Abstract
Subcritical extraction was optimized to maximize the extraction yield of flavoring compounds from cinnamon. The extracts of cinnamon were obtained at three different levels of extraction temperature (110–130 °C), time (20–60 min), and pressure (20–40 bar). Response surface methodology was used in order to optimize the subcritical extraction process. The suitability of each independent variable in the second-order polynomial regression model was evaluated on the extraction yield and flavoring compound contents. For optimum extraction yield, the optimum temperature, time, and pressure were determined as 130 °C, 60 min, and 26.63 bar, respectively. The contents of the flavoring compound predicted at optimum conditions were as follows: 10.01 mg/g at 110 °C, 20 min, and 20 bar for coumarin; 4.95 mg/g at 110 °C, 20 min, and 32 bar for cinnamic acid; 55 mg/g at 110 °C, 34.62 min, and 37 bar for cinnamldehyde; and 4.92 mg/g at 110.9 °C, 20 min, and 20 bar for cinnamyl alcohol.
Keywords: Subcritical extraction process, Cinnamon, Response surface methodology, Optimization, Regression equation
Introduction
The recent emergence of health-oriented social trends such as well-being and LOHAS (lifestyles of health and sustainability) has occurred due to the increase in life quality, life extension, the entry of a super-aged society, and improved living standards. Under the influence of this trend, the interest in and development of health functional foods have increased. Cinnamon is a medicinal herb made from the tree belonging to the genus Cinnamomum (Lin et al., 2015). It is used as herbal medicine for people who are weak in constitution and has been used to improve gastrointestinal disease and other ailments (Cao and Anderson, 2011; Kim et al., 2006; Qin et al., 2010). In addition, whole or ground cinnamon or extracts obtained from its leaves or bark can be added to food as a spice or for nutraceutical properties, such as antioxidant and preservative properties (Lv et al., 2012; Setthaaraksa et al., 2012; Van Haute et al., 2016).
Subcritical extraction can be used to extract active ingredients such as flavonoids and antioxidants from natural raw materials because it promotes the rate of mass transfer by involving phase transitions, which promotes cell permeability as well as the diffusion of secondary metabolites (He et al., 2012; Plaza et al., 2010). In addition, the application of subcritical extraction has been gradually increased as an alternative technology to conventional organic extraction for solving the problems such as low extraction rate, long extraction time, and remaining toxic organic matter (Khuwijitjaru et al., 2012). Optimum subcritical extraction conditions vary greatly depending on the raw materials. The optimum temperature for the extraction of anthocyanin from red grape skin was found to be 100–110 °C (Corrales et al., 2008). The maximum extraction rate of phenolic compound lignan from flaxseed happened at 160 °C and 5.2 MPa using subcritical extraction (Kanmaz, 2014).
Response surface methodology (RSM) is a combination applying mathematical and statistical techniques together to build an empirical model. RSM was developed to model one or more responses that varied depending on experimental parameters (Box and Wilson, 1951). One of the advantages of RSM is that it can statistically provide acceptable results with fewer experimental runs compared to a full factorial design (Tan et al., 2009). In addition, RSM can determine the combination of multiple factor levels that generate an optimum response. In the response surface analysis, when estimates varying in accordance with the parameters are expressed in a three-dimensional space, the maximum or minimum points on the surface are determined as the optimum conditions (Myers and Montgomery, 1995). The application of RSM to design optimization is aimed at reducing the cost expensive analysis methods and their associated numerical noise. RSM has been applied to establish optimal process conditions for the extraction processes in food-related studies (Kim et al., 2014; Lim et al., 2002; Nikrooz and Zandrahimi, 2011).
The objectives of this study were (1) to identify the effects of extraction conditions for extraction yield and the contents of flavoring compounds such as coumarin, cinnamic acid, cinnamaldehyde, and cinnamyl alcohol extracted from cinnamon using RSM, (2) to identify the regression equations to predict the extraction yield and the contents of flavoring compounds, and (3) to determine the optimum conditions of subcritical extraction for the extraction yield and the contents of flavoring compounds from cinnamon. The optimization parameters tested were extraction temperature, time, and pressure.
Materials and methods
Materials
The cinnamon (cultivated in Korea) used in this study was supplied from Cheonho Bio Co. (Seoul, Korea). The reagents used for the flavoring compound analysis were purchased from Sigma (Sigma Aldrich Co., St. Louis, Mo., USA) as standard products of coumarin, cinnamic acid, cinnamaldehyde, and cinnamyl alcohol.
