Abstract
Benign prostatic hyperplasia (BPH) is a noncancerous growth of the prostate. BPH commonly occurs in elderly men. Lower urinary tract symptoms (LUTS) secondary to BPH (LUTS/BPH) have significant impacts on their health. Saw palmetto (Serenoa repens) extract (SPE) has been evaluated for its effectiveness in improvement of LUTS/BPH at preclinical and clinical levels. Potential mechanisms of actions include anti-androgenic, pro-apoptotic, and anti-inflammatory effects. However, SPE efficacy was inconsistent, at least partly due to a lack of a standardized SPE formula. A hexane extract (free fatty acids, > 80%) provided more consistent results. Free fatty acids (lauric acid) were effective in inhibition of 5α-reductase, and phytosterol (β-sitosterol) reduced prostatic inflammation. Multiple actions derived from different constituents may contribute to SPE efficacy. Evaluation of the clinical relevance of these bioactive components is required for standardization of SPE, thereby enabling consistent efficacy and recommendations for the use in the prevention and treatment of BPH.
Keywords: Benign prostatic hyperplasia, Fatty acids, Phytosterol, Saw palmetto extract, Standardized formula
Introduction
Benign prostatic hyperplasia (BPH) refers to nonmalignant growth of the prostate. Histologically, BPH is described as a proliferative process of both the stromal and epithelial elements of the prostate gland. BPH commonly occurs in elderly men. Nearly all men develop histologic evidence of BPH by 80 years of age (Lepor, 2005).
BPH arises in the periurethral and transition zones of the prostate. Human prostate grows with age. This continued growth (hyperplasia) can enlarge the prostate to the extent that it compresses the urethra and limits the flow of urine, causing urinary symptoms (Lepor, 2005). However, prostatic enlargement varies in degree in different individuals (Lepor, 2005). Therefore, not all men with histologic BPH will develop significant symptoms (Roehrborn, 2005). The most common manifestation of BPH is lower urinary tract symptoms (LUTS), a group of clinical symptoms comprising obstructive symptoms including hesitancy, poor stream, incomplete voiding, urinary retention, and overflow incontinence as well as irritative symptoms including frequent urination, urgent urination, nocturia, and urge incontinence (Roehrborn, 2005).
A recent systemic review estimated that the prevalence of symptomatic BPH increases from 14.8% in males aged 40 to 36.8% in males aged 80 and above, although marked differences in estimated prevalence among different studies were observed largely due to the heterogeneity in BPH definition and the variation in case definitions used (reviewed in Lee et al., 2017). As the numbers of the elderly increase rapidly worldwide, the incidence and prevalence of BPH will increase and LUTS secondary to BPH will have a significant impact on the health of older men and on associated health-care costs.
The reasons causing the prostate to enlarge are still poorly understood. In general, dihydrotestosterone (DHT), a metabolite of testosterone, is thought to be a critical mediator of prostatic growth (Rhodes et al., 1993). DHT is derived from testosterone in specific tissues including the prostate gland via the action of 5α-reductase. DHT binds to nuclear androgen receptors and activates the transcription of androgen-regulated genes that are mitogenic to both epithelial and stromal cells (Saad et al., 2011).
Treatment options are medication and surgery. Medication for the treatment of mild to moderate BPH has become a standard of care since well-designed clinical studies showed that finasteride, a 5α-reductase inhibitor (5-ARI), and terazosin, an α-blocker, significantly improved LUTS and increased peak urinary flow rates in men with BPH (Lepor, 1989). Subsequently, many clinical trials have validated the effectiveness of two 5-ARIs (finasteride and dutasteride) and five α-blockers (terazosin, doxazosin, tamsulosin, alfuzosin, and silodosin), which were subsequently approved by the US Food and Drug Administration for the treatment of BPH (Lepor, 2011).
Alpha-blockers, also known as α-adrenergic receptor antagonists, relax smooth muscles in the prostate and the bladder neck, thus decreasing the blockage of urine flow and allowing urine to flow more easily. Alpha-blockers begin to work quickly with no effect on prostate size and are usually recommended as a first-line treatment for men with mild to moderate symptoms (Lepor, 2016). 5-ARIs, also known as DHT blockers, inhibit the 5α-reductase enzyme, and thus can prevent the prostate from growing further. In addition, phytotherapy including extract of the fruit from saw palmetto (Serenoa repens), the American dwarf palm tree has been used. Although many physicians remain skeptical regarding its therapeutic use, there is continued growing interest in the use of saw palmetto extract (SPE) in patients with BPH due to drug-related adverse effects associated with α-blockers (e.g., postural hypotension and retrograde ejaculation) and with 5-ARIs (e.g., impairment of erection) (Debruyne et al., 2004).
The present study reviewed the effects and potential mechanisms of action of SPE and putative bioactive components in SPE. The objective of the review was to manifest the importance of standardization of formula in preparation of dietary supplements such as SPE in order to recommend their preventive and therapeutic use based on their consistent efficacy.
