Abstract
Bacterial infection is the most common cause of sepsis. In this study, Phascolosoma esculenta oligosaccharides (PEOs) were prepared to evaluate their resistance against E. coli-induced sepsis. HPLC–MS and FT-IR indicated that PEOs were composed of d-glucosyl, d-galactosyl, with small amount of d-mannosyl, d-arabinosyl and residues with α- and β-type linkage. Different dosage administrations of PEOs for 30 days significantly improved ICR mice survival rate and bacterial clearance ability (P < 0.01) after as E. coli injection. Moreover, PEOs significantly reduced the secretion of IL-1β and TNF-α and enhanced that of IL-10 in sepsis mice, enhanced the antioxidant enzyme activities and total antioxidant capacity, decreased MDA level in the serum, and upregulated mRNA expression of Nrf2 (P < 0.01). All these results indicate that PEOs could improve the resistance of ICR mice against E. coli-induced sepsis that attributed to anti-inflammatory and anti-oxidative stress.
Keywords: Phascolosoma esculenta oligosaccharides, Sepsis, Oxidative stress, Inflammation
Introduction
Sepsis has been defined as a systemic inflammatory reaction syndrome caused by a dysregulated host response to a microbial infection (Lu et al., 2013; Vincent et al., 2002). Multiple factors could induce the incidence of sepsis, including gram-negative bacteria, gram-positive bacterial and fungal (Martin, 2012; Ramachandran, 2014; Riedemann et al., 2003). During the sepsis infection, endotoxin from bacterial pathogens triggers the release of a variety of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-10, and IL-1β in immune cells, which occurs rapidly in the early stage of sepsis (Eichacker et al., 2002; Kang et al., 2017). Inhibition of these proinflammatory cytokine productions has been developed as important targets to manage sepsis (LaRosa and Opal, 2008). Moreover, excessive levels of reactive oxygen species (ROS) and other free radicals would be generated, which leads to an imbalance in the redox system, induces oxidative stress and contributes to high mortality rates in the sepsis progress (Lowes et al., 2008). Malondialdehyde (MDA) is a product of lipid peroxidation induced by excessive ROS and is widely used as a marker for oxidative stress (Urso and Clarkson, 2003). Otherwise, some enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) can metabolize free radicals and protect cells from oxidative stress. Nrf2, an important transcriptional activator of antioxidant genes, can recognize the antioxidant response element (ARE), and regulate important antioxidant protective responses. In addition, Nrf2 also has been identified as a novel modifier gene of sepsis, that determines survival by mounting an appropriate innate immune response (Xue et al., 2015).
As a seafood with high nutritive value, Phascolosoma esculenta (commonly known as ‘Xing Chong’) is widely distributed along the southern coast of China, especially in Zhejiang and Fujian province. Polysaccharides extracted from Phascolosoma esculenta exhibit various functions, such as antioxidant improvement and neuro-protection (Liu et al., 2016). Oligosaccharides are the degradation products of polysaccharides. Compared with polysaccharides, oligosaccharides are usually water-soluble, easily absorbed substance through the intestine and exhibit multiples of physiological functions. In the previous studies, oligosaccharides derived from the enzymatic hydrolysis in human gastro-intestine could promote a more active immune function (Gibson et al., 2004). In the previous reports, oligosaccharides demonstrated the ability to prevent endothelial injury and suppress inflammation (Li et al., 2016), furthermore oligosaccharides from chitosan could protect mice during the lipopolysaccharide challenge, by its anti-inflammatory effects and anti-oxidation properties (Qiao et al., 2011). Human milk oligosaccharides which are associated with secretor mothers may have a protective effect by decreasing pathogens associated with sepsis (Underwood et al., 2015). In this study, Phascolosoma esculenta oligosaccharides (PEOs) were firstly prepared by enzymatic hydrolysis from Phascolosoma esculenta polysaccharide and characterized by HPLC–MS and FT-IR. PEO was orally administrated to several groups of bacteria-free Institute of Cancer Research (ICR) male mice, to improve the immunity of these mice. Then, the protection of PEO on the mice with E. coli-induced Sepsis was determined, by detection inflammatory regulation, antioxidant activity (SOD, TAC, GSH-Px, and MDA) and Nrf2 expression in E. coli-induced sepsis mice.
