Abstract
This study aimed to investigate the efficacy of ginseng vinegar (GV) for preventing and ameliorating the obesity and inflammation. Oral administrations of GV with different doses were conducted for 10 weeks in the preventive model and therapeutic model. In the preventive model, above GV-medium dose significantly reduced body weight gain, epididymal fat weight, triglycerides, and total cholesterol compared to control. GV-high dose effectively improved the inflammatory factors (tumor necrosis factor-alpha (TNF-α) and interleukin 6) in serum, liver, and adipose tissue. In the therapeutic model, all GV groups showed significantly decreased body weight gain, epididymal fat weight, triglycerides, and total cholesterol. Reductions of the TNF-α level in the serum and liver were observed in all GV groups, and the CRP levels in the liver of all GV groups were significantly decreased with different trend from the preventive model. These results suggest that GV is more effective in therapeutic model and is a potential food for obesity and associated inflammation.
Keywords: Ginseng vinegar, High fat diet, Obesity, Inflammation, Pro-inflammatory cytokine
Introduction
Obesity is well-recognized as an important risk factor for the development of metabolic syndrome, type 2 diabetes, cardiovascular disease, and cancer (Nam et al., 2015; Seo et al., 2014). Moreover, enlarged fat mass and excess adipose tissue in obesity reduce the ability to stimulate an inflammatory signal or to induce inflammatory cytokines, resulting in a low-grade inflammatory state and obesity related dysfunctions (Bastard et al., 2006). These inflammatory responses include elevated levels of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-a), interleukin 6 (IL-6), and the C-reactive protein (CRP) signaling pathway (Cesari et al., 2003; Koukkunen et al., 2001; Lin and Karin, 2007). In addition, pro-inflammatory cytokines activate various signal transduction cascades, including the pathways of insulin and leptin action, and the central nervous and immune systems (Hotamisligil, 2006). Therefore, the modulation of elevated pro-inflammatory cytokines is considered a key factor in reducing obesity and various related inflammatory diseases.
Vinegar, a representative fermented food, has been utilized as a traditional seasoning containing organic acids as well as various nutrients such as amino acids, minerals, and vitamins. Several preceding studies reported the efficacy of vinegar, describing its antihypertensive, antitumor, and antimicrobial properties (Sengun and Karapinar, 2005). In recent years, the anti-obesity and anti-inflammatory effects of vinegar through improving lipid metabolism have been investigated (Chou et al., 2015; Lee et al., 2013; Seo et al., 2014; Yun et al., 2007). As the demand to utilize the functionality of vinegar has increased, new various vinegars prepared from persimmon, tomato, pomegranate, and ginseng have been reported (Lee et al., 2013; Samad et al., 2016). In particular, many studies have been reported in terms of active compounds on ginseng vinegar (Ko et al., 2005), as well as its effect on anti-hyperglycemic activity (Lim et al., 2009), and lipid metabolism (Yun et al., 2007). But ginseng has not been used as a fermentation source due to the antimicrobial activity of saponin, it can only be used as an additive after microbial fermentation. Yun et al. (2007) examined the potential of ginseng ethanol extract mixed with fermented vinegar, and Kim et al. (2012) manufactured red ginseng vinegar based on rice wine and red ginseng concentrate. However, few studies have been carried out to manufacture the vinegar using ginseng from alcohol fermentation and its health-related effects.
Therefore, the purpose of this study was to investigate the effects of ginseng vinegar fermented with brown rice and ginseng in terms of preventing the development of obesity and associated inflammation and ameliorating the elevated serum lipid levels and inflammatory cytokines in established obesity. This study was designed to distinguish the effects of ginseng fermentation and acetic acid on obesity and related inflammation in HFD-fed mice.