Effect of saw palmetto extract on lower urinary track symptoms
Historically, Native Americans were known to use the fruit of saw palmetto to treat urinary and reproductive system problems (Suzuki et al., 2009). SPE is commonly used to treat BPH in Europe (Suzuki et al., 2009). SPE use in the treatment of BPH-related LUTS has been extensively evaluated. However, many of the reported studies (Champault et al., 1984; Reece Smith et al., 1986) were short-term studies conducted for 3 months or less and were performed before the development of validated symptom scores such as the international prostatic symptoms scale (IPSS) and the international index of erectile function (IESS) (MacDonald et al., 2012). This review included SPE clinical trials that used standardized screening tools such as IPSS to monitor the severity of the symptom of LUTS secondary to BPH.
Some non-comparative observational studies suggested that daily intake of 320 mg SPE for 24 months was effective in improving IPSS and IESS scores as well as quality of life (Sinescu et al., 2011) and that treatment for 15 years prevented progression of BPH (Vinarov et al., 2018). In contrast, a randomized and controlled study found that taking 320 mg SPE extract daily did not improve symptoms or urinary flow compared to placebo (Bent et al., 2006). Moreover, longer follow-up (72 weeks) and use of higher doses (640 or 960 mg) did not improve LUTS compared to placebo (Barry et al., 2011; Tacklind et al., 2012). Therefore, recommendations for the use of SPE in BPH cannot be proposed currently.
However, of note, disparity in composition of SPE may contribute to heterogeneity in efficacy (Habib and Wyllie, 2004). Different products derived from the same plant can have different activity due to differences in the composition of bioactive components in the plant extracts (Novara et al., 2016). Permixon is a hexane extract of saw palmetto and is the most studied. Many SPE products were found to have significantly different relative contents of putative active components, fatty acids and phytosterols (Habib and Wyllie, 2004; Penugonda and Lindshield, 2013). Permixon contained the highest content (> 80%) of free fatty acids among 14 products available in Europe (Habib and Wyllie, 2004). Permixon also showed the highest efficacy in inhibition of 5α-reductase (Scaglione et al., 2012). Consistently, clinical studies with Permixon have produced reproducible results of improvement of urinary symptoms (MacDonald et al., 2012; Pytel et al., 2002). A meta-analysis that includes all available randomized control trials and observational studies showed that Permixon reduced nocturia and improved maximum urinary flow compared with placebo (Vela-Navarrete et al., 2018). Moreover, Permixon had a similar efficacy to tamsulosin (a commonly prescribed α-blocker) (Debruyne et al., 2004) and 5-ARIs (Vela-Navarrete et al., 2018) in relieving LUTS. These data suggested that the hexane extract of saw palmetto (Permixon) is an efficacious therapeutic option for the long-term treatment of LUTS secondary to BPH. Different brands of SPE may show marked variation in content. Therefore, standardization of SPE formula, and control of the content of active components in SPE may be critical to achieving consistent efficacy of SPE and recommendations for its use in prevention and treatment of BPH (Fagelman and Lowe, 2001).
In general, saw palmetto extracts are well tolerated in men with BPH. There is agreement that adverse effects of saw palmetto extracts are few and mild, and that their incidences are not significantly different compared to placebo (MacDonald et al., 2012; Tacklind et al., 2012; Wilt et al., 2002).
Mechanisms of action of saw palmetto extract
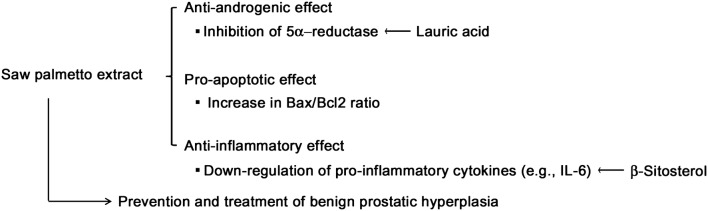
The exact mechanisms of action of SPE are unknown in relieving LUTS associated with BPH. Suggested mechanisms include anti-androgenic, pro-apoptotic, and anti-inflammatory effects (Fig. 1) as described below.
Fig. 1.
Putative mechanisms of action of saw palmetto extract (SPE) and its constituents in relief of lower urinary track symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH)
Anti-androgenic effect
Inhibition of receptor binding of androgens has also been studied (Sultan et al., 1984) but is less evident. The most studied area is the inhibitory activity of SPE on 5α-reductase. SPE non-selectively inhibited both type I and type II isoenzymes of 5α-reductase in comparison to finasteride, which selectively inhibits the type 2 isoform (Iehle et al., 1995). Inhibitory effects of SPE on 5α-reductase were also reported in human foreskin fibroblasts (Sultan et al., 1984) and a human prostate cancer cell line (Anderson, 2005).
