Abstract
Bacteria can survive and persist in food processing environments by attachment and biofilm formation and transfer to food products, causing serious foodborne illness. In this study, we investigated natural substances that belong to the family Brassicaceae to determine whether they have potential anti-attachment activities against Escherichia coli O157:H7. The inhibition of biofilm formation was evaluated by crystal violet and resazurin assays at different stages of biofilm formation (initial attachment, biofilm formation, and after biofilm development) of E. coli O157:H7. The sessile cells were reduced to a range of 13.8–31.3% by young radish, radish, radish sprout, red cabbage, and kale extracts, and the viability was reduced to between 5.83 and 51.5%. The radical scavenging activities and the presence of polyphenolic compounds were compared. The presence of phenolic compounds such as gallic acid, caffeic acid, and phenylethyl ITC in the Brassicaceae family verified the potential use as a natural anti-biofilm substituent against E. coli O157:H7.
Keywords: Brassicaceae, Anti-attachment, Anti-biofilm, Radical scavenging, E. coli O157:H7
Introduction
Microbial contamination in food service facilities and food processing plants can be a potential health threat since the food products are exposed to the transfer and cross-contamination of bacteria via food handlers, food preparation surfaces, and contaminated equipment (Verraes et al., 2013). This type of contamination is caused by the adherence and persistence of bacteria on food-contact surfaces. Bacteria can build a bacterial community on food-contact surfaces, to provide greater resistance against various stress conditions such as heat, humidity, pH, disinfectants, and other antimicrobials. Almost all (over 99%) of bacteria in nature exist as a biofilm and about 80% of bacterial infections are associated with biofilms (Costerton et al., 1987; Srey et al., 2013). Fish and shrimp processing plant showed bacterial biofilms on the processing line between 103 and 107 CFU/cm2, which can result in readily deleterious food (Martínez-Córdova et al., 2015). Effective control measures therefore should be developed to protect consumers from related health threats.
Biofilm formation can be divided into four common steps: attachment to surface, formation of a microcolony, biofilm maturation, and bacterial dispersion. The initial attachment step is the only reversible process that is initiated by the physico-chemical interaction between the bacteria and surface, such as hydrophobicity, van der Waals, acid–base, and electrostatic interaction (Bos et al., 1999). Once strongly attached, the bacteria start to replicate and produce exopolymeric substances (EPS), including extracellular polysaccharides, proteins, lipids, and extracellular DNA (eDNA) as a protective barrier to survive in the environment (Flemming and Wingender, 2010). The microcolonies further mature and stabilize. Bacteria in the microcolonies then disperse and travel to other surfaces to start a new life cycle (Abdallah et al., 2014).
Plants produce bioactive compounds, which have positive implications beyond their nutritional value to human health. These compounds include natural antioxidants that protect against damage by reactive oxygen species and antimicrobials that inhibit bacterial growth by damaging the cell membranes (Kim et al., 2013). Cruciferous vegetables in the family Brassicaceae have played an important role in the human diet worldwide because of their nutritional value as well as health-promoting phytochemicals. This group of vegetables includes radish, kale, broccoli, cauliflower, and cabbage. They are rich in vitamins C and E, soluble fiber, and phenolic compounds, including flavonoids, carotenoids, and various sulfur compounds (Ko et al., 2016). Numerous studies have focused on the protective effects of cruciferous vegetables against cancer, cardiovascular diseases, and immune dysfunction (Blekkenhorst et al., 2018; Wirth et al., 2017). Recently, researchers have demonstrated the antimicrobial effects of these vegetables: the radish root against Bacillus subtilis, Staphylococcus aureus, and Salmonella Typhimurium (Beevi et al., 2009), kale leaves against B. subtilis, S. aureus, Enterobacter faecalis, and Moraxella catarrhalis (Ayaz et al., 2008), and mustard seeds against S. aureus, Listeria monocytogenes, B. subtilis, and Escherichia coli (Engels et al., 2012). Several studies on the inhibition of biofilm formation using organic acids that are known to be present in cruciferous vegetables also have been reported (Amrutha et al., 2017; Lee et al., 2011). However, few studies on using vegetable extracts for biofilm inhibition have been reported. The purpose of this study therefore was to evaluate the potential anti-attachment and anti-biofilm activities of cruciferous vegetable extracts in order to determine which stage of biofilm formation is effectively controlled by the plant extracts and to determine the mechanism and the potential phenolic compounds that are involved in the controlling measures.
Materials and methods
Bacterial strains and growth conditions
Escherichia coli O157:H7 ATCC 43894 were used from the culture collection of the Food Safety Lab at Gyeongsang National University. The bacteria were inoculated in a tryptic soy broth (TSB, Becton–Dickinson Co., Franklin Lakes, New Jersey, USA) and incubated at 37 °C for 16-18 h in a shaking incubator. The cultures were maintained in 15% glycerol at − 80 °C until use.
