Abstract
Ultrasound processing can result in cell wall disruption, facilitating the release of the cellular content. Therefore, we hypothesized that sonication of vegetables could be used as a pre-treatment to increase the bioaccessibility of phenolic and antioxidant compounds. Overall, sonication (40 kHz, 250 W, 4 °C, 20 min) did not affect the main physicochemical parameters of tomato, lettuce, zucchini, and green and red pepper (p < 0.05). The polyphenolic content and antioxidant activity of digestive enzymatic extracts was higher than that of water:methanol extracts (p < 0.05). In addition, sonication resulted in increased bioaccessibility of phenolic compounds in lettuce and green pepper (p < 0.05), while no effect was observed for tomato, red pepper, and zucchini samples suggesting a matrix-dependent effect. The amount of phenolic compounds and antioxidants released by vegetables during a simulated gastrointestinal digestion may be higher than the one that can be expected from measurements in usual aqueous-organic extracts.
Keywords: Ultrasound processing, Processed fruits and vegetables, Bioaccessibility, Antioxidant activity, Polyphenols
Introduction
Minimally processed vegetables include ready-to-eat salads containing lettuce and other vegetables, which are generally disinfected using chlorine before being packed and distributed. However, chlorine has been prohibited in Belgium, Denmark, Germany, the Netherlands, and other European countries because it has been identified as a concern to public health (Meireles et al., 2016). Moreover, vegetable processing generally decreases the nutritional value and bioactive properties of foods in comparison to fresh produce. Previous studies reported, for example, the loss of antioxidant capacity, vitamin C, or phenolic compounds in vegetables after processing (Lafarga et al., 2018a). For these reasons, several non-thermal strategies, which are environmentally friendly and safe, have been developed for the disinfection of vegetables.
Ultrasound (US) processing alone, or in combination with chemical aids, has been proposed as an initial step to improve safety while maintaining the sensory and nutritional characteristics of vegetables (São José et al., 2014). For example, Sagong et al. (2013) compared the effectiveness of US treatment alone or in combination with surfactants as an alternative method to chlorine for reducing numbers of Bacillus cereus spores on lettuce and carrots. The authors of that study reported that operating at 40 kHz and 30 W/L for 5 min, in combination with 0.1% (w/v) Tween 20, resulted in no damage on the products surface and reduced B. cereus spores by 2.49- and 2.22-log CFU/g on lettuce and carrots, respectively. In addition, Lafarga et al. (2019b) and Tiwari et al. (2010) suggested that sonication could be utilized as a preservation technique for fruit juice processing where retention of polyphenols is desired.
Vegetables are rich in phytochemicals with antioxidant and other bioactive properties. In order to exert their physiological effect in vivo, bioactive compounds found in fruits and vegetables must be resistant to food processing and digestion. Bioavailability quantifies the proportion of a compound which is absorbed and available to produce systemic effects upon ingestion (Toutain and Bousquet-Mèlou, 2004). Bioaccessibility is one of the main factors affecting bioavailability, and is defined as the amount of a certain compound that is released from the food matrix into the gastrointestinal tract and is thus, available for absorption (Žugčić et al., 2019). In vitro approaches are of key importance, not only to predict in vivo effects after ingestion of foods, but to better understand the relationship between processing and bioactive compound bioavailability (van Buggenhout et al., 2010). However, although large amount of information is available on the effects of US processing on the physicochemical properties of vegetables and on the bioactive and nutritional properties of water:organic vegetable-derived extracts, data on the effects of processing on the bioaccessibility of these compounds are scarce. It is known that processing can modify the bioaccessibility of health-promoting compounds. For example, Rodríguez-Roque et al. (2015) demonstrated that high pressure processing of a milk-fruit juice increased the bioaccessibility of vitamin C by 8%. However, the effect of processing on the bioaccessibility of a certain compound or groups of compounds needs to be assessed independently as it highly depends on factors including food matrix and the bioactive compounds’ structure (Barba et al., 2017).
The amount of polyphenols and other antioxidant compounds in the in vitro digestive enzymatic extracts, obtained by enzymatic treatments that mimic conditions in the gastrointestinal tract, might be higher than the amounts expected from determinations in aqueous:organic extracts (Chen et al., 2014). Although these non-extractable antioxidant compounds are generally overlooked in the literature, these may have an antioxidant role in the human body. Therefore, the main aim of this study was to assess the effect of sonication of whole vegetables on their total phenolic content (TPC) and antioxidant capacity after a simulated gastrointestinal digestion. In addition, the current paper also compared the TPC and antioxidant capacity of enzymatic and water:methanol extracts of vegetables.
Materials and methods
Vegetable processing
Vegetables, listed in Table 1, were washed with tap water to remove dirt and left to dry at room temperature. Samples were further divided into two lots. One was dipped in tap water at 4 °C for 20 min. The other one was processed using an ultrasonic bath (JP Selecta S.A., Barcelona, Spain) operating at 4 °C, 40 kHz, and 250 W for 20 min. Immediately after processing, samples were left to dry at room temperature for approximately 1 h. The edible parts of selected vegetables were cut into small pieces, frozen using liquid nitrogen, and blended using a MINIMOKA GR-020 grinder (Taurus Group, Barcelona, Spain). Samples were stored at − 20 °C until further analysis.
