Abstract
The purpose of this study is to investigate the effect of fermented ginseng extract by Lactobacillus brevis (FGE) on lipopolysaccharide (LPS)-activated macrophages and 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated dermatitis in mice. FGE showed better anti-inflammatory activities than ginseng extract on the formation of nitric monooxide, IL-6, TNF-α, and IL-10 within non-cytotoxicity range in LPS-activated RAW264.7 cells. In addition, FGE reduced the expression of cyclooxygenase-2 and inducible nitric oxide synthase through inhibiting nuclear translocation of NF-κB. Consistent with in vitro experiments, FGE dose-dependently suppressed ear edema, and formation of TNF-α and IL-6, and it (50 mg/mL) significantly enhanced IL-10 level in ear tissues of TPA-treated mice. In conclusions, FGE has anti-dermatitic activity through inhibiting the activation of macrophages. Such effects of FGE are associated with suppressing nuclear translocation of NF-κB. Therefore, the features of FGE may provide the information for its application for therapy and prevention of dermatitis.
Keywords: Compound K, Contact dermatitis, Fermented ginseng, Lactobacillus brevis, Macrophage
Introduction
Contact dermatitis is one of the skin diseases, and is associated with acute inflammation by various pathologic determinants such as foods, drugs and chemicals (Dhingra et al., 2013). The disease according to the pathologic determinants is divided to allergic and irritant contact dermatitis (Dhingra et al., 2013). When various immune cells such as Langerhans cells and inflammatory dendritic epidermal cells in skin tissues are activated by the exposure of the pathologic determinants, they produce and liberate various inflammatory inducers such as cytokines and chemokines to surrounding tissues (Dhingra et al., 2013). The released inflammatory inducers initiate inflammation and recruit other immune cells such as mast cells, macrophages, and neutrophils in the skin tissues (Dhingra et al., 2013). As a result, they aggravate inflammatory response and progress the chronicization of inflammation. Therefore, inhibiting the activation of immune cells is a very important target for prevention and treatment of contact dermatitis.
Ginseng (Panax ginseng) has been used as a functional food and herbal medicine in the world. Ginseng exerts various beneficial effects, such as prevention of cancer and inflammation (Hofseth and Wargovich, 2007). Various processed goods, such as red ginseng and fermented ginseng, have been distributed currently, and possess stronger bioactive effects than raw ginseng. The reason is that the processed goods include rich ginsenosides, bioactive compounds, than raw ginseng (Shibata, 2001). The fermentation for phytomedicines using microorganisms improves their bioactive activities, such as beneficial action against allergy (Yoo et al., 2016), inflammation (Kim et al., 2017) and neurodegeneration (Park et al., 2016), through the increment of bioactive components. Recently, we found when fine roots of ginseng were fermented by Lactobacillus brevis (L. brevis), physiochemical properties of the extract of fermented ginseng fine root (FGE) were improved than extract of ginseng fine root (GE), and compound K level in FGE is increased (Yoo et al., 2018a). Furthermore, compound K is known to have anti-inflammatory activity (Joh et al., 2011). Nevertheless, effect of FGE on contact dermatitis is unknown.
In this study, we investigated effect of FGE on lipopolysaccharide (LPS)-activated macrophages and 2-O-tetradecanoylphorbol-13-acetate (TPA)-mediated contact dermatitis in mice. To evaluate anti-inflammatory effects of GE and FGE in LPS-activated RAW 264.7 cells, the level of nitrite, an indicator of nitric monoxide (NO), was determined and the amounts of interleukin-6 (IL-6), IL-10, and tumor necrosis factor-α (TNF-α) were measured by ELISA kits. To elucidate the anti-inflammatory mechanisms of FGE, the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), IκBα, and NF-κB in cytosol and nucleus of LPS-activated RAW264.7 cells was analyzed by immunoblotting analysis. To confirm the anti-inflammatory properties of FGE in a contact dermatitis model, the action of FGE was examined using TPA-induced contact dermatitis model. Herein, we report that FGE has stronger anti-inflammatory action than GE in LPS-activated macrophages, and demonstrates that FGE attenuates contact dermatitis in TPA-treated mice. These results bestow novel information for the feasible use of FGE as a functional food and herbal medicine for prevention and therapy of dermatitis.
