Abstract
Waste management is a major part of the food industry. The present study was designed to utilize the discarded byproduct of Schisandra chinensis Baillon. The antioxidant and anti-inflammatory effects of a 30% ethanol fraction (RPG-OM-30E) from the fermented hot water extraction of the Schisandra chinensis Baillon byproduct were investigated using RAW 264.7 cells and zebrafish larvae. RPG-OM-30E reduced lipopolysaccharide (LPS)-induced nitric oxide production in the RAW 264.7 cells. Additionally, RPG-OM-30E inhibited mRNA expression and protein secretion of pro-inflammatory cytokines, such as interleukin-6 (Il-6) and interleukin-1β (Il-1β). The anti-inflammatory effects of RPG-OM-30E were tested in Tg(mpx::EGFP)i114 zebrafish larvae. Neutrophil migration to a wound site was decreased by RPG-OM-30E. Neutrophil aggregation was also inhibited by RPG-OM-30E after induction of an LPS-induced immune response in the yolk. Finally, the antioxidant and hepatoprotective effects of RPG-OM-30E were examined in vivo. Mice with induced oxidative damage recovered from the stress following RPG-OM-30E treatment.
Keywords: Schisandra chinensis Baillon, Antioxidant, Anti-inflammatory, Zebrafish, RAW 264.7
Introduction
Omija (the fruit of Schisandra chinensis Baillon), which grows wild in Korea, Russia, China, and Japan, has been known as a herbal plant in some Asian countries (Chang et al., 2005). It is round and oblate in shape, dark red in color with a glossy surface, and sweet and sour in flavor. More than 80% of the red color of Schisandra chinensis Baillon is due to its anthocyanin content; the anthocyanin is reported to be peonidin 3-glucoside, which has been shown to exhibit anti-inflammatory, anti-hepatotoxic, anti-cancer, and antioxidant effects (Guo et al., 2008; Ko et al., 1995; Min et al., 2008). Products made by processing omija have been popularly sold as functional foods and drinks, but only the omija flesh is used to make these products. Although it contains a lot of bioactive substances, the byproduct containing the husks and seed production has been discarded as garbage (Choi et al., 2006; Kim et al., 2017).
Fermentation has been used to increase the amount of active compounds extracted and to convert macromolecules, such as polysaccharides and peptides, into simple materials in the byproducts (Chen et al., 2009). Previous studies have shown that fermentation of plants can enhance their bioactive properties, such as antioxidant and anti-immune effects (Fernandez-Orozco et al., 2008; Matsushita et al., 2008). However, few studies have analyzed the bioactive components of fermented omija and their properties. The lactic acid bacterium Lactobacillus paracasei is widely utilized in probiotic supplements to improve clinical outcomes. Previous studies of this bacterium have shown improved gastrointestinal conditions, inhibition of the adhesion of Escherichia coli and Salmonella typhimurium to Caco-2 cells, and induction of pro-inflammatory cytokines by human peripheral blood mononuclear cells (Maragkoudakis et al., 2006).
Inflammation is a defensive process that is initiated against pathogens, foreign stimuli, and various systemic damages (Bistrian, 2007; Mogensen, 2009; Lawrence and Gilroy, 2007). Numerous inflammatory cells are activated during this process, including macrophages, lymphocytes, monocytes, platelets, and fibroblasts. Reactive oxygen species (ROS), nitric oxide (NO), and prostaglandin E2 (PGE2) were known for inflammatory mediators in these cells. In particular, macrophages eliminate invading foreign pathogens and produce tumor necrosis factor-α (TNF-α), Il-6, and Il-1β to protect the damaged tissue (Choi and Hwang, 2005; Zhang and Ghosh, 2000). However, long-term exposure to these inflammatory mediators causes organ damage (Laskin and Pendino, 1995). It was known that chronic activation of macrophage induces various inflammatory diseases such as autoimmune diseases, neurodegenerative disorders, osteoporosis, and cardiovascular disease (Jou et al., 2013). Therefore, excessive oxidative stress or inflammation must be inhibited to prevent the progression of inflammatory diseases.
In this study, we obtained the hot water extract of the omija byproduct and fermented the extract with Lactobacilius paracasei subsp. tolerans. We investigated the antioxidant and anti-inflammatory effects of a 30% ethanol fraction from the fermented extract in LPS-induced RAW 264.7 cells and a transgenic (TG) zebrafish line (mpx:: EGFP)i114.