Table 1.
Effect of ultrasound processing on the physicochemical parameters of selected vegetables
| Raw material | Treatment | L* | a* | b* | Ch | δE | pH | TTA (mg/L) | SSC (° Brix) |
|---|---|---|---|---|---|---|---|---|---|
| Tomato | Untreated | 43.25 ± 1.41 | 14.90 ± 0.14 | 22.47 ± 1.38 | 26.97 ± 1.22 | – | 4.87 ± 0.06 | 2.94 ± 0.05 | 5.10 ± 0.00 * |
| Sonicated | 42.88 ± 0.76 | 14.84 ± 0.18 | 22.13 ± 1.13 | 26.65 ± 1.03 | 1.06 ± 0.76 | 4.83 ± 0.06 | 2.94 ± 0.02 | 5.15 ± 0.03 * | |
| Lettuce | Untreated | 59.94 ± 2.49 | − 20.88 ± 0.64 | 31.95 ± 1.17 | 38.17 ± 1.33 | – | 6.42 ± 0.01 | 0.33 ± 0.00 | 2.17 ± 0.06 |
| Sonicated | 60.76 ± 2.41 | − 21.13 ± 0.18 | 32.87 ± 0.27 | 39.08 ± 0.30 | 2.26 ± 0.79 | 6.42 ± 0.00 | 0.33 ± 0.01 | 2.22 ± 0.02 | |
| Green pepper | Untreated | 42.21 ± 1.95 | − 15.48 ± 0.98 | 21.47 ± 2.17 | 26.47 ± 2.27 | – | 6.48 ± 0.01 | 0.66 ± 0.02 | 4.87 ± 0.06 |
| Sonicated | 42.33 ± 1.03 | − 15.31 ± 1.37 | 21.80 ± 2.39 | 26.65 ± 2.68 | 2.39 ± 0.53 | 6.48 ± 0.01 | 0.65 ± 0.03 | 4.95 ± 0.05 | |
| Red pepper | Untreated | 41.79 ± 1.83 | 26.91 ± 2.35 | 22.39 ± 5.55 | 35.16 ± 4.50 | – | 5.03 ± 0.01 | 1.82 ± 0.05 | 6.03 ± 0.06 |
| Sonicated | 41.17 ± 0.94 | 26.82 ± 2.17 | 21.86 ± 1.68 | 36.64 ± 1.65 | 2.49 ± 0.34 | 5.03 ± 0.01 | 1.84 ± 0.04 | 6.07 ± 0.06 | |
| Zucchini | Untreated | 36.96 ± 0.49 | − 8.75 ± 1.33 | 12.37 ± 1.68 | 15.16 ± 2.14 | – | 6.40 ± 0.01 | 0.67 ± 0.07 | 4.87 ± 0.06 |
| Sonicated | 36.41 ± 1.05 | − 9.00 ± 1.75 | 11.57 ± 0.40 | 14.69 ± 1.39 | 1.95 ± 1.22 | 6.41 ± 0.01 | 0.65 ± 0.02 | 4.93 ± 0.03 |
TTA, titratable acidity; SSC, soluble solids content; δE, difference from the control; and Ch, chroma
*Significant differences between treatments. The criterion for statistical significance was p < 0.05
Colour measurement
Surface colour was recorded using a Minolta CR-200 colorimeter (Minolta INC, Tokyo, Japan). Calibration was made using a standard white tile (Y:92.5, x:0.3161, y:0.3321). The D65 illuminant, which approximates to daylight, was used. Chroma (Ch) and difference from the control (δE) were calculated using the following equations:
where , , and are the colour parameters of the untreated vegetables and , , and the colour parameters of the samples after sonication. Results are the average of 10 measurements per replicate.
Soluble solids content, pH, and titratable acidity
Soluble solids content (SSC) was measured at 20 °C with a handheld refractometer (Atago Co., Ltd., Tokio, Japan) in juices extracted using an Infinity Cold Press Revolution Juicer (Groupe SEB Iberica, Barcelona, Spain). Measurements were performed in triplicate per treatment and replicate and results were expressed in ºBrix. The pH was measured using a Basic 20 pH meter (Crison Instruments S.A., Barcelona, Spain). To measure titratable acidity (TTA), 10 mL of juice were diluted in 10 mL of distilled water and were titrated with 0.1 N NaOH up to pH 8.2. Results were expressed as g of malic acid per L and were determined in triplicate.
Total phenolic content
The TPC was determined by the Folin Ciocalteu method as described by Lafarga et al. (2018b). Samples were homogenized for 1 min using a T-25 digital ULTRA-TURRAX® homogenizer (IKA, Staufen, Germany) at 14,000 rpm. Extraction was held using 70% (v/v) methanol at room temperature and constant shaking for 30 min. TPC was determined in triplicate using a GENESYS™ 10S-UV Vis spectrophotometer (Thermo Fisher Scientific, MA, USA) and results were expressed as mg of gallic acid equivalents per 100 g of fresh weight (FW).