Materials and methods
Materials
Phascolosoma esculenta was purchased in a local marine market of Ningbo (Zhejiang, China). Standards (Glucose, galactose, mannose, arabinose), IL-1β, IL-10 and TNFα assay kit were purchased from BD Pharmingen (San Diego, CA, USA). Papain and trypsin were brought from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (TAC) assay kit were bought from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). RNAiso, Oligo (dT), AMV reverse transcriptase, Ex Taq DNA polymerase, 100 bp DNA Ladder Marker and SYBR Premix Ex Taq were bought from Takara Biotechnology (Dalian) Co., Ltd. Gel Extraction Kit was bought from Sigma-Aldrich. All other reagents were analytical reagents bought from Sinopharm Group Co., Ltd (Shanghai, China).
Preparation of Phascolosoma esculenta oligosaccharides
Fresh Phascolosoma esculenta without internal organs was washed, drained and homogenized, then grinded into substrate. Phascolosoma esculenta polysaccharide was obtained through bi-enzymatic hydrolysis by papain and trypsin (1:1) at a substrate/enzyme ratio of 40:1 (w/w, pH 6.5, 65 °C for 7 h). Phascolosoma esculenta polysaccharide was obtained through bi-enzymatic hydrolysis of papain and trypsin. The detailed parameters were papain/trypsin of 1:1, substrate/enzyme ratio of 40:1 (w/w), pH 6.5, temperature 65 °C, and the hydrolysis time of 7 h. After enzyme inactivation at 100 °C, pH was adjusted to 7.0. The protein was removed by trichloroacetic acid and ethanol with the final concentration of 75%, which was used for polysaccharide precipitation. After washing repeatedly with ethanol and acetone, membrane filtration of 3KDa was applied, to obtain polysaccharides (Millipore Corporation, Billerica, MA, USA) (Liu et al., 2016). The oligosaccharide mixture of Phascolosoma esculenta was produced through acid hydrolysis of polysaccharides with 0.1 M HCl at 65 °C for 5 h and was fractionated by gel filtration chromatography on a Superdex 30 column (1.6 × 100 cm), eluted with 0.3 M NH4 HCO3, at a flow rate of 0.3 mL/min and the pooled oligosaccharide was evaporated in vacuo followed by freezing at -20 °C until use.
Identification of composition of Phascolosoma esculenta oligosaccharides
High performance liquid chromatography-mass spectrometry (HPLC–MS) with gel permeation chromatography, was carried out to measure the monosaccharide component and obtain the molecular weight analysis of PEOs (Yamamoto et al., 1995). The column was calibrated with standard T-series dextran (T-500, T-110, T-70, T-40, and T-10). The data was analyzed with water gel permeation chromatography Millennium 32 software (Millennium Software Developers, Inc., NY, USA).
FT-IR spectroscopy
FT-IR spectra of PEOs were obtained using KBr disks containing 1% finely ground sample on Nicolet 6700 (Nicolet Instrument Company, USA). The spectra were running in the frequency range of 4000–400 cm−1 at a resolution of 2 cm−1 in the transmittance mode (Auddy et al., 2003).
Phascolosoma esculenta oligosaccharides (PEOs) administration
Institute of Cancer Research (ICR) male mice (14 ± 2 g, 3–4 weeks) were bought from the Zhejiang Academy of Medical Science (Hangzhou, Zhejiang, China) and were cared for according to the National Institutes of Health (NIH) guidelines. Mice were acclimatized for 1 week prior to use. All mice were housed under standard conditions at 24 ± 1 °C, with humidity of 50 ± 10%, and a 12-h light/12-h dark cycle and acclimatized for a 7 d period before the experiments. All procedures were carried out in strict accordance with PRC legislation, on the use and care of laboratory animals, and was approved by the Ningbo University committee for animal experiments (Yu et al., 2009). Mice were randomly assigned to four experiment groups. Saline was orally administered to the control group daily. PEOs at a dosage of 1 mg/kg (low dose, PEOs1), 10 mg/kg (middle dose, PEOs2), and 50 mg/kg (high dose, PEOs3) were orally administered to the other three groups. During the administration, the weight of mice was recorded. The PEOs were administered for a period of 30 days before the E. coli challenge.