General assay
The moisture content of the sample was measured using a dry oven method (AOAC, 1995). The samples were dried in a dry oven at 105 °C for 16 h, then cooled in a desiccator for 30 min before the moisture content was measured. According to the semi-micro Kjeldahl method, Se (selenium) mixed catalyst and 95% sulfuric acid solution were added to the sample. Next, the sample solution was disassembled until it became completely transparent. The crude protein content was measured using a Tecator digestion system (2006 Digestor, Foss, Hilleroed, Denmark) and Kjeltec auto sampler system (1035 analyzer, Foss, Hilleroed, Denmark). According to the Soxhlet extraction method, the crude fat was extracted with solvent ether for 16 h using an EAM 9202-03 extraction device (Injae Scientific Co., Seoul, Korea). The ether was then completely evaporated. Finally, after drying for 2 h at 105 °C dryer, the crude fat content was measured.
Subcritical extraction process
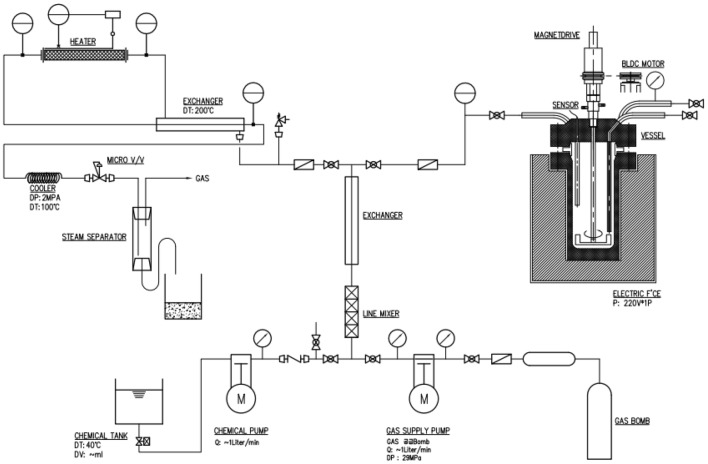
The subcritical extraction system, which was composed to set the optimal extraction conditions for cinnamon, was designed and fabricated by a manufacturing company (Innoway Co., Seoul, Korea). Figure 1 shows the schematic diagram of the subcritical extraction system. Extractions were performed at 13 different extraction conditions by combining three different levels of extraction temperature (°C), time (min), and pressure (bar). Thirty grams of the pulverized cinnamon was mixed with 10 times distilled water (w/v) to the amount of dried cinnamon in the sealed extraction pressure vessel connected to a heat exchanger and gas pump. After setting the specified pressure of the extraction vessel with distilled water and air using a pressure pump, the extraction vessel was heated to specified extraction temperature by an electric heating chamber around the vessel. The electric heating chamber was controlled using a temperature sensor equipped to the vessel. The extraction system was rinsed between extractions in order to overcome any extract carryover. The extraction condition of each experimental run is shown in Table 1. The extracts were centrifuged at 11,000×g and 4 °C for 5 min, then filtered through a filter paper (Whatman No. 4, Sigma-Aldrich, Maidstone, England). The supernatant obtained was lyophilized and used as the sample for analysis.
Fig. 1.
The schematic diagram of the subcritical extraction system
Table 1.