Antioxidant activity
Antioxidant activity was assessed using two different methods: the ferric reducing antioxidant power (FRAP) and the DPPH· scavenging activity assays following the methodologies previously described by Lafarga et al. (2018b). Antioxidant activity was determined in triplicate and results were expressed as mg of ascorbic acid equivalents per 100 g of FW.
Simulated gastrointestinal digestion
A simulated gastrointestinal digestion of processed and unprocessed samples was carried at following the methodology previously described by Minekus et al. (2014), which is an international consensus and includes three sequential phases: (i) Oral phase (pH 7.0, α-amylase, 2 min); (ii) Gastric phase (pH 3.0, pepsin, 2 h); and (iii) Intestinal phase (pH 7.0, pancreatin and fresh bile, 2 h). The pancreatin used contained enzymatic components which included trypsin, amylase and lipase, protease, and ribonuclease which allow hydrolysing proteins, fats, and starch. The simulated digestion was carried out at 37 °C and constant agitation at 150 rpm. A blank was prepared by using distilled water instead of the sample and following the exact same procedure.
Statistical analysis
Results are the average of three independent experiments ± standard deviation (S.D.). Difference between extraction methods, as well as processing were analysed using analysis of variance (ANOVA) with JMP 13 (SAS Institute Inc., Cary, USA). A Tukey pairwise comparison of the means was conducted when differences were present (p < 0.05).
Results and discussion
Effect of US processing on the physicochemical properties of selected vegetables
Table 1 lists physicochemical parameters of control and US processed vegetables. Surface colour parameters suggested that US processing had no effect on the lightness of selected vegetables as compared with fresh vegetables. In addition, US processing of selected vegetables did not affect Ch, a quantitative indicator of colourfulness. This indicates a comparable colour intensity after and before processing of vegetables. Previous studies suggested that although US processing does not generally affect colour parameters of fruits and vegetables, sonication can prevent loss of colour attributes during storage. Indeed, US processing of lettuce at 25 kHz for 3 min resulted in no significant differences on colour parameters including L* of the lettuce surface and cutting edge 90 h after processing (Yu et al., 2016). Luna-Guevara et al. (2015) observed a total colour change in US processed tomatoes after a 5-day storage period when compared with the untreated control. Moreover, the δE combines the change in L*, a*, and b* values to quantify the colour deviation from a standard reference sample, in this case, untreated and US processed vegetables. As expected, in the current study, all the control and processed samples showed a δE < 3, suggesting no visible colour deviation between treatments. Overall, results obtained herein compare well with previous reports which suggested no major changes in colour parameters of fruit and fruit-derived products after sonication. Similar results were observed for firmness values (data not shown).
Regarding pH and TTA, US processing did not affect the pH or the TTA of selected vegetables (Table 1). However, a significant increase was measured in the SSC of tomatoes when compared with untreated controls (p < 0.05). Although this trend was also observed for other vegetables, the observed differences were not statistically significant. Similar results were obtained by Ding et al. (2015), who observed no differences in the pH and other physicochemical properties of cherry tomatoes after US processing (40 kHz, 10 min). Moreover, Pokhrel et al. (2017) recently reported no effect on the pH, TTA, and SSC of carrots after US processing (24 kHz, 10 min) and Adekunte et al. (2010) reported no differences between the pH, SSC, and TTA of US processed and control tomato samples. Alexandre et al. (2013) also reported comparable results after US processing (35 kHz, 120 W) of red bell peppers.
Effect of US processing on the nutritional value of selected vegetables
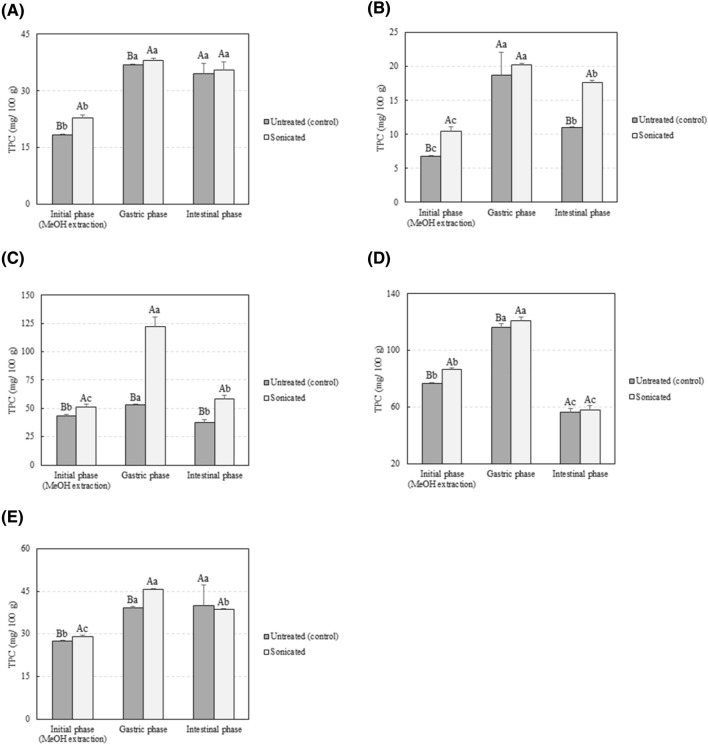