In agreement with in vitro studies, a 3-month randomized study reported that Permixon (320 mg/day) effectively decreased DHT and increased testosterone in the periurethral region of the prostate in patients with BPH (Di Silverio et al., 1998), suggesting the inhibition of 5α-reductase by SPE in vivo. However, Permixon did not inhibit serum levels of either testosterone or DHT in healthy men consuming 80 mg SPE twice a day for up to a week, in contrast to the effective reduction of serum DHT level by a single dose of 5 mg finasteride (Strauch et al., 1994). SPE may require longer treatment or higher dose to achieve effective inhibition of 5α-reductase. It is also possible that SPE may alter testosterone or DHT metabolism differently in men with or without LUTS, although mechanisms are unknown. Therefore, studies suggest that SPE may inhibit 5α-reductase in men with symptomatic BPH. However, of note, many studies (Opoku-Acheampong et al., 2016; Talpur et al., 2003) indicate that SPE may modulate androgen metabolism and/or block the effects of androgens because of mitigation of androgen-induced increase in prostatic mass in castrated or non-castrated rodent models. However, more recently, prostate hyperplasia has been suggested to be induced by prostatic inflammation mediated by androgens as well as other factors including proinflammatory cytokines (Chughtai et al., 2011). Hence, inhibition of androgen-induced growth of the prostate by SPE may also be attributed to anti-inflammatory effect.
Pro-apoptotic effect
Studies also suggested that SPE treatment can inhibit the growth of prostate by increase of apoptosis as described below. Treatment with SPE inhibited cell growth via inhibition of insulin growth factor-1-induced proliferation and increased apoptotic index [cleavage of poly (ADP-ribose) polymerase] in the P69 prostate epithelial cell line (Wadsworth et al., 2004). SPE induced apoptotic cell death in PC3 and LNCaP human prostate cancer cells through the intrinsic apoptotic pathway (Baron et al., 2009). SPE-induced apoptosis was also observed in other types of cancer cell lines including MCF-7 breast cancer cells and HCT116 colon cancer cells (Hostanska et al., 2007).
In addition, apoptotic index (Bax-to-Bcl2 ratio) was significantly increased in transurethral prostate tissues obtained from men with symptomatic BPH by surgery after treatment with 320 mg Permixon daily for 3 months compared to those obtained from untreated controls (Vela-Navarrete et al., 2005). Therefore, consumption of SPE may reduce prostate size by increasing apoptosis. However, most studies have conducted in cancer cell lines and more studies are required to prove its effect in vivo.
Anti-inflammatory effect
More recent studies have highlighted the observation that intraprostatic inflammation mediates the development and/or progression of BPH (Gandaglia et al., 2013; Mishra et al., 2007). The risk of urinary retention due to BPH was significantly greater in men with prostatic inflammation than in those without prostatic inflammation (Mishra et al., 2007). Chronic prostatic inflammation was related to larger prostate volumes, more severe LUTS, and poorer response to BPH medical treatment (Gandaglia et al., 2013). These findings suggested that anti-inflammatory effect of SPE can be beneficial in treatment of BPH symptoms.
Treatment with Permixon significantly reduced the expression of inflammation-regulated genes including IL-6, CCL-5, CCL-2, COX-2, and iNOS in the human prostate cancer cell lines LNCaP and PC3, as well as in primary human prostate cancer cells (Silvestri et al., 2013). A oral dose (100 mg/kg body weight/day) of Permixon for 28 days significantly decreased tissue weight and proliferation index (Ki-76 immunostaining) in mouse model of prostate hyperplasia involving prostate-specific over-expression of a prolactin (Prl) transgene, namely probasin (Pb)-Prl model (Bernichtein et al., 2015). In this Pb-Prl model, administration of Permixon reduced prostatic inflammation both histologically and molecularly (e.g., down-regulation of pro-inflammatory cytokine profiles with significant reduction of CCR7, CXCL6, IL-6, and IL-17 expression) (Bernichtein et al., 2015).
At a clinical level, patients receiving a daily oral dose of Permixon (320 mg) for 3 months showed reduced levels of inflammatory marker at mRNA levels in the lumen of the prostate gland and this effect was more effective compared to that of tamsulosin (0.4 mg) (Latil et al., 2015). In addition, in the same study, the number of patients who expressed urinary CCL2 and CXCL10 proteins was decreased and expression of urinary macrophage migration inhibitory factor was significantly reduced in the Permixon-treated group than in the tamsulosin-treated group (Latil et al., 2015). Therefore, preclinical and clinical studies indicated that SPE, particularly, Permixon may effectively relieve BPH symptoms through anti-inflammatory mechanisms. However, different studies used different inflammatory markers and the relevance of these markers in BPH needs to be validated. Moreover, it should be further evaluated whether inflammation causes development of LUTS/BPH and vice versa.
In summary, anti-androgenic effect of SPE has been primarily studied in relation to its efficacy in BPH-related LUTS. As described above, studies also suggested that pro-apoptotic and anti-inflammatory effects are involved in beneficial effect of SPE (Fig. 1). Unfortunately, reported studies were largely conducted in vitro and the relevance of the mechanisms described above in prevention and treatment of BPH-related LUTS should be further validated at a clinical level (Fagelman and Lowe, 2001). It was also suggested that SPE efficacy may related to relaxation of lower urinary tract smooth muscles. SPE inhibited ligand binding to human α1-adrenergic (Goepel et al., 1999; Suzuki et al., 2007) and muscarinic (Suzuki et al., 2007) receptors. However, there are only few studies that evaluate the effects of SPE on relaxation of smooth muscle.