Box–Behnken design and the response for the extraction yield and the content of cormarin, cinnmic acid, cinnamaldehyde, and cinnamyl alcohol from Cinnamomum cassia blume by subcritical extraction process
| Run no. | Independent variables | Dependent variables (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coded values | Uncoded values | ||||||||||
| X1 | X2 | X3 | X1 (°C) | X2 (min) | X3 (bar) | Y1 | Y2 | Y3 | Y4 | Y5 | |
| 1 | − 1 | − 1 | 0 | 110 | 20 | 40 | 7.61 | 15.02 | 4.91 | 53.73 | 4.49 |
| 2 | 1 | − 1 | 0 | 130 | 20 | 40 | 12.79 | 8.34 | 2.86 | 23.00 | 3.32 |
| 3 | − 1 | 1 | 0 | 110 | 60 | 40 | 10.22 | 12.87 | 4.14 | 45.23 | 3.92 |
| 4 | 1 | 1 | 0 | 130 | 60 | 40 | 15.11 | 7.54 | 2.74 | 15.75 | 3.24 |
| 5 | − 1 | 0 | − 1 | 110 | 40 | 20 | 9.35 | 15.53 | 4.62 | 53.68 | 4.29 |
| 6 | 1 | 0 | − 1 | 130 | 40 | 20 | 13.99 | 7.84 | 2.68 | 18.29 | 3.15 |
| 7 | − 1 | 0 | 1 | 110 | 40 | 60 | 8.83 | 13.78 | 4.46 | 41.49 | 4.05 |
| 8 | 1 | 0 | 1 | 130 | 40 | 60 | 13.45 | 6.80 | 2.50 | 17.46 | 3.04 |
| 9 | 0 | − 1 | − 1 | 120 | 20 | 20 | 9.79 | 12.29 | 3.65 | 37.89 | 3.69 |
| 10 | 0 | 1 | − 1 | 120 | 60 | 20 | 11.70 | 8.88 | 3.13 | 19.91 | 3.36 |
| 11 | 0 | − 1 | 1 | 120 | 20 | 60 | 11.64 | 9.96 | 3.27 | 25.67 | 3.37 |
| 12 | 0 | 1 | 1 | 120 | 60 | 60 | 11.78 | 9.80 | 3.35 | 25.71 | 3.47 |
| 13 | 0 | 0 | 0 | 120 | 40 | 40 | 13.03 | 9.34 | 3.59 | 38.50 | 2.92 |
| 14 | 0 | 0 | 0 | 120 | 40 | 40 | 11.52 | 9.80 | 3.99 | 35.05 | 2.91 |
| 15 | 0 | 0 | 0 | 120 | 40 | 40 | 10.62 | 8.98 | 3.83 | 40.66 | 2.86 |
X1 = extraction temperature (°C), X2 = extraction time (min), X3 = extraction pressure (bar), Y1 = extraction yield (%), Y2 = content of coumarin (%), Y3 = content of cinnamic acid (%), Y4 = content of cinnamaldehyde (%), Y5 = content of cinnamyl alcohol (%)
Measurement of extraction yield and flavoring compound analysis
The extraction yield was determined by dividing the weight of the lyophilized extract by the dry weight of the sample and it is expressed as a percentage. The lyophilized cinnamon extract was dissolved in distilled water to a concentration of 1 mg/mL, then sonicated for 60 min. After filtering through a 0.45 μm PVDF syringe filter, it was used as extract sample for flavoring compound analysis. The standard products of coumarin, cinnamic acid, cinnamaldehyde, and cinnamyl alcohol were dissolved in methanol to a concentration of 1 mg/mL and diluted to 0, 20, 40, 60, and 80 μg/mL. The diluted solution was then filtered through a 0.45 μm PVDF syringe filter and used for the analysis. As flavoring compounds, four kinds of coumarin, cinnamic acid, cinnamaldehyde, and cinnamyl alcohol were analyzed through HPLC (Agilent Technologies 1260 infinity, Santa Clara, CA, USA). The analytical column used was ZORBAX Eclipse XDB-C18 (250 mm × 4 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA), while acetonitrile (A) and 0.02% aqueous acetic acid in water (B) were used as mobile phase. The gradient program for the HPLC was as follows: 90–50% B for 0–60 min, 50–90% B for 60–65 min, and 90% B for 65–70 min, and the flow rate was 1 mL/min. The injection volume was 20 μL, and the column temperature was maintained at 20 °C of the column temperature. Coumarin, cinnamic acid, and cinnamaldehyde were all detected at 280 nm and cinnamyl alcohol was detected at 250 nm.
Experiment design using response surface methodology
Response surface methodology (RSM) was used to optimize the subcritical extraction process for cinnamon. The optimum extraction conditions were determined using Box–Behnken design (BBD) (Ferreira et al., 2007), which is an experimental design to fit the second-order response surface based on the structure of balanced incomplete block designs (Lim et al., 2002; Myers and Montgomery, 1995; Wang et al., 2008). For the experimental design of the three factors and three levels, extraction temperature (X1), time (X2), and pressure (X3) were encoded as independent variables affecting the extraction process, while the dependent variables were extraction yield and the contents of the four flavoring compounds of coumarin, cinnamic acid, cinnamaldehyde, and cinnamyl alcohol. Table 1 shows the symbols and levels of this RSM design. The BBD consisted of 12 different levels of the independent variables (Runs No. 1–12) and three central point runs (13–15). The central point runs were fitted with a second-order response surface and used to provide a measurement of process stability and inherent variability for the analysis of the experimental error (Nikrooz and Zandrahimi, 2011). RSM was performed using MiniTab (MiniTab 16, Minitab Inc., State college, PA, USA). All experiments were repeated three times, and the mean values were used for regression analysis. Polynomial regression modeling informs a mathematical relationship between the dependent variables and independent variables. The suitability of each independent variable in the reaction model was evaluated on the extraction yield and flavoring compound contents in the extraction process. The independent variable (Xn) and the dependent response (Yn) are shown in the second-order polynomial regression equation below, and bn are the fixed constant and regression coefficients of the equation.