Figure 1 shows the effect of US processing on the TPC of selected vegetables. The TPC of the analysed samples was comparable to that obtained in previous studies. Fresh red pepper followed by green pepper showed the highest TPC calculated as 76.76 ± 0.38 and 43.56 ± 0.92 mg/100 g, respectively (p < 0.05). Results suggest an increase in the TPC of the water:methanol extracts obtained from processed vegetables when compared to those obtained from untreated samples (p < 0.05). The observed increase in polyphenols can be attributed to an improved extraction efficiency. The cavitation and disrupting properties of US processing have been repeatedly used for the enhancement of polyphenol extraction or solubilisation (Zinoviadou et al., 2015). Sonication has been suggested to be a useful technique for increasing the extraction yield of valuable compounds such as lycopene from tomatoes (Eh and Teoh, 2012). Indeed, the TPC in the water:methanol extracts of US processed tomato, zucchini, lettuce, green pepper, and red pepper was 25.1, 9.8, 54.6, 17.6, and 12.6% higher regarding the amount obtained in control samples. Similar results were observed by Yu et al. (2016) after US processing of lettuce at 25 kHz. In that study, the TPC of lettuce extracts increased by 35.3 and 26.7%, when compared with untreated samples, after processing for 1 or 2 min, respectively. Results were also comparable to those previously reported by Aadil et al. (2013), who found that the TPC of grapefruit juices was improved after US treatments (28 kHz, 90 min), and to those reported by Zafra-Rojas et al. (2013), who observed a higher release of phenolic compounds after sonication of purple cactus pear.
Fig. 1.
Total phenolic content of (A) tomato, (B) lettuce, (C) green pepper, (D) red pepper, and (E) zucchini samples before and after the in vitro gastroinstestinal digestion. Values represent mean values of three independent experiments ± S.D. Different capital letters indicate significant differences between sonicated and unprocessed samples. Different lower case letters indicate significant differences between digestive phases. The criterion for statistical significance was p < 0.05
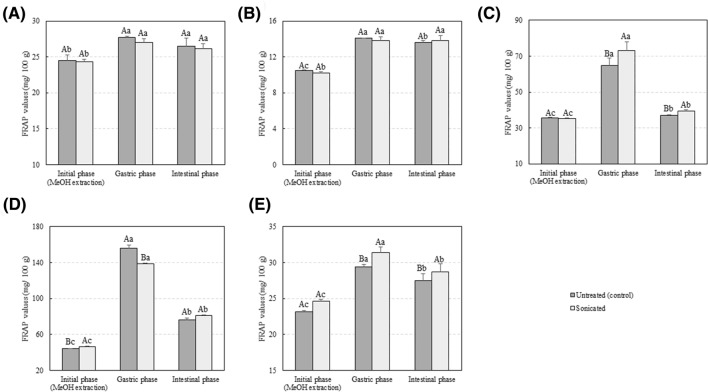
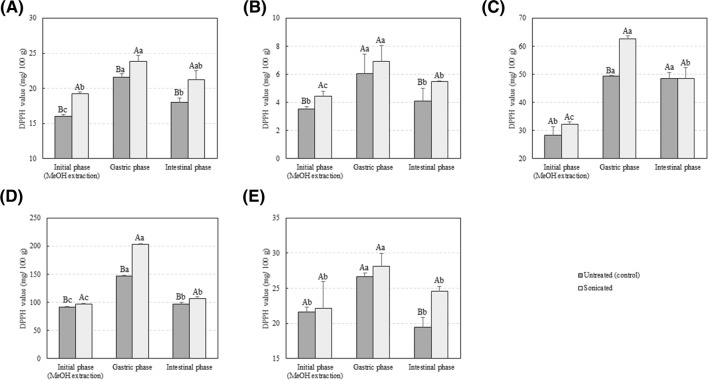
In addition, Figs. 2 and 3 show the effect of US processing on the antioxidant activity of extracts from selected vegetables when assessed using the FRAP or DPPH assay, respectively. US processing did not affect the FRAP values for tomato, lettuce, and green pepper extracts. However, a significant increase in the antioxidant activity of the water:methanol extracts, when assessed using the FRAP assay was observed after processing of red pepper and zucchini (p < 0.05). Results obtained after assessment of antioxidant activity using the DPPH· revealed higher DPPH· activity values after processing of tomato, lettuce, and red pepper (p < 0.05). Although the same trend was detected after US processing of zucchini and green pepper, differences were not statistically significant. Results were in line with those published by Yu et al. (2016) who observed an increase in the DPPH· activity of lettuce after US processing (25 kHz, 1–3 min). Selected vegetables are rich in phenolic compounds. The observed increase in antioxidant activity could be caused by a higher TPC in the extracts of processed samples when compared with the controls. Phenolic compounds comprise a wide variety of phytochemicals, which can be divided into flavonoids, phenolic acids, tannins (hydrolysable and condensed), stilbenes, and lignans. Tomatoes are also rich in lycopene, which has strong antioxidant capacity and has been associated with the prevention of cardiovascular diseases (Müller et al., 2015). Juániz et al. (2016) identified a total of 21 polyphenolic compounds (free and bound) in raw green pepper in concentrations ranging from 0.043 µmol/g of dry weight for quercetin-3-glucoside-7-rhamnoside to 7.912 µmol/g for quercetin rhamnoside, respectively. Pérez-López et al. (2018) also identified several phenolic compounds in lettuce. The most relevant phenols identified in that study of were the flavonoids quercetin-3-O-glucoside, querceting-3-O-glucuronide, and luteolin-7-O-glucoside. Other flavonoids and phenolic acids were identified at lower concentrations including 3,5,7,4′-tetrahydroxyflavone (kaempferol), 3,5,7,3′,4′,5′-hexahydroxyflavone (myricetin), and quercetin-3-O-rutinoside (rutin). Lettuce and pepper are rich sources of flavonoids (Ignat et al., 2011). In the current study, a positive correlation was observed between FRAP or DPPH· values and the TPC of unprocessed vegetables (r2 = 0.9082 and r2 = 0.9316, respectively). Similar results were obtained for US processed samples: r2 = 0.9322 and r2 = 0.9336 between the TPC and the antioxidant activity when assessed using the FRAP and DPPH· assay, respectively. The correlations between TPC and antioxidant activity were in accordance with the results reported by other researchers (Chen et al., 2014; Fu et al., 2011; Muñiz-Márquez et al., 2013). Overall, results suggest that US processing can promote the extraction of polyphenolic compounds from tomato, lettuce, green pepper, red pepper, and zucchini and that these compounds largely contribute to the antioxidant capacities measured in vitro. However, both the FRAP and DPPH assays are radical scavenging methods which measure single electron transfer from potential antioxidants to free radicals in simple and lipid-free systems. These methods are cheap, simple, and quick and provide important information on the intrinsic antioxidant capacity of potential antioxidants with minimum environmental impact. For these reasons these methods are widely used for initial screening and evaluation of novel antioxidant compounds or extracts. However, these methods are based on their scavenging activity against radical species which can be artificial or biologically irrelevant, and have been criticized for not fully reflecting what happens in in vivo situations (Shahidi and Zhong, 2015). Therefore, further studies in food model systems are needed in order to fully understand the real antioxidant capacity of the obtained extracts.
