Abstract
A well-established literature demonstrates executive function (EF) deficits in obese children and adults relative to healthy weight comparisons. EF deficits in obesity are associated with overeating and impulsive consumption of high calorie foods leading to excess weight gain and to problems with metabolic regulation and low-grade inflammation that detrimentally affect the structure and function of prefrontal cortex. Here, we test a complementary explanation for the relation between EF and body mass index (BMI) grounded in the energy demand of the developing brain. Recent work shows that the brain accounts for a lifetime peak of 66% of resting metabolic rate in childhood, and that developmental changes in brain energetics and normative changes in body weight gain are closely inversely related. This finding suggests a trade-off in early childhood between energy used to support brain development versus energy used to support physical growth and fat deposition. To test this theorized energetic trade-off, we analyzed data from a large longitudinal sample (N=1,292) and found that change in EF from age 3–5 years, as a proxy for brain development in energetically-costly prefrontal cortex, is inversely related to change in BMI from age 2–5 years. Greater linear decline in BMI predicted greater linear increase in EF. We interpret this finding as tentative support for a brain-body energetic trade-off in early childhood with implications for lifetime obesity risk.
Keywords: body mass index, executive function, early childhood development, obesity risk, brain energetics
Executive function (EF) deficits in obese children and adults relative to healthy weight controls have been demonstrated in a number of studies. A recent meta-analysis (Yang et al., 2018) indicated deficits in performance of moderate effect size (0.33–0.44) in obese vs. normal weight comparisons for each aspect of executive function examined, including inhibitory control, working memory, cognitive flexibility, decision making, verbal fluency, and planning. Over one-third of the studies in the meta-analysis included children; however, age was not found to moderate effects of obesity on any aspect of EF. Further, neuroimaging studies have documented relations between poor performance on EF tasks and altered structure and function of prefrontal cortex (PFC) in obese vs. healthy weight comparisons in children and adolescents (Kamijo et al., 2014; Ross, Yau, & Convit, 2015).
To some extent, the association between EF deficits and obesity is attributable to failures of dietary self-regulation and the impulsive consumption of high calorie foods (Hall, 2016). EF deficits associated with obesity are also attributable to alterations to PFC and associated brain areas resulting from insulin resistance and other characteristics of the metabolic syndrome (MetS) (Rusinek and Convit, 2014). Human neuroimaging and animal models have demonstrated that metabolic dysregulation affects multiple brain areas through processes related to decreased vascular reactivity and increased neuroinflammation and oxidative stress (Bocarsly et al., 2015; Thaler et al., 2012; Yates et al, 2012; Yau et al., 2012). Obesity and the consumption of a high-fat diet lead to increases in circulating cytokines and free fatty acids resulting in microglial proliferation, synaptic remodeling, and reductions in surface area and volume of cortical and subcortical structures (Miller and Spencer, 2014).
A number of studies, however, have demonstrated an inverse association between EF and body mass index (BMI) in typically developing populations of children and young adults, some of which include obese or overweight participants but none that exhibit MetS (Kamijo et al., 2012; Yang et al., 2018; Yau et al., 2014). A unique example is seen in a positron emission tomography (PET) study with healthy adults (N=21) in which resting glucose metabolism in PFC was negatively correlated with BMI and positively correlated with EF (Volkow et al., 2009). Further examples in children are seen in studies in which fat mass was assessed by dual-energy X-ray absorptiometry (DXA) in addition to BMI and that also controlled for aerobic fitness. In one analysis, both fat mass assessed by DXA and BMI were inversely related to executive inhibitory control as well as academic achievement in N=126 children 7 to 9 years of age (Kamijo et al., 2012). In the second analysis with N=233 children, also 7 to 9 years of age, whole body adiposity was associated with increased intraindividual variability in inhibitory control, an indicator of lapses in attention (Chojnacki et al., 2018).
BMI and the brain.