Establish of infection model
For sepsis models with a single bacterial species, mice (n = 10) were injected intraperitoneally (i.p.) with 100 μL of 2.5 × 109 colony-forming units (CFU) of live E. coli (DH5α). DH5α E. coli challenge in the mice was performed as described previously (He et al., 2013; Lu et al., 2011). Brief, overnight cultures of DH5α E. coli were diluted 1:100 in Luria–Bertani liquid medium, and grown at 37 °C with continuous shaking, and harvested when the OD values were at 0.6. After 18 h of DH5α E. coli injection, the animals were killed and analyzed.
Survival rate
The survival rates of mice were recorded at 12 h intervals for up to 96 h after the bacterial challenge.
Bacterial burden
After 12 h of injection with DH5α E. coli., the blood, livers, spleens and kidneys of mice were aseptically harvested (Lu et al., 2013). Tissue homogenates and blood were serially diluted in sterile PBS and plated onto separate LB agar plates. The plates were incubated overnight at 37 °C, and after counting, it was found that each plate yielded 30–300 colonies.
Cytokine determination
Cytokines IL-10, IL-1β, and TNF-α in the serum were detected by using ELISA kits from BD Pharmingen (San Diego, CA) according to the instructions.
Oxidative stress status assessment
Activities of SOD, GSH-Px and levels of TAC and MDA in the serum were assayed using commercially available assay kits (Nanjing Jiancheng Bio Company, Nanjing, China), under the guidelines provided. SOD, GSH-Px, and TAC were determined based on the protein content in the blood. While, MDA was determined based on the protein content in the liver.
Detection Nrf2 mRNA expression by qRT- PCR
The total RNA was extracted from the aortic endothelial cells using TRIzol plus RNA purification kit (Invitrogen, Life Technologies, Carlsbad, CA, USA), with the optical density ratio (OD260/OD280) 1.93 by the UV spectrophotometer, and 3 μg RNA was used to synthesize cDNA using the GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s protocol. 1 μl cDNA was taken for the purpose of real time-PCR with SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). 352 bp fragment of Nrf2 was amplified with specific upstream primer 5′-ATTGCCTG-TAAGTCCTGGTCA-3′ and downstream primer 5′-ACTGCTCTTTGGACAT-CATTTCG-3′. As a control, a 231-bp fragment of the housekeeping β-actin gene was amplified from the same cDNA preparations using pActin-F: 5′-TCGTGCGTGACATCAAGGAG-3′ and pActin-R: 5′-CGCACTTCATGATGCTGTTG-3′ primers. PCR reactions were analyzed using the software provided, with the Mx3000P QPCR system and mRNA expression of Nrf2 was calculated using the 2−ΔΔCT method and normalized against the expression of β-actin.
Statistical analysis
All experiments were carried out in triplicate, with the exception of the survival rate which was performed only once. Results were expressed as a mean ± standard error (SEM) and analyzed using an analysis of variance (ANOVA) and a comparison of the means was performed using Duncan’s multiple range test at a 95% confidence level with the SPSS package (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, USA). P value (two-tailed) < 0.05 was considered significant and < 0.01 was considered extremely significant.
Results and discussion
Composition of PEOs determined by HPLC
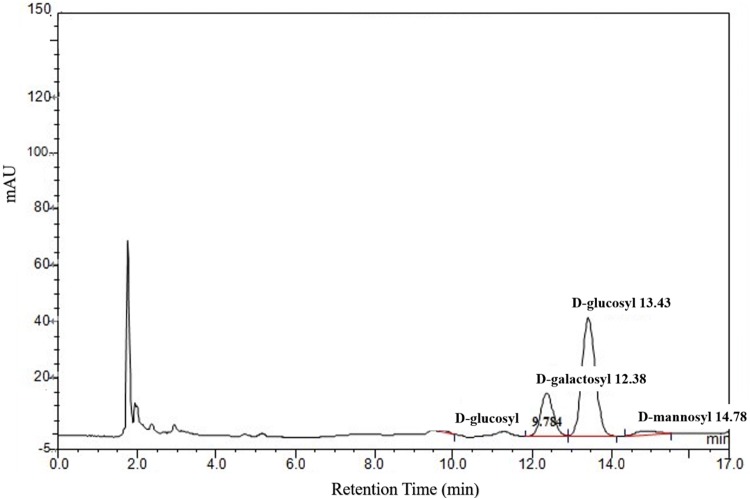
In this study, monosaccharide composition and content of PEOs were detected by HPLC, which were showed in Fig. 1 and Table 1. The results indicated that PEOs were mainly composed of d-glucosyl and d-galactosyl, with small amount of d-mannosyl and d-arabinosyl.
