Abstract
The current study compared the physicochemical properties, antioxidant compounds and activities, of unripe and ripe fruits of three cultivars (Seolhyang, Janghee, and Maehyang) of strawberries grown in Korea. As fruits matured, their soluble solids content increased and their organic acid content decreased. Total phenolic content (TPC) and total flavonoid content (TFC) did not differ between Seolhyang and Maehyang fruits, regardless of maturity, whereas unripe Janghee fruits showed higher TPC and TFC than ripe fruits. Total anthocyanin content was higher in ripe fruits than in unripe fruits. For total antioxidant activity, ripe and unripe Seolhyang fruits showed no differences, whereas unripe Janghee fruits showed significantly higher activity than ripe fruits. TPC and TFC were highly correlated, as were DPPH and ABTS radical scavenging activities. Thus, antioxidant contents and total antioxidant activities differed with variety and fruit ripeness at harvest. Unripe fruits show strong potential for use in functional food manufacturing.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00610-y) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant, Cultivar, Ellagic acid, Ripening stage, Strawberry
Introduction
Strawberries (Fragaria × ananassa Duch.) are perennials in the family Rosaceae that show excellent aroma and color. The varieties of strawberries grown in Korea prior to 2000 consisted mostly of Japanese varieties, such as the Janghee and Redpearl cultivars. Recently, the Korean government led a strawberry cultivar development program to address royalty issues, which has resulted in over 72% of strawberries grown in Korea being replaced by domestic varieties, such as the Seolhyang and Maehyang cultivars (Choi et al., 2013).
Strawberries are rich in nutrients and bioactive compounds, and they have long been known to provide health benefits such as fatigue recovery, detoxification, and better blood circulation (Kim and Shin, 2015; Naemura et al., 2005). With the increasing interest in phytochemicals in recent decades, many individuals have increased their consumption of fruits and vegetables to prevent cancer and aging. Strawberries have very high antioxidant activities, and have been reported to inhibit production of free radicals, such as peroxyl and superoxide radicals (Wang and Lin, 2000). Formation of free radicals can be induced by smoking, stress, and environmental hormones, and such free radicals can induce oxidative stress that can cause cancer, cardiovascular diseases, obesity, aging, and Alzheimer’s disease (AD). According to Singh et al. (2008), reactive oxygen species (ROS) cause neurodegenerative diseases in the human brain, including AD. Despite extensive research, the causes of AD have not yet been completely identified. However, a decrease in the level of acetylcholine, a neurotransmitter, is known to induce AD, and the enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are known to play a primary role in causing AD by reducing the level of acetylcholine (Adsersen et al., 2007; Orhan et al., 2004). Phenolic compounds contained in foods have an antioxidant effect, and as such, they are expected to have a significant impact on improving memory function compromised by AD; this potential has been suggested by the findings of several studies (Kim et al., 2005; Orhan et al., 2004). AChE and BChE have been performed by Korean research groups, including: comparative analysis of the AChE inhibitory activities of edible plant extracts, including Angelica gigas and Siberian ginger (Jung et al., 2012a; 2012b); analysis of the AChE inhibitory effects of green, puer, oolong, and black teas sold in Korea (Jeong et al., 2009); analysis of the AChE inhibitory effects of ginger, turmeric, and garlic (Jung et al., 2012a; 2012b). Although these studies analyzed the AChE inhibitory effects of edible plants, most studies have been limited to medicinal plants. Studies of the AChE and BChE inhibitory activities of fruits or vegetables grown in Korea are limited; comparative analyses based on different varieties or ripeness are also lacking. Furthermore, studies of the BChE inhibitory effects of edible plants grown in Korea are very rare.
Accordingly, the objective of the present study was to investigate the physicochemical properties, antioxidant compositions and activities, and anti-dementia activities of strawberries based on ripeness and cultivar.
Materials and methods
Strawberry samples
The strawberries used in the present study consisted of three cultivars, Seolhyang, Janghee, and Maehyang grown in a greenhouse in Gyeonggi-Province. Unripe (50% of fruit surface turns into red color) and ripe (100% of fruit surface turns into deep red color) fruits were harvested and sorted to eliminate damaged fruits. All fruits used in the experiment had a uniform size.
Evaluation of physicochemical qualities
To measure color change in the fruits, a colorimeter (Chroma Meter CR-400, Minolta, Japan) was used to determine the Hunter Lab values. The Chroma Meter was calibrated regularly using the white calibration plate (Y = 87.8, x = 0.3156, y = 0.3229). For each measurement, ten strawberries were measured three times and the mean value was calculated. Firmness of fruits was measured using a fruit hardness tester (FHM-1, Demetra, Tokyo, Japan), which measured the maximum resistance at the moment a 5.0-mm diameter probe penetrated the fruit from the side, expressed in Newton (N). The soluble solids content of the strawberry juice was measured three times using a digital glucose refractometer (PAL-1, Atago, Tokyo, Japan).
Sugar compositions
The quantification of sugars was performed using high-performance liquid chromatography (HPLC) (Kim and Shin, 2015). Strawberry extracts were diluted ten-fold in distilled water and then the samples were filtered through a 0.45 μm syringe filter. HPLC analyses were carried out using an Ultimate 3000 Dionex HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a Refractomax 520 refractive index (RI) detector (ERC Inc., Saitama, Japan). For individual sugar separation, a Carbohydrate high-performance column (4.6 mm i.d. × 250 mm, 4 μm; Waters Corp., Dublin, Ireland) was used at 30 °C. The mobile phase was 79% acetonitrile in distilled water with a 1.0 mL/min flow rate. The determination was performed three times for each sample and the results were shown as mg/100 g of fresh weight (FW).