The statistical significance level was set at p = 0.05.
Statistical analysis
The experimental results in Table 1 were analyzed using SPSS program (IBM SPSS 22 for windows, SPSS INC., Chicago, IL, USA). Data was analyzed by ANOVA and Duncan’s multiple range test, which was used to resolve the difference among treatment means. A value of p < 0.05 was used to indicate significant difference.
Results and discussion
The general components of the cinnamon used in this study were 10.86 in moisture, 3.52 in crude protein, 2.22 in crude fat, 4.49 in crude protein, and 78.91% in carbohydrate. The general components of cinnamon were mostly composed of carbohydrates.
RSM analysis for extraction yield
The extraction yield (Y1) of cinnamon subcritical extract was measured in 15 runs according to the Box–Behnken design, and the results ranging from 7.61 to 15.11% are shown in Table 1. The highest extraction yield was obtained at 130 °C and 40 bar for 60 min, and the lowest yield was obtained at 110 °C and 40 bar for 20 min. The extraction yield increased at the same extraction time and pressure (run 1–4 and 6–8) as extraction temperature increased from 110 to 130 °C (Table 1). Although it was not significantly different at most extraction conditions with the increase of extraction pressure, it significantly increased at 120 °C for 20 min (run 9 and 11) as the pressure increased from 20 to 60 bar (p < 0.05). Kim et al. (2014) reported that the extraction yield of kirenol increased slightly as the pressure increased from 100 to 500 MPa using high hydrostatic pressure (HHP) extraction; however, this difference was not significant at the 5% level. They believed that this result may have been due to other factors such as solvent type and feed-to-solvent ration, affecting the extraction of kirenol. However, Bi et al. (2009) reported that the solubility of salidroside from Rhodiola sachalinensis was improved as the pressure increased. The extraction yield increased at the conditions of 110 °C and 40 bar (run 1 and 3), 130 °C and 40 bar (run 2 and 4), and 120 °C and 20 bar (run 9 and 10) as the extraction time increased from 20 to 60 min (Table 1).
The regression equation for the extraction yield is shown in Table 2. The coefficient of determination (R2), which indicates the general validity and accuracy of the polynomial regression equation, was 0.912. In addition, Table 3 shows the coefficients and significance of each term of the regression equations so as to indicate the fitness of the model through an essential part of the data analysis. As a result, the first-order term of extraction temperature was significant, whereas the quadratic terms and reciprocal terms were not significant and relatively high (p > 0.05). It is thought that the independent variables did not interact with each other. The p value of extraction temperature among the first-order terms was 0.001 (Table 3). This result shows that this term was the most important in the reaction model of extraction yield. The lack of fit indicates that the p value of the model would be less than 0.05 if the function relationship between the dependent response and independent variables is not adequately explained in the response model. The p value of the correct model is greater than 0.05. It is thus considered that the regression equation of the extraction yield was appropriate because the p value of this equation was 0.718, as obtained in the variance analysis.
Table 2.
Quadratic polynomial equations of extraction yield and the contents of cormarin, cinnmic acid, cinnamaldehyde, and cinnamyl alcohol from Cinnamomum cassia blume by subcritical extraction process
| Response | Quadratic polynomial equation | R2 | Lack of fit (p value) |
|---|---|---|---|
| Extraction yield (%) | Y1 = 11.7233 + 2.4163X1 + 0.8725X2 + 0.1088X3 − 0.0567X21 − 0.2342X22 − 0.2617X23 − 0.0725X1X2 − 0.0050X1X3 − 0.4425X2X3 | 0.912 | 0.718 |
| Content of coumarin (%) | Y2 = 9.3733 − 3.3350X1 − 0.8150X2 − 0.5250X3 + 1.1621X21 + 0.4071X22 + 0.4521X23 + 0.3375X1X2 + 0.1775X1X3 + 0.8125X2X3 | 0.986 | 0.316 |
| Content of cinnaic acid (%) | Y3 = 3.80333 − 0.91875X1 − 0.16625X2 − 0.06250X3 + 0.03708X21 − 0.17792X22 − 0.27542X23 + 0.16250X1X2 − 0.00500X1X3 + 0.15000X2X3 | 0.982 | 0.744 |
| Content of cinnamaldehyde (%) | Y4 = 38.0700 − 14.9538X1 − 4.2113X2 − 2.4300X3 + 0.8962X21 − 4.5388X22 − 6.2362X23 + 0.3125X1X2 + 2.8400X1X3 + 4.5050X2X3 | 0.990 | 0.855 |
| Content of cinnamyl alcohol (%) | Y5 = 2.89667 − 0.50000X1 − 0.11000X2 − 0.070000X3 + 0.50292X21 + 0.34292X22 + 0.23292X23 + 0.12250X1X2 + 0.03250X1X3 + 0.10750X2X3 | 0.990 | 0.810 |
X1 = extraction temperature (°C), X2 = extraction time (min), X3 = extraction pressure (bar), Y1 = extraction yield (%), Y2 = content of coumarin (%), Y3 = content of Cinnamic acid (%), Y4 = content of cinnamaldehyde (%), Y5 = content of cinnamyl alcohol (%)
Table 3.