Materials and methods
Materials
Ginseng vinegar (GV) was provided from Sempio Fermentation Research Center (Osong, Korea). The GV procedure was consisted of two-stage fermentation as described Choi et al. (2017). Briefly, ginseng (4–5 years, Eumseong, Chungbuk, Korea) and brown rice were ground and mixed at a ratio of 1:25. Aspergillus oryzae SMF-138 (KCTC 11989BP) and Rhizopus delemer SMF136 (KCTC 11990BP) were then inoculated at 0.5% of their total weight. The mixture was inoculated with Lactobacillus casei (0.1% of the total weight, w/w) at 30 °C for 4 h and further fermented with Saccharomyces cerevisiae (0.1% of the total weight, w/w) at 30 °C for 3 days for alcohol fermentation. In the acetic acid fermentation process, ginseng and brown rice wine were filtered with a Whatman filter paper (no. 2) and the remaining was inoculated with Acetobacter aceti (1%, w/w) in the ginseng wine at 30 °C for 5 days. Consequently, GV titrated to 5.2% acetic acid was then obtained and the total ginsenoside contents of GV was 0.100 mg/g, which was analyzed using high-performance liquid chromatography (Ko et al., 2005) and mainly contained Re (0.023 mg/g), Rb 1 (0.018 mg/g), and Rg 1 (0.008 mg/g). All other chemicals used in this study were of the highest pure grade available.
Animals and diets
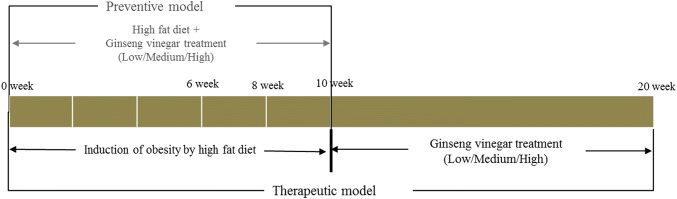
Five-week-old male C57/BL6 mice were purchased from DooYeol Biotech. (Seoul, Korea). The mice were housed individually with a 14 h light/dark cycle at a temperature of 23 ± 1 °C and a humidity of 45 ± 5% with access to water for 1 week prior to the experiment. As shown in Fig. 1, the C57/BL6 mice were randomly divided into six groups (n = 10) and fed the designated experimental diet for 10 weeks as followed: normal diet (ND), high-fat diet (HFD; AIN-93G diet containing 60% energy from fat) with saline, HFD with low dose of GV (HFD + GVL: 0.13 mL/kg), HFD with medium dose of GV (HFD + GVM: 0.40 mL/kg), HFD with high dose of GV (HFD + GVH: 4.00 mL/kg), and HFD with acetic acid [HFD + acetic acid: 6.5% (w/w) acetic acid, 0.40 mL/kg]. In the preventive experiment, HFD-feeding and GV treatment were simultaneously conducted for 10 weeks. On the other hand, in the therapeutic experiment, five-week-old mice were fed with HFD for 10 weeks to induce obesity and then divided into six groups, which received identical treatment to those with the GV treatment for 10 weeks. GV was administered using an oral feeding needle, and normal and high fat diet mice received an equivalent amount of saline. The composition of the experimental diet was based on the AIN-93G semisynthetic diet (Reeves et al., 1993). The body weight and food intakes were measured weekly. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the Korean Preclinical Center (IACUC 14-055).
Fig. 1.
Experimental design of ginseng vinegar treatments
Collection of serum, liver, and epididymis of experimental mice
At the sixth, eighth, and tenth week of the experimental period, the blood from each mouse was collected with a capillary tube after overnight fasting, and quickly centrifuged at 4 °C for 10 min. At the end of the treatment, tissues were collected and weighed individually including those of the liver, kidney, spleen, and epididymal fat. The serum and tissue were collected and stored at − 80 °C prior to analysis.
Biochemical analysis of serum samples
Serum lipids [triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol] and protein were assayed using a commercial kit (Vitalab Selectra, Merck, Darmstadt, Germany). Serum concentrations of TNF-α, IL-6, and CRP were measured via immunoassay using an ELISA kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s protocol.
Determination of TNF-α, IL-6, and CRP levels
The liver tissue was weighed and homogenized in lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1% Tripton) and then extracted with an equal volume of chloroform. The chloroform layers were dried and dissolved in isopropyl alcohol to measure the lipid levels. The epididymal fat tissue was homogenized in a lysis buffer. The lysate was centrifuged (13,000×g, 4 °C) for 15 min, and the supernatant was transferred to 96-well ELISA plates. TNF-α, IL-6, and CRP concentrations were determined as described above.