Organic acid compositions
Organic acids were analyzed using a method described by Kim and Shin (2015) with some modifications. Agilent 1100 series (Agilent, Palo Alto, CA, USA) with a diode array detector was used for the individual organic acid analysis. The extract was diluted ten-fold in distilled water, and the samples were filtered through a 0.45 μm syringe filter, and the samples were run through a Prevail organic acid column (4.6 mm i.d. × 250 mm, 5 μm; Alltech Associates Inc., Deerfield, IL, USA) at 25 °C. The HPLC mobile phase was 25 mM KH2PO4 adjusted to pH 2.1 using H3PO4 and the diode array detector was set at 210 nm with a 1.0 mL/min flow rate. An injection volume of 10 μL was used for the analysis. To calculate the calibration curves, three different points were obtained using standard solutions. The results are expressed in mg/100 g of FW.
Extraction for measurement of antioxidant activities
After slicing, the strawberries were frozen with liquid nitrogen. After applying 80% acetone to 40 g of frozen strawberries, a commercial blender (HR20011, Philips, Carson, NV, USA) was used for 3 min of homogenization. The homogenized solution was filtered with a Whatman #1 paper filter and the filtered solution was concentrated in a rotary evaporator (N-1000, Eyela, Tokyo, Japan) for 30 min at 45 °C. The concentrated samples were stored at − 20 °C and subsequently used for measurement of TAC, TFC, TPC, ellagic acid content, antioxidant activity, and AChE and BChE inhibitory activities.
Total anthocyanin analysis
The TAC of the strawberry extract was determined using a modified pH differential method (Boyles and Wrolstad, 1993; Meyers et al., 2003). A spectrophotometer (Optizen POP, Mecasys, Daejeon, Korea) was used to measure absorbance at 510 and 700 nm in buffers at pH 1.0 and 4.5. Anthocyanin content is expressed in cyanidin 3-glucoside equivalents, calculated using a MW of 449.2 and a molar absorptivity of 26,900, and the results are expressed in mg/100 g FW.
Total flavonoid analysis
The TFC in the extracted sample was measured by colorimetric assay (Jia et al., 1999; Meyers et al., 2003). After adding 0.3 mL of 5% NaNO2 to a 15-mL test tube containing 4 mL of distilled water and 1 mL of sample, the mixture was vortexed and left to sit for 5 min at room temperature. Subsequently, 0.3 mL of 10% AlCl3 was added and the mixture was vortexed and left to sit for 6 min at room temperature. After adding 2.4 mL of distilled water to 2 mL of 1 N NaOH, the mixture was vortexed and the final volume was brought to 10 mL, after which the absorbance at 510 nm was measured. Catechin was used as the standard and the results are expressed in mg catechin equivalent (CE)/100 g FW.
Total phenolic analysis
The TPC of the extracted sample was measured by the Folin–Ciocalteu colorimetric method (Meyers et al., 2003; Singleton et al., 1999). After adding 0.2 mL of the sample to a 15-mL test tube containing 2.6 mL of deionized water, 0.2 mL of Folin–Ciocalteu reagent was added and the mixture was vortexed and left to sit for 6 min at room temperature. Subsequently, 2 mL of 7% Na2CO3 was added to the mixture and vortexed. After leaving the mixture to sit for 90 min in a dark room, the absorbance at 750 nm was measured. Gallic acid was used as the standard and the results are expressed as mg gallic acid equivalent (GAE)/100 g FW.
Determination of ellagic acid compounds
The determination of ellagic acid was analyzed using HPLC (Bala et al., 2006). Strawberry extracts were diluted 10-fold in 1 N NaOH and distilled water (8:1, v/v) solutions. Prior to analysis, a 0.45 μm syringe filter was used to filter the sample. An Ultimate 3000 Dionex HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) was used with a Zorbax Eclipse plus C18 column (4.6 mm i.d. × 150 mm, 5 μm; Agilent, Palo Alto, CA, USA). The mobile phase gradients of 0.1% phosphoric acid in distilled water (A) and methanol (B) were used. The gradient was as follows: 0–8.5 min, 70–65%; 8.5–12 min, 65–55%; 12–15 min, 55–45%; and 15–20 min 45–30% (for solution A). HPLC operating conditions were flow rate 0.5 mL/min, column temperature 35 °C, injection volume 10 μL, and detection was at 254 nm. The determination was performed three times for each sample and the results were shown as mg/100 g of FW.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity analysis
The DPPH radical scavenging activity was measured using a modified version of the method described by Brand-Williams et al. (1995). After preparing 100 µM DPPH using 80% methanol, 80% methanol was used again to dilute this solution to an O.D. value of 0.63–0.67 at 517 nm. After adding 2.95 mL of DPPH solution to 50 µL of the extracted sample, it was allowed to stand for 30 min in a dark room, after which the absorbance at 517 nm was measured. Antioxidant activity by DPPH radical scavenging activity is expressed as mg vitamin C equivalent (VCE)/100 g FW.