If the association between BMI and brain structure and function in areas of PFC that underlie EF precedes as well as follows MetS, it is likely that an association between EF and BMI is developmental and detectable in early childhood, prior to the onset of any metabolic symptoms associated with excess adiposity. Executive function is developing rapidly in the preschool period at a time of declining BMI, prior to what is known as the adiposity rebound (Rolland-Cachera et al 1984). The adiposity rebound refers to the fact that infants are born with fat deposits that increase over the first year prior to decreasing throughout early childhood. Body fat mass tends to reach its lowest point at 4–6 years of age at which time relative weight and adiposity increase. Children reaching this inflection point early or at high BMI are at elevated lifetime risk for obesity and MetS (Peneau et al., 2016).
An inverse developmental association between BMI and EF is suggested by an analysis using cross-sectional PET and magnetic resonance imaging data to estimate the energy demand of the developing brain (Kuzawa et al., 2014). This analysis found that the percent of resting metabolic rate (RMR) accounted for by the brain increases rapidly in early childhood and peaks, at 66% of RMR, as normative weight gain velocity is decreasing in advance of the adiposity rebound. This finding inspired the hypothesis of an individual level brain-body energetic tradeoff between brain development and fat deposition during childhood (AUTHORS). Although little is currently known about between-person variability in the energy demands of the developing brain, an inverse association in early childhood between change in BMI and change in EF, as a proxy for brain development in energetically-costly prefrontal cortex (PFC), would be consistent with the hypothesis of a brain-body energetic tradeoff in childhood. Increases in grey matter and increases in the proliferation and density of synapses are key energetically costly characteristics of early brain development (Atwell and Laughlin, 2001; Gilmore et al., 2018). The development of PFC is a prominent aspect of brain development in early childhood, exhibiting both grey matter increase (Sowell et al., 2003) and synaptic proliferation (Elston et al., 2009; Petanjek et al., 2011), and as such is a primary contributor to the high developmental peak in brain energy demands during childhood (Goyal et al., 2014). The development of PFC in early childhood manifests behaviorally in the emergence of EF abilities. Accordingly, given the prominent role of PFC development in the childhood brain energetics peak, the development of EF in early childhood could provide a useful proxy for evaluating the hypothesis of a brain-body energetic trade-off.
Current study.
Data with which to estimate between-child variability in the energetic demand of the developing brain are rare. Children vary markedly, however, in trajectories of the development of EF. Normatively, EF develops rapidly in early childhood (Willoughby et al., 2012) at an age when BMI is typically declining, pointing to the likelihood of energetic links between these two aspects of development. However, we are aware of no study that has assessed children longitudinally to determine whether BMI change in early childhood is inversely related to the development of EF. Accordingly, we hypothesized that the rate of increase in EF, as an indicator of the energy required by the developing brain when controlling for well-known influences on EF development, would be related to the rate of decline in BMI in early childhood. Such an association would be consistent with a close biological linkage between brain development and physical growth.
Method
Participants
The Family Life Project (FLP) was designed to study child development and family ecology in areas of high rural poverty in two states (North Carolina and Pennsylvania). Complex sampling procedures were used to recruit a representative sample of 1,292 families at the time of the target child’s birth, with low-income families in both states being over-sampled and African Americans oversampled in NC. FLP recruiters identified 5471 (59% NC, 41% PA) women who gave birth to a child during a 12-month period spanning September 2003 to September 2004. A total of 1515 (28%) of all identified families were determined to be ineligible for participation for three primary reasons: not speaking English as the primary language in the home, residence in a non-target county, and intent to move within three years. Of the 2691 eligible families who agreed to the randomization process, 1571 (58%) families were selected to participate using the sampling fractions that were continually updated from our data center. Of those families selected to participate in the study, 1292 (82%) completed a home visit at 2 months of child age, at which point they were formally enrolled in the study. Seventy percent of enrolled families had an average income of less than twice the poverty threshold for the US; 99% of primary caregivers were the index child’s biological mother; 41% of mothers had 12 years of schooling or less, while only 16% had at least 4 years of post-secondary education. The sample was 43% African American and 51% male. Further details of FLP sampling plan and recruitment procedures are available in Vernon-Feagans, Cox and the FLP Investigators (2013).
Procedures
The data for this analysis were collected from home visits at child ages 7, 15, 24, 36, 48, and 60 months At all home visits for data collection, primary caregivers provided demographic information and information on numerous aspects of family life. The visit procedures at all time points included collection of physical measurements and administration of questionnaires relating to the composition of the household and relationships and resources. At 36, 48 and 60 months of age, children participated in task batteries to assess executive function. At age 36 months, children were administered a brief measure of IQ.
