Abstract
Tea catechins have attracted strong interests in pharmacological field for their extensive biological activities; however, their bioavailability in vivo is relatively low. Recent studies have shown tea catechins can modulate the composition of intestinal microbiota and help to improve hosts’ health. Meanwhile, the gut flora plays a crucial role in regulating the production of the metabolites of tea catechins and their biological activity. Although the activities of tea catechins to promote intestinal micro-ecology have been extensively studied, little is known about the two-way phenol-microbial interactions. This review focuses on the modulatory effect of tea catechins on intestinal microbiota as well as the microbial degradation of tea catechins and the metabolites formed. Finally, the potential effects of tea catechins on chronic intestinal inflammation are emphasized.
Keywords: Tea catechins, Intestinal microbiota, Bioavailability, Health benefits
Introduction
Tea is the second most popular beverage in the world, preceded only by water (Pan et al., 2016). In China, the consumption of tea has a long history for over 5000 years. Tea is not only one of the necessities in ordinary people’s life, but also often used as a medicine (Yang and Hong, 2013). In addition to attractive color and fragrance, it also has many beneficial biological activities to human health, including anti-oxidative, anti-allergy and anti-obesity (Cheng et al., 2017).
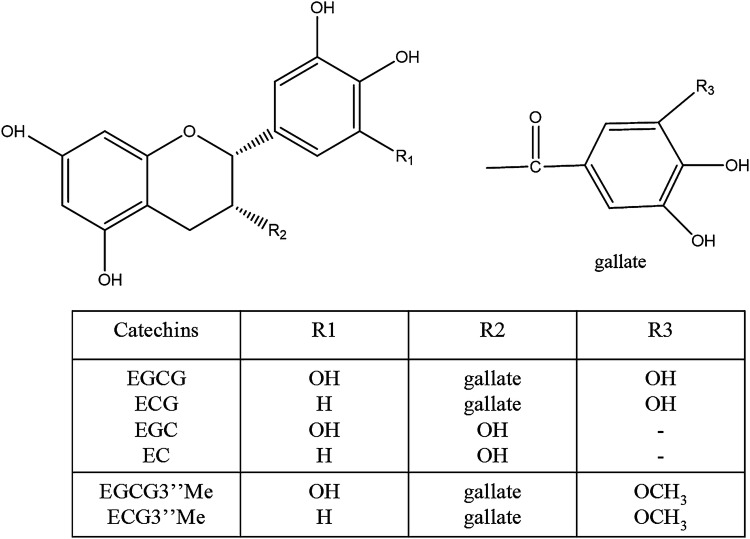
According to the production methods, tea is mainly divided into unfermented (green tea), semi-fermented (oolong tea), fermented (black tea), and post-fermented (puer tea). Tea polyphenols are a mixture of phenolic compounds extracted from tea. In terms of the concentration, tea catechins account for 60–80% of the total polyphenols, being one of the most important bioactive substances in tea. The levels of (−)-epicatechin (EC) and (−)-epigallocatechin (EGC) are less than half of the contents of ester type catechins, (−)-Epicatechin gallate (ECG) and (−)-Epigallocatechin gallate (EGCG) (Fig. 1). Among these catechins, EGCG shows the largest content and the highest biological activity. Extensive studies have demonstrated that only a small fraction of tea catechins is absorbed by the small intestine of human body (Zhang et al., 2019), most tea catechins are brought into the large intestine, where they are decomposed and metabolized into a series of absorbable metabolites.
Fig. 1.
Chemical structures of tea catechins
The human intestinal tract, especially the large intestine, inhabits over trillion microbes, containing hundreds or thousands of bacterial taxa (Human Microbiome Project Consortium, 2012). It has been shown that the intestinal flora mainly has nine bacterial phyla, 98% of which can be attributed to the following 4 categories: Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%) (Ley et al., 2006). The human genome consists of approximately 23, 000 genes, and the microbiome encodes more than 3 million genes, covering many metabolic functional genes that are not available to the host, and thus influences host’s adaptation, phenotype and health (Valdes et al., 2018). Studies have shown that the status of the human gut ecosystem mainly depends on factors such as postnatal environment and eating habits (Rothschild et al., 2018). It is estimated that only 1.9% of the gut microbiota are heritable, and more than 20% of the biodiversity of the microbial population can be inferred from environmental factors related to diet. David et al. (2014) investigated the response of the human intestinal flora to short-term macronutrient changes and demonstrated that the gut microbiome could respond quickly to altered diets. As the largest and most complex micro-ecological system, the gut microbiome is closely related to the health of the host. The relationships of gut microbiota and certain diseases have received attentions over the past century, such as liver disorders, diabetes, obesity, inflammatory bowel disease, and cancers (Okubo et al., 2018). In fact, the aromatic metabolites of tea catechins formed by the intestinal flora through different metabolism pathways, may in turn regulate microbiota structure via selective prebiotic effects and anti-microbial activities (Zhang et al., 2018). Consequently, the alternative microbes may lead to enhanced metabolic activity of the orally administered polyphenols. However, the effects of tea catechins on the intestinal micro-ecology, including the potential mechanisms and practical benefits of these biologically active agents are still unclear. In this review, we will outline the two-way phenol-microbial interactions (tea catechins are metabolized to a variety of derivative products of different structures by intestinal flora, which may accumulate to exert physiological effects, and modulate intestinal bacterial diversity and richness) and their impact on host health.