Materials and methods
Materials
Fine roots of dried ginseng were obtained from Wooshin Co., Ltd (Geumsan-gun, Korea). L. brevis (KCTC 3498) was purchased from the Korean Collection for Type Cultures (Jeongeup, Korea). Dulbecco’s modified Eagle’s minimum essential medium (DMEM), and 1 × DPBS were procured from WelGENE (Gyeongsan, Korea). Antibiotics and fetal bovine serum (FBS) were obtained from Gibco Life Technologies (Grand Island, NY, USA). Specific antibodies against COX-2 (#4842), iNOS (#13120), IκBα (#4812), NF-κB (#8242), Lamin B (#13435), and β-actin (#4970) were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). ELISA kits for IL-6, IL-10, and TNF-α were procured from e-Bioscience, Inc. (Science Center Drive, San Diego, CA, USA). LPS (Escherichia coli O55:B5), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), TPA, and all other chemicals of analytical grade were obtained from Sigma-Aldrich (St Louis, MO, USA).
Preparation of ginseng and fermented ginseng extracts
GE and FGE were prepared according to the previous method (Yoo et al., 2018a). Briefly, GE was the concentrations of 70% ethanol extract (1 L) at 75 °C for 24 h, repeated triplicate. Collected supernatant was concentrated under vacuum at 40 °C using a rotary evaporator (MG-2100, Buchi, Flawil, Switzerland).
To prepare FGE, 5% GE solution containing 7% α-herbzyme was sterilized at 95 °C for 10 min. The sterilized solution, adjusted to pH 6.5 with 1 N NaOH, was inoculated with L. brevis (1 × 105–107 CFU/mL), and then incubated at 37 °C for 5 days. Supernatant was concentrated using a rotary evaporator after the fermented solution was centrifuged (1900×g, 4 °C) for 20 min.
High performance liquid chromatography analysis
HPLC analyzed by the previously reported method (Yoo et al., 2018a). Briefly, the standards of ginsenoside Rb1, ginsenoside Rg1, and compound K as well as the samples (200 mg/mL) were dissolved and diluted in 50% methanol. All the sample solutions were used after filtering with a syringe filter (0.45 μm). Calibration curves constructed using linear least-squares regression were linear over the concentration range of the standards used. The relative standard deviation of the measured concentrations was used to assess the precision. A comparison of the mean measured concentration versus the corresponding nominal concentration was used to assess the accuracy. Data acquisition was performed using Empower 3 Chromatography Data Software (Waters Co., Milford, MA, USA).
Animals
ICR mice, Swiss CD-1 mice (Schwartz et al., 1974), (6 weeks, male and 25–30 g) were obtained from Nara Biotech Co. (Pyeongteak, Korea), and housed in cages (5 mice per cage) under specific pathogen-free conditions (21–24 °C and 40–60% relative humidity) with a 12 h light/dark cycle. They were given free access to water and standard rodent food (Orientbio Inc., Seongnam, Korea). All the animal studies were approved by the Committee of Animal Care and Experiment of Chungnam National University, Korea (CNU-00089), and performed according to the guidelines of the Animal Care and Use Committee at Chungnam National University.
12-O-tetradecanoylphorbol-13-acetate-induced contact dermatitis
TPA-induced contact dermatitis was evaluated according to the previously reported method (Gupta et al., 1988). Briefly, mice were applied with FGE (0–50 mg/mL) to the back of ears once a day. After 7 days, they were spread with TPA dissolved in acetone (0.1 mg/mL) on the ears after FGE application. Next day, the ears were isolated from sacrificed mice after measurement of ear thickness. The ear tissues were frozen in liquid nitrogen, and then quickly pulverized. The powder of ear tissue was lysed with a lysis buffer (1 mL), and then centrifuged (15,000×g, 15 min) at 4 °C. The lysates were stored at − 80 °C until use.