Fig. 2.
Antioxidant capacity, assessed using the FRAP assay, of (A) tomato, (B) lettuce, (C) green pepper, (D) red pepper, and (E) zucchini samples before and after the in vitro gastroinstestinal digestion. Values represent mean values of three independent experiments ± S.D. Different capital letters indicate significant differences between sonicated and unprocessed samples. Different lower case letters indicate significant differences between digestive phases. The criterion for statistical significance was p < 0.05
Fig. 3.
Antioxidant capacity, assessed using the DPPH assay, of (A) tomato, (B) lettuce, (C) green pepper, (D) red pepper, and (E) zucchini samples before and after the in vitro gastroinstestinal digestion. Values represent mean values of three independent experiments ± S.D. Different capital letters indicate significant differences between sonicated and unprocessed samples. Different lower case letters indicate significant differences between digestive phases. The criterion for statistical significance was p < 0.05
Resistance of health-promoting compounds to gastrointestinal degradation in vitro
Little is known on the effect of novel non-thermal technologies such as US processing on the bioaccessibility of phenolic compounds and other antioxidants. Previous studies suggested that literature data may underestimate the actual antioxidant capacity of foods as, for example, the TPC of digestive enzymatic extracts of baked goods and fruits was higher to that of their conventional extracts obtained using organic solvents (Chen et al., 2014; Lafarga et al., 2019a). Overall, the TPC and the antioxidant activity of sonicated and control samples were both higher after the gastric phase when compared with the initial value measured from a methanol:water extract (p < 0.05). For example, the TPC of fresh lettuce increased from 6.80 ± 0.03 mg/100 g in the water:methanol extract to 18.69 ± 3.37 mg/100 g after the gastric phase (p < 0.05). Results were comparable to those reported by Chen et al. (2014) who obtained the TPC of cantaloupe at the initial, gastric, and intestinal phases of digestion as 56.27 ± 0.73, 131.55 ± 1.72, and 106.64 ± 1.05 mg/100 g, respectively. Different pH values can affect the biological activities of polyphenols and may render antioxidants more reactive particularly at acidic pH values (Jamali et al., 2008). The longer extraction process, if compared to values prior to digestion (those obtained after a methanol:water extraction), may also partially explain these findings. FRAP and DPPH· values correlated well to those obtained for TPC. The higher antioxidant activity after the gastric and intestinal phases could be attributed to the increase in the polyphenolic content when compared to with the initial stage. Indeed, a positive correlation was observed between FRAP or DPPH· values and the TPC of unprocessed vegetables after the gastric stage of digestion (r2 = 0.9545 and r2 = 0.8908, respectively). Similar correlation results were obtained for US processed samples (r2 = 0.7975 and r2 = 0.6143) between the TPC and the antioxidant activity when assessed using the FRAP and DPPH· assays, respectively. In addition, correlation results demonstrated a positive correlation between FRAP values and the TPC of unprocessed vegetables after the intestinal stage of digestion (r2 = 0.9545). Similar results were observed for US-treated samples: r2 = 0.6135 and r2 = 0.8093 between FRAP or DPPH· values and the TPC after the intestinal phase, respectively. Chen et al. (2014) also observed higher DPPH· and FRAP values after the in vitro intestinal phase of digestion when compared with those measured for ethanol:water (50:50; v/v) extracts. In that study, the DPPH· values for tomato were 18.2 ± 0.4 and 41.1 ± 0.4 µmol/g when measured from the ethanol:water extract and the digestive enzymatic extract, respectively. Antioxidant compounds not extractable using conventional organic:water extractions could have an antioxidant role in the gastrointestinal tract. The TPC and antioxidant activity decreased after the intestinal phase, when compared with the gastric phase of digestion. Polyphenols are highly sensitive to alkaline conditions and could have been degraded by the alkaline pH, thus leading to the observed loss in the TPC and the antioxidant capacity (Bermúdez-Soto et al., 2007).