The regulation effects of tea catechins on intestinal micro-ecology
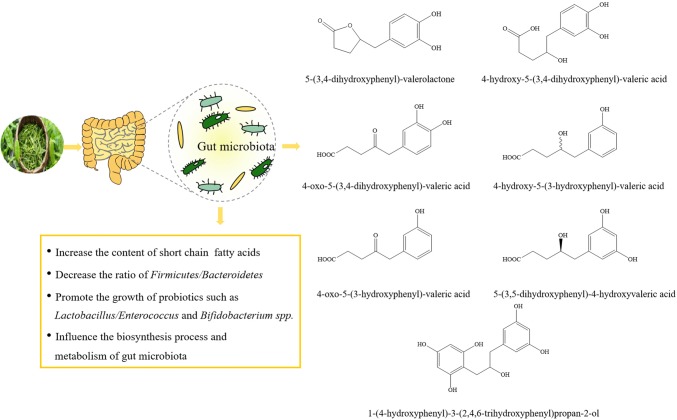
In the gut, Lactobacillus and Bifidobacterium have been observed to inhibit multifarious pathogens, improve lactose digestion, enhance intestinal barrier function and regulate colonic microbiota (Čitar et al., 2015). However, certain endogenous and environmental factors can disrupt the structure of the gut microbiota, and thus gastrointestinal diseases such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and antibiotic-associated diarrhea may occur. A multitude of studies have stated clearly that dietary polyphenols dramatically affected the biodiversity of human intestinal microbiota (Fig. 2) (Chen et al., 2019; Goodrich et al., 2014).
Fig. 2.
The modulatory effect of tea catechins on human intestinal micro-ecology
Studies on polyphenolic compounds in black tea and green tea have shown that they can inhibit the growth of many pathogens in vitro, including Helicobacter pylori, Staphylococcus aureus, Escherichia coli O157:H7, Salmonella typhimurium DT104, Pseudomonas aeruginosa and so on (Bancirova, 2010). Lee et al. (2006) studied the effects of EC, catechin, 3-O-methyl gallic acid and gallic acid on bacterial growth and found that different intestinal bacterial strains had varying degrees of growth sensitivity to tea phenolics. It is well known that short-chain fatty acids (SCFAs) play a central part in regulating host metabolism, immune system and cell proliferation (Koh et al., 2016). Among them, butyrate is the most important member of the family of SCFAs and the main energy source for human colon cells (Devadder et al., 2014). Propionate is the substrate of gluconeogenesis, which regulates intestinal gluconeogenesis through GPR41 signaling pathway and protects the host from obesity-related glucose intolerance (de and Mithieux, 2018). According to our previous study in vitro, the growth of Bacteroides-Prevotella, Clostridium histolyticum and Eubacterium-Clostridium groups were significantly inhibited by tea catechins, including EGCG, (+)-gallocatechin gallate (GCG) and (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3”Me), while the growth of Bifidobacterium spp. and Lactobacillus/Enterococcus groups were less affected. In addition, the concentration of SCFAs in cultures with tea catechins was relatively higher than the control (Zhang et al., 2013). Liao et al. (2016) found that tea polyphenols significantly increased the number of Bifidobacteria while reducing serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels in mice. Similar results were observed in a clinical study, 10 volunteers showed an increase of Bifidobacterium in their feces after drinking green tea for 10 days (Jin et al., 2012).