Bioactive components in saw palmetto extract
The bioactive components of SPE are considered to be fatty acids and phytosterols (Table 1). The fruit of saw palmetto typically contains 70–90% free fatty acids of caprylic, capric, lauric, myristic, palmitic, stearic, oleic, linoleic, and linolenic acids (Booker et al., 2014). The composition of the various brands of saw palmetto varies depending on extraction methods, source of the plant, and additional ingredients (Penugonda and Lindshield, 2013). The content of total free fatty acids was found to range from 40.7 to 80.7% (Table 1) in 14 SPE extracts commercially available for the treatment of LUTS (Habib and Wyllie, 2004). When 57 different products containing saw palmetto as mono-preparations or as a part of multicomponent supplements from nine countries were analyzed using gas chromatography, total fatty acid was found to be in the range of 8.43–1473 mg in a manufacturer-claimed therapeutic dose (Booker et al., 2014). In addition, the contents of free fatty acids and phytosterols were also found to vary depending on supplement types such as liquid, powder, dried berry, or tincture (Penugonda and Lindshield, 2013).
Table 1.
Composition of fatty acids and phytosterols in saw palmetto extract (SPE)
| Content in SPE | Proportion for each category (Penugonda and Lindshield, 2013) | |
|---|---|---|
|
Fatty acids (Habib and Wyllie, 2004) (40–80%) |
Oleic acid | > 30% |
| Lauric acid | 30% | |
| Myristic acid | 10% | |
| Linolenic acid | 10% | |
| Palmitic acid | 10% | |
| Stearic acid | < 5% | |
|
Phytosterols (Penugonda and Lindshield, 2013; Weisser et al., 1996) (< 1%) |
β-Sitosterol | 80% |
| Campesterol | < 15% | |
| Stigmasterol | < 10% | |
In spite of various concentrations of total fatty acid depending on SPE products, the composition of saw palmetto prepared by a similar extraction method was found to be comparable (Booker et al., 2014). The percentage of the nine different single fatty acids (capric acid, caprylic acid, lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid) was similar in products prepared by a similar extraction method (Booker et al., 2014). Saw palmetto fruits contained high amounts of oleic acid as well as lauric acid (Table 1) and each fatty acid was comprised approximately 30–40% of the total fatty acids (Booker et al., 2014). Oleic acid and lauric acid were also the predominant fatty acids irrespective of supplement types of liquid, powder, dried berry, or tincture (Penugonda and Lindshield, 2013). β-Sitosterol was the most abundant phytosterol, although phytosterol content varied in different supplement types (Penugonda and Lindshield, 2013).
SPE is a mixture of various compounds. One of the main difficulties in assessing the efficacy of SPE is the absence of standardization in composition (Fagelman and Lowe, 2001), which may result in varying efficacy of SPE in BPH treatment. Therefore, a standard formulation is essential to ensure a consistent biological effect of SPE. Permixon, the n-hexane lipidosterolic extract of saw palmetto, is the most studied form of SPE and studies with Permixon have produced relatively consistent beneficial effects on LUTS at preclinical and clinical levels. Therefore, it is important to identify major biologically active components of SPE in treatment of BPH and create a standardized formula for consistent beneficial effects on health. So far, only the European Pharmacopoeia has a specified requirement for saw palmetto preparations, requiring lauric acid to be at least 20% of total fatty acids (Booker et al., 2014).
Fatty acids
Fatty acids are the major constituents of SPE. The main fatty acids in SPE showed α1-adrenergic receptor-binding activity as well as inhibitory effects on 5α-reductase (Abe et al., 2009a; 2009b), suggesting that fatty acids in SPE contribute to relieving BPH symptoms via relaxation of muscle tone and inhibition of testosterone metabolism. Sub-fractionation of SPE further demonstrated that free fatty acids are largely responsible for inhibitory effects of SPE on 5α-reductase. When SPE was sub-fractionated into saponifiable, nonsaponifiable, and hydrophilic fractions, the saponifiable subfraction that consists mainly of lauric acid, oleic acid, myristic acid, and palmitic acid showed non-competitive and dose-dependent inhibition of 5α-reductase in the prostatic epithelium and stroma homogenates (Weisser et al., 1996). In contrast, the nonsaponifiable and hydrophilic subfractions showed only a slight and no inhibition of 5α-reductase, respectively (Weisser et al., 1996). 5α-Reductase derived from rat liver was also inhibited by the fatty acids (Liu et al., 2009). Inhibition of type 1 and type 2 5α-reductase activity was only observed with fatty acids whereas esterified fatty acids, alcohols, and sterols were inactive in inhibition of either type 1 or type 2 isoforms (Raynaud et al., 2002). Consistently, compared with fatty acids active in inhibition of 5α-reductase, methyl ester and alcohol analogs of these inhibitory fatty acids were either inactive or only slightly active in rat liver, suggesting that the free –COOH group is important for the inhibition of 5α-reductase (Liu et al., 2009).