Statistical analysis
All data were presented as mean ± standard deviation. Statistical analyses were carried out with SPSS software (version 19; SPSS Inc., Chicago, IL, USA) using a Duncan’s multiple range test by an analysis of variance and Student’s t test (p < 0.05). A p value < 0.05 was considered to indicate statistical significance.
Results and discussion
Body weight change and food intake
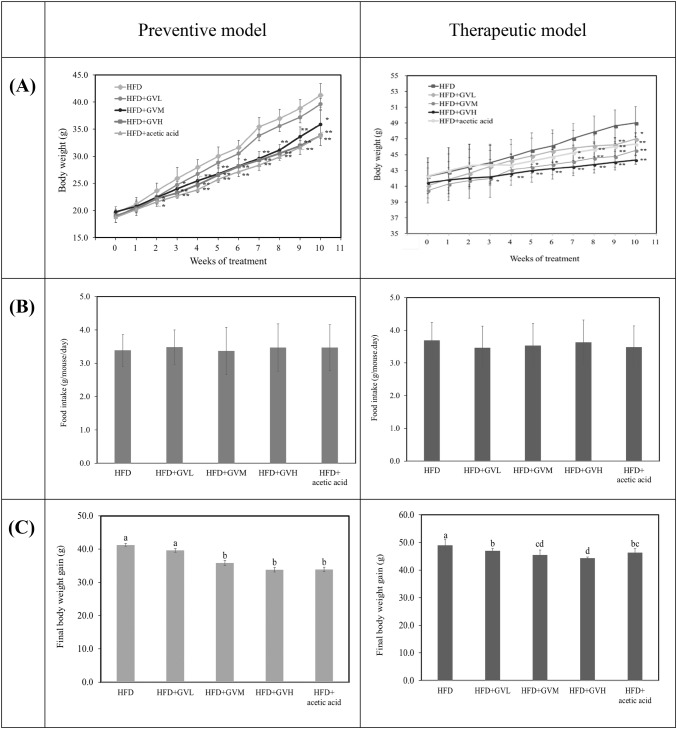
In order to elucidate the preventive effects of GV on obesity, the mice were fed on HFD with GV via oral feeding of 0.13, 0.40, and 4.0 mL/kg for GVL, GVM, and GVH for 10 weeks, respectively. At the initial stage, no significant difference was observed in body weight between the groups, but the GVM, GVH, and acetic acid treatment significantly inhibited HFD-induced increases in body weight from the second week (Fig. 2A, p < 0.05). No difference in food intake during the experimental period was observed among all groups (Fig. 2B), indicating that GV decreased body weight without decreasing dietary intake. At the end of the experimental period, the GVM, GVH, and acetic acid groups showed significantly decreased body weights compared to the HFD group (p < 0.01). The GVH group showed the highest body weight reduction of 18% compared to the HFD group (Fig. 2C). The changes of body weight and food intake in the therapeutic model are shown in Fig. 2. A reduction of body weight was observed in the GVH group at 3 weeks and the significant differences of the final body weight gain were observed at above low level of GV treatment. In addition, there was significant difference between acetic acid and GVH in therapeutic model, however, that was not obviously shown in preventive model.
Fig. 2.
Effects of ginseng vinegar on body weight change (A), final body weight gain (B), and food intake (C) compared to preventive and therapeutic models. All values mean ± SD (n = 10/group). Means with different letters differ significantly by Duncan’s test (p < 0.05). *p < 0.05 versus HFD group, **p < 0.01 versus HFD group by t-test
Effect of ginseng vinegar on fat accumulation in various organs
The effects of GV on fat accumulation in various organs (liver, kidney, spleen, epididymal fat) are shown in Table 1. The preventive effects of GV on fat accumulation were presented in the liver and the epididymal fat weight. The epididymal fat weights were decreased in all GV groups compared to HFD group and the highest reduction of epididymal fat weight was shown in GVH group. In addition, the therapeutic effects of GV were observed in the liver, kidney (left), and epididymal fat weight. Unlike the preventive model, the fat in the epididymal tissue was accumulated in a dose-dependent manner and the reductions of epididymal weight in the GV groups were higher than in the acetic acid group. Comparing the epididymal fat weight between the two models, showed that GV was more effective in the therapeutic model; significant (p < 0.01) differences were observed from the low dose group, moreover the epididymal fat weight of GVL was lower than that of acetic acid group. This study was designed to distinguish the effects of ginseng ingredient and acetic acid on health benefit effect in HFD mice, since acetic acid is the main component (Fushimi and Sato, 2005; Moon et al., 2010) of ginseng vinegar. This study presented the synergy effects of GV on the body weight and fat accumulation loss.