Cell culture
RAW264.7 cells were procured from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in DMEM medium containing 10% (v/v) FBS, 100 units/mL penicillin and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2. All the in vitro experiments contain a vehicle control group (0.1% ethanol).
Cell viability assay
Cell viability was determined following a modified method previously reported (Yoo et al., 2018b). Briefly, RAW264.7 cells were seeded on a 96-well plate (1 × 104 cells/well) in DMEM containing 10% FBS, and then incubated for 24 h. The cells were preincubated with GE or FGE (0–50 µg/mL) for 1 h, stimulated by LPS (2 µg/mL) for 22 h, and then further incubated with 200 μL culture media containing 500 μg/mL MTT reagent for 2 h. 100 µL of dimethyl sulfoxide was added to the plate after supernatnat was removed, and then incubated for 15 min. Cell viability was determined at 570 nm using a micro-plate reader.
Nitrite assay
The nitrite level in sample was assayed as described previously (Jeon et al., 2016). The absorbance at 540 nm was measured using a micro-plate reader.
Enzyme-linked immunosorbent assay of IL-6, IL-10, and TNF-α
To determine the levels of IL-6, IL-10, and TNF-α in cultured media and ear tissue lysates, all the tissue lysates and cultured media were centrifuged, and then stored at − 80 °C until use. The amounts of IL-6, IL-10 and TNF-α were assayed by ELISA kits according to the manufacturer’s instructions.
Extraction of nuclear and cytosolic protein
Nuclear and cytosolic proteins were separated according to the previously reported method (Jeon et al., 2016) with a Nuclear Extraction Kit from (Cayman Chemical Company, Inc., Ann Arbor, MI, USA). Nuclear and cytosolic proteins were fractionated following the instructions of the manufacturer.
Immunoblotting analysis
Immunoblotting analysis was evaluated following the previously reported method (Jeon et al., 2016). Briefly, the blotted proteins on PVDF membrane were visualized by WEST One™ western blot detection system (iNtRON Biotechnology, Inc., Seongnam, Korea). The level of target proteins was compared with that of the control (β-actin or Lamin B), and the results were presented as a ratio of density of each protein identified by a protein standard size marker (BIOFACT Co., Ltd., Daejeon, Korea). The density of each inverted band was determined using ImageJ software (version 1.51j8 for Windows; NIH, USA).
Statistical analyses
All the data were listed as mean ± SD for in vitro experiments or SEM for in vivo experiments. One-way and two-way analysis of variance (ANOVA) was used for multiple comparisons (GraphPad Prism version 5.03 for Windows, San Diego, CA, USA). The Dunnett test for one-way ANOVA used for significant variations between the LPS-treated groups or TPA-treated groups, and the Bonferroni post-test for two-way ANOVA for significant variations between GE and FGE groups. Differences at the *p < 0.05 and **p < 0.01 levels were considered statistically significant.