Measures
Executive Function.
The battery included three inhibitory control tasks (Simon-like Spatial Conflict, Stroop-like Silly Sounds, and Farm Animal go no-go), two working memory tasks (a span-like task and a self-ordered pointing task), and one attention shifting task (item selection modeled on the Dimensional Change Card Sort task; Jacques & Zelazo, 2001). Full details regarding the tasks, administration rules, psychometric properties, and scoring approach for the battery are available in a number of publications (e.g., Willoughby, Wirth, Blair & the FLP Investigators, 2012). Item response theory was used to generate expected a posteriori (EAP) scores for each task (see Willoughby, Wirth, Blair, & FLP Investigators, 2012). EAP scores were averaged to form a composite measure of EF ability at 36, 48, and 60 months of age.
Body Mass Index.
Child weight was measured to the nearest 0.1 kg and child height to the nearest 0.1 cm. BMI was calculated as kg/m2. Raw scores for BMI were used in data analysis.
Weight Status.
Weight status at each time point (obese, overweight, normal weight, and underweight) was calculated using CDC reference data, adjusted for age and sex. [Note: CDC reference data for BMI are not available for ages below 24 months. N=419 participants were younger than 24 months (22.2 – 23.9 months) at the 24 month time point.]
Cumulative Risk Index.
Informed largely by extensive prior work with these data (see Vernon Feagans et al., 2013), we created a longitudinal cumulative risk composite comprising 8 variables—family income, maternal education, constant spouse/partner living in the home, hours of employment, occupational prestige, household density, neighborhood noise and neighborhood safety. Full details are available in Vernon Feagans et al., (2013)
Covariates
included race (African American = 1, White = 0), state (PA = 1, NC = 1), child sex (male = 1) and estimated full scale IQ measured at age 36 months using the Wechsler Preschool and Primary Scales of Intelligence (Wechsler, 2002).
Missing Data
To assess possible differential attrition in the sample at each time point we examined variables for which we had complete information collected at infant age 2 months. Few variables indicated differences between families who were present and those who were missing. Participants missing data on EF were more likely to be male (χ2 = 14.8, p < .0001) and more likely to be living in North Carolina (χ2 = 12.5, p < .0001). Participants missing data on BMI were more likely to live in North Carolina (χ2 = 25.7, p < .0001).
Data Analysis
Latent growth curve (LGC) models were estimated to test key questions. All models were estimated in Mplus version 8 using a robust full information maximum likelihood estimator that uses all available data and is superior to listwise deletion and mean replacement missing data methods (Enders, 2001). A series of unconditional univariate and multivariate LGCs were estimated to characterize patterns of change in EF and BMI raw scores. The LGCs for EF and BMI were parameterized such that the intercept represented the level of each construct at age 36 and 24 months, respectively and the slope terms represented change from 36–60 months and 24–60 months. Given overlapping time points for both constructs but an earlier time point (24 months) for the measurement of BMI, the intercept for EF was regressed on the intercept for BMI and the linear slope for EF was regressed on the intercept and linear and quadratic slopes for BMI. The covariance between the EF and BMI linear slope parameters in the multivariate LGC was of primary interest. Following standard practice, model fit was evaluated using likelihood ratio tests and global fit indices (CFI > .95, RMSEA ≤ .05 were indicative of good fit).
Results
Descriptive statistics are presented in Table 1 and correlations among variables in the analysis are presented in Table 2. Table 2 indicates moderate to large correlations among measures of EF and IQ. Cumulative risk exhibited comparatively smaller correlations with these cognitive measures. Notably, measures of BMI are uncorrelated with measures of EF, IQ, and cumulative risk. Table 2 does indicate that BMI maintained high rank order stability in early childhood, particularly at ages 36, 48, and 60 months. Associations of BMI at these time points with BMI at age 24 months are somewhat reduced, particularly the association between BMI at 60 months and BMI at 24 months (r = .56, p < .0001). The number of participants and percent of the sample considered obese (≥95%), overweight (≥85%), normal weight, and underweight (< 5%) at each time point according to CDC reference data are reported in Table 3.