2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity analysis
The ABTS radical scavenging activity of the extracted sample was measured using ABTS radicals (Kim and Shin, 2015). After mixing 100 mL of phosphate buffered saline (PBS) solution with 1 mM 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) and 2.5 mM ABTS, the mixture was allowed to react for 40 min in a 70 °C water bath to prepare an ABTS radical solution, after which PBS solution was used to dilute the mixture to an O.D. value of 0.63–0.67 at 734 nm. After adding 980 µL of ABTS radical to 20 µL of the extracted sample it was allowed to react for 10 min at 37 °C, the absorbance was measured at 734 nm. The results are expressed as mg vitamin C equivalent (VCE)/100 g FW.
AChE and BChE inhibitory activity
The AChE inhibitory activity was measured using the Ellman’s method (Ellman et al., 1961). For the enzymatic reaction, after dispensing 150 µL of PBS buffer, the sample and 20 µL of 0.2 U AChE were added. Subsequently, 30 µL of 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB) and 20 µL of 15 mM acetylthiocholine iodide (ATCI) were added and allowed to react for 30 min at 37 °C. Then, the absorbance was measured at 415 nm with a microplate reader (Versa max, Molecular Devices, Sunnyvale, CA, USA). Tacrine was used as the positive control and the results are expressed as inhibition rate (%). The BChE inhibitory activity was also measured using a modified Ellman’s method (Ellman et al., 1961). Tacrine was used as the positive control and the results are expressed as inhibition rate (%).
Statistical analysis
For statistical analysis, analysis of variance was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and Duncan’s multiple range test. Significant differences are indicated using 95% confidence intervals. Pearson correlations were used to quantify relationships between parameters. The data are expressed as mean ± standard deviation for triplicate determinations.
Results and discussion
Physicochemical properties
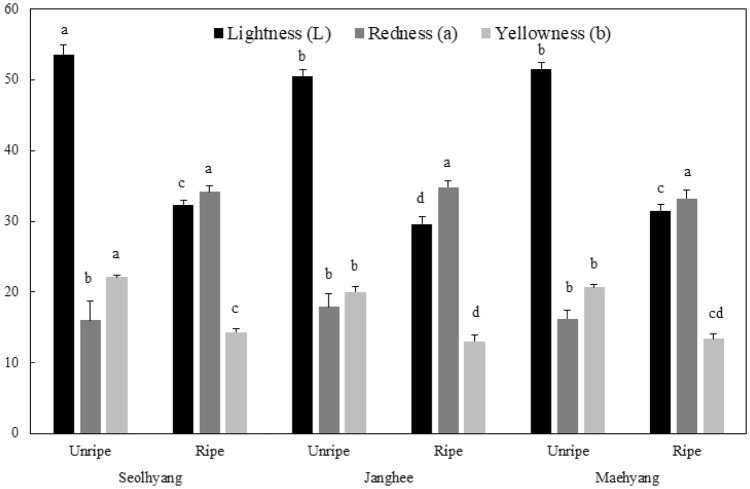
The ripe fruits of Seolhyang, Janghee, and Maehyang cultivars showed Hunter a values of 34.16, 34.82, and 33.27, respectively, which were significantly higher than the values for unripe fruits, which were 16.05, 17.94, and 16.23, respectively. For Hunter L value, representing lightness, the ripe fruits of Seolhyang, Janghee, and Maehyang cultivars showed values of 32.37, 29.62, and 31.51, respectively, which were significantly lower than the values of 53.57, 50.53, and 51.58, respectively, shown by the unripe fruits (Fig. 1).
Fig. 1.
Hunter Lab color of unripe and ripe strawberries. Vertical bars indicate standard deviation. Different letters are significant differences among the same color parameter by Duncan’s multiple range test (p < 0.05)
Firmness is one of the key factors that determine the quality of strawberries. The unripe and ripe fruits of the Maehyang cultivar showed values of 6.50 N and 4.83 N, respectively, which were significantly higher than the values shown by the unripe and ripe fruits of the Seolhyang and Janghee cultivars (Table 1). Soluble solids content and acidity are important factors that influence the sensory qualities of fruit. The ripe fruits of the Seolhyang cultivar showed a high value of 10.13 °Brix. The unripe fruits showed no significant differences among the three cultivars. Overall, ripe fruits showed a higher soluble solids content than unripe fruits (Table 1).
Table 1.
Firmness, soluble solid content (SSC), titratable acidity (TA), SSC/TA ratio, and pH of unripe and ripe strawberries
| Cultivars | Ripening stage | Firmness (N/5 mmØ) | Soluble solid content (°Brix) | Titratable acidity (%) | SSC/TA ratio | pH |
|---|---|---|---|---|---|---|
| Seolhyang | Unripe | 5.14 ± 0.46b | 8.13 ± 0.45c | 0.88 ± 0.05bc | 9.27 ± 0.29d | 3.52 ± 0.02d |
| Ripe | 3.88 ± 0.56c | 10.13 ± 0.64a | 0.79 ± 0.05c | 12.93 ± 1.23b | 3.77 ± 0.04b | |
| Janghee | Unripe | 5.23 ± 0.42b | 8.23 ± 0.06c | 0.82 ± 0.06bc | 10.04 ± 0.67 cd | 3.65 ± 0.01c |
| Ripe | 3.17 ± 0.22d | 9.00 ± 0.01b | 0.50 ± 0.09d | 18.51 ± 2.66a | 4.00 ± 0.00a | |
| Maehyang | Unripe | 6.50 ± 0.56a | 8.13 ± 0.42c | 1.09 ± 0.03a | 7.49 ± 0.15d | 3.50 ± 0.01d |
| Ripe | 4.83 ± 0.42b | 9.67 ± 0.45ab | 0.92 ± 0.03b | 10.54 ± 0.39bc | 3.62 ± 0.02c |
Results are mean values ± standard deviation from three measurements (n = 3); means in the same column with superscript with different letters (a, b, c, and d) are significantly different at p < 0.05
SSC/TA ratio is calculated by SSC (°Brix)/Titratable acidity (%)
The quantification of individual sugars (fructose, glucose, and sucrose) was analyzed by cultivars and ripening stages. The results showed that sucrose content increased as strawberries matured in all cultivars. Total sugar contents of the Janghee and Seolhyang cultivars at ripe stage were significantly higher (8417.93 and 8080.38 mg/100 g FW, respectively) than that of Maehyang cultivar (7031.96 mg/100 g FW). Kim and Shin (2015) reported that total sugar contents of Yukbo, Seolhyang, and Janghee cultivars were between 5450 and 8420 mg/100 g FW depending on harvest locations. They concluded that the harvest locations may also affect the sugar content of strawberry (Table 2).