p value of each parameter in the quadratic polynomial equations of extraction yield and the contents of cormarin, cinnmic acid, cinnamaldehyde, and cinnamyl alcohol from Cinnamomum cassia blume by subcritical extraction process
| Parameter | p value of each parameter in quadratic polynomial eqations | ||||
|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | |
| Constant | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| X1 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| X2 | 0.060 | 0.009 | 0.036 | 0.002 | 0.002 |
| X3 | 0.775 | 0.043 | 0.332 | 0.022 | 0.022 |
| X21 | 0.919 | 0.010 | 0.683 | 0.415 | 0.415 |
| X22 | 0.678 | 0.214 | 0.093 | 0.009 | 0.009 |
| X23 | 0.643 | 0.175 | 0.024 | 0.002 | 0.002 |
| X1X2 | 0.893 | 0.274 | 0.106 | 0.778 | 0.778 |
| X1X3 | 0.993 | 0.547 | 0.954 | 0.043 | 0.043 |
| X2X3 | 0.425 | 0.032 | 0.128 | 0.008 | 0.008 |
X1 = extraction temperature (°C), X2 = extraction time (min), X3 = extraction pressure (bar), Y1 = extraction yield (%), Y2 = content of coumarin (%), Y3 = content of Cinnamic acid (%), Y4 = content of cinnamaldehyde (%), Y5 = content of cinnamyl alcohol (%)
RSM analysis for flavoring compounds using HPLC
The results of quantitative analysis of coumarin, cinnamic acid, cinnamaldehyde, and cinammyl alcohol in cinnamon subcritical extracts at the extraction conditions of 15 runs are shown in Table 1. The highest content of coumarin was 15.53 mg/g extracted at 110 °C and 20 bar for 40 min, and the lowest content was 6.80 mg/g at 130 °C and 60 bar for 40 min. The content of coumarin decreased at the same extraction time and pressure (run 1–8) as the extraction temperature increased from 110 to 130 °C; it also decreased at the conditions of 110 °C and 40 bar (run 1 and 3), 130 °C and 40 bar (run 2 and 4), and 120 °C and 20 bar (run 9 and 10) as the extraction time increased from 20 to 60 min. In addition, it decreased at the same extraction temperature and time (run 5–7) as the extraction pressure increased from 20 to 60 bar. The highest content of cinnamic acid obtained was 4.91 mg/g extracted at 110 °C and 40 bar for 20 min, and the lowest one was 2.50 mg/g at 130 °C and 60 bar for 40 min. The content of cinnamic acid decreased at the same extraction time and pressure (run 1–8) as the extraction temperature increased from 110 to 130 °C. However, it was not significantly different at most extraction conditions with the increase of extraction time and pressure (p > 0.05). The highest content of cinnamaldehyde was 53.73 mg/g extracted at 110 °C and 40 bar for 20 min, and the lowest content was 15.75 mg/g at 130 °C and 40 bar for 60 min. The content of cinnamaldehyde decreased to more than half at the same extraction time and pressure (run 1–8) as the extraction temperature increased from 110 to 130 °C; it also decreased at the conditions of 110 °C and 40 bar (run 1 and 3), 130 °C and 40 bar (run 2 and 4), and 120 °C and 20 bar (run 9 and 10) as the extraction time increased from 20 to 60 min. The highest content of cinnamyl alcohol obtained was 4.49 mg/g extracted at 110 °C and 40 bar for 20 min, and the lowest one was 2.86 mg/g at 120 °C and 40 bar for 40 min. The content of cinnamyl alcohol decreased at the same extraction time and pressure (run 1–8) as the extraction temperature increased from 110 to 130 °C. However, it was not significantly different at most extraction conditions with the increase of extraction time and pressure (p > 0.05). Based on these results, it is considered that the contents of flavoring compounds decreased with increasing extraction temperatures over 110 °C due to evaporation during extraction.