Fig. 1.
Monosaccharide Component of Phascolosoma esculenta oligosaccharides (PEOs)
Table 1.
Monosaccharide Component of Phascolosoma esculenta oligosaccharides (PEOs) by HPLC–MS
| No. | Retention time (min) | Components | Content (%) |
|---|---|---|---|
| 1 | 9.78 | d-arabinosyl | 2.80 |
| 2 | 12.38 | d-galactosyl | 77.6 |
| 3 | 13.43 | d-glucosyl | 213.0 |
| 4 | 14.78 | d-mannosyl | 17.4 |
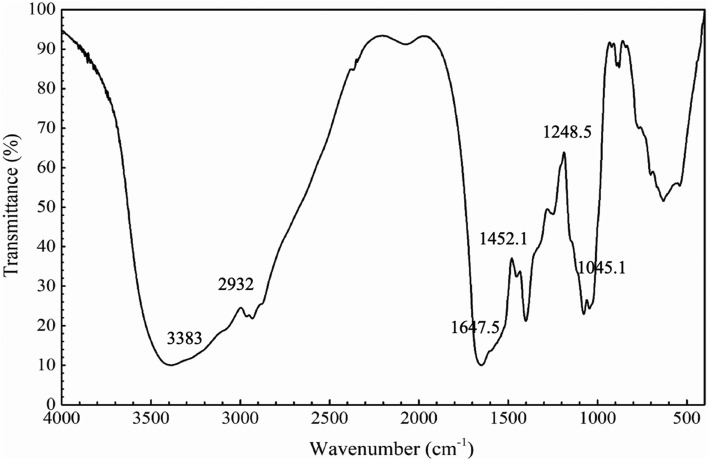
Characterization of PEOs by FT-IR
Saccharide strongly absorb in the so-called “fingerprint region” (1200–900 cm−1) of FT-IR spectra (Santos et al., 2014). As shown in Fig. 2, PEOs showed a typical saccharide FT-IR spectrum. The absorption bands in the region of 1077.2 cm−1 and 1045.1 cm−1, showed the monosaccharides in glucopyranosides (1100–1010 cm−1). The bands in the region of 3383 cm−1 were due to an O–H stretching vibration, and the bands in the region of 2932 cm−1 were due to a C–H stretching vibration (Cao et al., 2006). Absorption bands at 1647.5 cm−1 were due to a hydrated vibration or C=O stretching vibration. The absorption bands at 1452.1 cm−1 were due to a C–H bending vibration, the bands in the region of 1400.7 cm−1 were due to a C–N stretching vibration, and the bands in the region of 1248.5 cm−1 were due to a C–O stretching vibration. The characteristic bands of α-type glycosidic linkages were 844 ± 8 cm−1 (Sun et al., 2018), and the peaks ranged of 900–890 cm−1 indicated the presence of dominant β-glycosidic linkages (Bian et al., 2012). Thus, the small sharp absorption peaks at 841.5 cm−1 and 892.5 cm−1 showed that PEOs contained both α- and β-type linkages.
Fig. 2.
FT-IR spectra of Phascolosoma esculenta oligosaccharides (PEOs)
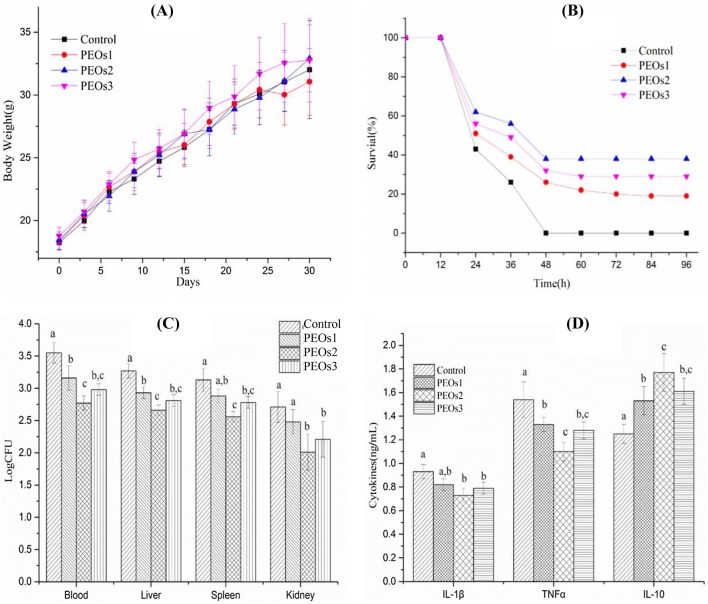
Effects of PEOs administration on body weight of ICR mice
All mice were in good health status throughout the PEOs administration period. Body weight of the mice in each group is shown in Fig. 3(A). There were no significant differences (P > 0.05) among all the groups, which indicated that PEOs administration had no significantly negative effect on the body weight of mice.
