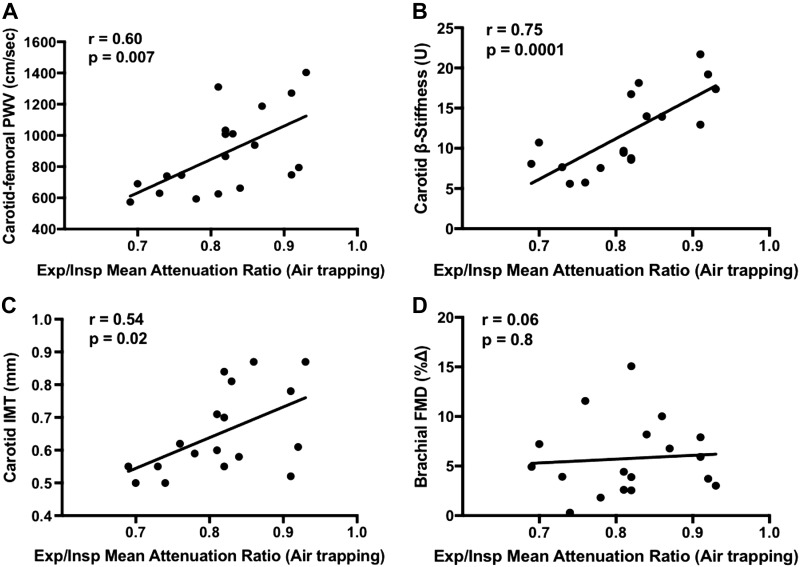
In the entire cohort (n = 19), lung air-trapping was associated with higher CFPWV (r = 0.60, P = 0.007) and carotid β-stiffness (r = 0.75, P = 0.0001) (Fig. 1). After adjusting for age, pack-years and FEV1, the correlation between lung air-trapping and carotid β-stiffness remained significant (r = 0.64, P = 0.01) (Table 3). There was no need to statistically adjust for BP in this partial correlation because carotid β-stiffness determines stiffness of the vessel wall independent of changes in carotid artery pressure (17). The relation between lung air-trapping and CFPWV remained significant after adjustment for BP and pack-years (r = 0.53, P = 0.03). However, when also adjusting for age and FEV1, the relation between lung-air trapping and CFPWV was abolished (r = 0.12, P = 0.67) (Table 3). Lung air-trapping was also associated with carotid intima-media thickness (IMT) (r = 0.54, P = 0.02); however, after adjusting for BP, age, pack-years, and FEV1, this relation was abolished (r = −0.12, P = 0.69). Lung air-trapping was not associated with brachial artery FMD (r = 0.06, P = 0.80) (Fig. 1). Carotid β-stiffness was not associated with 6-min walk test (6MWT) total distance walked (r = −0.42, P = 0.09), body mass index (BMI; r = −0.17, P = 0.5) or waist-to-hip ratio (r = 0.02, P = 0.93). CFPWV was significantly associated with 6MWT total distance walked (r = −0.67, P = 0.002), but not with BMI (r = 0.15, P = 0.55) or waist-to-hip ratio (r = −007, P = 0.79).
Fig. 1.
Unadjusted bivariate correlations between CT-measured lung air-trapping and carotid-femoral pulse wave velocity (PWV) (r = 0.60, P = 0.007) (A), carotid β-stiffness index (r = 0.75, P = 0.0001) (B), carotid intimal-medial thickness (IMT) (r = 0.54, P = 0.02) (C), and brachial artery flow-mediated dilation (FMD) (r = 0.06, P = 0.8) (D) in the entire cohort (n = 19).
Table 3.
Partial correlations between lung air-trapping on CT with arterial stiffness in COPD patients and controls
| Carotid β-Stiffness Index | Carotid-Femoral PWV | Carotid IMT | |
|---|---|---|---|
| COPD and Controls | |||
| Air-trapping (Exp/Insp mean attenuation ratio) adjusting for age, MAP, pack-years and FEV1 | 0.64* | 0.12 | −0.12 |
| Air-trapping (% of lung voxels <−865 HU at RV) adjusting for age, MAP, pack-years, and FEV1 | 0.71** | −0.10 | −0.39 |
| COPD only | |||
| Air-trapping (Exp/Insp Mean attenuation ratio) adjusting for age, MAP, pack-years, and FEV1 | 0.82* | 0.12 | 0.05 |
| Air-trapping (% of lung voxels <−865 HU at RV) adjusting for age, MAP, pack-years, and FEV1 | 0.91* | −0.16 | −0.22 |
Data are partial correlation coefficients for β-stiffness and pulse wave velocity (PWV). Carotid β-stiffness was not adjusted for mean arterial pressure (MAP) because it is pressure independent. COPD, chronic obstructive pulmonary disease; CT, computed tomography; Exp, expiratory; FEV1, forced expiratory volume; HU, Hounsfield units; IMT, intima-media thickness; Insp, inspiratory; RV, residual volume.