Preparation of plant extracts
Radish, young radish, kale, vegetable mustard, radish sprout, red cabbage, and beet were purchased from local grocery stores in Jinju, Gyeongsangnam-do, Korea. The plants were freeze-dried and blended. Methanol extraction was processed two times with 25 g of freeze-dried vegetables in 500 ml of methanol in a shaking incubator at 30 °C and 100 rpm. After the extraction process, the samples were evaporated at 30 °C and the extraction yield of each plant was between 9.04 and 47.5% (w/w).
Biofilm formation assessment
Biofilm formation of E. coli O157:H7 was evaluated using a crystal violet assay and a resazurin reduction assay based on Lim et al. (2017). Briefly, the bacterial strain was inoculated in TSB and incubated for 16–18 h at 37 °C. The overnight culture was inoculated in a 96-well plate to approximately 107 CFU/mL in TSB and was then incubated at 37 °C for 2 h to initiate the attachment. After incubation, the culture medium was carefully removed and washed with PBS (phosphate-buffered saline, pH 7.0) to remove non-attached cells. A fresh medium was added and incubated for 24 h to allow biofilm formation. The crystal violet (CV) and resazurin (Res) assays were then performed using the following methods:
Crystal violet assay
After 24 h incubation, the microtiter plate was washed to remove any planktonic cells. One percent of CV solution (bioWORLD, Dublin, Ohio, USA) was added, and the cells were incubated for 30 min at room temperature (RT) under dark. After washing with PBS three times, absolute ethanol was added and incubated for 15 min at RT to destain the CV. The destained solution was transferred to a new 96-well plate, and the absorbance was measured at 595 nm using a SpectraMax® M2 (Molecular Devices®, Sunnyvale, CA, USA).
Resazurin assay
After 24 h incubation, the microtiter plate was washed to remove any planktonic cells. Then, 0.001% (wt/vol) resazurin (Sigma-Aldrich) was added and incubated for 60 min at 37 °C under dark conditions. The fluorescence (λex 570 nm, λem 590 nm) was measured using a SpectraMax® M2 (Molecular Devices®).
Anti-attachment and anti-biofilm activities
The reduction of bacterial attachment and biofilm formation using botanical extracts was assessed based on four different models mimicking bacterial attachment and biofilm development: Anti-attachment (AA), anti-attachment and anti-biofilm (AA + AB), anti-biofilm (AB), and post anti-biofilm treatment (pAB). Anti-attachment (AA) treatment was performed by adding plant extract during the initial 2 h of the attachment process. After the incubation, each well was washed to remove any non-attached bacteria, and the plant extract was added with a fresh medium for anti-biofilm (AB) treatment. Post treatment (pAB) was carried out after completing the biofilm formation of E. coli O157:H7. The plant extract was then added and incubated at 25 °C for 2 h. Afterwards, the wells were washed, and the CV and resazurin assays were performed.
Confocal laser scanning microscopy (CLSM)
Biofilm formation of the bacteria and inhibition of its formation by plant extracts was tested on confocal dish glass bottom 6-well plates (SPL Life-sciences, Gyeonggi-do, Korea), which was carried out as described above. After the incubation, each well was washed three times with sterile PBS, and the bacteria were stained using a Live/Dead BacLight bacterial viability kit (Invitrogen, Grand Island, NY, USA). Staining was carried out for 30 min at RT under dark conditions and each well was washed two times with PBS. The attached bacteria were observed using an Olympus Fluo View FV1000 (Olympus, Tokyo, Japan). Microscopic images were captured at excitation/emission wavelengths of 480/530 (488) nm for SYTO 9 and 520/580 (543) nm for propidium iodide. The images were analyzed using FV10-ASW Viewer software (ver. 4.0; Olympus).
Radical scavenging activity
DPPH (1,1-diphenyl-2-picrylhydrazyl) and ABTS [2,2′-azinobis-(3-ethylbenzo-thiazoline-6-sulfonate)] radical scavenging activities were tested to evaluate the antioxidant capacity of the plants. The DPPH method from Blois (1958) was applied with slight modifications. Fifty μg/mL of the methanolic DPPH solution was mixed with the same volume of the sample and incubated at RT for 10 min under dark conditions. The electron donor activity was measured with the absorbance at 517 nm using a spectrophotometer (Asys Hiteck GmbH, UVM 340, Austria). For the ABTS method, 7 mM ABTS solution was mixed with 2.4 mM potassium persulfate and incubated for 12–16 h at a refrigerated temperature under dark conditions. Water was added to the mixture until the optical density was read to be about 1.5 at 415 nm. Each sample was added to the diluted mixture and incubated for 5 min at RT. The absorbance was measured at 415 nm (Re et al., 1999). Both radical scavenging activities were calculated as the ratio of the absorbance of each sample to the control using deionized water.