Table 1.
Descriptive statistics and number of participants for raw score and z-score height, weight, and BMI, Descriptive statistics and number of participants for analysis variables
| Variable | N | Mean | SD |
|---|---|---|---|
| BMI 24mos raw score | 1068 | 17.36 | 1.68 |
| BMI 24mos z-score* | 649 | .52 | 1.07 |
| BMI 36mos raw score | 1049 | 16.61 | 1.67 |
| BMI 36mos z-score | 1049 | .46 | 1.12 |
| BMI 48mos raw score | 1011 | 16.55 | 2.83 |
| BMI 48mos z-score | 1011 | .56 | 1.24 |
| BMI 60mos raw score | 1036 | 16.60 | 2.39 |
| BMI 60mos z-score | 1036 | .62 | 1.14 |
| Weight 24mos kg raw score | 1096 | 12.89 | 1.70 |
| Weight 24mos z-score | 1096 | .17 | 1.15 |
| Weight 36mos kg raw score | 1057 | 15.23 | 2.23 |
| Weight 36mos z-score | 1057 | .42 | 1.11 |
| Weight 48mos kg raw score | 1011 | 17.53 | 3.77 |
| Weight 48mos z-score | 1011 | .45 | 1.09 |
| Weight 60mos kg raw score | 1036 | 20.26 | 4.04 |
| Weight 60mos z-score | 1036 | .51 | 1.11 |
| Height 24mos cm raw score | 1075 | 86.11 | 3.95 |
| Height 24mos z-score** | 894 | −.08 | 1.04 |
| Height 36mos cm raw score | 1055 | 95.61 | 4.24 |
| Height 36mos z-score | 1055 | .13 | 1.03 |
| Height 48mos cm raw score | 1016 | 102.66 | 4.48 |
| Height 48mos z-score | 1016 | .22 | 1.01 |
| Height 60mos cm raw score | 1042 | 110.14 | 5.68 |
| Height 60mos z-score | 1042 | .32 | 1.07 |
| Child age 24mos | 1144 | 24.88 | 1.95 |
| Child age 36mos | 1123 | 37.05 | 1.75 |
| Child age 48mos | 1049 | 48.31 | 1.49 |
| Child age 60mos | 1099 | 60.62 | 3.26 |
| EF EAP score 36mos | 973 | −0.54 | 0.54 |
| EF EAP score 48mos | 1009 | −0.13 | 0.51 |
| EF EAP score 60mos | 1038 | 0.29 | 0.48 |
| WPPSI-III Estimated Full-Scale IQ | 1046 | 93.64 | 16.50 |
CDC reference data for BMI z-score are not available for children less than 24 months of age.
CDC reference data for z-score height are available only for 23.5 months of age and above.
Table 2.
Correlation among variables in the analysis.
| EF36 | EF48 | EF60 | IQ | Risk | BMI 24m | BMI 36m | BMI 48m | |
|---|---|---|---|---|---|---|---|---|
| EF 36mos | --- | |||||||
| EF 48mos | .37 | --- | ||||||
| EF 60mos | .32 | .59 | --- | |||||
| IQ 36mos | .40 | .54 | .47 | --- | ||||
| Risk mean | −.29 | −.36 | −.33 | −.45 | --- | |||
| BMI 24mos | −.03 | −.04 | −.01 | −.02 | .01 | --- | ||
| BMI 36mos | .01 | −.02 | −.02 | .02 | −03 | .69 | --- | |
| BMI 48mos | .01 | −.03 | −.03 | .02 | −.02 | .62 | .83 | --- |
| BMI 60mos | −.02 | −.03 | −.08 | .01 | −.01 | .56 | .77 | .86 |
Correlations in bold are p < .0001
Table 3.