The regression equation for the amount of coumarin in the sample extracted at various extraction conditions is shown in Table 2. This equation fits well because its coefficient of determination was 0.986. The significance for the amount of coumarin in the first-order terms was shown for this equation, whereas that in the quadratic terms and the reciprocal terms were shown not to be statistically significant (p > 0.05). Among the first-order terms, the p values of extraction temperature and time were 0.000 and 0.009, respectively (Table 3), indicating that they have great importance in the regression equation for the amount of coumarin. The p value for the lack of fit was 0.316, which indicated that the model was appropriate.
The regression equation for the amount of cinnamic acid in the sample extracted at various extraction conditions is shown in Table 2. This equation fits well because its coefficient of determination was 0.982. In addition, the statistical significance for the amount of cinnamic acid in the first-order terms and quadratic terms was shown for this equation, whereas the reciprocal terms were shown not to be statistically significant (p > 0.05). The p value of the extraction temperature among the first-order terms was 0.001 (Table 3), showing that this term was the most important in the regression equation for the amount of cinnamic acid. The lack of fit had a p value of 0.744, which indicated that the model was appropriate.
The regression equation for the amount of cinnamaldehyde in the sample extracted at various extraction conditions is shown in Table 2. The coefficient of determination was 0.982. In addition, the significance for the amount of cinnamaldehyde in all of the terms except for the quadratic term of extraction temperature and the reciprocal term between extraction temperature and time, was shown for this equation statistically (p < 0.05). The first-order terms of the extract temperature and time had p values of 0.000 and 0.002 (Table 3), respectively, indicating a great importance in the regression equation of cinnamaldehyde. The p value for the lack of fit was 0.855, which indicated that the model was appropriate.
The regression equation for the amount of cinnamyl alcohol in the sample extracted at various extraction conditions is shown in Table 2. This equation fits well because its coefficient of determination was 0.990. The first-order term and the reciprocal terms of the extract pressure were not statistically significant (p > 0.05). The first-order terms of the extract temperature had a p value of less than 0.0001 (Table 3), indicating a great importance in the regression equation of cinnamyl alcohol. The p value for the lack of fit was 0.810, which indicated that the model was appropriate.
Optimization of extraction condition using RSM
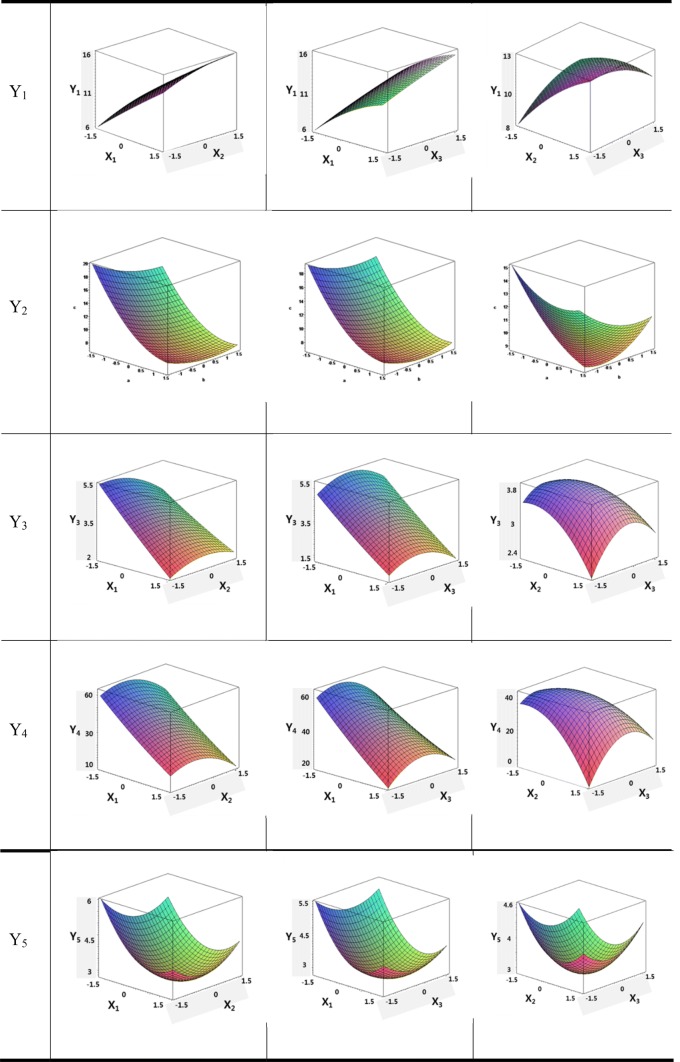
The optimal variables of extraction temperature, time, and pressure were determined by the regression equation of the reaction model using MiniTaP. The optimized values and the interaction between the variables were analyzed and expressed as a three-dimensional surface response graph, shown in Fig. 2. The extraction yield (Y1) increased with increasing extraction time and temperature, while it decreased with increasing extraction pressure. For the optimum extraction yield using the regression equation, the optimum temperature, time, and pressure were determined as 130 °C, 60 min, and 26.628 bar, respectively. The extraction yield was predicted to be 14.76% in this optimum extraction condition. In the case of coumarin (Y2), the content of coumarin decreased