Table 1.
Effects of ginseng vinegar (GV) in various organ weight
| Model | Group | Liver | Kidney (Lt) | Kidney (Rt) | Epididymal fat | Spleen |
|---|---|---|---|---|---|---|
| Preventive model | ND | 1.96 ± 0.23a | 0.17 ± 0.02c | 0.16 ± 0.01b | 0.39 ± 0.08d | 0.11 ± 0.03a |
| HFD | 1.52 ± 0.24b | 0.21 ± 0.02b | 0.23 ± 0.03a | 2.71 ± 0.40a | 0.09 ± 0.01a | |
| HFD + GVL | 1.43 ± 0.23bc | 0.24 ± 0.03a | 0.25 ± 0.03a | 2.27 ± 0.31b | 0.10 ± 0.01a | |
| HFD + GVM | 1.29 ± 0.24c | 0.22 ± 0.02ab | 0.24 ± 0.03a | 1.63 ± 0.60c | 0.10 ± 0.01a | |
| HFD + GVH | 1.30 ± 0.13c | 0.23 ± 0.02ab | 0.25 ± 0.03a | 1.25 ± 0.41c | 0.09 ± 0.01a | |
| HFD + acetic acid | 1.24 ± 0.14c | 0.23 ± 0.03ab | 0.25 ± 0.04a | 1.53 ± 0.32c | 0.10 ± 0.03a | |
| Therapeutic model | HFD | 2.61 ± 0.48a | 0.23 ± 0.03a | 0.21 ± 0.02a | 2.08 ± 0.17a | 0.12 ± 0.02b |
| HFD + GVL | 2.50 ± 0.35a | 0.20 ± 0.04b | 0.21 ± 0.04a | 1.55 ± 0.22c | 0.11 ± 0.01b | |
| HFD + GVM | 2.34 ± 0.74ab | 0.20 ± 0.04b | 0.19 ± 0.05a | 1.45 ± 0.29 cd | 0.14 ± 0.03a | |
| HFD + GVH | 1.90 ± 0.56b | 0.21 ± 0.01ab | 0.21 ± 0.02a | 1.34 ± 0.18d | 0.12 ± 0.04ab | |
| HFD + acetic acid | 2.27 ± 0.24ab | 0.20 ± 0.02b | 0.20 ± 0.02a | 1.88 ± 0.14b | 0.11 ± 0.02b |
All values mean ± SD (n = 10/group)
ND normal diet, HFD high fat diet, GVL ginseng vinegar low dose, GVM ginseng vinegar medium dose, GVH ginseng vinegar high dose, Lt left, Rt right
Effect of ginseng vinegar on serum lipid level
The effects of GV administration on serum lipid level were also investigated and are presented in Table 2. The improvements in the lipid metabolism of the GV treatments showed both preventive and therapeutic models. For the preventive model of GV, the differences in the serum TG and TC levels were observed between all GV administered groups and the HFD group, and the levels of TG and TC in GV decreased with increasing the GV administration contents. GVH and acetic acid treatment decreased the LDL-cholesterol level, however GV treatment had no effect on HDL-cholesterol levels. Particularly, the GVH group had the highest reduction in TG, TC, and LDL levels. The pattern in the changes of serum lipid in the therapeutic model was similar to those of the preventive model. In this study, GV was more effective in suppressing obesity than acetic acid, which has been suggested to have an anti-obesity effects (Lee et al., 2013; Seo et al., 2014). These effects can be considered to be due to the active ingredient of ginseng.