Table 2.
Individual sugar and organic acid contents of unripe and ripe strawberries
| Cultivars | Ripening stage | Fructose (mg/100 g FW) | Glucose (mg/100 g FW) | Sucrose (mg/100 g FW) | Total sum (mg/100 g FW) |
|---|---|---|---|---|---|
| Individual sugar contents | |||||
| Seolhyang | Unripe | 214.29 ± 5.68b | 200.79 ± 3.70ab | 175.35 ± 9.27d | 590.42 ± 15.87c |
| Ripe | 265.68 ± 18.38a | 244.55 ± 16.22a | 297.80 ± 36.44bc | 808.04 ± 67.19a | |
| Janghee | Unripe | 211.78 ± 14.48b | 193.71 ± 14.26b | 253.18 ± 22.85c | 658.68 ± 32.50bc |
| Ripe | 265.77 ± 7.85a | 248.42 ± 7.26a | 327.60 ± 31.26ab | 841.79 ± 24.72a | |
| Maehyang | Unripe | 153.32 ± 8.50c | 168.24 ± 16.08b | 296.35 ± 30.19bc | 617.91 ± 23.85bc |
| Ripe | 165.00 ± 7.85c | 175.61 ± 45.36b | 362.59 ± 10.92a | 703.20 ± 48.54b | |
| Cultivars | Ripening stage | Malic acid (mg/100 g FW) | Citric acid (mg/100 g FW) | Total sum (mg/100 g FW) |
|---|---|---|---|---|
| Individual organic acid contents | ||||
| Seolhyang | Unripe | 281.79 ± 4.13ab | 650.00 ± 24.75ab | 931.79 ± 22.18a |
| Ripe | 198.96 ± 34.13c | 563.50 ± 77.68b | 762.46 ± 90.49b | |
| Janghee | Unripe | 103.48 ± 9.40d | 554.02 ± 99.21b | 657.50 ± 81.39b |
| Ripe | 77.24 ± 2.68d | 440.89 ± 41.73c | 518.13 ± 32.22c | |
| Maehyang | Unripe | 267.68 ± 18.54b | 768.12 ± 70.54a | 1035.80 ± 69.97a |
| Ripe | 305.60 ± 19.35a | 695.42 ± 31.45a | 1001.03 ± 11.96a | |
Results are mean values ± standard deviation from three measurements (n = 3); means in the same column with superscript with different letters (a, b, c, and d) are significantly different at p < 0.05
Analysis of organic acids showed that most of the organic acids were citric acid and malic acid, with a higher content of citric acid than malic acid in all cultivars. Organic acid content is highly associated with the acidity of strawberries, and is also closely linked to their taste. The results showed that citric acid and malic acid content decreased as strawberries matured (Table 2). In particular, the citric acid content of the Janghee cultivar was significantly higher in unripe fruits than in ripe fruits, whereas the malic acid content of the Maehyang cultivar was significantly higher in ripe fruit than in unripe fruit.
Antioxidant compounds analysis
Ripe strawberries showed a higher TAC than unripe strawberries (Table 3). The TAC of ripe fruits of the Seolhyang, Janghee, and Maehyang cultivars was 13.88, 16.14, and 12.72 mg/100 g FW, respectively. There was no significant difference in TAC between the Seolhyang and Janghee cultivars or between the Seolhyang and Maehyang cultivars, whereas the TAC in ripe fruits of the Janghee cultivar was significantly higher than that of the Maehyang cultivar. Moreover, the TAC of unripe fruits of the Seolhyang, Janghee, and Maehyang cultivars was 2.95, 3.31, and 3.73 mg/100 g FW, respectively, showing no significant differences. Overall, Hunter a values were higher in ripe fruits, due to the increase in anthocyanins, which cause red coloration, showing a pattern similar to that observed in the TAC results. Shin et al. (2008) conducted TAC analysis with unripe (white tip) and ripe (red ripe tip) strawberries grown in the US. The results showed that TAC at harvest day was higher in ripe fruits than in unripe fruits, and as the storage period increased, unripe fruits tended to show an increase in TAC. Wang and Lin (2000) also analyzed TAC in strawberries based on ripeness and found that the TAC of unripe strawberries was 0.2 mg/100 g and increased to 38.9 mg/100 g as the fruits reached full maturity. Siriwoharn et al. (2004) reported that TAC in unripe blackberries was 69.9 mg/100 g, which increased to 317.0 mg/100 g as they ripened.