Polyphenols can act as a promoting factor for the proliferation of beneficial members of the intestinal microbiota. The mechanism for the modulatory effect of tea catechins on the gut flora includes the interactions with the basic development and metabolic aspects of bacteria, as well as interference with cell membrane function and bacterial energy metabolism (Barbieri et al., 2017). The effects of tea catechins on the bacterial growth and metabolism depends on the structure of polyphenols, dosimetry and the microorganism strain, which can interact with bacterial cell surfaces to inhibit enzyme activity and thereby affect energy metabolism (Hervert-Hernández and Goñi, 2011). Our previous studies have shown that when microorganisms were stressed by exposure to polyphenols, they may up-regulate proteins related to defensive mechanisms which protect cells, while simultaneously down-regulate various proteins involved in metabolism and biosynthesis (Cheng et al., 2018). It has been reported that ECG can make methicillin-resistant S. aureus sensitive to β-lactam antibiotics, promote staphylococcal cell aggregation and increase cell wall thickness (Stapleton et al., 2007).
EGCG also up-regulated certain stress responses and survival proteins, while down-regulated the energy metabolism, cell envelope, DNA metabolism and biosynthetic proteins in Klebsiella pneumonia (Daglia et al., 2014). Akkermansia muciniphila (A. muciniphila) is a mucin-degrading bacterium commonly found in human gut. Due to its highly promising probiotic activities against obesity and diabetes, it has drawn intensive interest recently (Zhou et al., 2017). A. muciniphila could generate SCFAs by decomposing mucins to stimulate the goblet cells to produce more mucus, and thus replenishing or preserving the intestinal barrier integrity. Besides, A. muciniphila may decrease the abundance of Firmicutes and Clostridia, therefore promoting the gut homeostasis (Hänninen et al., 2017). It has been reported dietary polyphenols could increase the relative abundance of A. muciniphila and attenuate high-fat diet-induced metabolic syndrome (Roopchand et al., 2015). The most enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in terms of differentially expressed genes (DEGs) after oolong tea polyphenol (OTP) intervention included the amino acids biosynthesis, carbon metabolism and the ribosome (Cheng et al., 2018), while treatment with EGCG3”Me, the results displayed that genes enrichment involved in amino acid biosynthesis, two-component system, ATP-binding cassette (ABC) transporters, purine metabolism, and carbon metabolism (Zhang et al., 2018).
In general, as a prebiotic supplement, tea catechins can regulate intestinal microbiota composition by enriching beneficial bacteria and inhibiting certain pathogenic bacteria. Therefore, the application of tea catechins may be benefit for the prevention and treatment of intestinal dysfunction.
Microbial metabolism of tea catechins
The metabolic absorption of tea catechins by the human body is mainly dependent on the biotransformation of intestinal microorganisms. Part of the tea catechins ingested by the human body directly enter the colon, and another part may undergo extensive Phase I (oxidation, reduction and hydrolysis) and especially Phase II (conjugation) biotransformations in intestinal cells and then the hepatocytes, resulting in the rapid release of a series of water-soluble conjugate metabolites (methyl, glucuronide and sulfate derivatives) into the systemic circulation. In the colon, tea catechins are glycosylated by bacterial enzymes, then dehydroxylated and demethylated by intestinal microbes into intermediate metabolites, and further converted into small molecular compounds, which enter the hepatoenteral circulation or systemic circulation to exert various physiological functions (Pastoriza et al., 2017). Tea catechins are converted into small molecular phenolic acids by intestinal microbes and then methylated, glucuronated, sulfated or nucleated into the blood. Valerolactone is converted into valeric acid by isomerization, and continues to oxidize or glycinate in the liver to become phenylacetic acid, benzoic acid, hydroxypropionic acid, and hippuric acid (Williamson and Clifford, 2017). The ability of tea catechins to pass through biofilms depends on their size, hydrophobicity, and any intracellular responses that promote diffusion by maintaining a concentration gradient (Williamson et al., 2018). The multiple hydroxyl groups in the molecules of tea catechins form a hydration layer outside the molecules, making them difficult to be directly absorbed by the enterocytes. The flavan-3-ol monomers are easily absorbed by the small intestine, or converted into glucuronic acid and oxymethyl derivative under the action of Phase II enzyme in the small intestine, and then enter the systemic circulation; its oligomer or polymer needs to enter the colon through intestinal microbial transformation before they can be absorbed into the blood. Extensive research has been carried out on the transformation of tea catechins in the intestine, and the results have showed that the intestinal flora may enhance the biological activity of dietary polyphenols (Chiou et al., 2014).