P < 0.05,
P < 0.01.
In the individuals with COPD only (n = 10), the relation between lung air-trapping and carotid β-stiffness was stronger and remained significant after adjusting for age, pack-years, and FEV1 (r = 0.82, P = 0.05). Similar to the findings in the whole cohort, after adjusting for age, BP, pack-years, and FEV1, the relation between lung air-trapping and CFPWV was nonsignificant in this group (r = 0.12, P = 0.83) (Table 3). Lung air-trapping was also not associated with carotid IMT after adjusting for BP, age, pack-years, and FEV1 (r = 0.05, P = 0.94). Additionally, there was no relation between lung air-trapping and brachial artery FMD in the COPD group (r = 0.14, P = 0.71). Carotid β-stiffness was not associated with 6MWT total distance walked (r = −0.35, P = 0.35), BMI (r = −0.23, P = 0.55) or waist-to-hip ratio (r = 0.04, P = 0.93) in individuals with COPD. CFPWV was not associated with 6MWT total distance walked (r = −0.57, P = 0.08), BMI (r = 0.20, P = 0.59), or waist-to-hip ratio (r = −0.21, P = 0.55).
DISCUSSION
The major and novel finding of the present study was that CT-quantified lung air-trapping was positively associated with carotid artery stiffness independent of BP, age, pack-years, and FEV1 among individuals with COPD. Furthermore, in individuals with and without COPD, lung air-trapping was positively associated with both aortic and carotid artery stiffness and remained significant after adjusting for BP and smoking history. However, only the association between lung air-trapping and carotid artery stiffness remained significant after adjustment for age and FEV1. Overall, these data suggest a potential link between the amount of lung air-trapping and high CVD risk among individuals with COPD with little or no emphysema.
Although individuals with COPD are typically considered one clinical phenotype varying only by the degree of airway obstruction and GOLD criteria, COPD is now recognized as a heterogeneous disorder, including both emphysema and airway-predominant phenotypes (21). Therefore, it is necessary to determine to what extent large elastic artery stiffness contributes to overall CVD risk in specific COPD phenotypes. Previous studies have demonstrated that among individuals with an emphysema-predominant phenotype of COPD, aortic stiffness is greater than non-COPD controls and is associated with emphysema severity (12, 22, 24). In the present study, we demonstrate that even before overt CT-quantified emphysema manifests, lung air-trapping is strongly associated with carotid artery stiffness in COPD patients.
Among individuals with an airway-predominant phenotype of COPD, the small conducting airways experience structural remodeling, including increased airway wall thickness, heightened airway inflammation, and a greater concentration of mucus exudates in the airway lumen. This structural remodeling causes a narrowing of the small airways and, therefore, becomes a major site of resistance to airflow (18, 23). Importantly, individuals with an airway-predominant phenotype of COPD are at greater CVD risk compared with patients with a predominant emphysema phenotype because of the higher prevalence of CVD-related comorbidities in the airway-predominant phenotype (6). Thus, the findings from the present study suggest that carotid artery stiffness may contribute to the greater CVD risk demonstrated among individuals with COPD with an airway-predominant phenotype, including an increased risk of stroke. Previous studies have demonstrated that carotid artery stiffness is a known independent predictor of first incident stroke and that individuals with COPD have both a higher prevalence and incidence of stroke compared with non-COPD controls (28, 36). Although speculative, the present findings suggest that lung air-trapping may be associated with an increased risk of stroke in airway-predominant phenotypes of COPD because of the association between lung air-trapping and carotid artery stiffness in the present study. However, further studies are needed to support this relation.
Endothelial dysfunction is a common feature of COPD, and its presence in the pulmonary vasculature has been proposed as a mechanism that contributes to disease progression (31). In addition, systemic endothelium-dependent dilation, measured by brachial artery FMD, is lower in individuals with COPD compared with controls and is suggested to contribute to the heightened CVD risk in this group (19, 31). Importantly, the relation between brachial artery FMD and FEV1 is linear across all stages of lung function, including those with both mild and severe COPD (3). Therefore, given the evidence suggesting that endothelial dysfunction is present, even in mild COPD, we hypothesized that greater lung air-trapping would be independently associated with lower endothelial function. However, in contrast to our hypothesis, there was no evidence of an association between lung air-trapping and endothelial function. A possible explanation for this is the influence of current smoking status on endothelial function. In the present study, 2 non-COPD controls and 4 COPD patients were current smokers. Thus, it is plausible that current smoking in non-COPD patients with less air-trapping could have confounded the results because smoking is a known, independent risk factor for endothelial dysfunction (2, 13). Unfortunately, this could not be determined in the present study.