Table 3.
Antioxidant contents and activities of unripe and ripe strawberries
| Cultivar | Ripening stage | Total anthocyanin (mg/100 g FW) | Total flavonoids (mg CE/100 g FW) | Total phenolics (mg GAE/100 g FW) | Ellagic acid (mg/100 g FW) | DPPH (mg VCE/100 g FW) | ABTS (mg VCE/100 g FW) |
|---|---|---|---|---|---|---|---|
| Seolhyang | Unripe | 2.95 ± 0.29c | 45.77 ± 5.63b | 164.62 ± 14.12b | 0.24 ± 0.01c | 162.57 ± 17.69b | 287.03 ± 6.83b |
| Ripe | 13.88 ± 1.63ab | 37.44 ± 1.75bc | 168.59 ± 3.42b | 0.17 ± 0.01d | 161.64 ± 10.63b | 286.60 ± 6.04b | |
| Janghee | Unripe | 3.31 ± 0.34c | 62.93 ± 1.67a | 211.68 ± 1.95a | 0.34 ± 0.01b | 237.49 ± 7.41a | 398.79 ± 13.24a |
| Ripe | 16.14 ± 0.59a | 40.52 ± 4.07bc | 182.37 ± 7.56b | 0.22 ± 0.01c | 171.38 ± 26.31b | 327.54 ± 29.49b | |
| Maehyang | Unripe | 3.73 ± 0.56c | 41.90 ± 7.21bc | 186.79 ± 23.56b | 0.65 ± 0.04a | 227.76 ± 40.08a | 333.99 ± 40.73b |
| Ripe | 12.72 ± 2.52a | 35.57 ± 4.12c | 179.41 ± 10.89b | 0.37 ± 0.00b | 175.24 ± 24.75b | 299.50 ± 33.69b |
Results are mean values ± standard deviation from three measurements (n = 3); means in the same column with superscript with different letters (a, b, c, and d) are significantly different at p < 0.05
Flavonoid compounds account for a relatively significant portion of the natural antioxidants found in plants, and flavonoids are known to prevent or delay aging and cancer by playing a role in preventing oxidative stress, ROS scavenging, and lipid oxidation (Lim et al., 1996). With respect to TFC, the Seolhyang and Maehyang cultivars showed no significant differences between unripe and ripe fruits, whereas in the Janghee cultivar, the unripe fruits (62.93 mg (CE)/100 g FW) had a significantly higher TFC than ripe fruits (40.52 mg (CE)/100 g FW) (Table 3). Shin et al. (2008) also reported that TFC is higher in unripe strawberries than in ripe strawberries. Kim et al. (2010) reported that TFC in Jeju mangos is 8.14 mg (CE)/g in unripe fruits and 3.30 mg (CE)/g in ripe fruits, indicating that unripe mangos have a higher TFC than ripe mangos. Thus, TFC in strawberries varies with variety and ripeness, with unripe Janghee fruits showing the highest TFC content.
TPC of the Seolhyang and Maehyang cultivars showed no significant differences between unripe and ripe fruits, whereas the unripe fruits of the Janghee cultivar had a significantly higher TPC (211.68 mg (GAE)/100 g FW) than ripe fruits (182.37 mg (GAE)/100 g FW) (Table 3). According to Shin et al. (2008), unripe Jewel strawberries show a higher TPC than ripe fruits, at 300–350 mg (GAE)/100 g FW and 230–280 mg (GAE)/100 FW, respectively. Yoo et al. (2005) reported that TPC is higher in unripe fruits (231.5 mg (GAE)/100 g FW) of yuja than ripe fruits (227.2 mg (GAE)/100 g FW), while Moon et al. (2015) reported that TPC of yuja from different production areas showed a tendency to decrease, from 2.48 mg (GAE)/g FW to 1.45 mg/g FW, as harvest was delayed. Meanwhile, Oh et al. (2011) reported that TPC in Jeju kiwi tended to decrease as ripeness increased, from 56.10 mg (GAE)/g FW to 8.64 mg (GAE)/g FW. However, Kim et al. (2010) reported that TPC in Jeju mango showed no significant difference between unripe and ripe fruits, at 27.8 and 26.9 mg (GAE)/g, respectively. Moreover, Siriwoharn et al. (2004) reported that among blackberries, the Marion cultivar showed a gradual increase in TPC from 975 mg (GAE)/100 g to 1541 mg (GAE)/100 g as they matured from the unripe to the ripe stage, whereas the TPC of the Evergreen cultivar showed a tendency to decrease from 1090 mg (GAE)/100 g to 960 mg (GAE)/100 g when maturing from the unripe to the half-ripe stage, increasing to 1035 mg (GAE)/100 g at full ripeness. Thus, TPC in unripe and ripe strawberries vary according to cultivars.
Interestingly, unripe strawberries showed a higher ellagic acid content than ripe strawberries in all cultivars (Table 3). The ellagic acid contents of unripe fruits of the Seolhyang, Janghee, and Maehyang cultivars was 0.24, 0.34, and 0.65 mg/100 g FW, respectively. The ellagic acid content in unripe fruits of the Maehyang cultivar was significantly higher than that of the Janghee and Seolhyang cultivars. It is known that the most abundant phytochemicals in strawberries are ellagic acid, anthocyanin, catechin, quercetin, and kaempferol (Hakkinen et al., 2000; Wang and Lin, 2000). Less ripe strawberries contain higher total phenolic concentrations (Wang and Lin, 2000) and ellagic acid was higher in green stage than in red stage (Kosar et al., 2004).