HPLC analysis
HPLC was performed on a 1100 series system (Agilent Technologies, Santa Clara, CA, USA). A Capcell Pak C18 column (MGII S5, 4.6 × 250 mm, 5 μm; Shiseido, Tokyo, Japan) set at 30 °C was used. An injection volume of 10 µL was used in each experiment and the detector was PDA at 240 nm. The binary solvent system was composed of water and acetonitrile (ACN) and used at a flow rate of 1 mL/min. The gradient began at 70%, increased to 90% after 15 min, and then to 95% after 25 min.
Statistics
Each experiment was repeated at least twice with duplicate samples, and the statistical analyses were conducted using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The significant difference of each sample was analyzed by a Duncan’s multiple range test and a Student-t test at p < 0.05.
Results and discussion
Antimicrobial activity of cruciferous vegetable extracts
The antimicrobial activity of the cruciferous vegetable extracts was compared in vitro on E. coli O157:H7 and L. monocytogenes. The minimum inhibitory concentrations (MICs) of radish, radish sprouts, mustard greens, beet, red cabbage, kale and radish leaves were compared (data not shown). Kale extract demonstrated the strongest activity on E. coli O157:H7 with MIC of 2 mg/mL, while the MIC of L. monocytogenes was over 16 mg/mL. Mustard green extract inhibited with the MIC of 4 and 8 mg/mL against E. coli O157:H7 and L. monocytogenes, respectively. Radish sprout and radish leave extracts showed MICs of 16 mg/mL against E. coli O157:H7. However, the MICs of the remaining samples were over 16 mg/mL. Overall, in the planktonic cell condition, kale and mustard green extracts were able to inhibit bacterial growth, while other samples required a MIC of 16 mg/mL or over.
Anti-attachment and anti-biofilm activities of cruciferous vegetable extracts
To test whether cruciferous vegetable extracts can inhibit biofilm development, the treatment took place at each stage of biofilm formation. During the initial attachment, many physicochemical interactions occur among bacteria, surface material, and the environment. After the bacteria attach to the surface, a biofilm is irreversibly developed. The samples were treated with the extracts during the initial phase of attachment (anti-attachment), along with the biofilm development (anti-biofilm) and after the biofilm formation (post anti-biofilm) stages. While the CV assay has been the most widely accepted method for quantifying bacterial attachment to various surfaces, qualitative evaluation for the bacterial viability cannot be performed. Therefore, a resazurin assay was jointly carried out to observe the changes in the attached bacterial viability.
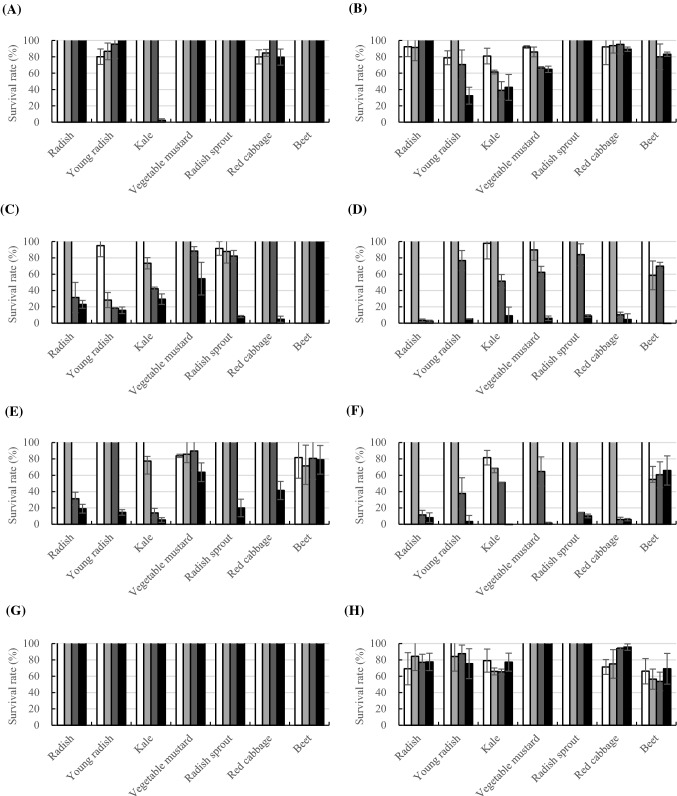
In this study, complete cell removal during the attachment stage (AA) was observed in 8 mg/mL of kale extract, and the viability reduced to 80.9% at 1 mg/mL (Fig. 1). Red cabbage extract reduced the cell amount up to 79.9% at 1 mg/mL. However, in most cases, the initial attachment was not completely controlled when the treatment was stopped after the first 2 h. When the plant extracts were treated after the attachment stage, anti-biofilm activities (AB) were observed at 4 mg/mL in radish and kale extracts with 31.3% and 13.8% of remaining bacteria, respectively. The biofilms of young radish and radish sprout extracts at 8 mg/mL with 14.5% and 20.0% remained attached. Cell viability was efficiently reduced to 11.4%, 37.7%, 14.4%, 5.83%, and 51.5% in radish, young radish, radish sprout, red cabbage, and kale extracts at 4 mg/mL, respectively. Anti-biofilm activities were not affected by whether the plant extracts were treated after (AB) or at the initial attachment stage (AA + AB) during the biofilm development. Young radish extract showed more effective control at lower concentrations by 2 mg/mL with 28.3% during the attachment and biofilm development (AA + AB) than during the biofilm formation (AB). Once the biofilm was formed, none of the plant extracts could remove the bacteria (pAB), while viability was reduced to 53.7% at 4 mg/mL with beet extract to 69.2% at 1 mg/mL with radish extract.