The results of the present study should be interpreted in the context of several limitations. First, the cross-sectional nature of the present study prevented determination of the causal relation between lung air-trapping and carotid artery stiffness. Second, by design, the sample size of the study was limited and only included participants with lung air-trapping and little or no emphysema. Additional studies are needed to compare large elastic artery stiffness and endothelial function between airway and emphysema-predominant phenotypes. Third, the control group in the present study had significantly lower pack-years than individuals with COPD. The relation between lung air-trapping and carotid artery and aortic stiffness remained significant after statistically adjusting for pack-years. However, further studies are needed to compare this relation between individuals with COPD and non-COPD controls that are matched for smoking history. Finally, although participation of minorities was encouraged, our study included non-Hispanic Caucasians; therefore, further studies among persons of non-Caucasian and Hispanic race/ethnicity are needed.
In summary, the results of the present study demonstrate that lung air-trapping is significantly associated with carotid artery stiffness in individuals with COPD, independent of age, BP, pack-years, and FEV1. Among individuals with and without COPD, lung air-trapping was associated with carotid artery and aortic stiffness; however, the relation between lung air-trapping and aortic stiffness was mediated by age and FEV1. In contrast, there was no evidence of an association between lung air-trapping and endothelial function. Overall, our results suggest that carotid artery stiffness may be a mechanism linking, in part, the higher CVD risk observed with airway-predominant phenotypes of COPD. Further studies are needed to determine the intermediary mechanism linking lung air-trapping with carotid artery stiffness, such as inflammation and sympathetic nerve activity.
GRANTS
This work was supported by National Institutes of Health grants K23 HL-095658 (to K. F. Hoth), R01 HL-08989 (to James Crapo and Edwin Silverman), U54TR001356 (University of Iowa), R21 AG043722 (to G. L. Pierce), American Heart Association 13SDG143400012 (to G. L. Pierce) and University of Iowa Departments of Psychiatry and Radiology; Magnetic Resonance Research Facility pilot award (to K. F. Hoth) and Environmental Health Science Research Center career development award and NIH P30 ES-005605 (to G. L. Pierce).
DISCLOSURES
Dr. John D Newell, Jr, is a paid consultant for VIDA Diagnostics. Eric Hoffman is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software, which was developed, in part, at the University of Iowa. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.F.H. and G.L.P. conceived and designed research; R.E.L., L.E.D., and G.L.P. analyzed data; R.E.L., J.D.N., A.P.C., G.L.P., and K.F.H. interpreted results of experiments; R.E.L. prepared figures; R.E.L. drafted manuscript; R.E.L., J.D.N., A.P.C., E.A.H., K.W., A.C., L.E.D., P.N., V.M., S.A., G.L.P., and K.F.H. edited and revised manuscript; R.E.L., J.D.N., A.P.C., E.A.H., K.W., A.C., L.E.D., P.N., V.M., S.A., G.L.P., and K.F.H. approved final version of manuscript; K.W., L.E.D., G.L.P., and K.F.H. performed experiments.
ACKNOWLEDGMENTS
We thank Joel Bruss, Dr. Abbi Lane, Deb O'Connell-Moore, Eric Axelson, Harold Winnike, Jered Siren, Melissa Taylor, and Mark Escher and the University of Iowa Institute for Clinical and Translational Science Clinical Research Unit nurses/staff for assistance.