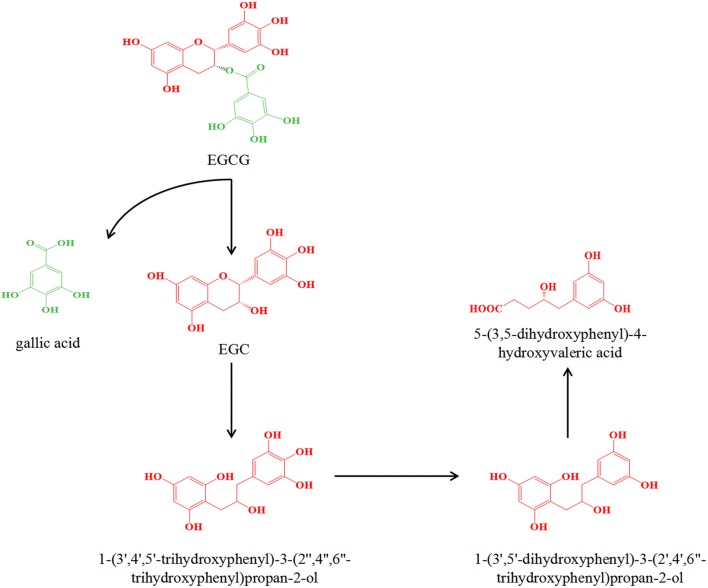
Kutschera et al. (2011) isolated two strains Eggerthella lenta rK3 and Flavonifractor plautii aK2 from an EC-converting human feces suspension. They found that Eggerthella lenta rK3 converted (+)-catechin or EC to 1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol, Flavonifractor plautii aK2 further converted 1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol to 5-(3,4-dihydroxyphenyl)-valerolactone and 4-hydroxy-5-(3,4-dihydroxyphenyl)-valeric acid. It is worth noting that the conversion of (+)-catechin was five times faster than that of EC. The catabolism of (+)-catechin and EC was detected in vitro by the rat intestinal microbiota. As a result, 4-hydroxy-5-(3-hydroxyphenyl)-valeric acid, 4-oxo-5-(3,4-dihydroxyphenyl)-valeric acid, 1-4-oxo-5-(3-hydroxyphenyl)-valeric acid, and 1-(4-hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol were identified as new metabolites (Takagaki and Nanjo, 2013). Besides, the anaerobic metabolism of EGCG by rat intestinal microbes was also investigated (Takagaki and Nanjo, 2010). Firstly, intestinal strains are able to hydrolyze EGCG to EGC and gallic acid. Then EGC was converted to 1-(3′,4′,5′-trihydroxyphenyl)-3-(2″,4″,6″-trihydroxyphenyl)propan-2-ol by reductive cleavage between 1 and 2 positions of EGC, and subsequently the metabolite was converted to 1-(3′,5′-dihydroxyphenyl)-3-(2′,4′,6″-trihydroxyphenyl)propan-2-ol followed by the conversion to 5-(3,5-dihydroxyphenyl)-4-hydroxyvaleric acid by the decomposition of the phloroglucinol ring in 1-(3′,5′-dihydroxyphenyl)-3-(2′,4′,6″-trihydroxyphenyl)propan-2-ol, which is the main pathway for EGCG metabolism (Fig. 3). EGCG was found to be relocated to the cecum and large intestine and then degraded by the intestinal microbiota to 5-(3’,5’-dihydroxyphenyl)-γ-valerolactone with EGC as an intermediate product (Kohri et al., 2001). Typically, these metabolites formed by the colonic microbiota are taken up by the portal vein and transported to the liver where a Phase II conjugation reaction can occur and a conjugate or combination thereof can be produced. These metabolites then circulate in the blood and are subsequently expelled in the urine, while the unabsorbed ones are excreted in the feces. The effective absorption of polyphenolic microbial metabolites into the intestine is a key pathway for tea catechins to be considered to exert health benefits, but their biological activities are still under investigation. Collectively, these conclusions demonstrate the importance of intestinal microbial metabolism of polyphenols and the various biotransformation possibilities encoded in the microbiome.
Fig. 3.