Fig. 1.
Anti-attachment and anti-biofilm activity of Brassicaceae extracts on E. coli O157:H7. Extracts of (A) radish, (B) young radish, (C) kale, (D) vegetable mustard, (E) radish sprout, (F) red cabbage, (G) beet extracts were used at different concentrations (white square: 1 mg/mL, light grey square: 2 mg/mL, grey square: 4 mg/mL, black square: 8 mg/mL). Results represent the survival rate (%) compared to the negative control by mean ± SD of the crystal violet assay (A, C, E, G) and resazurin test (B, D, F, H) experiments and are expressed as relative percent of negative control. Test with different method by AA (A, B) (anti-attachment activity, the extracts were added before cell attachment), AA + AB (C, D) (anti-attachment activity + anti-biofilm activity, the extracts were added before cell attachment and cultivation), AB (E, F) (anti-biofilm activity, the extracts were added after initial attachment) and pAB (G, H) (the extracts were added after biofilm formation)
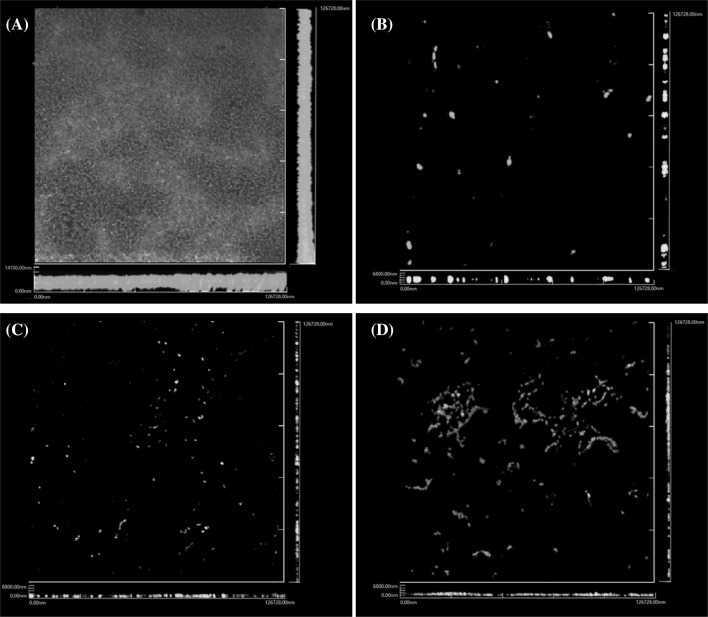
Overall, the anti-attachment and anti-biofilm activities were concentration-dependent and the most effective treatment condition was application during the biofilm development, but not on the preformed biofilm. In respect to the microtiter plate assay, we evaluated the removal of the biofilm by confocal laser scanning microscopy. While initial attachment inhibition and removal of bacteria were not achieved, cruciferous vegetable extracts were able to remove and inhibit the pathogen during biofilm development (Figs. 1, 2).
Fig. 2.
Confocal laser scanning microscopy (CLSM) of E. coli O157:H7 biofilm. E. coli O157:H7 biofilm was formed at 37 °C for 24 h in (A) BHI medium only, or BHI medium supplemented with (B) radish, (C) radish sprouts and (D) kale extracts. The biofilm formation was visualized by CLSM at excitation wavelengths of 488 and 543 nm after staining with live/dead BacLight bacterial viability kit (Invitrogen)
A comparison of CV and RES shows that the biofilm was inactivated, but the dead cells remained attached to the surface. This phenomenon was also observed by Borges et al. (2014a), who have shown that isothiocyanates (ITCs) inhibited the biofilm formation than the removal activities (Borges et al., 2014a). Bacteria can remain in the biofilm in an inactivated condition, killed or metabolically inactivated due to the EPS in the biofilm (Borges et al., 2012). This shows that the removal of bacteria from the biofilm and the killing of bacteria are separate phenomena (Borges et al., 2014b). It is more challenging for the anti-biofilm agents to penetrate and inhibit the already established biofilm than in the initial stage of the cell attachment (Sandasi et al., 2010). We tested whether the plant reduces the motility for biofilm inhibition. However, bacterial motility was not affected (data not shown).