Fig. 3.
Effect of PEOs administration on body weight, survival rate, bacterial burden and cytokine levels. Mice were treated with PEOs1 (low dose, 1 mg/kg), PEOs2 (middle dose, 10 mg/kg), PEOs3 (high dose, 50 mg/kg) and saline (control) for 30 days before the E. coli challenge. (A) Body weight of mice during the 30 days. (B) Survival rate of mice after 2.5 × 109 viable E. coli infection for 96 h. (C) Bacterial burden from blood and organs of mice after 2.5 × 109 viable E. coli infection for 12 h. (D) Levels of IL-1β, TNF-α and IL-10 of mice after 2.5 × 109 viable E. coli injection for 12 h. The different letter on Fig. 2(C), (D) means significant difference between groups (P < 0.05)
Effects of PEOs on mice survival rates and bacterial burden after E coli injection
Sepsis is a severe fatal illness via an excessive inflammation reaction to microbial infections (Yang et al., 2015). In this study, sepsis model induced by E. coli were applied to examine the effects of PEOs on mice survival rates. The survival rate of saline-treated control animals dramatically decreased, and 100% mortality was observed at 48 h after E. coli injection (Fig. 3B). However, administration of PEOs at all three different dosages enhanced the survival rates compared to the control group (saline-treatment) (P < 0.01). Moreover, the groups, administered with middle dose (PEOs2, 10 mg/kg) and high dose (PEOs3, 50 mg/kg), exhibited higher survival rates compared to the low dose-administrated group (PEOs1, 1 mg/kg). 10 mg/kg administration displayed the best protective effect during the E. coli challenge.
To verify that the survival beneficial effect of PEOs administration was related to enhanced bacterial clearance, bacterial burden from the blood and organs from the E. coli-induced sepsis experiment were also detected. Bacterial burden from the blood and liver were significantly inhibited with all three doses of PEOs treatment (Fig. 3C). The middle dose (10 mg/kg) displayed better bacterial clearance than the low dosage (P < 0.01), whereas, the high dose group had no significant difference compared with the other two treated groups (P > 0.05). Bacterial burden of spleen and kidney were dramatically decreased with the middle (10 mg/kg) and high (50 mg/kg) doses of PEOs administration (P < 0.01), but not in the low dose of PEOs administration (1 mg/kg). All these results suggested that PEOs administration could enhance systemic bacterial clearance, which was partially contributed to the increased survival rate.
Effects of PEOs on cytokine determination
Sepsis involves the overexpression of many inflammatory factors, such as TNF-α and IL-1β, as well as anti-inflammatory IL-10 (Hatherill et al., 2000; King et al., 2014). TNF-α and IL-1β play a critical role in the pathogenesis in the early stages of sepsis, contributing to excessive levels of inflammation and multiple organ failure (Kim et al., 2003). As shown in Fig. 3D, it could be found that the secretion of TNF-α and IL-1β in the PEOs-administrated mice (10 and 50 mg/kg) was significantly decreased (P < 0.05), which means that PEOs play a protective role for inflammation reaction in the early development of E-coli induced sepsis. In the previous study, IL-10 was reported to show some contribution in the secondary infection during sepsis development as it could down regulate pro-inflammatory cytokines, such as TNF-α and IL-1β (Muenzer et al., 2010). In our results, anti-inflammatory cytokine IL-10 was elevated with all three doses of PEOs treated mice10 (P < 0.01). Thus, PEOs pretreatment may act by up-regulating the IL-10 expression which might in turn, reduce the expression of TNF-α and IL-6, thereby mitigating systemic inflammation and organ damage induced by E-coli infection.