The metabolic pathway and common metabolites of EGCG
Potential use of tea catechins in IBD
IBDs are a group of chronic inflammatory bowel diseases, including Crohn’s disease (CD) and ulcerative colitis (UC) (Zhang and Merlin, 2018), which generally considered to be caused by host inheritance, environment, immune response and intestinal microbes (Wlodarska et al., 2015). In genetics and immunology studies, the imbalance between cytokine production and T cell dysfunction is the main cause of IBD (Geremia et al., 2014). Upon the disruption of the barrier function, symbiotic bacteria and microbial products enter the intestinal wall from the intestinal lumen, resulting in the activation of immune cells and production of cytokines. The chronic intestinal inflammation is triggered by uncontrolled mucosal immune system activation (Fig. 4). The chronic inflammation can give rise to disease complications and tissue destruction, both of which are driven by mucosal cytokine responses. Subsequently, an abnormal and excessive response to this environment leads to subclinical or acute mucosal inflammation of genetically susceptible hosts (Peery et al., 2018).
Fig. 4.
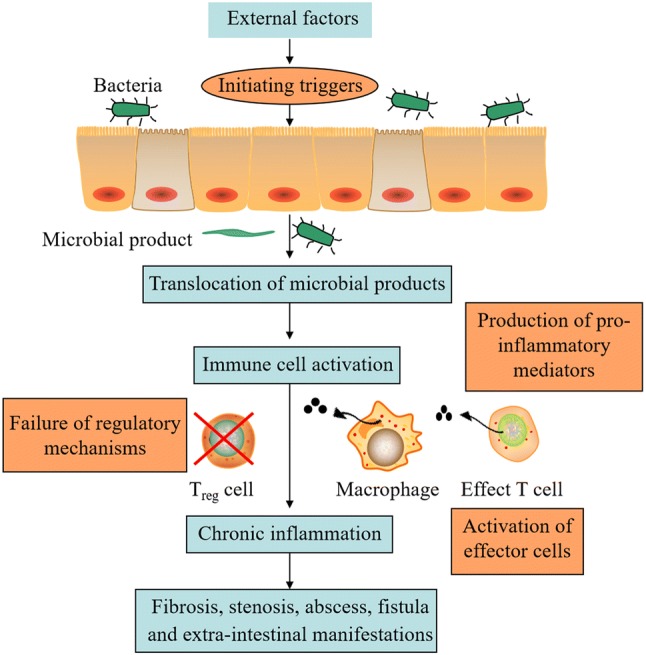
The formation of chronic intestinal inflammation
NF-κB is an important immunoregulatory factor that responds to the stimulation of harmful cells in the first place. Once activated, NF-κB induces the expression of genes involved in inflammation and immunity, such as pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, and IL-12), chemokines, adhesion molecules and inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (Park et al., 2017). In addition to the NF-kB pathway, the cytokine signaling pathway involved in transcription factors of the signal transducer and transcriptional activator (STAT) family is another related pro-inflammatory signaling pathway in the IBD range (Mizoguchi et al., 2018). Current treatments for patients with IBD are mainly by anti-TNFα antibodies for down-regulating abnormal immune responses and inflammatory cascades (Rutgeerts et al., 2009). However, these therapies are subject to many restrictions due to the poor tolerance and adverse effects. Therefore, preventive/adjuvant therapy has become particularly important.
For the unique structure, polyphenols have shown significant ability to modulate inflammation and immune response (Bhooshan and Ibrahim, 2009). It has been shown that polyphenols can prevent or delay the development of IBDs by the antioxidant activity, anti-inflammatory activity as well as the action on intestinal microbiota (Kaulmann and Bohn, 2016). The administration of green tea polyphenol (GTP) and EGCG significantly reduced the increase of inflammatory markers TNFα, IL-6 and serum amyloid A, and lowered the severity of colitis (Oz et al., 2013). However, it has been reported that a high EGCG dose (5% diet) may enhance the inflammation in the colon and produce deleterious effects (Guan et al., 2012). Although antioxidant effects play an important role in the origin and progression of IBD, more attention has been paid to anti-inflammatory aspects. GTP could promote the regulation of inflammatory responses by reducing the production of final pro-inflammatory mediators and interfering with the induction of the NF-κB and MAPKs signaling pathways (Varilek et al., 2001). It has been reported in IEC-6 cells pretreated with GTP, the TNFα-induced IKK and NF-κB activity were dramatically reduced (Yang et al., 2001). In addition, in the Mdr1a−/− IBD mouse model, the activation of STAT1 or NF-κB pathways by innate immune responses was inhibited by the treatment of GTP (Barnett et al., 2013). Our previous work has demonstrated that tea catechins might have prebiotic-like activity and potential therapeutic utility in manipulating the gut microbiota (Zhang et al., 2018). It is worth noting a significant reduction in the number of Bacteroides and Firmicutes was shown on the intestinal mucosa of patients with IBD, while the population of Proteobacteria and Actinobacteria increased significantly (Barnett et al., 2013). Therefore, understanding the potential cross-talk mechanism may help us further elucidate the clinical values of tea catechins in the prevention and treatment of IBDs.