Although the TPC of processed vegetables was higher in the water:methanol extract in comparison to that of the controls, no differences were observed in the TPC of processed and unprocessed samples after the intestinal stage of digestion of tomato, red pepper, and zucchini. In turn, the TPC after the intestinal phase of US-processed lettuce and green pepper was higher when compared to the untreated samples (p < 0.05). Juániz et al. (2016) observed that some polyphenolic compounds (not all of them) in raw green pepper were resistant to a simulated gastrointestinal digestion, using the same in vitro methodology utilized in the current paper. For example, in the study of Juániz et al. (2016), no differences were observed in the content of quercetin-3-O-rutinoside or luteolin 6,8-di-C-glucoside before and after in vitro digestion. Moreover, Goñi et al. (2006) demonstrated that 91% of the β-carotene, lutein, and lycopene contained in vegetables, all of them known for their high antioxidant capacities, was available in the gut during the entire digestion. Results suggest that US processing of fresh lettuce and green pepper can increase the bioaccessibility of phenolic compounds after a simulated gastrointestinal digestion. The antioxidant capacity of the enzymatic extracts obtained after the intestinal phase, assessed using the FRAP assay, was in line with the values expected from the TPC at the same stage. Indeed, no differences were observed in FRAP values for tomato, zucchini, and red pepper suggesting that the increased liberation of antioxidant compounds during the simulated digestion could be matrix-dependent. However, DPPH· values showed higher antioxidant activity in US-processed tomato, lettuce, red pepper, and zucchini after the intestinal phase (p < 0.05). Results suggest that US processing of these samples could facilitate the liberation of antioxidant compounds from the food matrix during a simulated digestion, potentially increasing their bioaccessibility. As mentioned previously, the main mechanism responsible for the membrane or cell wall disruption efficacy of sonication is the acoustic cavitation phenomena (Zinoviadou et al., 2015). Fava et al. (2017) recently studied the structure changes of cherry tomatoes after processing using sonication (20 kHz, 95.2 µm amplitude, 2 min) combined with hydrogen peroxide and observed several effects including epicuticular wax layer alteration, accentuated transition between the cellulose layer and the cuticular membrane of the epidermal cell wall, swelling of walls, aggregation of cellulose microfibrils, and contraction of epicarp, among others. Similar results were observed by Rajewska and Mierzwa (2017), who reported that the microstructure of plant tissues was significantly influenced after sonication both in terms of structure (intercellular spaces and porosity) and constitution (cell organelles). Results obtained herein are consistent with those presented in a review paper by Barba et al. (2017), who concluded that processing could either increase or decrease the bioaccessibility of bioactive compounds depending on both, food matrix and target compound.
Overall, US processing had no effect on the pH, colour, TTA, and SSC of tomato, lettuce, zucchini, and green and red pepper with respect to fresh vegetables. Results suggest that the amount of phenolic compounds and antioxidants released by vegetables into the human intestine may be higher than the one that can be expected from measurements in the usual aqueous-organic extracts. However, this is a hypothesis that needs further in vitro and in vivo studies to be confirmed. In addition, US processing at 4 °C, 40 kHz, and 250 W for 20 min resulted in increased bioaccessibility of phenolic compounds in lettuce and green pepper (p < 0.05), while no effect was observed for tomato, red pepper, and zucchini samples. Differences in the antioxidant activity were also observed between different vegetables, suggesting that the increased liberation of polyphenols and other antioxidant compounds during an in vitro digestion could be matrix-dependent. Results suggest that sonication of whole fresh vegetables could be used as a novel strategy to facilitate the release of polyphenols from selected vegetables during digestion. Although the methodology followed aims at simulating human digestion, we would like to highlight that results must be taken with caution. For example, the authors of the in vitro methodology utilised herein suggested that modifications should be needed to simulate digestion in infants or the elderly, which may vary considerably in enzyme concentration (Minekis et al., 2014). Authors of that study also suggested that dynamic in vitro models would better simulate in vivo conditions. Future studies will include the characterisation of the obtained extracts to identify which compounds are responsible for the observed antioxidant activity.