as the extraction time, temperature, and pressure increased. The optimum conditions using the regression equation were found to be 110 °C, 20 min, and 20 bar. The content of coumarin was predicted to be 10.01 mg/g at the optimum condition. The content of cinnamic acid (Y3) decreased with increasing extraction time and temperature. In the case of extraction pressure, the content tended to increase up to 32 bar, and then decreased. The optimal temperature, time, and pressure using the regression equation were determined as 110 °C, 20 min, and 32 bar, respectively. The content of cinnamic acid was predicted to be 4.95 mg/g in this optimum extraction condition. The content of cinnamaldehyde (Y4) tended to decrease as the extraction time increased. However, it did first increase to a certain range before decreasing. The optimum temperature, time, and pressure using the regression equation, were determined as 110 °C, 34.62 min, and 37 bar, respectively. The content of cinnamaldehyde was predicted to be 55 mg/g in this optimum extraction condition. The content of cinnamyl alcohol (Y5) showed a tendency to decrease, then showed a tendency to increase in a certain range as the extraction time, temperature, and pressure increased. The optimum conditions using the regression equation were determined to be 110.9 °C, 20 min, and 20 bar, respectively. The content of cinnamyl alcohol was predicted to be 4.92 mg/g in this optimum condition.
Fig. 2.
Response surface plots for the effects of the extraction temperature (°C, X1), time (min, X2), and pressure (bar, X3) on the extraction yield (%, Y1) and the contents of coumarin (mg/g, Y2), cinnamic acid (mg/g, Y3), cinnamaldehyde (mg/g, Y4), and cinnamyl alcohol (mg/g, Y5) from from Cinnamomum cassia blume by subcritical extraction process
In conclusion, cinnamon is used as an herbal medicine and a food ingredient due to its functional properties. The subcritical extraction method was optimized in order to maximize the extraction yield and flavoring compounds from cinnamon (Cinnamomum cassia blume). Four flavoring compounds of the extracts were analyzed by HPLC. Response surface methodology, which applied Box–Behnken design, was used in order to optimize the subcritical extraction process for cinnamon. The contents of the flavoring compound predicted at optimum conditions were as follows: 10.01 mg/g at 110 °C, 20 min, and 20 bar for coumarin; 4.95 mg/g at 110 °C, 20 min, and 32 bar for cinnamic acid; 55 mg/g at 110 °C, 34.62 min, and 37 bar for cinnamldehyde; and 4.92 mg/g at 110.9 °C, 20 min, and 20 bar for cinnamyl alcohol. For the optimum extraction yield using the regression equation, the optimum temperature, time, and pressure were determined as 130 °C, 60 min, and 26.63 bar, respectively. It was validated that the regression equations for extraction yield and four flavoring compounds were accurate according to the high values of the determination coefficients over 0.91. Under the influence of health-oriented social trends, the extraction process developed in the study will be used for encouragement of small businesses in the field of food and agriculture.
Acknowledgements
This research was supported by the research grant of the Ministry for Agriculture, Food and Rural Affairs for the 2016 joint research and development of industry-academy-research cooperation technology. This support is appreciated.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaeyoon Cha, Email: chajaeyoon@dau.ac.kr.
Chong-Tai Kim, Email: ctkim@ieasthill.com.
Tae-Eun Kim, Email: tekim@kfri.re.kr.
Yong-Jin Cho, Phone: +82-63-219-9136, Email: yjcho@kfri.re.kr.