Antioxidant activity analysis
With respect to the DPPH radical scavenging activity of strawberries, the Seolhyang cultivar did not show significant differences between unripe and ripe fruits, at 162.57 and 161.64 mg/100 g FW, respectively, whereas the Janghee and Maehyang cultivars showed significantly higher antioxidant activity in unripe fruits (237.49 and 227.76 mg/100 g FW, respectively) than in ripe fruits (171.38 and 175.24 mg/100 g FW, respectively) (Table 3). Kim and Shin (2015) reported that the DPPH radical scavenging activity in ripe fruits of the Seolhyang and Janghee cultivars is 1.7–2.3 g/kg FW and 1.8–2.7 g/kg FW, respectively, similar to the results of the current study. They also reported that antioxidant activity varied with cultivation area and variety. Kim et al. (2010) reported that DPPH radical scavenging activity in Jeju mangos was higher in ripe fruits than in unripe fruits, while Oh et al. (2011) reported that the DPPH activity of Jeju kiwis decreased with ripening, from 89.37% in unripe fruits to 43.94% in ripe fruits. Thus, in strawberries DPPH differs with variety, and in the Janghee and Maehyang cultivars, unripe fruits tended to have higher antioxidant activity.
With respect to the ABTS radical scavenging activity of strawberries, the Seolhyang and Maehyang cultivars did not show significant differences in antioxidant activity between unripe and ripe fruits, whereas the Janghee cultivar showed significantly higher antioxidant activity in unripe fruits (398.79 mg/100 g FW) than in ripe fruits (327.54 mg/100 g FW), similar to the pattern observed for DPPH scavenging activity (Table 3). Shin et al. (2008) reported that Jewel strawberries that are 30–40% ripe have higher antioxidant activity than fully ripe strawberries, with unripe Jewel strawberries showing antioxidant activity of approximately 7–9 mmol/kg FW. Based on these results, antioxidant activity varies according to variety and ripeness at harvest time. Moreover, Choi et al. (2013) reported that in unripe Daewang strawberries (50% ripe), ABTS radical scavenging activity decreases as storage time increases, but increases again after a certain point, whereas Seolhyang strawberries show a gradual increase in ABTS radical scavenging activity during maturation from the unripe to the ripe stage. Thus, differences between unripe and ripe fruits may vary according to variety. Furthermore, Kim and Shin (2015) reported that the ABTS radical scavenging activity in Seolhyang and Janghee cultivars is 2.5–3.6 and 2.6–4.2 g/kg FW, respectively, which indicates that ABTS radical scavenging activity may also vary according to cultivation area.
AChE and BChE inhibitory activities
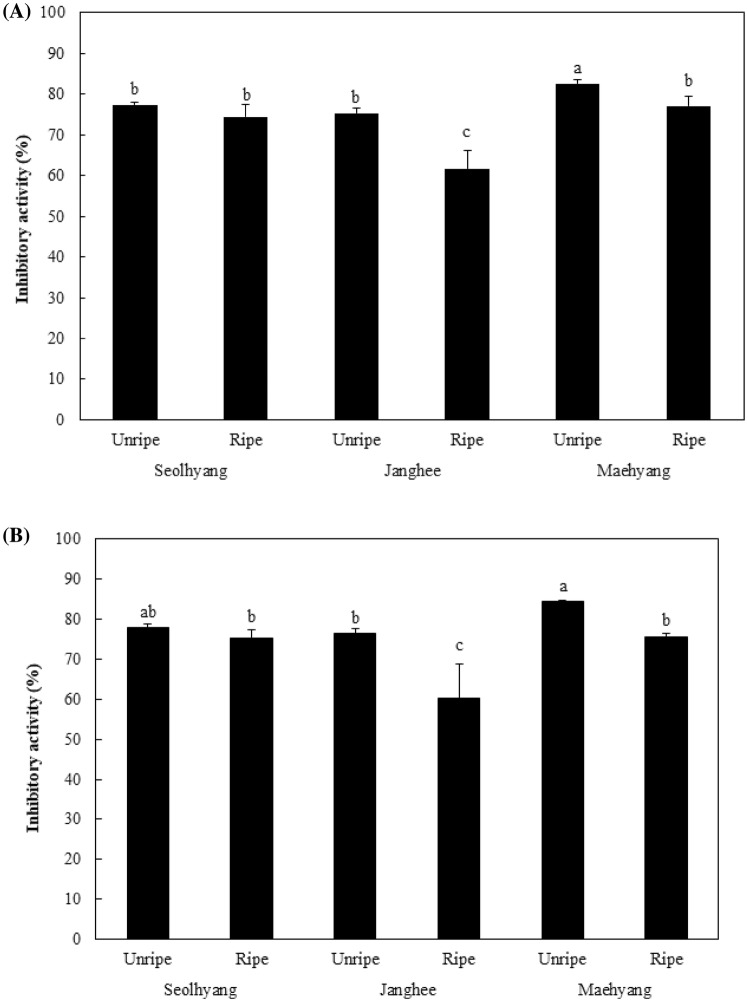
AChE inhibitory activity of the Janghee cultivar showed a significant difference between unripe and ripe fruits, at 75.41% and 61.71%, respectively, the Maehyang cultivar also showed significant differences between unripe and ripe fruits, at 82.56% and 77.09%, respectively. In both cultivars, unripe fruits showed significantly higher inhibition rate than ripe fruits. However, the Seolhyang cultivar showed no significant difference in AChE inhibitory activity between unripe and ripe fruits (Fig. 2A). Thus, inhibition rates differed between unripe and ripe fruits according to cultivar, but unripe fruits of the Maehyang cultivar showed the highest inhibitory activity. Yun et al. (2010) measured the AChE and BChE inhibitory activities of white ginseng and black ginseng and found that black ginseng had higher inhibitory activity. Thus, in