Inhibitory activity of individual fatty acids has been found to vary in different studies as described below, probably due to the use of different assay systems. Lauric acid and myristic acid were the most effective in inhibition of 5α-reductase present in the prostatic epithelium and stroma homogenates whereas oleic acid and palmitic acids had almost no inhibitory effect (Weisser et al., 1996). When two isoforms of 5α-reductase were tested separately, lauric acid was active against both isoforms whereas palmitic acid and stearic acid were inactive against both isoforms (Raynaud et al., 2002). Oleic acid and linolenic acid were selectively effective against type 1 isoform (Raynaud et al., 2002). Myristic acid had a strong inhibitory effect against type 2 with its effect against type 1 being unevaluated (Raynaud et al., 2002). Similar results were observed in 5α-reductase derived from rat liver; among the saturated fatty acids, the inhibitory effects of shorter fatty acids such as lauric and myristic acids were greater (Liu et al., 2009). Inhibitory activity against 5α-reductase in the liver was similar for lauric acid, oleic acid, myristic acid, linoleic acid, but no inhibitory effect of palmitic acid was found among the tested fatty acids (Abe et al., 2009a). Therefore, the main free fatty acid constituents in SPE, lauric acid, oleic acid, and myristic acid, appear to be effective in inhibition of 5α-reductase.
In addition, lauric acid, myristic acid, and oleic acid significantly inhibited the contraction induced by phenylephrine in isolated rat vas deferens (a thick-walled tube in the male reproductive system) with lauric acid showing the highest at the same relative concentration present in SPE (Arruzazabala et al., 2011). Alpha-1 adrenergic receptors mediate smooth muscle contraction. Fatty acids such as lauric acid and oleic acid significantly inhibited the specific binding of radiolabeled ligands to α1-adrenergic, muscarinic, and 1,4-dihydropyridine calcium channel (Abe et al., 2009a). Thus, as described above, studies have suggested that activity of 5α-reductase and α1-adrenergic receptor-binding can be effectively inhibited by free fatty acids, particularly lauric acid, the main constituent of total fatty acids in SPE. However, inhibitory effect on 5α-reductase were mostly performed in vitro and their relevance in vivo needs to be validated. In addition, the bioavailable concentration of these fatty acids and SPE in the prostate tissue needs to be evaluated.
Phytosterols
Phytosterols are relatively minor constituents (Table 1) of SPE (Penugonda and Lindshield, 2013; Weisser et al., 1996). Studies have suggested that phytosterols may be beneficial in BPH treatment due to their anti-inflammatory and cholesterol-lowering effects (Freeman and Solomon, 2011; Scholtysek et al., 2009). β-Sitosterol inhibited proliferation of human prostate cancer cells (von Holtz et al., 1998) and growth of tumors derived from PC-3 human prostate cancer cells (Awad et al., 2001).
In a randomized, double-blind, and placebo-controlled clinical trial, treatment with 130 mg free β-sitosterol daily for 6 months significantly improved IPSS and quality of life index with an increase in peak urinary flow rates and decrease in post-void residual urinary volume over placebo (Klippel et al., 1997). Similar results were observed with another randomized, double-blind, and placebo-controlled multicentric study that a mixture of pytosterols (60 mg β-sitosterol per day) was treated for 6 months in patients with symptomatic BPH (Berges et al., 1995). Moreover, results for the 18-month follow-up of the trial suggested that the beneficial effects of β-sitosterol were maintained in patients who continued β-sitosterol treatment for 18 months after the initial 6-month trial (Berges et al., 2000). In addition, taking phytosterol prepared from pumpkin seeds and saw palmetto for 3 months significantly improved BPH symptoms with no side effects in a randomized, double-blind study (Carbin et al., 1990). Therefore, well-designed clinical trials supported beneficial effects of phytosterols in treating BPH symptoms. However, clinical studies mentioned above were mostly observational studies and were conducted in 1990’s. SPE contains only small amount of phytosterols (< 1%) and the quantity may not be sufficient to exert the beneficial effect of phytosterol treatment. Moreover, the phytosterol-rich extracts were often prepared from various plants that also contain other components in small quantities, leaving open the possibility that components other than phytosterols may have been involved in the observed beneficial effects.
In conclusion, saw palmetto extract has therapeutic potential. However, efficacy of SPE has been found to be inconsistent, at least partly due to a lack of standardization of the SPE formula. Free fatty acids such as lauric acid, the main constituents of SPE, have been shown to be effective in inhibition of 5α-reductase and phytosterol (β-sitosterol), minor constituents of SPE, was found to effectively reduce prostatic inflammation. Multiple rather than single mechanisms may be involved in the beneficial effects of SPE in BPH treatment. Therefore, it is important to standardize SPE products for consistent efficacy and to enable recommendations for SPE use in prevention and treatment of BPH.