Materials and methods
Sample preparation
The byproduct of Schisandra chinensis Baillon was collected after preparing a drink and extracted using a hot water extractor. The extracted fraction was fermented by the patented bacterial strain Lactobacilius paracasei subsp. tolerans, which is a type of lactic acid bacteria. Lactic acid bacteria have a function of inhibiting microbial growth by producing lactic acid and decomposing proteins using protease. Therefore, Lactobacillus paracasei subsp. tolerans was used for fermentation in this study. The bacteria were precultured in Lactobacilli MRS broth (Difco, USA) for 48 h at 30 °C and inoculated into a 10% hot water extract solution. The fermentation was carried out at 30 °C for 48 h. The fermented extract was centrifuged to remove the bacterial cells. The supernatant was subjected to column chromatography using column (80 × 150 mm) packed with PB-600 resin and eluted with a stepwise gradient of 0% (DDW), 10, 30, 50, 80 and 100% of EtOH (500 mL each). The scheme for RPG-OM-30E preparation from the omija byproduct is shown in Fig. 1A.
Fig. 1.
Effects of RPG-OM-30E on NO production in LPS-stimulated RAW 264.7 cells. (A) Scheme for fraction of RPG-OM-30E from Omija byproduct. (B) LPS-stimulated RAW 264.7 cells were used for testing the inhibition of NO production. Inhibitory effects of fermented and unfermented omija hot water extract on NO production were compared in the concentration of 5 and 10%. (C) The inhibition of NO production by ethanol fractions of fermented omija hot water extract was tested at the concentration of 0.1 mg/mL. (D) Cell viability was determined through the WST assay. Cells were treated with each concentration of RPG-OM-30E for 24 h. Values are expressed as percentages of the control (0.1% DMSO). (E) The effects of RPG-OM-30E on LPS-induced NO production were measured using the Griess reagent. Cells were stimulated with 1 μg/mL of LPS in the presence of each concentration of RPG-OM-30E. NO production was measured after 24 h. Significant compared to the group treated with LPS alone, *p < 0.05, **p < 0.01, and ***p < 0.001
Cell culture
RAW 264.7 mouse macrophage (Korea Cell Line Bank, Seoul, Korea) was cultured in Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT, USA) containing 10% fetal bovine serum (HyClone), and 1% Penicillin–Streptomycin Solution (HyClone). Cells were maintained in an incubator supplied with humidified 5% CO2 atmosphere at 37 °C.
Animals
All experiments using zebrafish and mice were conducted with the approval of the Animal Care and Use Committee at the Korea Research Institute of Chemical Technology. Adult zebrafish were administered at 28 °C in a water-circulating tank facility (Techniplast; ZebTEC, West Chester, PA, USA) and exposed to a 14-h light/10-h dark cycle. The Tg(mpx::EGFP)i114 zebrafish line, which express green fluorescence in neutrophils, was used for observation of neutrophils at inflammatory areas. The embryos were maintained in an incubator (HB-302 M; HANBAEK, Gwangju, Republic of Korea) under a 14-h light/10-h dark cycle at 28 °C.
Healthy, 7-week-old, male ICR mice were purchased from NARA-Bio Co. (Seoul, Korea) and maintained in a room with a temperature of 24 ± 1 °C in a 12-h light/12-h dark cycle. Food and water were freely supplied to the mice during the experimental period. The following treatments were performed on mice (n = 5): blank control (treated with phosphate-buffered saline [PBS]), negative control (10 mg/kg of LPS), 1 mg/kg of RPG-OM-30E + 10 mg/kg of LPS, 10 mg/kg of RPG-OM-30E + 10 mg/kg of LPS and 20 mg/kg of RPG-OM-30E + 10 mg/kg of LPS. RPG-OM-30E was orally administered every day for three days. LPS (10 mg/kg) was administered via an intraperitoneal injection on the third day to induce oxidative stress. The mice were sacrificed 24 h later, and the collected liver tissues were stored in a deep freezer (-80 °C) until the subsequent experiments.
Cell viability assay
RAW 264.7 cells (1 × 105 cells) were seeded in 96-well plate (Costar, Corning, NY, USA). To determine cell viability, EZ-Cytox Cell Viability Assay Kit (Daeil Lab Service, Busan, Korea) was used in accordance with the manufacturer’s instructions. Briefly, 10 μL of EZ-Cytox solution was added to each well. After incubation for 1 h at 37 °C, the absorbance values at 450 nm were measured by a microplate reader (M1000pro; TECAN, Mannedorf, Switzerland). The survival rate was calculated as a percentage of the absorbance in the treated well compared to the absorbance in the well treated with only 1% dimethyl sulfoxide (DMSO).
Nitric oxide (NO) production assay
RAW 264.7 cells (1 × 105 cells) were seeded in 96-well plate (Costar, Corning, NY, USA). The cells were incubated with different RPG-OM-30E concentrations in the presence of 1 μg/mL of LPS (Sigma-Aldrich, St. Louis, MO, USA). The culture medium was used to measure the NO content after 24 h. The culture medium and Griess reagent were mixed with the same volume of 100 μL. The absorption of the mixture was measured at 540 nm using a microplate reader (M1000pro; Tecan). The NO production percentage was calculated as follows: (absorbance of treated cells/absorbance of LPS treated cells) × 100.