The number of participants and percent of the sample considered overweight (≥85%) and obese (≥95%) at each time point according to CDC reference data.
| Category | 24m N (%)* | 36m N (%) | 48m N (%) | 60m N (%) |
|---|---|---|---|---|
| underweight** | 18 (3%) | 17 (2%) | 16 (2%) | 9 (1%) |
| normal weight | 482 (74%) | 869 (83%) | 807 (80%) | 784 (76%) |
| overweight | 127 (20%) | 132 (13%) | 136 (13%) | 156 (15%) |
| obese | 40 (6%) | 48 (4%) | 68 (7%) | 96 (9%) |
39 children were 22 months old and 398 children were 23 months old at the 24 month time point. CDC reference data are not available for children younger than 24 months.
underweight defined as CDC reference data z-score <−2 SD for weight for age
Latent Growth Model relating change in Executive Function to change in BMI
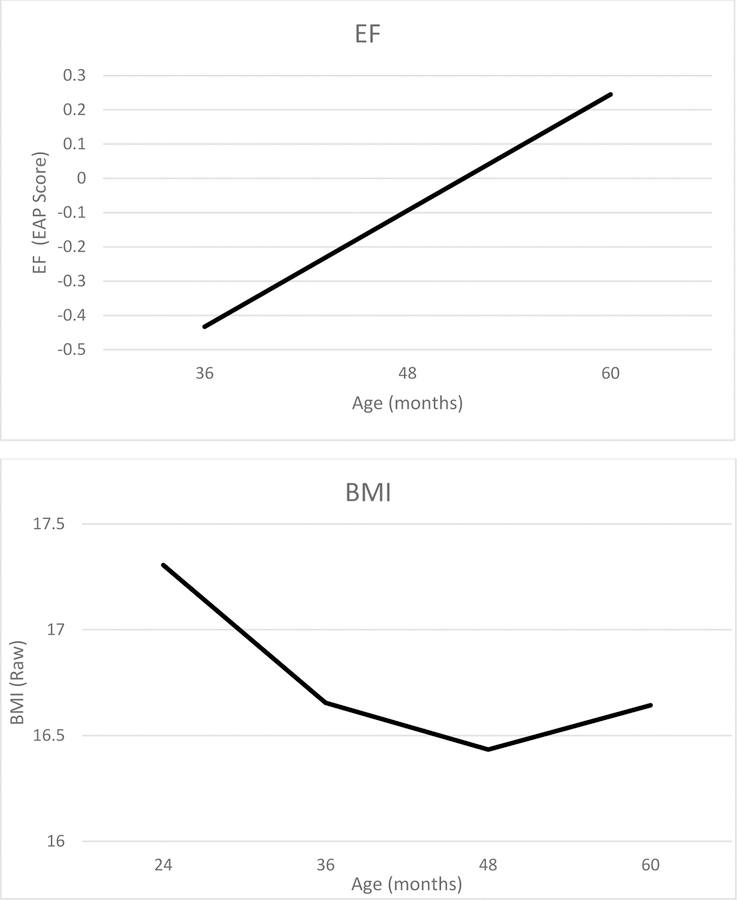
Preliminary unconditional models indicated that change in EF was adequately characterized by a linear model while change in BMI required a fixed quadratic slope in addition to a linear slope term to represent an initial decline followed by increase. Means and variances for all latent growth parameters from the fully conditional model are presented in Table 4. The intercept for EF is negative, reflecting the longitudinal scaling of the EAP score, and the linear slope is positive, indicating growth in EF from age 36 to 60 months. The intercept for BMI is positive, the linear slope is negative, and quadratic slope is positive, indicating that, on average, BMI declined linearly from age 24 to 60 months but that change in BMI was also characterized by positive acceleration. All growth parameters were significantly different from zero and variances for the intercept and linear slope terms for both constructs are significant. The model fit the data well (χ2(35) = 49.02, p = .06, CFI=.996, RMSEA=.018, SRMR=.016). Slope and intercept are positively correlated for BMI (φ=0.14, se=0.05, p < .01) but are uncorrelated for EF. The model implied trajectories for EF and BMI are shown in Figure 1.
Table 4.
Means and variances for all latent growth parameters.
| Parameter | M (SE) | Variance (SE) |
|---|---|---|
| Executive Function | ||
| Intercept | −1.75 (.09)*** | .036 (.012)** |
| Linear slope | 0.45 (.01)*** | .024 (.007)*** |
| BMI | ||
| Intercept | 17.05 (.14)*** | 1.77 (.151)*** |
| Linear slope | −0.52 (.11)*** | 0.26 (.038)*** |
| Quadratic slope | 0.22 (.01)*** | 0.00 (fixed) |
p < .01
p < .001
Figure 1.