Acknowledgements
This study was supported by a grant from the Ottogi Foundation (16-241).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe M, Ito Y, Oyunzul L, Oki-Fujino T, Yamada S. Pharmacologically relevant receptor binding characteristics and 5alpha-reductase inhibitory activity of free fatty acids contained in saw palmetto extract. Biol. Pharm. Bull. 2009;32:646–650. doi: 10.1248/bpb.32.646. [DOI] [PubMed] [Google Scholar]
- Abe M, Ito Y, Suzuki A, Onoue S, Noguchi H, Yamada S. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal. Sci. 2009;25:553–557. doi: 10.2116/analsci.25.553. [DOI] [PubMed] [Google Scholar]
- Anderson ML. A preliminary investigation of the enzymatic inhibition of 5alpha-reduction and growth of prostatic carcinoma cell line LNCaP-FGC by natural astaxanthin and saw palmetto lipid extract in vitro. J. Herb Pharmacother. 2005;5:17–26. [PubMed] [Google Scholar]
- Arruzazabala ML, Perez Y, Ravelo Y, Molina V, Carbajal D, Mas R, Rodriguez E. Effect of oleic, lauric and myristic acids on phenylephrine-induced contractions of isolated rat vas deferens. Indian J. Exp. Biol. 2011;49:684–688. [PubMed] [Google Scholar]
- Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur. J. Cancer Prev. 2001;10:507–513. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Baron A, Mancini M, Caldwell E, Cabrelle A, Bernardi P, Pagano F. Serenoa repens extract targets mitochondria and activates the intrinsic apoptotic pathway in human prostate cancer cells. BJU Int. 2009;103:1275–1283. doi: 10.1111/j.1464-410X.2008.08266.x. [DOI] [PubMed] [Google Scholar]
- Barry MJ, Meleth S, Lee JY, Kreder KJ, Avins AL, Nickel JC, Roehrborn CG, Crawford ED, Foster HE, Jr, Kaplan SA, McCullough A, Andriole GL, Naslund MJ, Williams OD, Kusek JW, Meyers CM, Betz JM, Cantor A, McVary KT, CAMUS Study Group Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA. 2011;306:1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006;354:557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- Berges RR, Kassen A, Senge T. Treatment of symptomatic benign prostatic hyperplasia with beta-sitosterol: An 18-month follow-up. BJU Int. 2000;85:842–846. doi: 10.1046/j.1464-410x.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol study group. Lancet. 1995;345:1529–1532. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Pigat N, Camparo P, Latil A, Viltard M, Friedlander G, Goffin V. Anti-inflammatory properties of lipidosterolic extract of Serenoa repens (Permixon(R)) in a mouse model of prostate hyperplasia. Prostate. 2015;75:706–722. doi: 10.1002/pros.22953. [DOI] [PubMed] [Google Scholar]
- Booker A, Suter A, Krnjic A, Strassel B, Zloh M, Said M, Heinrich M. A phytochemical comparison of saw palmetto products using gas chromatography and (1) H nuclear magnetic resonance spectroscopy metabolomic profiling. J. Pharm. Pharmacol. 2014;66:811–822. doi: 10.1111/jphp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. Br. J. Urol. 1990;66:639–641. doi: 10.1111/j.1464-410x.1990.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Champault G, Patel JC, Bonnard AM. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br. J. Clin. Pharmacol. 1984;18:461–462. doi: 10.1111/j.1365-2125.1984.tb02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011;13:147–150. [PMC free article] [PubMed] [Google Scholar]
- Debruyne F, Boyle P, Silva F, Gillenwater JG, Hamdy FC, Perrin P, Teillac P, Vela-Navarrete R, Raynaud JP, Schulman CC. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur. Urol. 2004;45:773–780. doi: 10.1016/j.eururo.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Di Silverio F, Monti S, Sciarra A, Varasano PA, Martini C, Lanzara S, D’Eramo G, Di Nicola S, Toscano V. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate. 1998;37:77–83. doi: 10.1002/(sici)1097-0045(19981001)37:2<77::aid-pros3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fagelman E, Lowe FC. Saw palmetto berry as a treatment for BPH. Rev. Urol. 2001;3:134–138. [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Solomon KR. Cholesterol and benign prostate disease. Differentiation. 2011;82:244–252. doi: 10.1016/j.diff.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, Sciarra A, Montorsi F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) BJU Int. 2013;112:432–441. doi: 10.1111/bju.12118. [DOI] [PubMed] [Google Scholar]
- Goepel M, Hecker U, Krege S, Rubben H, Michel MC. Saw palmetto extracts potently and noncompetitively inhibit human alpha1-adrenoceptors in vitro. Prostate. 1999;38:208–215. doi: 10.1002/(sici)1097-0045(19990215)38:3<208::aid-pros5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Habib FK, Wyllie MG. Not all brands are created equal: A comparison of selected components of different brands of serenoa repens extract. Prostate Cancer Prostatic Dis. 2004;7:195–200. doi: 10.1038/sj.pcan.4500746. [DOI] [PubMed] [Google Scholar]