Results and discussion
Inhibitory actions of GE and FGE against inflammatory inducers in LPS-stimulated RAW264.7 cells: comparison between GE and FGE
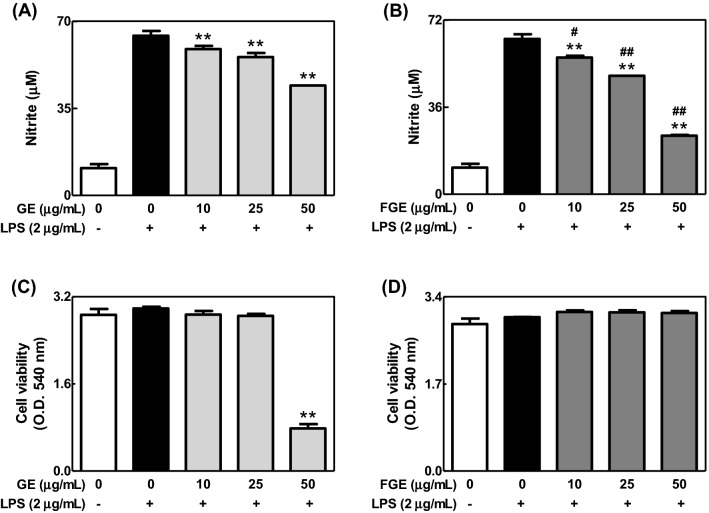
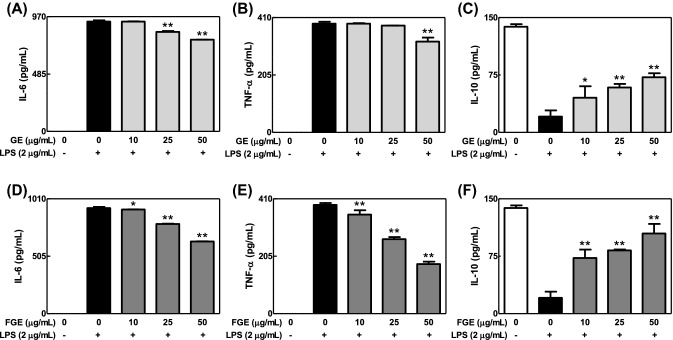
To compare effects of GE and FGE on LPS-mediated inflammatory response in macrophages, we investigated effects of GE and FGE on the formation of NO and cytokines in LPS-activated RAW264.7 cells. GE and FGE dose-dependently inhibited the formation of NO in LPS-activated RAW264.7 cells [Fig. 1(A), (B)]. Additionally, they not only reduced the levels of IL-6 and TNF-α, proinflammatory cytokines, but also increased that of IL-10, an anti-inflammatory cytokine (Fig. 2). Moreover, such anti-inflammatory effects of FGE were better than that of GE. Furthermore, FGE did not induce cytotoxicity [Fig. 1(D)], whereas GE at 50 μg/mL showed cytotoxicity in LPS-activated RAW264.7 cells [Fig. 1(C)]. These results suggest that the fermentation of ginseng by L. brevis improves anti-inflammatory activity of ginseng within non-cytotoxic ranges in LPS-activated macrophages. In addition, the anti-inflammatory action of FGE may be closely associated with elevated compound K (Yoo et al., 2018a). In support of this, FGE included rich compound K (0.42 ± 0.03 mg/g dry weight) (Yoo et al., 2018a). In addition, fermented red ginseng and compound K possess anti-inflammatory activities (Joh et al., 2011; Jung et al., 2012).
Fig. 1.
Effects of GE and FGE on the formation of NO and cell viability in LPS-activated RAW264.7 cells. RAW 264.7 cells were seeded on 96-well plate (5 × 104 cells/well) in DMEM with 10% FBS at 37 °C overnight. The cells were simultaneously incubated with GE or FGE (0–50 μg/mL) with or without LPS (2 μg/mL) for 24 h. The level of NO and cell viability were determined as described in the Materials and Methods section. The data are expressed as the mean ± SD values of triple or quadruple determinations. **p < 0.01 versus the LPS-treated group; #p < 0.05 and ##p < 0.01 versus each concentration group between (A) and (B). (A) and (B) NO; (C) and (D) cell viability
Fig. 2.