Real-time PCR
Total RNA was isolated from the RAW 264.7 cells and zebrafish larvae using the TRIzol™ Reagent (Invitrogen, NJ, USA). RNA concentration and purity were measured by a microplate reader (M1000pro; Tecan) using NanoQuant plate™ (Tecan). For real-time PCR (RT-qPCR), 100 ng of total RNA was added in each well of 96-well reaction Plate (Life Technologies, Carlsbad, CA, USA). The sequence of primers used were as follows: mouse Il-6 forward primer: 5′-AGCAGTCCCTCCACATTT-3′, reverse primer: 5′-CATAGACACAGAAAGTAG-3′; mouse Il-1β forward primer: 5′-CTCACATTTTCCCTTCCC-3′, reverse primer: 5′-ACAGAGTATGGAGAATAG-3′; zebrafish il-6 forward primer: 5′-GATTTGTGTGGGAGAGGG-3′, reverse primer: 5′-CAGGAGTTGTGTCAAGGT-3′; zebrafish il-1β forward primer: 5′-TGGCTGACCTGTTCTCTG-3′, reverse primer: 5′-CGATCTCCTGTTGGACAC-3′; β-actin forward primer: 5′-GCGAGAAGATGACCCAGA-3′, reverse primer: 5′-ATCACGATGCCAGTGGTA-3′. RT-qPCR measurements were performed using the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The amplifications were monitored using the Verso™ SYBR Green 1-Step qRT-PCR Low ROX Mix (Thermo Scientific, Waltham, MA, USA). Expression was analyzed using the ΔΔCT method and normalized to β-actin.
Cytokine measurements
Cell culture medium was collected at the indicated times. Amount of cytokines was determined by mouse IL-6 and IL-1β enzyme-linked immunosorbent assay (ELISA) kits (Elabscience, Wu Han, Hubei Province, China) according to the manufacturers’ protocols. The concentrations of IL-6 and IL-1β were caculated using their respective standard curve.
Tail wounding in zebrafish larvae
As previously reported, zebrafish larvae’s tail-fin was injured to lead inflammation (Tauzin et al., 2014). Zebrafish larvae at 3 days post fertilization (dpf) were anesthetized in the egg-water containing 0.02% tricaine. Tailfin wounding was performed using a 33-gauge × 1/2-inch needle. Ten larvae were immediately transferred to a 24-well plate containing 1 mL of test compound and incubated for six hours. Fluorescent images were acquired using an automated live imager (LionHeart; Bio-Tek, Winooski, VT, USA) after anesthesia in the egg-water containing 0.02% tricane.
Acute inflammation induction in zebrafish larvae
Microinjection method was performed in accordance with the previously reported protocols to induce acute inflammatory response in Tg(mpx::EGFP)i114 zebrafish larvae (3 dpf) (Yang et al., 2014). Microinjection was performed with a volume of 2 nL per larva using a microinjector (PV830 Pneumatic PicoPump; World Precision Instruments, Sarasota, FL, USA) after anesthetizing the larvae with 0.02% tricaine. LPS (1 mg/mL) and RPG-OM-30E (1 mg/mL) stock solution were used for microinjection. PBS injected larvae was used as the negative control group. Fluorescence images of the larvae were acquired us-ing an automated live imager (LionHeart; Bio-Tek).
Determination of hepatic biochemical parameters
The livers were immediately removed from the sacrificed animals and frozen at − 18 °C prior to the biochemical analyses. To prepare a homogenate, the liver was chopped in an Eppendorf tube and then homogenized with ice-cold PBS in a 1:10 ratio. The supernatants were collected after centrifuging the homogenates for 5 min at 10,000 × g. The alanine transaminase (ALT) and reactive aldehyde levels in the liver tissues were assayed according to the manufacturers’ protocols. To determine lipid peroxidation in the liver, the reactive aldehyde levels were quantified with the OxiSelect™ thiobarbituric acid-reactive substances (TBARS) Assay Kit (Cell Biolabs Inc., CA, USA). To assess liver function, the ALT levels were measured using the Alanine Transaminase Activity Assay Kit (Abcam, Cambridge, UK).
Data analysis
Images were quantitated using ImageJ (National Institutes of Health, Bethesda, MD, USA). Data for all experiments were expressed as the mean ± standard deviation (SD). Significant differences were analyzed using the unpaired t test on the GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA). A p value < 0.05 was considered statistically significant.