Acknowledgements
The CERCA Programme (Generalitat de Catalunya) supported this study. T. Lafarga and I. Aguiló-Aguayo are in receipt of Juan de la Cierva (FJCI-2016-29541) and Ramon y Cajal (RYC-2016-19949) contracts, both funded by the Spanish Ministry of Economy, Industry, and Competitiveness.
Abbreviations
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- US
Ultrasound
- TPC
Total phenolic content
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- SSC
Soluble solids content
- TTA
Titratable acidity
- DW
Dry weight
- FRAP
Ferric reducing antioxidant power
- S.D.
Standard deviations
- ANOVA
Analysis of variance
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tomás Lafarga, Email: tomas.lafarga@irta.cat.
Maria Janeth Rodríguez-Roque, Email: mjrodriguez@uach.mx.
Gloria Bobo, Email: gloria.bobo@irta.cat.
Silvia Villaró, Email: silvia.villaro@irta.cat.
Ingrid Aguiló-Aguayo, Phone: +34 973003431, Email: Ingrid.Aguilo@irta.cat.
References
- Aadil RM, Zeng XA, Han Z, Sun DW. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013;141:3201–3206. doi: 10.1016/j.foodchem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Adekunte AO, Tiwari BK, Cullen PJ, Scannell AGM, O’Donnell CP. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500–507. doi: 10.1016/j.foodchem.2010.01.026. [DOI] [Google Scholar]
- Alexandre EMC, Brandão TRS, Silva CLM. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innov. Food Sci. Emerg. 2013;17:99–105. doi: 10.1016/j.ifset.2012.11.009. [DOI] [Google Scholar]
- Barba FJ, Mariutti LRB, Bragagnolo N, Mercadante AZ, Barbosa-Cánovas GV, Orlien V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Tech. 2017;67:195–206. doi: 10.1016/j.tifs.2017.07.006. [DOI] [Google Scholar]
- Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–874. doi: 10.1016/j.foodchem.2006.06.025. [DOI] [Google Scholar]
- Chen GL, Chen SG, Zhao YY, Luo CX, Li J, Gao YQ. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crop Prod. 2014;57:150–157. doi: 10.1016/j.indcrop.2014.03.018. [DOI] [Google Scholar]
- Ding T, Ge Z, Shi J, Xu YT, Jones CL, Liu DH. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT-Food Sci. Technol. 2015;60:1195–1199. doi: 10.1016/j.lwt.2014.09.012. [DOI] [Google Scholar]
- Eh ALS, Teoh SG. Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrason. Sonochem. 2012;19:151–159. doi: 10.1016/j.ultsonch.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Fava J, Nieto A, Hodara K, Alzamora SM, Agueda Castro M. A study on structure (micro, ultra, nano), mechanical, and color changes of Solanum lycopersicum L. (cherry tomato) fruits induced by hydrogen peroxide and ultrasound. Food Bioprocess Technol. 2017;10:1324–1336. doi: 10.1007/s11947-017-1905-4. [DOI] [Google Scholar]
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Goñi I, Serrano J, Saura-Calixto F. Bioaccessibility of β-carotene, lutein, and lycopene from fruits and vegetables. J. Agric. Food Chem. 2006;54:5382–5387. doi: 10.1021/jf0609835. [DOI] [PubMed] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Jamali B, Bjørnsdottir I, Nordfang O, Hansen SH. Investigation of racemisation of the enantiomers of glitazone drug compounds at different pH using chiral HPLC and chiral CE. J. Pharm. Biomed. Anal. 2008;46:82–87. doi: 10.1016/j.jpba.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Juániz I, Ludwig IA, Bresciani L, Dall’Asta M, Mena P, del Rio D, Cid C, de Peña MP. Catabolism of raw and cooked green pepper (Capsicum annum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Foods. 2016;27:201–213. doi: 10.1016/j.jff.2016.09.006. [DOI] [Google Scholar]
- Lafarga T, Bobo G, Viñas I, Zudaire L, Simó J, Aguiló-Aguayo I. Steaming and sous-vide: Effects on antioxidant activity, vitamin C, and total phenolic content of Brassica vegetables. Int. J. Gastronom. Food Sci. 2018;13:134–139. doi: 10.1016/j.ijgfs.2018.05.007. [DOI] [Google Scholar]
- Lafarga T, Gallagher E, Bademunt A, Bobo G, Echeverria G, Viñas I, Aguiló-Aguayo I. Physicochemical and nutritional characteristics, bioaccessibility and sensory acceptance of baked crackers containing broccoli co-products. Int. J. Food Sci. Tech. 2019;54:634–640. doi: 10.1111/ijfs.13908. [DOI] [Google Scholar]
- Lafarga T, Ruiz-Aguirre I, Abadias M, Viñas I, Bobo G, Aguiló-Aguayo I. Effect of thermosonication on the bioaccessibility of antioxidant compounds and the microbiological, physicochemical, and nutritional quality of an anthocyanin-enriched tomato juice. Food Bioprocess Tech. 2019;12:147–157. doi: 10.1007/s11947-018-2191-5. [DOI] [Google Scholar]