Effects of GE and FGE on the formation of cytokines in LPS-activated RAW264.7 cells. RAW 264.7 cells were seeded on 96-well plate in DMEM with 10% FBS at 37 °C overnight. The cells were simultaneously exposed to GE or FGE (0–50 μg/mL) with or without LPS (2 μg/mL) for 24 h. The levels of IL-6, TNF-α and IL-10 were determined as described in the Materials and Methods section. The data are expressed as the mean ± SD values of triple determinations. *p < 0.05 and **p < 0.01 versus the LPS-treated group. (A) and (D) IL-6; B and E, TNF-α; (C) and (F) IL-10
Inhibitory actions of FGE against inflammatory mechanism in LPS-activated RAW264.7 cells
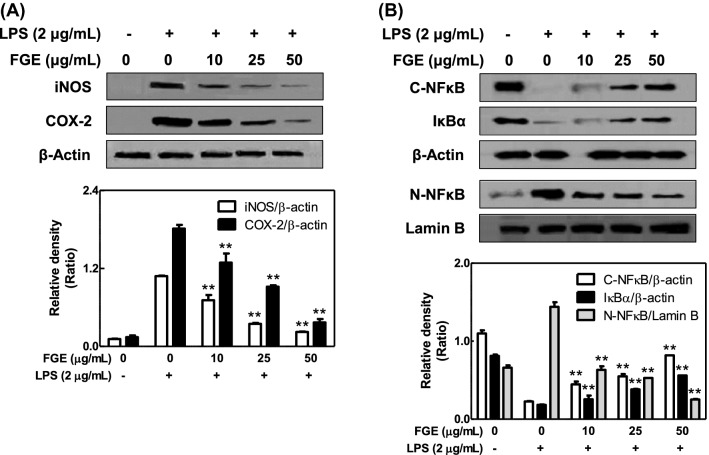
We further examined the effect of FGE on inflammatory mechanism in LPS-activated macrophages after we found that the anti-inflammatory action of FGE is better than that of GE. LPS-activated macrophages upregulate the expression of iNOS, related with NO production, and COX-2, a biomarker of inflammation (Hamidzadeh et al., 2017). FGE dose-dependently reduced the expression of iNOS and COX-2 in LPS-activated RAW 264.7 cells [Fig. 3(A)]. In addition, FGE decreased the level of NF-κB in nucleus but increased the level of NF-κB and IκBα in cytosol in the cells [Fig. 3(B)]. The results suggest that the anti-inflammatory effect of FGE is associated with directly inhibition of expression of iNOS and COX-2 through suppressing nuclear translocation of NF-κB in LPS-activated macrophages. In support of this, when macrophages are exposed to LPS, toll-like receptor 4 (TLR4), known as the LPS receptor, is activated by LPS-endotoxin binding protein complex (O’Neill, 2002). The reason is that macrophages express TLR4 on the extracellular plasma membrane (O’Neill, 2002). The activation of TLR4 leads to express iNOS and COX-2 as well as inflammatory inducers by the activation of myeloid differentiation factor 88/mitogen-activated protein kinases/NF-κB pathway (O’Neill, 2002). Consequently, LPS-activated macrophages induce acute imflammatory response in surrounding tissues, and aggravate through recruiting other immune cells (Dhingra et al., 2013). Therefore, the nuclear translocation of NF-κB is an important event in the activation of TLR4 signaling cascade in macrophages. Taken together, the inhibitory effect of FGE may be closely associated with the direct inhibition of IκB kinase or NF-κB translocation in activated macrophages.
Fig. 3.