Results and discussion
Antioxidant and anti-inflammatory effects of RPG-OM-30E on RAW 264.7 macrophages
A previous study showed that the anti-inflammatory effect was increased when adlay-soymilk was fermented using Lactobacillus plantarum (Wu et al., 2013). The antioxidant activity was assessed before and after fermentation of the omija extract, because previous studies showed that substances with antioxidant activity exhibited anti-inflammatory activity (Liang et al., 1999; Tsai et al., 1999). The extract fermented with Lactobacilius paracasei subsp. tolerans increased the inhibitory effect of NO production compared to that of the unfermented omija hot water extract (Fig. 1B). To identify the active fraction in the fermented mixture, NO production assay was performed with each ethanol fraction. The fraction called RPG-OM-30E, which was a 30% ethanol fraction of the fermented mixture of omija byproduct, had the best effect of inhibiting NO production (Fig. 1C). The antioxidant and anti-inflammatory effects by the RPG-OM-30E were subsequently studied in more detail. First, the cytotoxicity of RPG-OM-30E toward RAW 264.7 cells was evaluated as described in the Materials and Methods. RPG-OM-30E reduced the viability of RAW 264.7 cells by 22 and 30% at concentrations of 0.25 and 0.5 mg/mL, respectively (Fig. 1D). Then, its antioxidant activity was investigated in RAW 264.7 cells treated with LPS. RPG-OM-30E was applied at the same concentrations. LPS markedly increased NO production, whereas pretreatment with RPG-OM-30E led to the dose-dependent suppression of LPS-induced NO production (Fig. 1E). At the 0.25 mg/mL concentration, RPG-OM-30E inhibited NO production over 60%. When the concentration was increased to 0.5 mg/mL, NO production was inhibited to a level similar to control. Although the cytotoxic effect was shown when RPG-OM-30E was simultaneously treated with LPS, the inhibitory effect of NO production was much greater at these concentrations. It was known that LPS-induced inflammatory reactions could increase cytotoxicity by small-molecule materials (Stone et al., 2003). It was assumed that the stimulation of RAW 264.7 cells by LPS affected the reduction of cell viability by RPG-OM-30E treatment. Nevertheless, it is still possible that cytotoxicity by RPG-OM-30E affected NO production assay and further increased the inhibition effect of NO production.
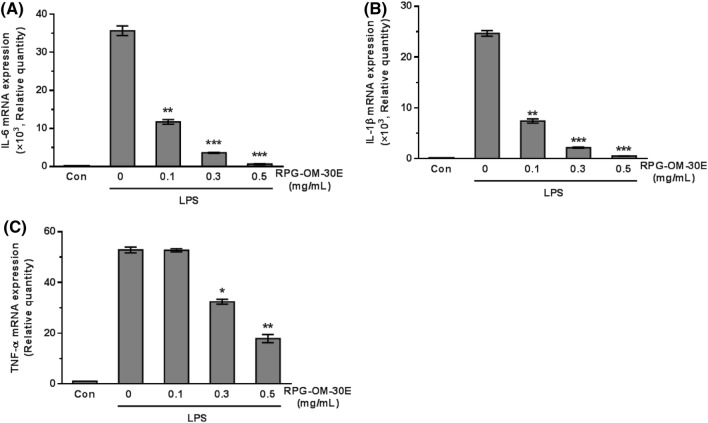
Previously, NO was shown to be produced by iNOS during the inflammatory process and was a known pro-inflammatory factor that promoted inflammation by stimulating the biosynthesis of inflammatory mediators (Mu et al., 2001; Ryu et al., 2003). NO reacts quickly with superoxide (O2−) to produce peroxynitrite (ONOO−), which promotes inflammatory reactions (Mittal et al., 2014). RPG-OM-30E, which most effectively inhibited NO production, was selected as the fraction that contained the most anti-inflammatory substances. The inhibition of NO production suggested that RPG-OM-30E had an anti-inflammatory effect, because NO is an inflammatory mediator. When the transcriptional level of cytokines was analyzed through RT-qPCR, LPS stimulation caused 36,000-, 25,000-, and 53-fold increases in Il-6, Il-1β, and TNF-α mRNA expression, respectively (Fig. 2). Pretreatment with RPG-OM-30E significantly inhibited the LPS-induced increase in the expression of these cytokines. At the 0.5 mg/mL concentration, RPG-OM-30E reduced the mRNA expression of Il-6, Il-1β, and TNF-α by 98.2, 97.9, and 68.9%, respectively.
Fig. 2.