- Lafarga T, Viñas I, Bobo G, Simó J, Aguiló-Aguayo I. Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. 2018;47:412–420. doi: 10.1016/j.ifset.2018.04.008. [DOI] [Google Scholar]
- Luna-Guevara M, Hernández-Carranza P, Luna-Guevara J, Flores-Sánchez R, Ochoa-Velasco C. Effect of sonication and blanching postharvest treatments on physicochemical characteristics and antioxidant compounds of tomatoes: fresh and refrigerated. Acad. J. Agric. Res. 2015;3:127–131. [Google Scholar]
- Meireles A, Giaouris E, Simões M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016;82:71–85. doi: 10.1016/j.foodres.2016.01.021. [DOI] [Google Scholar]
- Minekis M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Ménard O, Santos CN, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Brodkorb A. A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- Müller L, Caris-Veyrat C, Lowe G, Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases – a critical review. Crit. Rev. Food Sci. 2015;11:1868–1879. doi: 10.1080/10408398.2013.801827. [DOI] [PubMed] [Google Scholar]
- Muñiz-Márquez DB, Martínez-Ávila GC, Wong-Paz JE, Belmares-Cerda R, Rodríguez-Herrera R, Aguilar CN. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013;20:1149–1154. doi: 10.1016/j.ultsonch.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Pérez-López U, Sgherri C, Miranda-Apodaca J, Micaelli F, Lacuesta M, Mena-Petite A, Quartacci MF, Muñoz-Rueda A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Bioch. 2018;123:233–241. doi: 10.1016/j.plaphy.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Pokhrel PR, Bermúdez-Aguirre D, Martínez-Flores HE, Garnica-Romo MG, Sablani S, Tang J, Barbosa-Cánovas GV. Combined Effect of Ultrasound and Mild Temperatures on the Inactivation of E. coli in Fresh Carrot Juice and Changes on its Physicochemical Characteristics. J. Food Sci. 2017;82:2343–2350. doi: 10.1111/1750-3841.13787. [DOI] [PubMed] [Google Scholar]
- Rajewska K, Mierzwa D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. 2017;43:117–129. doi: 10.1016/j.ifset.2017.07.034. [DOI] [Google Scholar]
- Rodríguez-Roque MJ, de Ancos B, Sánchez-Moreno C, Cano MP, Elez-Martínez P, Martín-Belloso O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J Funct. Foods. 2015;14:33–43. doi: 10.1016/j.jff.2015.01.020. [DOI] [Google Scholar]
- Sagong HG, Cheon HL, Kim SO, Lee SY, Park KH, Chung MS, Choi YJ, Kang DH. Combined effects of ultrasound and surfactants to reduce Bacillus cereus spores on lettuce and carrots. Int. J. Food Microbiol. 2013;160:367–372. doi: 10.1016/j.ijfoodmicro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- São José JFB, Andrade NJ, Ramos AM, Vanetti MCD, Stringheta PC. Chaves JBP Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014;45:36–50. doi: 10.1016/j.foodcont.2014.04.015. [DOI] [Google Scholar]
- Shahidi F, Zhong Y. Measurement of antioxidant activity. J. Funct. Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- Tiwari BK, Patras A, Brunton N, Cullen PJ, O’Donnell CP. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010;17:598–604. doi: 10.1016/j.ultsonch.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Toutain PL, Bousquet-Mèlou A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 2004;27:455–466. doi: 10.1111/j.1365-2885.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- van Buggenhout S, Alminger M, Lemmens L, Colle I, Knockaert G, Moelants K, van Loey A, Hendrickx M. In vitro approaches to estimate the effect of food processing on carotenoid bioavailability need thorough understanding of process induced microstructural changes. Trends Food Sci. Tech. 2010;21:607–618. doi: 10.1016/j.tifs.2010.09.010. [DOI] [Google Scholar]
- Yu J, Engeseth NJ, Feng H. High intensity ultrasound as an abiotic elicitor—effects on antioxidant capacity and overall quality of romaine lettuce. Food Bioprocess Technol. 2016;9:262–273. doi: 10.1007/s11947-015-1616-7. [DOI] [Google Scholar]
- Zafra-Rojas QY, Cruz-Cansino N, Ramírez-Moreno E, Delgado-Olivares L, Villanueva-Sánchez J, Alanís-García E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013;20:1283–1288. doi: 10.1016/j.ultsonch.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Zinoviadou KG, Galanakis CM, Brnčić M, Grimi N, Boussetta N, Mota MJ, Saraiva JA, Patras A, Tiwari B, Barba FJ. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015;77:743–752. doi: 10.1016/j.foodres.2015.05.032. [DOI] [Google Scholar]
- Žugčić T, Abdelkebir R, Alcantara C, Collado MC, García-Pérez JV, Meléndez-Martínez AJ, Jambrak AR, Lorenzo JM, Barba FJ. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Tech. 2019;83:63–77. doi: 10.1016/j.tifs.2018.11.005. [DOI] [Google Scholar]