Antioxidant capacity of cruciferous vegetable extracts
As shown in Tables 1 and 2, with the exception of radish extract, the samples displayed a significant scavenging effect against the DPPH radicals in a dose-dependent manner. The DPPH radical scavenging activity of the radish sprout extract was 96.3% at 5 mg/mL, while the radish extract scavenged only 36.8% of DPPH. From the EC50 values of the extracts, which are defined as the concentration of the extract required for 50% radical scavenging ability, higher antioxidant activity was presented with lower EC50 values (Cuvelier et al., 1992). The EC50 of radish extract was 6874 μg/mL, while the radish sprout extract was only 654 μg/mL. Other extracts ranged between 1553 and 2450 μg/mL, and the scavenging effect on the DPPH radical by EC50 values increased in the order of radish sprout, kale, beet, mustard green, red cabbage, radish leaves, and radish extracts. The ABTS radical scavenging activity of the extracts varied with an EC50 value of 604 μg/mL for the radish sprout extract and 5834 μg/mL for radish extract. The radical scavenging activity for ABTS decreased in the order of radish sprout, radish leaves, kale, mustard green, red cabbage, beet, and radish extracts.
Table 1.
DPPH radical scavenging activity of different vegetable extracts
| Concentration (μg/mL) | DPPH (%)a | ||||||
|---|---|---|---|---|---|---|---|
| Radish | Red cabbage | Beet | Mustard leaves | Radish sprout | Kale | Radish leaves | |
| 5000 | 36.8 ± 1.32Ad‡ | 84.2 ± 1.67Ce | 81.2 ± 2.10Be | 80.5 ± 2.87Bd | 96.3 ± 0.26Ed | 97.1 ± 0.59Ed | 90.0 ± 1.1 9De |
| 2000 | 17.5 ± 4.56Ac | 44.9 ± 2.95BCd | 49.3 ± 1.37Cd | 43.9 ± 5.34Bc | 93.6 ± 1.20Ed | 63.9 ± 4.86Dc | 47.1 ± 5.70BCd |
| 1000 | 9.72 ± 3.67Ab | 24.8 ± 3.00Bc | 29.3 ± 2.27BCc | 34.1 ± 4.76CDb | 70.6 ± 5.63Ec | 38.6 ± 4.64Db | 26.0 ± 2.56Bc |
| 500 | 7.49 ± 1.41Aab | 14.5 ± 3.04Bb | 19.0 ± 2.41BCb | 21.1 ± 3.72Ca | 42.9 ± 4.97Db | 23.5 ± 4.47Ca | 19.1 ± 5.84BCb |
| 200 | 4.98 ± 2.19Aa | 6.73 ± 3.15ABa | 9.81 ± 3.74ABCa | 16.3 ± 4.43DEa | 20.0 ± 4.25Ea | 13.9 ± 5.66CDEa | 11.3 ± 6.35BCDa |
| EC50 (μg/mL)b | 6874 ± 173F | 2333 ± 178DE | 2024 ± 82.3C | 2137 ± 217CD | 654 ± 82.4A | 1553 ± 93.1B | 2450 ± 71.7E |
aAll values are mean ± SD (n = 4)
bEC50 values (Effective concentration for 50% radical scavenging activity)
‡Values in a row (A–F) and a column (a–e) sharing the same superscript letter are not significantly different at p < 0.05 by Duncan’s multiple range test
Table 2.