Effects of RPG-OM-30E on LPS-induced Il-6, Il-1β, and TNF-α mRNA expression in RAW 264.7 cells. Cells were incubated with each concentration of RPG-OM-30E for 1 h, followed by LPS (1 μg/mL) treatment for 6 h. Total RNA was isolated from the cells. Il-6 (A), Il-1β (B), and TNF-α (C) mRNA expression was analyzed using real-time PCR and normalized to GAPDH. Data represent the mean ± SD of three independent experiments. Significant difference compared to the group treated with LPS alone, **p < 0.01, and ***p < 0.001
Time-dependent inhibitory effects of RPG-OM-30E on pro-inflammatory cytokine production
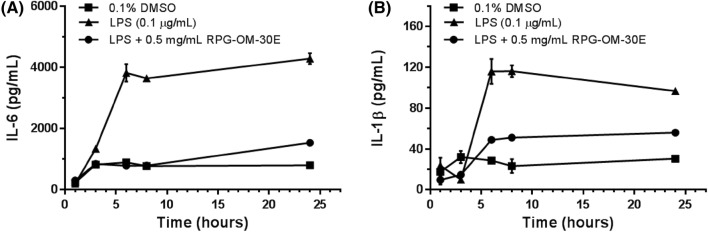
Previous studies showed that IL-6 and IL-1β acted as pro-inflammatory cytokines during the early stages of the immune response (Turrin et al., 2001). To determine the optimal test time for the anti-inflammatory effects of RPG-OM-30E, pro-inflammatory cytokine production was investigated in LPS-stimulated RAW 264.7 cells (Fig. 3). Peak IL-6 and IL-1β production occurred after 6 h of LPS stimulation. The production of IL-6 and IL-1β was increased by 19.6- and 4.9-fold, respectively, compared with 1 h. However, cotreatment with 0.5 mg/mL of RPG-OM-30E decreased IL-6 and IL-1β production after 6 h of treatment. At 6 h, RPG-OM-30E inhibited the production of IL-6 and IL-1β by up to 79.5 and 56.9%, respectively. For the subsequent anti-inflammatory testing, we fixed the treatment time with RPG-OM-30E at 6 h.
Fig. 3.
Time-dependent effects of RPG-OM-30E on LPS-induced cytokine production in RAW 264.7 cells. Cells were treated with 0.5 mg/mL of RPG-OM-30E, followed by LPS (1 μg/mL) treatment for the indicated times. After each time interval, the secreted cytokines were quantified using culture medium. The amounts of IL-6 (A) and IL-1β (B) were measured in the presence and absence of LPS
Anti-inflammatory effects of RPG-OM-30E in zebrafish larvae
Zebrafish (Danio rerio) have been employed as a model organism to test drug efficacy and toxicity due to several merits (Leite et al., 2012; Yang et al., 2012). Due to the transparency of the zebrafish embryo, both organs and intracellular fluorescence can be visualized with a microscope (Hu et al., 2013; Kang et al., 2014; Ko et al., 2014). Furthermore, it was previously reported that zebrafish and human immune system are similar (Li et al., 2017). Based on these studies, the anti-inflammatory effect of RPG-OM-30E was evaluated using Tg(mpx::EGFP)i114 zebrafish larvae. Induction of the inflammatory response was initiated through a tailfin wound, as described in the Materials and Methods. All treatments were performed immediately after tailfin wounding.
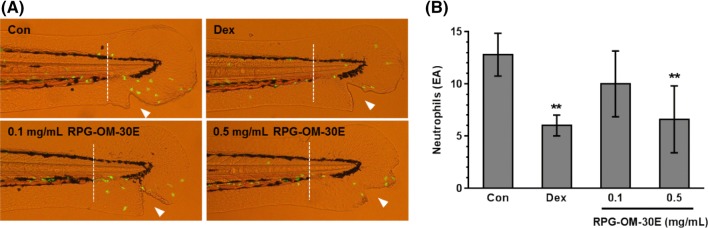
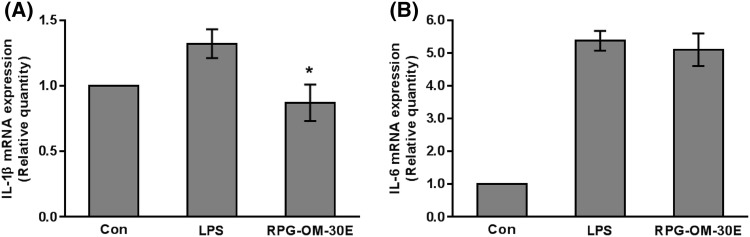
Zebrafish larvae were exposed to each treatment for 6 h, and then neutrophils were observed at the tailfin (Fig. 4A). Recruitment of neutrophils was increased around the wound site of tailfin. RPG-OM-30E treatment decreased the number of neutrophils migrating to the wound area (Fig. 4B). To confirm the anti-inflammatory effect of RPG-OM-30E, wild-type zebrafish larvae were microinjected with LPS in the yolk and treated with RPG-OM-30E. Total RNA was prepared from whole larvae, and mRNA expression was analyzed by real time PCR (Fig. 5A, B). LPS stimulation caused 5.4- and 1.3-fold increases in il-6 and il-1β mRNA expression, respectively. The il-1β mRNA expression was decreased significantly by RPG-OM-30E, whereas RPG-OM-30E had no significant effect on il-6 mRNA expression. Thus, RPG-OM-30E exhibited no effect on il-6 expression but had an effect on il-1β expression. Although slightly different from the results obtained using RAW 264.7 cells, RPG-OM-30E also showed an anti-inflammatory effect in the zebrafish model. These results suggest that zebrafish can be used efficiently to evaluate the anti-inflammatory effects of substances derived from natural products.