ginseng, inhibitory activity varied by cultivar, whereas in strawberries, it tends to vary by ripeness stage, except in the Seolhyang cultivar. Both AChE and BChE are enzymes known to reduce the level of acetylcholine, a neurotransmitter found in neurons in brain tissues. Here, the BChE inhibitory activity of strawberries showed no significant difference between unripe and ripe fruits of the Seolhyang cultivar, whereas a significant difference was observed between the unripe (76.40%) and ripe (60.20%) fruits of the Janghee cultivar and between the unripe (84.37%) and ripe (75.58%) fruits of the Maehyang cultivar, indicating that unripe fruits have a significantly higher inhibition rate than ripe fruits (Fig. 2B). Fewer studies have been published on BChE than on AChE, while studies on fruits have primarily focused on ripe fruits. In this finding that unripe fruits have high BChE inhibitory activity represents an interesting opportunity for further study.
Fig. 2.
Acetylcholinesterase and butyrylcholinesterase inhibitory activity [AChE (A) and BChE (B)] of unripe and ripe strawberries. Different letters are significant differences by Duncan’s multiple range test (p < 0.05)
Pearson correlation
Correlations among antioxidant compounds, antioxidant activities, and anti-dementia activities of strawberries according to cultivar and ripeness are shown in Table 4. Several studies have reported that antioxidant compounds and antioxidant activities of fruits and vegetables are highly correlated (Kim and Shin, 2015; Shin, 2012; Shin et al., 2008). The present study also found a high correlation (R = 0.787) between TFC and TPC. TPC also showed a strong correlation with total antioxidant activities (R = 0.883 with DPPH radical scavenging activity and R = 0.932 with ABTS radical scavenging activity). Moreover, the total antioxidant activities, DPPH and ABTS radical scavenging activities, also showed a high correlation with each other (R = 0.876). However, the total antioxidant activity and the anti-dementia activity were not correlated.
Table 4.
Pearson correlation (R) among antioxidant compounds, antioxidant activities, and anti-dementia activities of strawberries
| Total anthocyanins | Total flavonoids | Total phenolics | DPPH | ABTS | AChE | BChE | |
|---|---|---|---|---|---|---|---|
| Total flavonoids | − 0.581* | ||||||
| Total phenolics | − 0.242 | 0.787** | |||||
| DPPH | − 0.482* | 0.714** | 0.883** | ||||
| ABTS | − 0.333 | 0.836** | 0.932** | 0.876** | |||
| AChE | − 0.640** | 0.027 | − 0.053 | 0.242 | − 0.109 | ||
| BChE | − 0.699** | 0.106 | 0.023 | 0.294 | − 0.053 | 0.935** | |
| Ellagic acid | − 0.471* | 0.064 | 0.345 | 0.619** | 0.299 | 0.564* | 0.620** |
Pearson correlation (R): **significance at p < 0.01; *significance at p < 0.05
Thus, this research showed that some qualities, such as antioxidant content, varied by cultivar, whereas others, such as TAC and anti-dementia activity, varied by ripeness stage. TPC and TFC were found to be highly correlated, as were the DPPH and ABTS radical scavenging activities. Unripe fruits showed high levels of anti-dementia and antioxidant activities, and thus unripe fruits discarded due to damage show potential for use in functional foods.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The present study was conducted with support from the research fund of Dankook University in 2017.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyesung Hwang, Email: sug7764@hanmail.net.
Young-Jun Kim, Email: kimyj@seoultech.ac.kr.
Youngjae Shin, Phone: 041-550-3562, Email: ys234@dankook.ac.kr.