Model implied trajectories for EF and BMI. All covariates are grand mean centered.
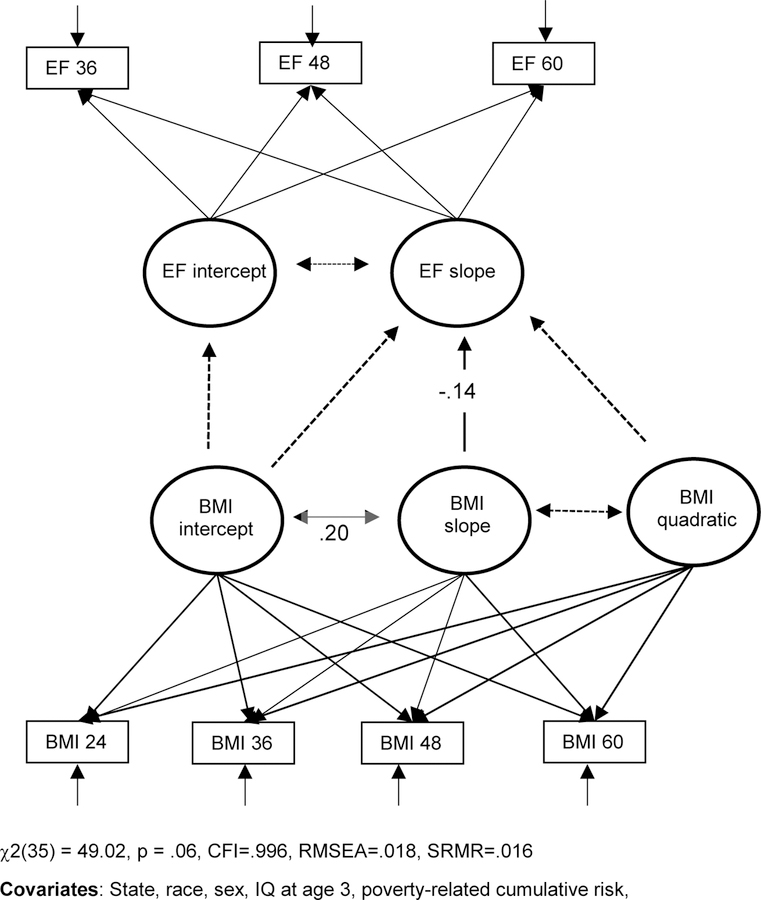
As hypothesized, the model in Figure 2 indicated a negative association between the linear slope for EF and the linear slope for BMI (b=−0.04, se=0.02, p < .05, β=−.14). This effect indicates that children exhibiting greater decline in BMI from age 24 to age 60 months exhibited greater increase in EF from age 36 to 60 months. The intercept for BMI and intercept for EF are not related.
Figure 2.
Association between change in BMI from age 2 to 5 years and change in EF from age 3 to 5 years.
Other predictors of the intercept for EF indicated that children in Pennsylvania had higher EF at 36 months, on average, than children in North Carolina (b=0.18, se=0.04, p < .0001, β=.26) and that mean EF at age 36 months for males was lower than that for females (b=−0.08, se=0.03, p < .005, β=−.12). Cumulative poverty-related risk was negatively (b=−0.08, se=0.02, p < .001, β=−.16) and IQ was strongly positively related to EF at 36 months (b=0.01, se=0.001, p < .0001, β=.56). No variable other than state of residence (b=−0.07, se=0.02, p < .01, β=−.19) predicted the slope for EF, with children in Pennsylvania exhibiting less growth from age 36 to 60 months.
The only predictor of the intercept, linear slope, and quadratic slope for BMI was sex, with males presenting higher BMI at 24 months (b=0.41, se=0.10, p < .0001, β=.15), greater linear decline in BMI, (b=−0.28, se=0.09, p < .005, β=−.26), and greater acceleration in change in BMI as indicated by a positive quadratic slope (b=0.06, se=0.03, p < .05, β=.58). Race was also associated with the quadratic slope (b=0.08, se=0.03, p < .01, β=.82), with African American children exhibiting greater acceleration in BMI.