Fig. 4.
Inhibitory effects of RPG-OM-30E on neutrophil migration toward the wound site. A neutrophil migration assay was carried out using Tg(mpx::EGFP)i114 larvae. (A) Fluorescent neutrophils were observed at the tailfin of zebrafish larvae (white arrows). Control and positive control larvae were treated with 0.1% DMSO and 100 μM dexamethasone, respectively. RPG-OM-30E was applied at concentrations of 0.1 and 0.5 mg/mL. (B) Quantification of neutrophils recruited to the injury site (n > 15). The number of neutrophils was counted at the tailfin of zebrafish larvae. The data represent the mean ± SD of three independent experiments. Significant differences compared to the control group, **p < 0.01
Fig. 5.
Effects of RPG-OM-30E on LPS-induced il-1β and il-6 mRNA expression in zebrafish larvae. The injected volume in each treatment was equal to 2 nL. PBS was injected as a control treatment. Other treatments were carried out in the presence of LPS. RPG-OM-30E and LPS were injected as a 1 mg/mL stock solution. Total RNA was extracted from whole larvae, and il-1β (A) and il-6 (B) mRNA expression was analyzed by real-time PCR and normalized to β-actin. Significant differences compared to the control group, *p < 0.05
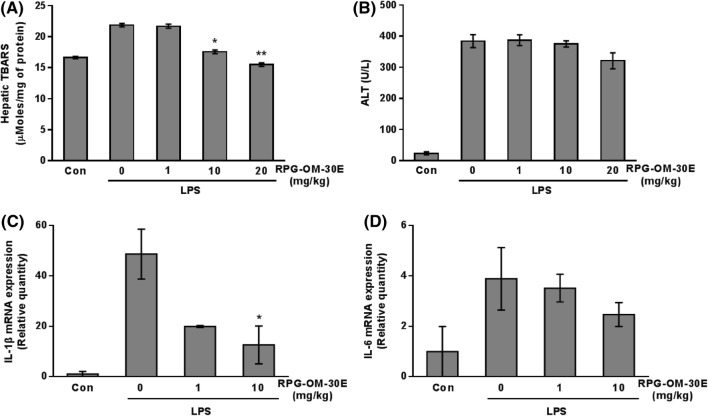
Antioxidant and anti-inflammatory effects of RPG-OM-30E in an LPS-induced oxidative stress mouse model
The antioxidant effect of RPG-OM-30E was further evaluated in the livers of ICR mice. Oxidative stress was induced through an intraperitoneal injection of LPS. The TBARS levels were measured in the liver fraction (Choi et al., 2016). The TBARS levels were enhanced in the LPS-induced group by approximately 1.3-fold compared with those of the control group. Administration of RPG-OM-30E at the 10 mg/kg concentration reduced the elevated TBARS levels by more than 80% (Fig. 6A). One hepatic enzyme, ALT, was also measured in the liver homogenates. Reactive metabolites produced during oxidative stress have been reported to disrupt cell membranes and release enzymes, such as aspartate transaminase, ALT, and alkaline phosphatase, into the blood (Hyder et al., 2013). Significant elevation of the ALT levels was detected in the LPS-induced group. In contrast, ALT was decreased slightly by administration of 20 mg/kg of RPG-OM-30E (Fig. 6B). The present study demonstrates the effects of RPG-OM-30E on oxidative stress. To confirm the anti-inflammatory effect by RPG-OM-30E in the livers of the LPS-induced oxidative mice, Il-1β and Il-6 mRNA expression was examined through real-time PCR (Fig. 6C, D). LPS (10 mg/kg) stimulation caused 48.7- and 3.89-fold increases in Il-1β and Il-6 mRNA expression, respectively. Conversely, 10 mg/mL of RPG-OM-30E caused 73.9 and 36.5% reductions in Il-1β and Il-6 mRNA expression, respectively.
Fig. 6.