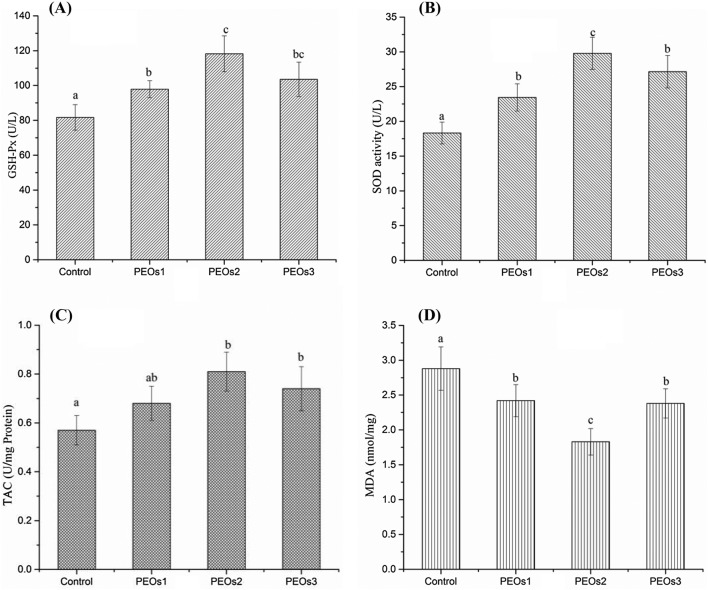
Effects of PEOs on oxidative stress status
Oxidative stress is one of the important mechanisms underlying the pathogenesis of sepsis and tissues would become hypoxic and simultaneously with an increase of free radical production and oxidative stress during the development of sepsis (Karapetsa et al., 2013). Increased ROS also leads to the activation of redox-sensitive transcription factors which further induce the transcription of a large range of genes involved in the inflammation, then to release varieties of pro-inflammatory cytokines (e.g. IL-6, IL-1, and TNF-α) (Chen and Lan, 2017). SOD and GSH-Px are the primary antioxidant enzymes minimizing the oxygen radical cascade and removing cytotoxic peroxides in the mammalian system (Asci et al., 2015). Our result showed that the enzyme activities of GSH-Px and SOD were enhanced within three PEOs administration groups and the level of TAC was elevated in the10 mg/kg and 50 mg/kg PEOs administration groups (P < 0.01), while the middle dose is more effective compared with the saline control group (Fig. 4A–C). Lipid peroxidation could greatly affect the physicochemical properties of membrane lipid bilayers, which cause the possibility of severe cellular dysfunction. MDA, the product of lipid peroxidation, is well-known for its role to induce protein oxidation (Catalá, 2009). Therefore, we also tested the lipid peroxidation, by measuring MDA in the blood samples, as shown in Fig. 4D, and the result demonstrated that all doses of PEOs administration significantly suppressed elevation of MDA levels. As the relationship between the pathogenesis of sepsis and oxidation stress become clearer, it is logical to try to overcome the nefarious effects of oxidative stress by increasing anti-oxidants for sepsis (Bar-Or et al., 2015). Based on the improvement of PEOs administration to reduce inflammation reaction and oxidation stress, PEOs could be used as protective dietary food against E. coli-induced sepsis.
Fig. 4.
Effect of PEOs administration on oxidative stress in E. coli-induced sepsis mice model. Mice were treated with saline (control) or different doses of PEOs, 1 mg/kg (low dose, PEOs1), 10 mg/kg (middle dose, PEOs2), and 50 mg/kg (high dose, PEOs3) for 30 days, then were intraperitoneally injected with 2.5 × 109 viable E. coli; blood was collected after 12 h and activities of (A) glutathione peroxidase (GSH-Px), (B) superoxide dismutase (SOD), levels of (C) total antioxidant capacity (TAC) and (D) malondialdehyde (MDA). The different letter on each figure means significant difference between groups (P < 0.05)
Effects of PEOs on Nrf2 mRNA expression
Nrf2 is a DNA-binding protein that recognizes ARE and it regulates the expression of most genes related to the ARE-driven antioxidant in response to noxious stimuli. Nrf2 plays a key role in the defense against oxidative stress via the induction of phase II and antioxidant enzymes (Chapple et al., 2012). Nrf2 mRNA expression level was significantly increased in three PEOs-administrated groups compared with the saline control group (P < 0.05) (Fig. 5). Our observations suggested that an accumulation of Nrf2 mRNA might contribute to the induction of ARE-mediated antioxidant gene expression, after the PEOs treatment. Previous reports demonstrated that some dietary antioxidative oligosaccharides could induce Nrf2-ARE mediated gene expression (Zhang et al., 2015), which was consistent with our results.