Effects of FGE on the expression of iNOS, COX-2, nuclear NF-κB, or cytosolic NF-κB and IκB. RAW 264.7 cells were seeded on 6-well plate (1 × 106 cells/well) in DMEM with 10% FBS at 37 °C overnight. The cells were simultaneously incubated with FGE with or without LPS for 15 min or 6 h. They were washed, and then lysed in a cell lysis buffer. The expression of iNOS, COX-2 and nuclear NF-κB as well as cytosolic NF-κB and IκBα was determined as described in Materials and Methods section. Similar results were obtained in three independent experiments. **p < 0.01 versus LPS-treated group. (A) iNOS and COX-2; (B) nuclear NF-κB, cytosolic NF-κB, and cytosolic IκBα
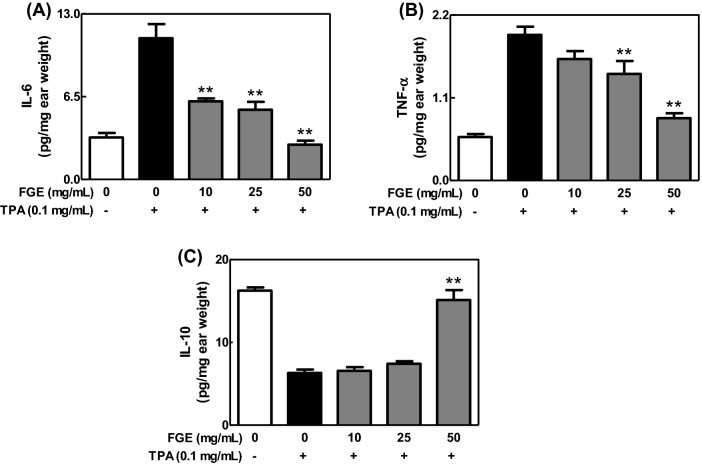
Inhibitory effect of FGE on TPA-induced contact dermatitis
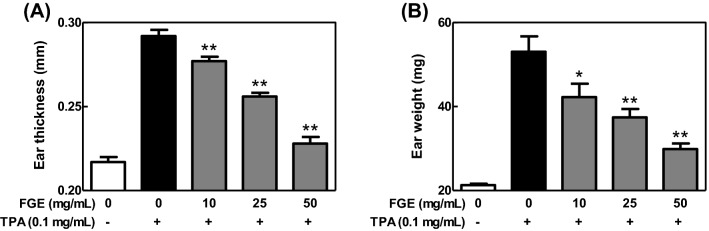
To confirm the anti-inflammatory activity of FGE in whole body system, we investigated effect of FGE on TPA-induced dermatitis model, a contact dermatitis model (Fowler et al., 2003). When mice were applied with FGE to ears prior to TPA treatment, FGE dose-dependently inhibited ear edema (Fig. 4), and the levels of IL-6 and TNF-α [Fig. 5(A), (B)] in TPA-treated mice. In addition, FGE at 50 mg/mL recovered the level of IL-10 as the same level in control group [Fig. 5(C)]. These findings suggest that FGE has anti-dermatitic activity through regulating the formation of cytokines in skin tissues of mice. The anti-dermatitic action of FGE may be associated with the direct inhibition of the activated macrophages in skin tissues. In addition, the strong anti-dermatitic action of FGE may be closely correlated with rich compound K (Joh et al., 2011; Jung et al., 2012). Overall, FGE has possibility as a phytomedicine and functional food for prevention and therapy of inflammatory skin diseases.
Fig. 4.
Inhibitory effects of FGE on ear edema in TPA-treated mice. ICR mice were spread with FGE (0–50 mg/mL) on the back of ears prior to TPA challenge. Ear thickness and ear weight were determined as described in the Materials and Methods section. The data are expressed as the mean ± SEM values of quintuple determinations. *p < 0.05 and **p < 0.01 versus the TPA-treated group. (A) Ear thickness; (B) ear weight
Fig. 5.
Effects of FGE on the formation of cytokines in TPA-exposed mice. ICR mice were spread with FGE on the back of ears prior to TPA treatment. The levels of IL-6, TNF-α and IL-10 in ear tissues were determined as described in the Materials and Methods section. The data are expressed as the mean ± SEM values of quintuple determinations. **p < 0.01 versus the TPA-treated group. (A) IL-6; (B) TNF-α; (C) IL-10
In summary, this study demonstrates that FGE including rich compound K has anti-dermatitic activity through directly inhibiting the activation of macrophages. The finding revealed a novel feature of FGE in skin inflammatory diseases. The inhibitory mechanisms for anti-dermatitic action of FGE may include several targets such as iNOS, COX-2 and NF-κB in macrophages. Such effects of FGE may be associated with rich total phenolic compounds and compound K, and may support further information for its application as a herbal medicine and functional food for prevention and treatment for inflammatory skin diseases including contact dermatitis.
Acknowledgements
This work was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant Number 2017R1D1A3B03027867).