References
- AOAC. Official Method of Analysis of the AOAC Intl. 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
- Bi HM, Zhang SQ, Liu CJ, Wang CZ. High hydrostatic pressure extraction of salidroside from Rhodiola sachalinensis. J. Food Process Eng. 2009;32:53–63. doi: 10.1111/j.1745-4530.2007.00202.x. [DOI] [Google Scholar]
- Box GEP, Wilson KG. On the experimental attainment of optimum conditions. J. R. Stat. Soc. 1951;13:1–45. [Google Scholar]
- Cao H, Anderson RA. Cinnamon polyphenol extract regulates tristetraprolin and related gene expression in mouse adipocytes. J. Agric. Food Chem. 2011;59:2739–2744. doi: 10.1021/jf103527x. [DOI] [PubMed] [Google Scholar]
- Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B. Extraction of anthocyanins from graph by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. 2008;9:85–91. doi: 10.1016/j.ifset.2007.06.002. [DOI] [Google Scholar]
- Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL. Box–Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597:176–189. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- He L, Zhang X, Xu H, Xu C, Yuan F, Knez Ž, Novak Z, Gao Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC-ABTS + assay. Food Bioprod. Process. 2012;90:215–223. doi: 10.1016/j.fbp.2011.03.003. [DOI] [Google Scholar]
- Kanmaz EÖ. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE) Eur. Food Res. Technol. 2014;238:85–91. doi: 10.1007/s00217-013-2088-5. [DOI] [Google Scholar]
- Khuwijitjaru P, Sayputikasikorn N, Samuhasaneetoo S, Penroj P, Siriwongwilaichat P, Adachi S. Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum) J. Oleo Sci. 2012;61:349–355. doi: 10.5650/jos.61.349. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J. Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Kim MB, Park JE, Woo SW, Lim SB, Hwang JK. Optimization of high hydrostatic pressure process for the extraction of kirenol from Siegesbeckia orientalis L. using response surface methodology. Food Sci. Biotechnol. 2014;23:731–738. doi: 10.1007/s10068-014-0099-z. [DOI] [Google Scholar]
- Lim SB, Jung SK, Jwa MK. Extraction of valuable substances from citrus peel by supercritical carbon dioxide. Food Sci. Biotchnol. 2002;11:644–648. [Google Scholar]
- Lin GM, Chen YH, Yen PL, Chang ST. Antihyperglycemic and antioxidant activities of twig extract from Cinamomum osmophloeum. J. Trad. Comp. Med. 2015;6:281–288. doi: 10.1016/j.jtcme.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Huang H, Yu L, Whent M, Niu Y, Shi H, Wang TTY, Luthria D, Charles D, Yu LL. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012;132:1442–1450. doi: 10.1016/j.foodchem.2011.11.135. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology. New York, NY, USA: John Wiley & Sons Inc; 1995. pp. 208–350. [Google Scholar]
- Nikrooz B, Zandrahimi M. Optimization of process variables and corrosion properties of a multi layer silica sol gel coating on AZ91D using the Box–Behnken design. J. Sol-Gel Sci. Techn. 2011;59:640–649. doi: 10.1007/s10971-011-2539-z. [DOI] [Google Scholar]
- Plaza M, Amigo-Benavent M, del Castillo MD, Ibánez E, Herrero M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010;43:1123–1129. doi: 10.1016/j.foodres.2010.02.005. [DOI] [Google Scholar]
- Qin B, Panickar KS, Anderson RA. Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J. Diabetes Sci. Tech. 2010;4:685–693. doi: 10.1177/193229681000400324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setthaaraksa S, Jongjareonrak A, Hmadhlu P, Chansuwan W, Siripongvutikorn S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012;19:1581–1587. [Google Scholar]
- Tan CH, Ghzali HM, Kuntom A, Tan CP, Ariffin AA. Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chem. 2009;113:645–650. doi: 10.1016/j.foodchem.2008.07.052. [DOI] [Google Scholar]
- Van Haute S, Raes K, Van der Meeren P, Sampers I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control. 2016;68:30–39. doi: 10.1016/j.foodcont.2016.03.025. [DOI] [Google Scholar]
- Wang L, Yang B, Du X, Yang Y, Liu J. Optimization of conditions for extraction of acid-soluble collagen from grass carp (Ctenopharyngodon idella) by response surface methodology. Innov. Food Sci. Emerg. 2008;9:604–607. doi: 10.1016/j.ifset.2008.03.001. [DOI] [Google Scholar]