References
- Adsersen A, Kjølbye A, Dall O, Jäger AK. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava Schweigg. Kort. J. Ethnopharmacol. 2007;113:179–182. doi: 10.1016/j.jep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Bala I, Bhardwaj V, Hariharan S, Kumar MR. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006;40:206–210. doi: 10.1016/j.jpba.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Boyles MJ, Wrolstad RE. Anthocyanin composition of red raspberry juice: influences of cultivar, processing, and environmental factors. J. Food Sci. 1993;58:1135–1141. doi: 10.1111/j.1365-2621.1993.tb06132.x. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Choi HG, Kang NJ, Moon BY, Kwon JK, Rho IR, Park KS, Lee SY. Changes in fruit quality and antioxidant activity depending on ripening levels, storage temperature, and storage periods in strawberry cultivars. Korean J. Hortic. Sci. Technol. 2013;31:194–202. doi: 10.7235/hort.2013.12151. [DOI] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Hakkinen SH, Karenlampi SO, Mykkanen HM, Heinonen IM, Torronen AR. Ellagic acid content in berries: influence of domestic processing and storage. Eur. Food Res. Technol. 2000;212:75–80. doi: 10.1007/s002170000184. [DOI] [PubMed] [Google Scholar]
- Jeong CH, Kang ST, Joo OS, Lee SC, Shin YH, Shim KH, Cho SH, Choi GS, Heo HJ. Phenolic content, antioxidant effect and acetylcholinesterase inhibitory activity of Korean commercial green, puer, oolong, and black teas. Korean J. Food Preserv. 2009;16:230–237. [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Jung YS, Park SJ, Kim JE, Yang SA, Park JH, Kim JH, Jhee KH, Lee SP, Lee IS. A comparative study of GABA, glutamate contents, acetylcholinesterase inhibition and antiradical activity of the methanolic extracts from 10 edible plants. Korean J. Food Sci. Technol. 2012;44:447–451. doi: 10.9721/KJFST.2012.44.4.447. [DOI] [Google Scholar]
- Jung YS, Park SJ, Park JH, Jhee KH, Lee IS, Yang SA. Effects of ethanol extracts from Zingiber officinale Rosc., Curcuma longa L., and Curcuma aromatica Salisb. on acetylcholinesterase and antioxidant activities as well as GABA Contents. J. Korean Soc. Food Sci. Nutr. 2012;41:1395–1401. doi: 10.3746/jkfn.2012.41.10.1395. [DOI] [Google Scholar]
- Kim YJ, Shin Y. Antioxidant profile, antioxidant activity, and physicochemical characteristics of strawberries from different cultivars and harvest locations. J. Korean Soc. Appl. Biol. Chem. 2015;58:587–595. doi: 10.1007/s13765-015-0085-z. [DOI] [Google Scholar]
- Kim DI, Lee SH, Hur EY, Cho SM, Park HJ. Screening of natural plant resources with acetylcholinesterase inhibition and antioxidant activity. J. Korean Soc. Food Sci. Nutr. 2005;34:427–432. doi: 10.3746/jkfn.2005.34.3.427. [DOI] [Google Scholar]
- Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi HK, Kim YS, Mosaddik A, Cho SK. Antioxidant and antiproliferative activities of mango (Mangiferaindica L.) flesh and peel. Food Chem. 2010;121:429–436. doi: 10.1016/j.foodchem.2009.12.060. [DOI] [Google Scholar]
- Kosar M, Kafkas E, Paydas S, Baser KHC. Phenolic composition of strawberry genotypes at different maturation stages. J. Agric. Food Chem. 2004;52:1586–1589. doi: 10.1021/jf035093t. [DOI] [PubMed] [Google Scholar]
- Lim DK, Choi U, Shin DH. Antioxidative activity of ethanol extract from Korean medicinal plants. Korean J. Food Sci. Technol. 1996;28:83–89. [Google Scholar]
- Meyers KJ, Watkins CB, Pritts MP, Liu RH. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003;51:6887–6892. doi: 10.1021/jf034506n. [DOI] [PubMed] [Google Scholar]
- Moon SH, Assefa AD, Ko EY, Park SW. Comparison of flavonoid contents and antioxidant activity of Yuzu (Citrus junos Sieb. ex Tanaka) based on harvest time. Korean J. Hortic. Sci. Technol. 2015;33:283–291. doi: 10.7235/hort.2015.14180. [DOI] [Google Scholar]
- Naemura A, Mitani T, Ijiri Y, Tamura Y, Yamashita T, Okimura M, Yamamoto J. Anti-thrombotic effect of strawberries. Blood Coagul. Fibrinolysis. 2005;16:501–509. doi: 10.1097/01.mbc.0000184737.50594.a8. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Jeon SB, Kang HY, Yang YJ, Kim SC, Lim SB. Chemical composition and antioxidative activity of Kiwifruit in different cultivars and maturity. J. Korean Soc. Food Sci. Nutr. 2011;40:343–349. doi: 10.3746/jkfn.2011.40.3.343. [DOI] [Google Scholar]
- Orhan I, Sener B, Choudhary MI, Khalid A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004;91:57–60. doi: 10.1016/j.jep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Shin Y. Correlation between antioxidant concentrations and activities of yuja (Citrus junos Sieb ex Tanaka) and other citrus fruit. Food Sci. Biotechnol. 2012;21:1477–1482. doi: 10.1007/s10068-012-0195-x. [DOI] [Google Scholar]
- Shin Y, Ryu JA, Liu RH, Nock JF, Polar-Cabrera K, Watkins CB. Fruit quality, antioxidant contents and activity, and antiproliferative activity of strawberry fruit stored in elevated CO2 atmospheres. J. Food Sci. 2008;73:339–344. doi: 10.1111/j.1750-3841.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–177. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004;52:8021–8030. doi: 10.1021/jf048619y. [DOI] [PubMed] [Google Scholar]
- Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- Yoo KM, Park JB, Seoung KS, Kim DY, Hwang IK. Antioxidant activities and anticancer effects of Yuza (Cirtus Junos) Food Sci. Ind. 2005;38:72–77. [Google Scholar]
- Yun BS, Lee MR, Oh CJ, Cho JH, Wang CY, Gu LJ, Mo EK, Sung CK. Characterization of black ginseng extract with acetyl- and butyrylcholin-esterase inhibitory and antioxidant activities. J. Ginseng Res. 2010;34:348–354. doi: 10.5142/jgr.2010.34.4.348. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.