Table 2.
Effects of ginseng vinegar (GV) on serum lipid level
| Model | Group | Triglyceride (mg/dL) | High-density lipoprotein cholesterol (mg/dL) | Low-density lipoprotein cholesterol (mg/dL) | Total cholesterol (mg/dL) |
|---|---|---|---|---|---|
| Preventive model | ND | 92.8 ± 21.9d | 84.3 ± 18.4b | 31.2 ± 3.4abc | 120.2 ± 20.1a |
| HFD | 172.8 ± 34.7a | 111.7 ± 11.9a | 37.3 ± 7.0a | 200.7 ± 11.6a | |
| HFD + GVL | 133.9 ± 19.9b | 112.6 ± 7.6a | 33.6 ± 8.7ab | 177.7 ± 17.3b | |
| HFD + GVM | 106.6 ± 20.4 cd | 102.5 ± 7.7a | 32.1 ± 7.8ab | 166.3 ± 12.0bc | |
| HFD + GVH | 95.5 ± 16.5d | 110.9 ± 8.3a | 24.3 ± 8.4c | 148.7 ± 9.6d | |
| HFD + acetic acid | 120.3 ± 18.7bc | 104.9 ± 5.9a | 26.8 ± 3.4bc | 156.2 ± 12.5cd | |
| Therapeutic model | HFD | 196.7 ± 33.9a | 122.0 ± 17.7a | 73.6 ± 22.4a | 211.5 ± 26.5a |
| HFD + GVL | 135.9 ± 22.1b | 132.7 ± 20.2a | 62.5 ± 28.4ab | 178.7 ± 26.2b | |
| HFD + GVM | 111.1 ± 31.7bc | 132.9 ± 36.1a | 53.2 ± 14.4b | 177.2 ± 12.7b | |
| HFD + GVH | 84.4 ± 26.5c | 140.2 ± 32.1a | 50.2 ± 9.3b | 151.1 ± 29.0c | |
| HFD + acetic acid | 113.7 ± 37.1b | 139.0 ± 27.3a | 60.2 ± 9.4ab | 152.6 ± 31.9c |
All values mean ± SD (n = 10/group)
ND normal diet, HFD high fat diet, GVL ginseng vinegar low dose, GVM ginseng vinegar medium dose, GVH ginseng vinegar high dose
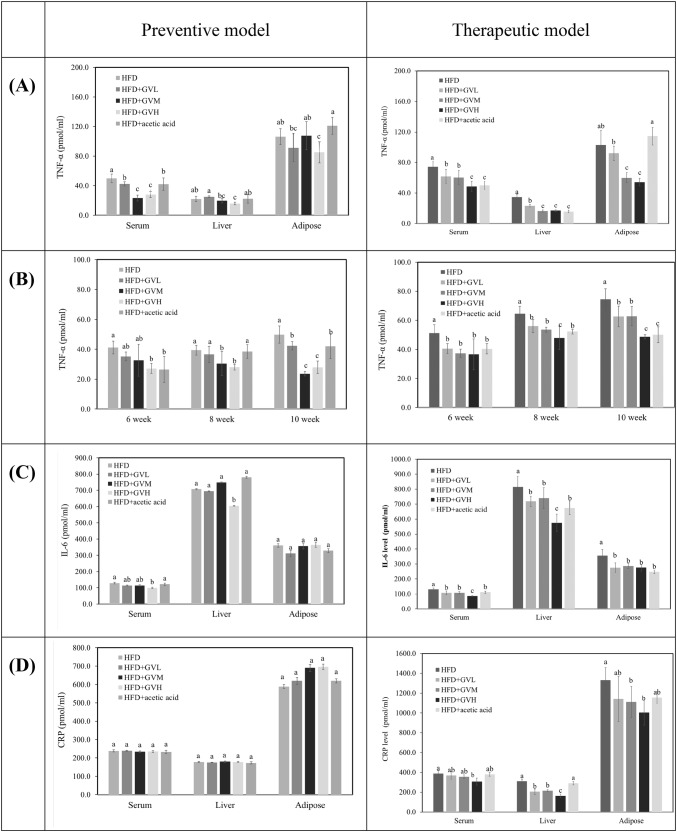
Effect of ginseng vinegar on TNF–α in serum, liver, and adipose tissue
The anti-inflammatory effect of ginseng vinegar is expressed with the levels of TNF-α expression in serum, liver, and adipose tissue in Fig. 3A. All GV treatments significantly reduced TNF-α expression in serum compared to the HFD group (p < 0.05). The GVH administration led to the reduction of the TNF-α level in liver and adipose tissue. The change of TNF-α in serum was examined over the time trends from 6 to 8 weeks (Fig. 3B). From 6 weeks, the TNF-α level of GVH in serum was significantly reduced, and all GV treatments positively influenced the reduction of the TNF-α level in serum after 10 weeks. For the therapeutic experiment, the dose-dependent pattern of TNF-α was presented in serum, liver and adipose tissue. The administration of GVL was effective in the level of TNF-α in serum and liver, while the GVM and GVH reduced the TNF-α level in adipose tissues (Fig. 3A). The levels of TNF-α in serum were decreased with all GV from 6 weeks in therapeutic model.