- Hostanska K, Suter A, Melzer J, Saller R. Evaluation of cell death caused by an ethanolic extract of Serenoae repentis fructus (Prostasan) on human carcinoma cell lines. Anticancer Res. 2007;27:873–881. [PubMed] [Google Scholar]
- Iehle C, Delos S, Guirou O, Tate R, Raynaud JP, Martin PM. Human prostatic steroid 5 alpha-reductase isoforms - a comparative study of selective inhibitors. J. Steroid Biochem. Mol. Biol. 1995;54:273–279. doi: 10.1016/0960-0760(95)00134-l. [DOI] [PubMed] [Google Scholar]
- Klippel KF, Hiltl DM, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-phyto study group. Br. J. Urol. 1997;80:427–432. [PubMed] [Google Scholar]
- Latil A, Petrissans MT, Rouquet J, Robert G, de la Taille A. Effects of hexanic extract of Serenoa repens (Permixon(R) 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate. 2015;75:1857–1867. doi: 10.1002/pros.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A systematic review and meta-analysis. Sci. Rep. 2017;7:7984. doi: 10.1038/s41598-017-06628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepor H. Nonoperative management of benign prostatic hyperplasia. J. Urol. 1989;141:1283–1289. doi: 10.1016/s0022-5347(17)41282-1. [DOI] [PubMed] [Google Scholar]
- Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev. Urol. 2005;7(Suppl 4):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- Lepor H. Medical treatment of benign prostatic hyperplasia. Rev. Urol. 2011;13:20–33. [PMC free article] [PubMed] [Google Scholar]
- Lepor H. Alpha-blockers for the treatment of benign prostatic hyperplasia. Urol. Clin. North Am. 2016;43:311–323. doi: 10.1016/j.ucl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Liu J, Shimizu K, Kondo R. Anti-androgenic activity of fatty acids. Chem. Biodivers. 2009;6:503–512. doi: 10.1002/cbdv.200800125. [DOI] [PubMed] [Google Scholar]
- MacDonald R, Tacklind JW, Rutks I, Wilt TJ. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated cochrane systematic review. BJU Int. 2012;109:1756–1761. doi: 10.1111/j.1464-410X.2012.11172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, Motiwala HG, Laniado ME. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100:327–331. doi: 10.1111/j.1464-410X.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- Novara G, Giannarini G, Alcaraz A, Cozar-Olmo JM, Descazeaud A, Montorsi F, Ficarra V. Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. Eur. Urol. Focus. 2016;2:553–561. doi: 10.1016/j.euf.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Opoku-Acheampong Alexander B., Penugonda Kavitha, Lindshield Brian L. Effect of Saw Palmetto Supplements on Androgen-Sensitive LNCaP Human Prostate Cancer Cell Number and Syrian Hamster Flank Organ Growth. Evidence-Based Complementary and Alternative Medicine. 2016;2016:1–10. doi: 10.1155/2016/8135135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penugonda K, Lindshield BL. Fatty acid and phytosterol content of commercial saw palmetto supplements. Nutrients. 2013;5:3617–3633. doi: 10.3390/nu5093617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytel YA, Vinarov A, Lopatkin N, Sivkov A, Gorilovsky L, Raynaud JP. Long-term clinical and biologic effects of the lipidosterolic extract of Serenoa repens in patients with symptomatic benign prostatic hyperplasia. Adv. Ther. 2002;19:297–306. doi: 10.1007/BF02853175. [DOI] [PubMed] [Google Scholar]
- Raynaud JP, Cousse H, Martin PM. Inhibition of type 1 and type 2 5alpha-reductase activity by free fatty acids, active ingredients of Permixon. J. Steroid Biochem. Mol. Biol. 2002;82:233–239. doi: 10.1016/s0960-0760(02)00187-5. [DOI] [PubMed] [Google Scholar]
- Reece Smith H, Memon A, Smart CJ, Dewbury K. The value of Permixon in benign prostatic hypertrophy. Br. J. Urol. 1986;58:36–40. doi: 10.1111/j.1464-410x.1986.tb05424.x. [DOI] [PubMed] [Google Scholar]
- Rhodes L, Primka RL, Berman C, Vergult G, Gabriel M, Pierre-Malice M, Gibelin B. Comparison of finasteride (Proscar), a 5 alpha reductase inhibitor, and various commercial plant extracts in in vitro and in vivo 5 alpha reductase inhibition. Prostate. 1993;22:43–51. doi: 10.1002/pros.2990220107. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005;7(Suppl 9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- Saad F, Yassin AA, Haider A, Gooren L. Effects of testosterone on the lower urinary tract go beyond the prostate: New insights, new treatment options. Arab J. Urol. 2011;9:147–152. doi: 10.1016/j.aju.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglione F, Lucini V, Pannacci M, Dugnani S, Leone C. Comparison of the potency of 10 different brands of Serenoa repens extracts. Eur. Rev. Med. Pharmacol. Sci. 2012;16:569–574. [PubMed] [Google Scholar]