Abbreviations
- COX-2
Cyclooxygenase-2
- FGE
Extract of fermented ginseng fine root
- GE
Extract of ginseng fine root
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- NO
Nitric monoxide
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- TPA
2-O-tetradecanoylphorbol-13-acetate
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ji Yeon Lee, Email: looneyjy@cnuh.co.kr.
Jae-Myung Yoo, Email: jmyoo@cnu.ac.kr.
Seong Yeon Baek, Email: qor7683@naver.com.
Mee Ree Kim, Phone: +82-42-821-8030, Email: mrkim@cnu.ac.kr.
References
- Dhingra N, Gulati N, Guttman-Yassky E. Mechanisms of contact sensitization offer insights into the role of barrier defects vs. intrinsic immune abnormalities as drivers of atopic dermatitis. J. Invest. Dermatol. 2013;133:2311–2314. doi: 10.1038/jid.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J. Invest. Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Fisher GJ, Elder JT, Nickoloff BJ, Voorhees JJ. Sphingosine inhibits phorbol ester-induced inflammation, ornithine decarboxylase activity, and activation of protein kinase C in mouse skin. J. Invest. Dermatol. 1988;91:486–491. doi: 10.1111/1523-1747.ep12476635. [DOI] [PubMed] [Google Scholar]
- Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu. Rev. Physiol. 2017;79:567–592. doi: 10.1146/annurev-physiol-022516-034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- Jeon HL, Yoo JM, Lee BD, Lee SJ, Sohn EJ, Kim MR. Anti-Inflammatory and Antioxidant Actions of N-Arachidonoyl Serotonin in RAW264.7 Cells. Pharmacology. 2016;97:195–206. doi: 10.1159/000443739. [DOI] [PubMed] [Google Scholar]
- Joh EH, Lee IA, Jung IH, Kim DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem. Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Choi H, Lim HW, Shin D, Kim H, Kwon B, Lee JE, Park EH, Lim CJ. Enhancement of anti-inflammatory and antinociceptive actions of red ginseng extract by fermentation. J. Pharm. Pharmacol. 2012;64:756–762. doi: 10.1111/j.2042-7158.2012.01460.x. [DOI] [PubMed] [Google Scholar]
- Kim DG, Lee MR, Yoo JM, Park KI, Ma JY. Fermented herbal formula KIOM-MA-128 protects against acute colitis induced by dextran sodium sulfate in mice. BMC Complement. Altern. Med. 2017;17:354. doi: 10.1186/s12906-017-1855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002;23:296–300. doi: 10.1016/S1471-4906(02)02222-6. [DOI] [PubMed] [Google Scholar]
- Park HR, Lee H, Park H, Cho WK, Ma JY. Fermented Sipjeondaebo-tang Alleviates Memory Deficits and Loss of Hippocampal Neurogenesis in Scopolamine-induced Amnesia in Mice. Sci. Rep. 2016;6:22405. doi: 10.1038/srep22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JN, Daniels CA, Shivers JC, Klintworth GK. Experimental cytomegalovirus ophthalmitis. Am. J. Pathol. 1974;77:477–492. [PMC free article] [PubMed] [Google Scholar]
- Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JM, Lee JY, Lee YG, Baek S, Kim MR. Enhanced production of compound K in fermented ginseng extracts by Lactobacillus brevis. Food Sci. Biotechnol. 2018;28:823–829. doi: 10.1007/s10068-018-0504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JM, Park KI, Yang J-H, Cho W-K, Lee B, Ma JY. Anti-allergic actions of F-PASA, a novel herbal cocktail, in IgE/antigen-mediated allergic responses in RBL-2H3 cells and passive cutaneous anaphylaxis in mice. Phytomedicine. 2018;55:229–237. doi: 10.1016/j.phymed.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Yoo JM, Yang JH, Yang HJ, Cho WK, Ma JY. Inhibitory effect of fermented Arctium lappa fruit extract on the IgE-mediated allergic response in RBL2H3 cells. Int. J. Mol. Med. 2016;37:501–508. doi: 10.3892/ijmm.2015.2447. [DOI] [PubMed] [Google Scholar]