Fig. 3.
Comparison to the effects of ginseng vinegar in preventive and therapeutic models: TNF-α levels (A), the change of TNF-α in serum for 6-10 weeks (B), IL-6 levels (C), and CRP levels (D) in serum, liver, and adipose tissue. All values mean ± SD (n = 10/group). Means with different letters differ significantly by Duncan’s test (p < 0.05)
Effect of ginseng vinegar on IL-6 and CRP in serum, liver, and adipose tissue
Determining the changes in the IL-6 level is an important parameter in the analysis of inflammation and immune disorders. The IL-6 levels in serum, liver, and adipose tissue from the preventive model and therapeutic model are shown in Fig. 3C. For the preventive model, only GVH treatment significantly reduced the IL-6 level in serum and liver, however, in the case of therapeutic model, the IL-6 level had tendency to decreased with increasing the levels of GV and all GV samples affected the IL-6 level in all tissues. Although there was no effect of GV treatment on CRP level in serum, liver, and adipose tissue in preventive model (Fig. 3D), the all GV groups in therapeutic model significantly decreased the CRP level in the liver and adipose tissue. The more obvious effect of GV treatment in the levels of TNF-α and IL-6 was observed compared to acetic acid treatment. The pro-inflammatory cytokines were not influenced by acetic acid treatment. The anti-inflammatory effects of ginseng, especially ginseng saponin (ginsenoside) have been previously conducted, and orally administered ginsenoside-Rb1 lowered serum IL-6 and TNF-α levels (Song et al., 2010; Wu et al., 2014). In the present study, the active compound of ginseng vinegar also decreased the levels of pro-inflammatory cytokines such as TNF-α, IL-6, and CRP than in the HFD group and presented the inflammatory effect. In conclusion, ginseng has received much attention as a functional plant due to its bioactive ingredient (Hwang et al., 2007; Shang et al., 2007; Wu et al., 2014), however, the physiological activities of ginseng vinegar have not been fully determined. This study particularly demonstrated the combined effects of ginseng vinegar on obesity induced inflammation in two different obese models as well as the potential for use in anti-obesity and anti-inflammation functional foods. Moreover, further studies are needed to extend potential for use in anti-obesity and anti-inflammation functional foods.
Acknowledgements
This research was financially supported by the Ministry of Trade, Industry, and Energy (MOTIE), Korea, under the “Regional Specialized Industry Development Program” (R0002329) supervised by the Korea Institute for Advancement of Technology (KIAT).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Imkyung Oh, Email: angeloik@naver.com.
Eun Jong Baek, Email: beunjong@sempio.com.
Dae-Hee Lee, Email: ldaehee@sempio.com.
Yong Ho Choi, Email: cyongho@sempio.com.
In Young Bae, Phone: +82-43-880-3160, Email: iybae@kdu.ac.kr.