ABTS radical scavenging activity of different vegetable extracts
| Concentration (μg/mL) | ABTS (%)a | ||||||
|---|---|---|---|---|---|---|---|
| Radish | Red cabbage | Beet | Mustard leaves | Radish sprout | Kale | Radish leaves | |
| 5000 | 41.5 ± 1.98Ae‡ | 96.1 ± 0.42Ce | 83.5 ± 1.84Be | 117 ± 2.16Fe | 110 ± 1.07De | 115 ± 3.76EFe | 113 ± 4.07Ed |
| 2000 | 23.0 ± 0.88Ad | 59.6 ± 4.68Cd | 41.4 ± 0.72Bd | 73.3 ± 3.55Dd | 99.8 ± 0.63Fd | 92.6 ± 0.81Ed | 110 ± 1.78Gd |
| 1000 | 15.6 ± 1.18Ac | 35.9 ± 0.61Cc | 26.9 ± 0.85Bc | 43.4 ± 2.74Dc | 73.4 ± 0.63Fc | 59.0 ± 2.37Ec | 70.9 ± 5.57Fc |
| 500 | 11.5 ± 1.08Ab | 23.4 ± 1.08Cb | 18.5 ± 0.99Bb | 23.8 ± 2.30Cb | 44.7 ± 2.95Fb | 35.7 ± 1.51Db | 39.1 ± 3.48Eb |
| 200 | 9.56 ± 1.16Aa | 14.3 ± 0.80Ca | 12.4 ± 0.73Ba | 12.8 ± 2.56BCa | 21.5 ± 2.13Ea | 16.5 ± 0.99 Da | 18.1 ± 1.40 Da |
| EC50 (μg/mL)b | 5834 ± 79.2G | 1646 ± 21.4E | 2597 ± 47.8F | 1264 ± 22.2D | 604 ± 13.7A | 811 ± 37.9C | 672 ± 46.0B |
aAll values are mean ± SD (n = 4)
bEC50 values (Effective concentration for 50% radical scavenging activity)
‡Values in a row (A–G) and a column (a–e) sharing the same superscript letter are not significantly different at p < 0.05 by Duncan’s multiple range test
Polyphenolic composition of cruciferous vegetables
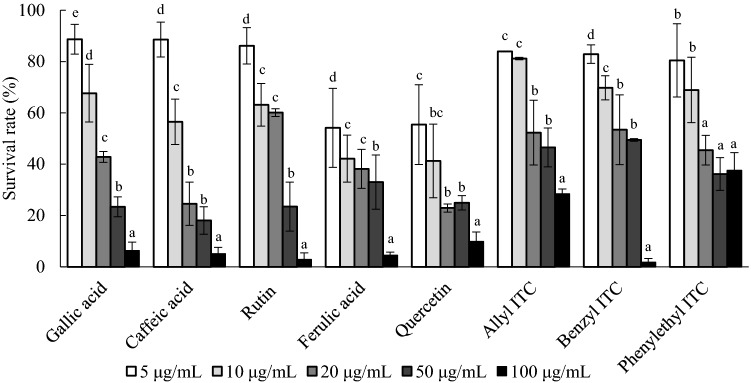
We investigated the presence of polyphenolic compounds, such as gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, rutin, ferulic acid, sinapic acid, diadzein, quercetin, genistein, allyl ITC, benzyle ITC, phenyl ITC, and phenylethyl ITC in radish, radish sprout, and kale (Table 3). Kale contained various phenolic compounds, including chlorogenic acid 818.6 μg/g, caffeic acid 503.2 μg/g, and sinapic acid 376.2 μg/g. Radish sprout showed the presence of chlorogenic acid 663.4 μg/g, rutin 238.1 μg/g, caffeic acid 135.9 μg/g, and others. Rutin was primarily detected in radish with 250.7 μg/g. Phenolic compounds have been shown to be present in rather smaller amounts in the roots than leaves, whereas strong anti-biofilm activities were observed in radish (Gutiérrez and Perez, 2004). For the isothiocyanates, radish only showed 70.4 μg/g of benzyl ITC, while radish sprouts showed the presence of 215.2 μg/g of allyl ITC and 59.8 μg/g of benzyl ITC. Phenylethyl ITC was detected only in 5.7 μg/g of kale. Anti-biofilm activities of gallic acid, caffeic acid, rutin, ferulic acid, quercetin, allyl ITC, benzyl ITC, and phenylethyl ITC detected in the Brassicaceae plants were evaluated on E. coli O157:H7 (Fig. 3). Inhibition of biofilm development was observed to occur in a dose dependent manner for most components. The removal of attached bacteria was most efficient on ferulic acid, followed by rutin and quercetin. Cruciferous vegetables that belong to the family Brassicaceae are rich in various bioactive compounds such as glucosinolate, polyphenols, carotenoids, tocopherols, and ascorbic acid. These compounds are present in a large amount of cruciferous vegetables and have a strong antioxidant capacity that is beneficial to human health (Hagen et al., 2009; Beecher, 1994). They also have a large group of glucosinolates that hydrolyze into ITCs and also have anticancer activities (Plumb et al., 1996; Podsędek, 2007). Inhibition of biofilm formation by ITCs can be derived from the effect of ITCs on damaging the cell membranes, depleting the intracellular ATP, and decreasing the intracellular pH (Turgis et al., 2009; Troncoso et al., 2005).
Table 3.