- Scholtysek C, Krukiewicz AA, Alonso JL, Sharma KP, Sharma PC, Goldmann WH. Characterizing components of the saw palmetto berry extract (SPBE) on prostate cancer cell growth and traction. Biochem. Biophys. Res. Commun. 2009;379:795–798. doi: 10.1016/j.bbrc.2008.11.114. [DOI] [PubMed] [Google Scholar]
- Silvestri I, Cattarino S, Agliano A, Nicolazzo C, Scarpa S, Salciccia S, Frati L, Gentile V, Sciarra A. Effect of Serenoa repens (Permixon(R)) on the expression of inflammation-related genes: Analysis in primary cell cultures of human prostate carcinoma. J. Inflamm. 2013;10:11. doi: 10.1186/1476-9255-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinescu I, Geavlete P, Multescu R, Gangu C, Miclea F, Coman I, Ioiart I, Ambert V, Constantin T, Petrut B, Feciche B. Long-term efficacy of Serenoa repens treatment in patients with mild and moderate symptomatic benign prostatic hyperplasia. Urol. Int. 2011;86:284–289. doi: 10.1159/000322645. [DOI] [PubMed] [Google Scholar]
- Strauch G, Perles P, Vergult G, Gabriel M, Gibelin B, Cummings S, Malbecq W, Malice MP. Comparison of finasteride (Proscar) and Serenoa repens (Permixon) in the inhibition of 5-alpha reductase in healthy male volunteers. Eur. Urol. 1994;26:247–252. doi: 10.1159/000475388. [DOI] [PubMed] [Google Scholar]
- Sultan C, Terraza A, Devillier C, Carilla E, Briley M, Loire C, Descomps B. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J. Steroid Biochem. 1984;20:515–519. doi: 10.1016/0022-4731(84)90264-4. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ito Y, Fujino T, Abe M, Umegaki K, Onoue S, Noguchi H, Yamada S. Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol. Sin. 2009;30:227–281. doi: 10.1038/aps.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Oki T, Sugiyama T, Umegaki K, Uchida S, Yamada S. Muscarinic and alpha 1-adrenergic receptor binding characteristics of saw palmetto extract in rat lower urinary tract. Urology. 2007;69:1216–1220. doi: 10.1016/j.urology.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Tacklind J, Macdonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2012;12:CD001423. doi: 10.1002/14651858.CD001423.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpur N, Echard B, Bagchi D, Bagchi M, Preuss HG. Comparison of saw palmetto (extract and whole berry) and cernitin on prostate growth in rats. Mol. Cell Biochem. 2003;250:21–26. doi: 10.1023/a:1024988929454. [DOI] [PubMed] [Google Scholar]
- Vela-Navarrete R, Alcaraz A, Rodriguez-Antolin A, Minana Lopez B, Fernandez-Gomez JM, Angulo JC, Castro Diaz D, Romero-Otero J, Brenes FJ, Carballido J, Molero Garcia JM, Fernandez-Pro Ledesma A, Cozar Olmos JM, Manasanch Dalmau J, Subirana Cachinero I, Herdman M, Ficarra V. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon((R))) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018;122:1049–1065. doi: 10.1111/bju.14362. [DOI] [PubMed] [Google Scholar]
- Vela-Navarrete R, Escribano-Burgos M, Farre AL, Garcia-Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. J. Urol. 2005;173:507–510. doi: 10.1097/01.ju.0000150533.94952.25. [DOI] [PubMed] [Google Scholar]
- Vinarov Andrey Zinovievich, Spivak Leonid Grigorievich, Platonova Darina Vladimirovna, Rapoport Leonid Mikhailovich, Korolev Dmitry Olegovich. 15 years’ survey of safety and efficacy of Serenoa repens extract in benign prostatic hyperplasia patients with risk of progression. Urologia Journal. 2018;86(1):17–22. doi: 10.1177/0391560318772466. [DOI] [PubMed] [Google Scholar]
- von Holtz RL, Fink CS, Awad AB. Beta-sitosterol activates the sphingomyelin cycle and induces apoptosis in lncap human prostate cancer cells. Nutr. Cancer. 1998;32:8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- Wadsworth TL, Carroll JM, Mallinson RA, Roberts CT, Jr, Roselli CE. Saw palmetto extract suppresses insulin-like growth factor-I signaling and induces stress-activated protein kinase/c-Jun N-terminal kinase phosphorylation in human prostate epithelial cells. Endocrinology. 2004;145:3205–3214. doi: 10.1210/en.2003-1716. [DOI] [PubMed] [Google Scholar]
- Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate. 1996;28:300–306. doi: 10.1002/(SICI)1097-0045(199605)28:5<300::AID-PROS5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2002;3:CD001423. doi: 10.1002/14651858.CD001423. [DOI] [PubMed] [Google Scholar]