References
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Harris TB. Inflammatory markers and onset of cardiovascular events results from the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim KO, Baek EJ, Hur BS. Simultaneous two-step fermentation ginseng black vinegar and method for manufacturing the same. Korea Patent 10-1749990 (2017)
- Chou CH, Liu CW, Yang DJ, Wu YHS, Chen YC. Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chem. 2015;168:63–69. doi: 10.1016/j.foodchem.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Fushimi T, Sato Y. Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Brit. J. Nutri. 2005;94:714–719. doi: 10.1079/BJN20051545. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, Kim MJ, Kwon DY. Anti-obesity effects of ginsenoside Rh 2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007;364:1002–1008. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- Kim DK, Baik MY, Kim HK, Hahm YT, Kim BY. Manufacture of the red ginseng vinegar fermented with red ginseng concentrate and rice wine, and its quality evaluation. Korean J. Food Sci. Technol. 2012;44:179–184. doi: 10.9721/KJFST.2012.44.2.179. [DOI] [Google Scholar]
- Ko SK, Lee KH, Hong JK, Kang SA, Sohn UD, Im BO, Han ST, Yang BW, Chung SH, Lee BY. Change of ginsenoside composition in ginseng extract by vinegar process. Food Sci. Biotechnol. 2005;14:509–513. [Google Scholar]
- Koukkunen H, Penttilä K, Kemppainen A, Halinen M, Penttilä I, Rantanen T, Pyörälä K. C-reactive protein, fibrinogen, interleukin-6 and tumour necrosis factor-α in the prognostic classification of unstable angina pectoris. Ann. Med. 2001;33:37–47. doi: 10.3109/07853890109002058. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho HD, Jeong JH, Lee MK, Jeong YK, Shim KH, Seo KI. New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chem. 2013;141:3241–3249. doi: 10.1016/j.foodchem.2013.05.126. [DOI] [PubMed] [Google Scholar]
- Lim S, Yoon JW, Choi SH, Cho BJ, Kim JT, Chang HS, Kim YB. Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin-resistant rat model. Metabolism. 2009;58:8–15. doi: 10.1016/j.metabol.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YJ, Choi DS, Oh SH, Song YS, Cha YS. Effects of persimmon-vinegar on lipid and carnitine profiles in mice. Food Sci. Biotechnol. 2010;19:343–348. doi: 10.1007/s10068-010-0049-3. [DOI] [Google Scholar]
- Nam YR, Won SB, Chung YS, Kwak CS, Kwon YH. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr. Res. Pract. 2015;9:235–241. doi: 10.4162/nrp.2015.9.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Samad A, Azlan A, Ismail A. Therapeutic effects of vinegar: a review. Curr. Opin. Food Sci. 2016;8:56–61. doi: 10.1016/j.cofs.2016.03.001. [DOI] [Google Scholar]
- Sengun IY, Karapinar M. Effectiveness of household natural sanitizers in the elimination of Salmonella typhimurium on rocket (Eruca sativa Miller) and spring onion (Allium cepa L.). Int. J. Food Microbiol. 98: 319–323 (2005) [DOI] [PubMed]
- Seo KI, Lee J, Choi RY, Lee HI, Lee JH, Jeong YK, Lee MK. Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced obese mice. Food Funct. 2014;5:1579–1586. doi: 10.1039/c4fo00135d. [DOI] [PubMed] [Google Scholar]
- Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, Chen M. Ginsenoside Rb 1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ 2 and C/EBPα gene expression. Life Sci. 2007;80:618–625. doi: 10.1016/j.lfs.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Song YB, An YR, Kim SJ, Park HW, Jung JW, Kyung JS, Hwang SY, Kim YS. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2010;92:388–396. doi: 10.1002/jsfa.4589. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yu Y, Szabo A, Han M, Huang XF. Central inflammation and leptin resistance are attenuated by ginsenoside Rb 1 treatment in obese mice fed a high-fat diet. PloS One. 2014;9:e92618. doi: 10.1371/journal.pone.0092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SN, Ko SK, Lee KH, Chung SH. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch. Pharmacal. Res. 2007;30:587–595. doi: 10.1007/BF02977653. [DOI] [PubMed] [Google Scholar]