Polyphenolic compounds and isothiocyanates detected in kale, radish sprouts and radish analyzed by HPLC chromatogram
| Polyphenols | Contents (μg/g of weight) | |||||
|---|---|---|---|---|---|---|
| Kale | Radish sprout | Radish | ||||
| Extractsa | Raw materialsb | Extracts | Raw materials | Extracts | Raw materials | |
| Gallic acid | 74.5 | 0.59 | ND | ND | 29.2 | 0.79 |
| Chlorogenic acid | 818.6 | 6.48 | 663.4 | 10.65 | ND | ND |
| Caffeic acid | 503.2 | 3.98 | 135.9 | 2.18 | ND | ND |
| p-Coumaric acid | 158.3 | 1.25 | ND | ND | ND | ND |
| Rutin | 239.1 | 1.89 | 238.1 | 3.82 | 250.7 | 6.75 |
| Ferulic acid | ND | ND | 57.0 | 0.92 | ND | ND |
| Sinapic acid | 376.2 | 2.98 | ND | ND | ND | ND |
| Daidzein | 74.3 | 0.59 | ND | ND | ND | ND |
| Quercetin | ND | ND | 50.5 | 0.81 | ND | ND |
| Genistein | ND | ND | ND | ND | ND | ND |
| Allyl ITC | ND | ND | 215.2 | 3.45 | ND | ND |
| Benzyl ITC | ND | ND | 59.8 | 0.96 | 70.4 | 1.90 |
| Phenyl ITC | ND | ND | ND | ND | ND | ND |
| Phenylethyl ITC | 5.70 | 0.05 | ND | ND | ND | ND |
| Moisture content (%) | 91.24 | 93.95 | 93.54 | |||
| Methanol extraction yield (%) | 9.04 | 26.53 | 41.70 | |||
aConcentration of polyphenolic compounds from methanolic extracts
bCalculation of each polyphenol and ITC in the vegetables based on moisture contents and methanol extraction yield. The formula is following: Raw materials contents = (1 − moisture content) × methanol extraction yield × content of each extract
Fig. 3.
Anti-biofilm activity of polyphenols on E. coli O157:H7. Polyphenolic compounds of gallic acid, caffeic acid, rutin, ferulic acid, quercetin, allyl ITC, benzyl ITC, phenyl ITC and phenylethyl ITC at different concentrations (white square: 5 ug/mL, light grey square: 10 ug/mL, grey square: 20 ug/mL, dark grey square: 50 ug/mL, black square:100 ug/mL). Results represent the survival rate (%) compared to the negative control by mean ± SD of the crystal violet assay (CV) experiments and are expressed as relative percent of negative control. Different alphabets within the same samples represent the significant difference at p < 0.05
Potential health concerns are related to the contamination of food service facilities and bacterial attachment, biofilm formation, persistence, and transfer of bacteria to other food products. Therefore, the control point is an important factor, where the initial attachment process is reversible, whereas after biofilm development, bacteria are irreversibly bound to the surface. Botanical antimicrobial agents can be a reliable choice for use in the food industry due to their natural ingredients, which are generally considered to be safe for humans. Essential oils (EO) from spices and herbs are the most widely studied antimicrobials, as they have a broad spectrum to control food spoilage bacteria and foodborne pathogens (Swamy et al., 2016). However, a large amount of these extracts is commonly used, which is challenging for food applications due to the undesirable sensory changes by the extracts (Engels et al., 2012). In this study, plant extracts from the family Brassicaceae prevented biofilm formation or inactivated the bacterial cells in preformed biofilms. Polyphenolic compounds and ITCs potentially contributed to the inactivation. However, further investigation of the active compounds may enhance the understanding of the inhibition mechanism.
Acknowledgements
This research was supported by Main Research Program (E0142104-05) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen Si Hu, Email: huwensi@naver.com.
Da Min Nam, Email: ekals9079@gmail.com.
Jin Young Choi, Email: cjy3541@naver.com.
Joo Sung Kim, Email: jskim@kfri.re.kr.
Ok Kyung Koo, Phone: +82-55-772-1441, Email: okoo@gnu.ac.kr.