Beneficial effects of tea catechins on obesity
In recent years, obesity has gradually become a global health burden (Bray et al., 2017). Obesity is the result of energy destruction and an important risk factor for many chronic diseases, including non-alcoholic fatty liver disease, type II diabetes, and cardiovascular disease (Karri et al., 2019). Therefore, the implementation of a dietary regimen to prevent obesity has becoming a public health goal.
It is currently recognized that adipocyte formation can be divided into two related stages (Cristancho and Lazar, 2011). First, the adipose-derived stem cells (ASC) contained in the stromal vascular fraction (SVF) of the adipose tissue matrix are differentiated into preadipocytes. Second, preadipocytes proliferate and differentiate into mature adipocytes, which are regulated by a variety of transcription factors and accompanied by the expression of lipid-related genes and lipid metabolism synthase genes. CCAAT/enhancer-binding protein family members (C/EBP) and peroxisome proliferator-activated receptors (PPARγ) are two important transcriptional factors promoting the early stage of adipogenesis. In the first step of this phase, preadipocytes undergo contact inhibition, exit the cell cycle and stop cell division. When the hormonal changes in the body initiate the pre-adipocyte proliferation and differentiation program, C/EBPβ and C/EBPδ are transiently expressed, and then PPARγ is induced, which further promotes the production of C/EBPα. Co-expression of C/EBPα and PPARγ produces a synergistic effect that up-regulates the expression of specific genes associated with differentiation. The second step of the stage is mainly characterized by a marked increase in lipid droplets, an increase in volume, and an increase in the expression of enzymes related to triglyceride synthesis, such as fatty acid synthase (FAS), lipoprotein lipase (LPL), acetyl-CoA carboxylase (ACCase), and ATP citrate lyase (ACLY) (Pan et al., 2016).
Tea has been used as an anti-obesity therapy in China for more than a millennium. The anti-obesity mechanism of tea catechins has been proposed including suppressing dietary fat absorption, enhancing fat oxidation in adipose tissue and skeletal muscle, decreasing de novo lipogenesis, and regulating intestinal microbiota (Chen et al., 2011; Sheng et al., 2018). Our previous study demonstrated that methylated tea catechins, including EGCG3’’Me and ECG3’’Me inhibited the proliferation and differentiation of 3T3-L1 preadipocyte, and the difference of inhibitory effects for tested compounds may be due to their structural difference (Yang et al., 2015). A host of evidence suggests that altering the intestinal flora by the supplement of specific prebiotics or probiotics may effectively alleviate metabolic dysfunction (Delzenne and Cani, 2011). It is worth noting that the Firmicutes to Bacteroidetes (F/B) ratio is higher in obese human than in lean ones. In our previous study, after 4 weeks of OTP administration in a high fat diet-induced obesity mouse model, a large increase in the relative abundance of Bacteroidetes and a significant decrease in Firmicutes was observed (Cheng et al., 2018). However, obese women who consumed EGCG (300 mg/d) for 12 weeks did not find significant changes in body weight, fat mass, and liver function biomarkers (Juan et al., 2014). These inconsistencies may depend on the bioavailability of tea catechins, intake, and ethnic and genetic factors. Consequently, long-term research on the use of tea catechins supplements is indispensable.
Tea catechins and their metabolites can regulate intestinal micro-ecological balance by the modulation of the component of intestinal flora. Researches focusing on the effects of two-way interactions between tea catechins and gut microbiota are critical because these results may provide new information about health. Furthermore, a combination of metagenomics and metabolomics studies will better understand the relationship between dietary polyphenols and intestinal microflora in order to advance knowledge in this area and provide evidence of the practical value of polyphenols.
Acknowledgements
This work was sponsored by Zhejiang Provincial Natural Science Foundation of China (LY19C200006), the Key Research and Development Project of Zhejiang Province (2017C02039 and 2018C02047), and K.C. Wong Magna Fund in Ningbo University.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tongtong Guo, Email: 919888018@qq.com.
Dan Song, Email: 1263577116@qq.com.
Lu Cheng, Email: lc894@scarletmail.rutgers.edu.
Xin Zhang, Email: zhangxin@nbu.edu.cn.