Fig. 5.

Effect of PEOs administration on Nrf2 mRNA expression in E. coli-induced sepsis mice model. Mice were treated with saline (control) or different doses of PEOs, 1 mg/kg (low dose, PEOs1), 10 mg/kg (middle dose, PEOs2), and 50 mg/kg (high dose, PEOs3) for 30 days, and were intraperitoneally injected with 2.5 × 109 viable E. coli and euthanized after 12 h
In conclusion, PEOs was mainly composed of d-glucosyl, d-galactosyl, with small amount of d-mannosyl, d-arabinosyl and residues with α- and β-type linkage. PEOs could enhance sepsis resistance, through the increase of bacterial clearance effect, decreasing pro-inflammatory and increasing anti-inflammatory cytokines in sepsis, and mediating Nrf2-ARE pathways via the antioxidative effect. PEOs administration might serve as an effective protective strategy to control sepsis and regulate immunity and anti-oxidative stress.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31601476), Zhejiang Provincial Natural Science Foundation of China (LQ15C200002), and sponsored by K. C. Wong Magna Fund in Ningbo University.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhihao Yang, Email: ynez_777@163.com.
Ying Pan, Email: panying_27@sina.cn.
Jiong Chen, Email: jchen1975@163.com.
Hao Zhang, Email: zhanghao@nbu.edu.cn.
Hua Wei, Email: weihua@nbu.edu.cn.
Zufang Wu, Email: wuzufang@nbu.edu.cn.
Lianliang Liu, Phone: +86-574-8760084, Email: hahaliang408@126.com.
References
- Asci A, Surmeli-Onay O, Erkekoglu P, Yigit S, Yurdakik M, Kocer-Gumusel B. Oxidant and antioxidant status in neonatal proven and clinical sepsis in relation to selenium status. Pediatr. Int. 2015;57(6):1131–1137. doi: 10.1111/ped.12698. [DOI] [PubMed] [Google Scholar]
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003;84:131–138. doi: 10.1016/S0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Bar-Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Rep. 2015;20(5):193–197. doi: 10.1179/1351000215Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian J, Peng F, Peng XP, Xu F, Sun RC, Kennedy JF. Isolation of hemicelluloses from sugarcane bagasse at different temperatures: structure and properties. Carbohydr. Polym. 2012;88:638–645. doi: 10.1016/j.carbpol.2012.01.010. [DOI] [Google Scholar]
- Cao W, Liu XQ, Liu L, Wang M, Fan HT, Li C, Lv Z, Wang X, Mei Q. Structural analysis of water-soluble glucans from the root of Angelica sinensis (Oliv.) Carbohydr. Res. 2006;341:1870–1877. doi: 10.1016/j.carres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Chapple SJ, Siow RCM, Mann GE. Crosstalk between Nrf2 and the proteasome: Therapeutic potential of Nrf2 inducers in vascular disease and aging. Int. J. Biochem. Cell B. 2012;44(8):1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Chen L, Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 2017;8(5):1785–1792. doi: 10.1039/C6FO01561A. [DOI] [PubMed] [Google Scholar]
- Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Resp. Crit. Care. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit. Care Med. 2000;28:2591–2594. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- He YQ, Chen J, Lu XJ, Shi YH. Characterization of P2X7R and its function in the macrophages of ayu. Plecoglossus altivelis. Plos ONE. 2013;8:e57505. doi: 10.1371/journal.pone.0057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DR, Yoon GY, Cho J, Lee SJ, Lee SJ, Park HJ, Kang TH, Han HD, Park WS, Yoon YK, Park YM, Jung ID. Neoagarooligosaccharides prevent septic shock by modulating A20-and cyclooxygenase-2-mediated interleukin-10 secretion in a septic-shock mouse model. Biochem. Bioph. Res. Co. 2017;486:998–1004. doi: 10.1016/j.bbrc.2017.03.152. [DOI] [PubMed] [Google Scholar]
- Karapetsa M, Pitsika M, Goutzourelas N, Stagos D, Becker AT, Zakynthinos E. Oxidative status in ICU patients with septic shock. Food Chem. Toxicol. 2013;61(4):106–111. doi: 10.1016/j.fct.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Kim GY, Roh SI, Park SK, Ahn SC, Oh YH, Lee JD, Park YM. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol. Pharm. Bull. 2003;26(10):1418–1423. doi: 10.1248/bpb.26.1418. [DOI] [PubMed] [Google Scholar]