References
- Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib NE. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014;196:453–472. doi: 10.1007/s00203-014-0983-1. [DOI] [PubMed] [Google Scholar]
- Amrutha B, Sundar K, Shetty PH. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017;111:156–162. doi: 10.1016/j.micpath.2017.08.042. [DOI] [PubMed] [Google Scholar]
- Ayaz FA, Hayırlıoglu-Ayaz S, Alpay-Karaoglu S, Grúz J, Valentová K, Ulrichová J, Strnad M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008;107:19–25. doi: 10.1016/j.foodchem.2007.07.003. [DOI] [Google Scholar]
- Beecher CW. Cancer preventive properties of varieties of Brassica oleracea: a review. Am. J. Clin. Nutr. 1994;59:1166S–1170S. doi: 10.1093/ajcn/59.5.1166S. [DOI] [PubMed] [Google Scholar]
- Beevi SS, Mangamoori LN, Dhand V, Ramakrishna DS. Isothiocyanate profile and selective antibacterial activity of root, stem, and leaf extracts derived from Raphanus sativus L. Foodborne Pathog. Dis. 2009;6:129–136. doi: 10.1089/fpd.2008.0166. [DOI] [PubMed] [Google Scholar]
- Blekkenhorst LC, Bondonno CP, Lewis JR, Woodman RJ, Devine A, Bondonno NP, Lim WH, Zhu K, Beilin LJ, Thompson PL, Prince RL, Hodgson JM. Cruciferous and total vegetable intakes are inversely associated with subclinical atherosclerosis in older adult women. J. Am. Heart Assoc. 2018;7:e008391. doi: 10.1161/JAHA.117.008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Borges A, Saavedra MJ, Simões M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling. 2012;28:755–767. doi: 10.1080/08927014.2012.706751. [DOI] [PubMed] [Google Scholar]
- Borges A, Simões LC, Saavedra MJ, Simões M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014;86:25–33. doi: 10.1016/j.ibiod.2013.01.015. [DOI] [Google Scholar]
- Borges A, Serra S, Cristina Abreu A, Saavedra MJ, Salgado A, Simões M. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling. 2014;30:183–195. doi: 10.1080/08927014.2013.852542. [DOI] [PubMed] [Google Scholar]
- Bos R, Van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol. Rev. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987;41(1):435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship. Biosci. Biotech. Biochem. 1992;56:324–325. doi: 10.1271/bbb.56.324. [DOI] [Google Scholar]
- Engels C, Schieber A, Gänzle MG. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MSn and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012;234:535–542. doi: 10.1007/s00217-012-1669-z. [DOI] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Gutiérrez RMP, Perez RL. Raphanus sativus (Radish): their chemistry and biology. Sci. World J. 2004;4:811–837. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen SF, Borge GIA, Solhaug KA, Bengtsson GB. Effect of cold storage and harvest date on bioactive compounds in curly kale (Brassica oleracea L. var. acephala) Postharvest Biol. Technol. 2009;51:36–42. doi: 10.1016/j.postharvbio.2008.04.001. [DOI] [Google Scholar]
- Kim SJ, Cho AR, Han JJ. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 2013;29:112–120. doi: 10.1016/j.foodcont.2012.05.060. [DOI] [Google Scholar]
- Ko MO, Kim MB, Lim SB. Relationship between chemical structure and antimicrobial activities of isothiocyanates from cruciferous vegetables against oral pathogens. J. Microbiol. Biotechnol. 2016;26:2036–2042. doi: 10.4014/jmb.1606.06008. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho MH, Lee J. 3-Indolylacetonitrile decreases Escherichia coli O157: H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ. Microbiol. 2011;13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- Lim ES, Lee JE, Kim JS, Koo OK. Isolation of indigenous bacteria from a cafeteria kitchen and their biofilm formation and disinfectant susceptibility. LWT-Food Sci. Technol. 2017;77:376–382. doi: 10.1016/j.lwt.2016.11.060. [DOI] [Google Scholar]
- Martínez-Córdova LR, Emerenciano M, Miranda-Baeza A, Martínez-Porchas M. Microbial-based systems for aquaculture of fish and shrimp: an updated review. Rev. Aquacult. 2015;7:131–148. doi: 10.1111/raq.12058. [DOI] [Google Scholar]
- Plumb GW, Lambert N, Chambers SJ, Wanigatunga S, Heaney RK, Plumb JA, Aruoma OI, Halliwell B, Miller NJ, Williamson G. Are whole extracts and purified glucosinolates from cruciferous vegetables antioxidants? Free Radic. Res. 1996;25:75–86. doi: 10.3109/10715769609145657. [DOI] [PubMed] [Google Scholar]
- Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT Food Sci. Technol. 2007;40:1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010;50:30–35. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- Srey S, Jahid IK, Ha SD. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31(2):572–585. doi: 10.1016/j.foodcont.2012.12.001. [DOI] [Google Scholar]
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid. Based Compl. Altern. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso R, Espinoza C, Sánchez-Estrada A, Tiznado ME, García HS. Analysis of the isothiocyanates present in cabbage leaves extract and their potential application to control Alternaria rot in bell peppers. Food Res. Int. 2005;38:701–708. doi: 10.1016/j.foodres.2005.02.004. [DOI] [Google Scholar]
- Turgis M, Han J, Caillet S, Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella typhi. Food Control. 2009;20:1073–1079. doi: 10.1016/j.foodcont.2009.02.001. [DOI] [Google Scholar]
- Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, De Schaetzen MA, Van Huffel X, Imberechts H, Dierick K, Daube G, Saegerman C, De Block J, Dewulf J, Herman L. Antimicrobial resistance in the food chain: a review. Int. J. Environ. Res. Public Health. 2013;10:2643–2669. doi: 10.3390/ijerph10072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MD, Murphy EA, Hurley TG, Hébert JR. Effect of cruciferous vegetable intake on oxidative stress biomarkers: differences by breast cancer status. Cancer Invest. 2017;35:277–287. doi: 10.1080/07357907.2017.1289218. [DOI] [PMC free article] [PubMed] [Google Scholar]