Inhibitory effects of RPG-OM-30E on hepatic oxidative damage in LPS-induced mice. The hepatic TBARS (A) and ALT (B) levels were measured as described in the Materials and Methods in the normal group, 10 mg/kg of LPS-treated group, and (1, 10, and 20 mg/kg) RPG-OM-30E-treated mice. Total RNA was extracted from the mouse liver, and Il-1β (C) and Il-6 (D) mRNA expression was analyzed by real-time PCR and normalized to β-actin. Values are the mean ± SD for n = 5 per group. Significant differences compared to the LPS-treated group, *p < 0.05, and **p < 0.01
Through this study, we intend to contribute to the economic and environmental benefits provided by utilizing omija byproducts. First, polysaccharides and active substances remaining in the omija byproducts were extracted using hot water. Next, we tried to increase the anti-inflammatory effect by fermenting the hot water extract with Lactobacilius paracasei subsp. tolerans. Although differences were noted in effectiveness among species, RPG-OM-30E showed an anti-inflammatory effect. Consequently, we predict that RPG-OM-30E will show an anti-inflammatory effect by reducing the generation of ONOO− via inhibition of NO production. In the future, we will study the detailed action mechanisms by identifying active molecules within RPG-OM-30E.
Acknowledgements
This research was supported by a grant from the Ministry of SMEs & Startups (S2374332) and the Ministry of Trade, Industry & Energy (2019-10063396), Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jung Yoon Yang, Email: yjy1608@krict.re.kr.
Geum Ran Kim, Email: goldegg@krict.re.kr.
Jin Sil Chae, Email: truth@krict.re.kr.
Hyemin Kan, Email: khm4029@krict.re.kr.
Seong Soon Kim, Email: firstsay@krict.re.kr.
Kyu-Seok Hwang, Email: kshwang@krict.re.kr.
Byung Hoi Lee, Email: bnhlee@krict.re.kr.
Sangcheol Yu, Email: yusc90@raphagen.com.
Seongcheol Moon, Email: moonsc@raphagen.com.
Byounghee Park, Email: park@raphagen.com.
Myung Ae Bae, Email: mbae@krict.re.kr.
Dae-Seop Shin, Phone: +82-42-860-7082, Email: dsshin@krict.re.kr.
References
- Bistrian B. Systemic response to inflammation. Nutr. Rev. 2007;65:170–172. doi: 10.1301/nr.2007.dec.S170-S172. [DOI] [PubMed] [Google Scholar]
- Chang GT, Kang SK, Kim JH, Chung KH, Chang YC, Kim CH. Inhibitory effect of the Korean herbal medicine, Dae-Jo-whan, on platelet-activating factor-induced platelet aggregation. J. Ethnopharmacol. 2005;102:430–439. doi: 10.1016/j.jep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Chen IN, Ng CC, Wang CY, Chang TL. Lactic fermentation and antioxidant activity of Zingiberaceae plants in Taiwan. Int. J. Food Sci. Nutr. 2009;60:57–66. doi: 10.1080/09637480802375531. [DOI] [PubMed] [Google Scholar]
- Choi Eun-Mi, Hwang Jae-Kwan. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005;76(7-8):608–613. doi: 10.1016/j.fitote.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Choi GH, Jung YS, Shin HC. The effects of haedoksamul-tang on oxidative stress and hyperlipidemia in LPS-induced ICR mouse. J. Korean Med. 2016;37:77–89. doi: 10.13048/jkm.16008. [DOI] [Google Scholar]
- Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. Schisandrene, a dibenzocyclooctadiene lignin from Schisandra chinensis: structure-antioxidant anctivity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod. 2006;69:356–359. doi: 10.1021/np0503707. [DOI] [PubMed] [Google Scholar]
- Fernandez-Orozco R, Frias J, Munoz R, Zielinski H, Piskula MK, Kozlowska H, Vidal-Valverde C. Effect of fermentation conditions on the antioxidant compounds and antioxidant capacity of Lupinus angustifolius cv. Zapaton. Eur. Food Res. Technol. 2008;227:979–988. doi: 10.1007/s00217-007-0809-3. [DOI] [Google Scholar]
- Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN, Kang SS, Kim HP, Kim YS. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008;591:293–299. doi: 10.1016/j.ejphar.2008.06.074. [DOI] [PubMed] [Google Scholar]
- Hu CX, Li DH, Liu YD. Lipid peroxidation and antioxidant responses in zebrafish brain induced by Aphanizomenon flos-aquae DC-1 aphantoxins. Aquatic Toxicol. 2013;144–145:250–256. doi: 10.1016/j.aquatox.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur. J. Exp. Biol. 2013;3(2):280–284. [Google Scholar]
- Jou IM, Lin CF, Tsai KJ, Wei SJ. Macrophage-mediated inflammatory disorders. Mediators Inflamm. 2013;2013:1–3. doi: 10.1155/2013/316482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MC, Kim SY, Kim YT, Kim EA, Lee SH, Ko SC. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydrate Polymers. 2014;99:365–371. doi: 10.1016/j.carbpol.2013.07.091. [DOI] [PubMed] [Google Scholar]