REFERENCES
- 1.Albu A, Fodor D, Bondor C, Suciu O. Carotid arterial stiffness in patients with chronic obstructive pulmonary disease. Acta Physiol Hung 98: 117–127, 2011. doi: 10.1556/APhysiol.98.2011.2.3. [DOI] [PubMed] [Google Scholar]
- 2.Amato M, Frigerio B, Castelnuovo S, Ravani A, Sansaro D, Tremoli E, Squellerio I, Cavalca V, Veglia F, Sirtori CR, Werba JP, Baldassarre D. Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis 228: 153–160, 2013. doi: 10.1016/j.atherosclerosis.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med 176: 1200–1207, 2007. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camiciottoli G, Bigazzi F, Magni C, Bonti V, Diciotti S, Bartolucci M, Mascalchi M, Pistolesi M. Prevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 11: 2229–2236, 2016. doi: 10.2147/COPD.S111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 133: 795–800, 1991. doi: 10.1093/oxfordjournals.aje.a115958. [DOI] [PubMed] [Google Scholar]
- 8.Clarenbach CF, Senn O, Sievi NA, Camen G, van Gestel AJ, Rossi VA, Puhan MA, Thurnheer R, Russi EW, Kohler M. Determinants of endothelial function in patients with COPD. Eur Respir J 42: 1194–1204, 2013. doi: 10.1183/09031936.00144612. [DOI] [PubMed] [Google Scholar]
- 9.Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, Hallstrand TS, Hankinson JL, Kaminsky DA, MacIntyre NR, McCormack MC, Rosenfeld M, Stanojevic S, Weiner DJ; ATS Committee on Proficiency Standards for Pulmonary Function Laboratories . Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 196: 1463–1472, 2017. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty TM. Quantitative computed tomography based measures of vascular dysfunction for identifying COPD phenotypes and subphenotypes (MS thesis). Iowa City: The University of Iowa, 2016. (http://www.ir.uiowa.edu/etd/2069). [Google Scholar]
- 11.DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova A, Sigurdsson G, Schmid P, Barlow PB, Pierce GL. Carotid β-stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle-aged/older adults. J Appl Physiol (1985) 122: 868–876, 2017. doi: 10.1152/japplphysiol.00769.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duckers JM, Shale DJ, Stockley RA, Gale NS, Evans BA, Cockcroft JR, Bolton CE. Cardiovascular and musculskeletal co-morbidities in patients with alpha 1 antitrypsin deficiency. Respir Res 11: 173, 2010. doi: 10.1186/1465-9921-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esen AM, Barutcu I, Acar M, Degirmenci B, Kaya D, Turkmen M, Melek M, Onrat E, Esen OB, Kirma C. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J 68: 1123–1126, 2004. doi: 10.1253/circj.68.1123. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh CP, Washko GR, Estépar RS, Lutz S, Friedman PJ, Han MK, Hokanson JE, Judy PF, Lynch DA, Make BJ, Marchetti N, Newell JD Jr, Sciurba FC, Crapo JD, Silverman EK; COPDGene Investigators . Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res 14: 42, 2013. doi: 10.1186/1465-9921-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80: 78–86, 1989. doi: 10.1161/01.CIR.80.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 19.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63: 459–467, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Symptoms of chronic bronchitis and the risk of coronary disease. Lancet 348: 567–572, 1996. doi: 10.1016/S0140-6736(96)02374-4. [DOI] [PubMed] [Google Scholar]
- 21.Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, Barr RG, Colby TV, Galvin JR, Gevenois PA, Coxson HO, Hoffman EA, Newell JD Jr, Pistolesi M, Silverman EK, Crapo JD. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 277: 192–205, 2015. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, Newby DE, Murchison JT, Macnee W. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176: 1208–1214, 2007. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 365: 1567–1575, 2011. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, Macnee W. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180: 513–520, 2009. doi: 10.1164/rccm.200903-0414OC. [DOI] [PubMed] [Google Scholar]
- 25.Mills NL, Miller JJ, Anand A, Robinson SD, Frazer GA, Anderson D, Breen L, Wilkinson IB, McEniery CM, Donaldson K, Newby DE, Macnee W. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax 63: 306–311, 2008. doi: 10.1136/thx.2007.083493. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 3: 56–64, 2009. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan AD, Sharma C, Rothnie KJ, Potts J, Smeeth L, Quint JK. Chronic obstructive pulmonary disease and the risk of stroke. Ann Am Thorac Soc 14: 754–765, 2017. doi: 10.1513/AnnalsATS.201611-932SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS; GOLD Scientific Committee . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 46: 798–825, 2001. [PubMed] [Google Scholar]
- 30.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polverino F, Celli BR, Owen CA. COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm Circ 8: 2045894018758528, 2018. doi: 10.1177/2045894018758528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qvist L, Nilsson U, Johansson V, Larsson K, Rönmark E, Langrish J, Blomberg A, Lindberg A. Central arterial stiffness is increased among subjects with severe and very severe COPD: report from a population-based cohort study. Eur Clin Respir J 2: 27023, 2015. doi: 10.3402/ecrj.v2.27023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD 7: 32–43, 2010. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J 28: 1245–1257, 2006. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 36.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Stehouwer CDA. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol 66: 2116–2125, 2015. doi: 10.1016/j.jacc.2015.08.888. [DOI] [PubMed] [Google Scholar]
- 37.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 38.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]