- King EG, Bauzá GJ, Mella JR, Remic DG. Pathophysiologic mechanisms in septic shock. Lab. Invest. 2014;94:4–12. doi: 10.1038/labinvest.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa SP, Opal SM. Sepsis strategies in development. Clin. Chest. Med. 2008;29:735–747. doi: 10.1016/j.ccm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang R, Guo J, Shao H, Yang X. Protective effect of R. glutinosa oligosaccharides against high L-carnitine diet-induced endothelial dysfunction and hepatic injury in mice. Int. J. Biol. Macromol. 2016;85:285–293. doi: 10.1016/j.ijbiomac.2015.12.092. [DOI] [PubMed] [Google Scholar]
- Liu L, Cao J, Chen J, Zhang X, Wu Z, Xiang H. Effects of peptides from Phascolosoma esculenta on spatial learning and memory via anti-oxidative character in mice. Neurosci. Lett. 2016;631:30–35. doi: 10.1016/j.neulet.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radical Biol. Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Lu XJ, Chen J, Huang ZA, Shi YH, Lv JN. Identification and characterization of a novel cathelicidin from ayu. Plecoglossus altivelis. Fish Shellfish Immun. 2011;31:52–57. doi: 10.1016/j.fsi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Lu XJ, Chen J, Yu CH, Shi YH, He YQ, Zhang RC, Huang ZA, Lv JN, Zhang S, Xu L. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J. Exp. Med. 2013;210:5–13. doi: 10.1084/jem.20121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev. Anti-Infe. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect. Immun. 2010;78(4):1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Bai XF, Du YG. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011;11:121–127. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5(1):196–201. doi: 10.4161/viru.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J. Clin. Invest. 2003;112:460–467. doi: 10.1172/JCI200319523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MI, Araujo-Andrade C, Tymczyszyn EE, Gómez-Zavaglia A. Determination of amorphous/rubbery states in freeze-dried prebiotic sugars using a combined approach of near-infrared spectroscopy and multivariate analysis. Food Res. Int. 2014;64:514–519. doi: 10.1016/j.foodres.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Sun HQ, Song WW, Zhang LJ, Yang XY, Zhu ZY, Ma RC, Wang DY. Structural characterization and inhibition on α-glucosidase of a novel oligosaccharide from barley malt. J. Cereal Sci. 2018;82:82–93. doi: 10.1016/j.jcs.2018.05.009. [DOI] [Google Scholar]
- Underwood MA, Gaerlan S, Leoz M, Dimapasoc L, Kalanetra KM, Lemay DG, German JB, Mills DA, Lebrilla CB. Human milk oligosaccharides in premature infants: absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015;78:670–677. doi: 10.1038/pr.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicol. 2003;189:41–54. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis. 2002;34:1084–1093. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- Xue WL, Bai XY, Zhang L. rhTNFR: Fc increases Nrf2 expression via miR-27a mediation to protect myocardium against sepsis injury. Biochem. Bioph. Res. Co. 2015;464:855–861. doi: 10.1016/j.bbrc.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nunome T, Yamauchi R, Kato K, Sone Y. Structure of an exocellular polysaccharide of Lactobacillus helveticus TN-4, a spontaneous mutant strain of Lactobacillus helveticus TY1-2. Carbohydr. Res. 1995;275:319–332. doi: 10.1016/0008-6215(95)00077-7. [DOI] [PubMed] [Google Scholar]
- Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43(5):923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Li HY, Qian Y, Yan L. Effect of Lentinus edodes polysaccharide on oxidative stress, immunity activity and oral ulceration of rats stimulated by phenol. Carbohydr. Polym. 2009;75:115–118. doi: 10.1016/j.carbpol.2008.07.002. [DOI] [Google Scholar]
- Zhang H, Wang J, Liu Y, Sun B. Wheat bran feruloyl oligosaccharides modulate the phase II detoxifying/antioxidant enzymes via Nrf2 signaling. Int. J. Biol. Macromol. 2015;74:150–154. doi: 10.1016/j.ijbiomac.2014.12.011. [DOI] [PubMed] [Google Scholar]