- Kim MS, Sung HJ, Park JY, Sohn HY. Evaluation of anti-oxidant, anti-microbial and anti-thrombosis activities of fruit, seed and pomace of Schizandra chinensis Baillon. J. Life Sci. 2017;27:131–138. doi: 10.5352/JLS.2017.27.2.131. [DOI] [Google Scholar]
- Ko JY, Kim EA, Lee JH, Kang MC, Lee JS, Kim JS. Protective effect of aquacultured flounder fish-derived peptide against oxidative stress in zebrafish. Fish Shellfish Immunol. 2014;36:320–323. doi: 10.1016/j.fsi.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Ko KM, Ip SP, Poon MK, Wu SS, Che CT, Ng KH, Kong YC. Effect of a lignin-enriched fructus schisandrae extract on hepatic glutathione status in rats: protection against carbon tetrachloride toxicity. Planta Med. 1995;61:134–137. doi: 10.1055/s-2006-958032. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int. J. Exp. Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite CE, da Cruz Teixeira A, Cruz FF, Concatto SC, Amaral JH, Bonan CD. Analytical method for determination of nitric oxide in zebrafish larvae: toxicological and pharmacological applications. Analytical Biochem. 2012;421:534–540. doi: 10.1016/j.ab.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Li Y, Li Y, Cao X, Jin X, Jin T. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cellular & Mol. Immunol. 2017;14:80. doi: 10.1038/cmi.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20(10):1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairyproducts. Int. Dairy J. 2006;16(3):189–199. doi: 10.1016/j.idairyj.2005.02.009. [DOI] [Google Scholar]
- Matsushita H, Kobayashi M, Tsukiyama R, Fujimoto M, Suzuki M, Yamamoto K. Stimulatory effect of shoyu polysaccharides from soy sauce on the intestinal immune system. Int. J. Mol. Med. 2008;22:243–247. [PubMed] [Google Scholar]
- Min HY, Park EJ, Hong JY, Kang YJ, Kim SJ, Chung HJ, Woo ER, Hung TM, Youn UJ, Kim YS, Kang SS, Bae KH, Lee SK. Antiproliferative effects of dibenzocyclootadiene lignans isolated from Schisandra chinensis in human cancer cells. Bioor. Med. Chem. Lett. 2008;18(2):523–526. doi: 10.1016/j.bmcl.2007.11.082. [DOI] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants and Redox Signaling. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu M.M., Chakravortty D., Sugiyama T., Koide N., Takahashi K., Mori I., Yoshida T., Yokochi T. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. Journal of Endotoxin Research. 2001;7(6):431–438. doi: 10.1179/096805101101533034. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Ahn H, Kim JY, Kim YK. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophage. Phytother. Res. 2003;17:485–489. doi: 10.1002/ptr.1180. [DOI] [PubMed] [Google Scholar]
- Stone WL, Qui M, Smith M. Lipopolysaccharide enhances the cytotoxicity of 2-chloroethyl ethyl sulfide. BMC Cell Biol. 2003;4:1–7. doi: 10.1186/1471-2121-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin S, Starnes TW, Becker FB, Lam PY, Huttenlocher A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J. Cell Biol. 2014;207:589–598. doi: 10.1083/jcb.201408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NF-kappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999;126(3):673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 2001;54:443–453. doi: 10.1016/S0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Wu S, Fang JY, Ng CC, Wang CY, Shyu YT. Anti-inflammatory activity of Lactobacillus-fermented adlay-soymilk in LPS-induced macrophages throughsuppression of NF-kB pathways. Food Res. Int. 2013;52(1):262–268. doi: 10.1016/j.foodres.2013.02.053. [DOI] [Google Scholar]
- Yang HM, Ham YM, Yoon WJ, Roh SW, Jeon YJ, Oda T. Quercitrin protects against ultraviolet B-induced cell death in vitro and in an in vivo zebrafish model. J. Photochem. Photobiol. 2012;114:126–131. doi: 10.1016/j.jphotobiol.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Yang LL, Wang GQ, Yang LM, Huang ZB, Zhang WQ, Yu LZ. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: a novel screening method for anti-inflammatory drugs. Molecules. 2014;19:2390–2409. doi: 10.3390/molecules19022390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through toll-like receptors. J. Endotoxin Res. 2000;6:445–453. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]