References
- Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 2010;43:1379–1382. [Google Scholar]
- Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Barnett MP, Cooney JM, Dommels YE, Nones K, Brewster DT, Park Z, Butts CA, Mcnabb WC, Laing WA, Roy NC. Modulation of colonic inflammation in Mdr1a(-/-) mice by green tea polyphenols and their effects on the colon transcriptome and proteome. J. Nutr. Biochem. 2013;24:1678–1690. doi: 10.1016/j.jnutbio.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Bhooshan PK, Ibrahim RS. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- Chen Tingting, Liu Anna B., Sun Shili, Ajami Nadim J., Ross Matthew C., Wang Hong, Zhang Le, Reuhl Kenneth, Kobayashi Koichi, Onishi Janet C., Zhao Liping, Yang Chung S. Green Tea Polyphenols Modify the Gut Microbiome in db/db Mice as Co‐Abundance Groups Correlating with the Blood Glucose Lowering Effect. Molecular Nutrition & Food Research. 2019;63(8):1801064. doi: 10.1002/mnfr.201801064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2011;59:11862–11871. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Zhang X, Guo XJ, Wu ZF, Weng PF. The interaction effect and mechanism between tea polyphenols and intestinal microbiota: Role in human health. J. Food Biochem. 2017;41:e12415. [Google Scholar]
- Cheng M, Zhang X, Zhu J, Cheng L, Cao J, Wu Z, Weng P, Zheng X. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. 2018;9:1079–1087. doi: 10.1039/c7fo01570d. [DOI] [PubMed] [Google Scholar]
- Chiou YS, Wu JC, Huang Q, Shahidi F, Wang YJ, Ho CT, Pan MH. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods. 2014;7:3–25. [Google Scholar]
- Čitar M, Hacin B, Tompa G, Štempelj M, Rogelj I, Dolinšek J, Narat M, Matijašić BB. Human intestinal mucosa-associated Lactobacillus and Bifidobacterium strains with probiotic properties modulate IL-10, IL-6 and IL-12 gene expression in THP-1 cells. Benef. Microbes. 2015;6:325–336. doi: 10.3920/BM2014.0081. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia M, Di Lorenzo A, Nabavi SF, Talas ZS, Nabavi SM. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr. Pharm. Biotechnol. 2014;15:362–372. doi: 10.2174/138920101504140825120737. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vadder F, Mithieux G. Gut-brain signaling in energy homeostasis: the unexpected role of microbiota-derived succinate. J. Endocrinol. 2018;236:R105–R108. doi: 10.1530/JOE-17-0542. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Cani PD. Gut microbiota and the pathogenesis of insulin resistance. Curr. Diab. Rep. 2011;11:154–159. doi: 10.1007/s11892-011-0191-1. [DOI] [PubMed] [Google Scholar]
- Devadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Geremia A, Biancheri P, Allan P, Corazza GR, Di SA. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F, Liu AB, Li G, Yang Z, Sun Y, Yang CS, Ju J. Deleterious effects of high concentrations of (-)-epigallocatechin-3-gallate and atorvastatin in mice with colon inflammation. Nutr. Cancer. 2012;64:847–855. doi: 10.1080/01635581.2012.695424. [DOI] [PubMed] [Google Scholar]
- Hänninen Arno, Toivonen Raine, Pöysti Sakari, Belzer Clara, Plovier Hubert, Ouwerkerk Janneke P, Emani Rohini, Cani Patrice D, De Vos Willem M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2017;67(8):1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- Hervert-Hernández D, Goñi I. Dietary polyphenols and human gut microbiota: a review. Food Rev. Int. 2011;27:154–169. [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JS, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012;56:729–739. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- Juan MA, Lurdes B, Pilar A, Eider L, Javier M, Idoia L. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014;111:1263–1271. doi: 10.1017/S0007114513003784. [DOI] [PubMed] [Google Scholar]
- Karri S, Sharma S, Hatware K, Patil K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019;110:224–238. doi: 10.1016/j.biopha.2018.11.076. [DOI] [PubMed] [Google Scholar]
- Kaulmann A, Bohn T. Bioactivity of polyphenols: preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016;2016:9346470–29. doi: 10.1155/2016/9346470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A, De VF, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N. Metabolic fate of (-)-[4-3H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001;49:4102–4112. doi: 10.1021/jf001491+. [DOI] [PubMed] [Google Scholar]
- Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011;111:165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Liao ZL, Zeng BH, Wang W, Li GH, Wu F, Wang L, Zhong QP, Wei H, Fang X. Impact of the consumption of tea polyphenols on early atherosclerotic lesion formation and intestinal Bifidobacteria in high-fat-fed ApoE-/- mice. Front. Nutr. 2016;3:42. doi: 10.3389/fnut.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018;53:465–474. doi: 10.1007/s00535-017-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo H, Nakatsu Y, Kushiyama A, Yamamotoya T, Matsunaga Y, Inoue MK, Fujishiro M, Sakoda H, Ohno H, Yoneda M, Ono H, Asano T. Gut microbiota as a therapeutic target for metabolic disorders. Curr. Med. Chem. 2018;25:984–1001. doi: 10.2174/0929867324666171009121702. [DOI] [PubMed] [Google Scholar]
- Oz HS, Chen T, de Villiers WJ. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013;4:132. doi: 10.3389/fimmu.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MH, Tung YC, Yang G, Li S, Ho CT. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 2016;7:4481–4491. doi: 10.1039/c6fo01168c. [DOI] [PubMed] [Google Scholar]
- Park Jun-Young, Chung Tae-Wook, Jeong Yun-Jeong, Kwak Choong-Hwan, Ha Sun-Hyung, Kwon Kyung-Min, Abekura Fukushi, Cho Seung-Hak, Lee Young-Choon, Ha Ki-Tae, Magae Junji, Chang Young-Chae, Kim Cheorl-Ho. Ascofuranone inhibits lipopolysaccharide–induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. PLOS ONE. 2017;12(2):e0171322. doi: 10.1371/journal.pone.0171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoriza S, Mesías M, Cabrera C, Rufián-Henares JA. Healthy properties of green and white teas: an update. Food Funct. 2017;8:2650–2662. doi: 10.1039/c7fo00611j. [DOI] [PubMed] [Google Scholar]
- Peery AF, Keku TO, Addamo C, McCoy AN, Martin CF, Galanko JA, Sandler RS. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin. Gastroenterol. Hepatol. 2018;16:884–891. doi: 10.1016/j.cgh.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. 2015;64:2847–2858. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Vermeire S, Assche GV. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sheng L, Jena PK, Liu HX, Hu Y, Nagar N, Bronner DN, Settles ML, Bäumler AJ, Wan YY. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 8: fj201800370R (2018) [DOI] [PMC free article] [PubMed]
- Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The beta-lactam-resistance modifier (-)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093–2103. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki A, Nanjo F. Catabolism of (+)-catechin and (−)-epicatechin by rat intestinal microbiota. J. Agric. Food Chem. 2013;61:4927–4935. doi: 10.1021/jf304431v. [DOI] [PubMed] [Google Scholar]
- Takagaki A, Nanjo F. Metabolism of (−)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010;58:1313–1321. doi: 10.1021/jf903375s. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varilek GW, Yang F, Lee EY, de Villiers WJ, Zhong J, Oz HS, Westberry KF, McClain CJ. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J. Nutr. 2001;131:2034–2039. doi: 10.1093/jn/131.7.2034. [DOI] [PubMed] [Google Scholar]
- Williamson G, Clifford MN. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017;139:24–39. doi: 10.1016/j.bcp.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. F. 2018;17:1054–1112. doi: 10.1111/1541-4337.12351. [DOI] [PubMed] [Google Scholar]
- Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17:577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu. Rev. Nutr. 2013;33:161–181. doi: 10.1146/annurev-nutr-071811-150717. [DOI] [PubMed] [Google Scholar]
- Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001;60:528–533. [PubMed] [Google Scholar]
- Yang Y, Qiao L, Zhang X, Wu Z, Weng P. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia. 2015;104:45–49. doi: 10.1016/j.fitote.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang M, Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm. Bowel Dis. 2018;24:1401–1415. doi: 10.1093/ibd/izy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang X, Ho CT, Huang Q. Chemistry and health effect of tea polyphenol (-)-epigallocatechin 3-O-(3-O-methyl)gallate. J. Agric. Food Chem. 2019;67:5374–5378. doi: 10.1021/acs.jafc.8b04837. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Zhu J, Zhang M, Ho CT, Huang Q, Cao J. Metagenomics analysis of gut microbiota in a high fat diet-induced obesity mouse model fed with (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3’’Me) Mol. Nutr. Food Res. 2018;62:e1800274. doi: 10.1002/mnfr.201800274. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu X, Sun Y, Hu B, Sun Y, Jabbar S, Zeng X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013;54:1589–1595. [Google Scholar]
- Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods. 2017;33:197–201. doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]