We next added birth weight, maternal BMI, paternal BMI, and whether the child spent time in the neonatal intensive care unit (NICU) as a robustness check to the LGM relating change in EF to change in BMI. The model continued to fit the data well, (χ2(52) = 63.6, p = .12, CFI=.997, RMSEA=.013, SRMR=.018). NICU stay was associated with a lower initial level of EF (b=−0.17, se=0.05, p< .001, β=−.11), and birth weight with a higher initial level of EF (b=0.04, se=0.02, p = .05, β=.06). Maternal BMI (b=0.03, se=.008, p < .05, β=.14), paternal BMI (b=0.03, se=0.01, p < .05, β=.11), and birth weight (b=0.29, se=0.09, p < .001, β=.13) were all positively related to the initial level of BMI. None of these additional variables were related to the linear slope for BMI or EF. Maternal BMI was related to greater acceleration in change in BMI as indicated by a positive effect on the quadratic slope for BMI (b=.006, se=.002, p < .01, β=.62). Addition of these variables to the model did not alter the association between the slope for BMI and the slope for EF or alter coefficients for predictors of interest on EF and BMI.
We also ran two additional robustness checks. We first ran the analysis using the CDC reference BMI z-scores instead of raw scores. Despite the reduced sample size available for this analysis at 24 months, the association between the linear slope for EF and the linear slope for BMI remained significant, with the standardized effect increasing slightly (b=−0.09, se=0.04, p < .05, β=−.16). Second, we reran both the z-score and the raw score analysis deleting z-score outliers for height (±3SD) at each time point using CDC reference data. Doing so reduced the sample size only minimally and had no effect on associations among variables (results not shown).
Discussion
This analysis confirmed a hypothesized inverse linear association between change in BMI and change in EF in early childhood. Children exhibiting greater linear decline in BMI from 2 to 5 years of age exhibited greater linear increase in EF from age 3 to 5 years. The intercepts for BMI and EF were unrelated, indicating that the association was not present at baseline, but emerged developmentally. Given the young age of the sample, we interpret this finding as support for the hypothesized energetic trade-off between brain development and fat deposition in early childhood (Kuzawa and Blair, in press). Specifically, we propose that inversely correlated change between BMI and EF reflects an aspect of development in which between-child variation in the energy demand of the developing brain affects between-child variation in fat deposition at a time when children are typically losing fat mass in advance of the adiposity rebound (Rolland-Cachera et al., 1984).
Although data with which to estimate between person variation in the energy requirement of the developing brain in childhood are rare, such variation and the hypothesized inverse relation of this variation to between person variation in fat mass is suggested by genetic analyses of BMI. Specifically, a large (N=339,224) genome-wide association meta-analysis found that genes associated with BMI are primarily expressed in the central nervous system and associated with neuronal function and development (Locke et al., 2015). Using a variety of approaches to determine which aspects of CNS function and where in the brain BMI-associated genes were expressed, the authors found that the largest category of BMI-relevant genes are associated with processes of neuronal transmission and development, and strongly expressed in brain areas associated with learning and memory, including the hippocampus, frontal cortex, and limbic structures.
In addition, three studies demonstrate evidence for genetic pleiotropy between BMI and grey matter volume of multiple cortical and subcortical structures, pointing to an energetic trade-off with body weight gain as central to the genetic architecture of obesity risk. The first of these (Ho et al., 2010) found that a single nucleotide polymorphism in the fat mass and obesity-associated (FTO) gene was pleiotropically associated with greater BMI and reduced grey matter volumes in frontal and occipital lobes in otherwise healthy older adults (N=206). The risk allele of FTO was not associated with the presence of MetS, indicating that the association was not attributable to metabolic dysregulation. Second, an analysis with a large pedigreed adult Mexican American sample (N=839) reported evidence for pleiotropic genetic effects linking elevated BMI with widespread reductions in surface area throughout the cortex (Curran et al., 2013). Third, a multiple cohort study found that the polygenic risk score derived from the Locke et al. (2015) meta-analysis was associated with reduced grey matter volume in orbital frontal cortex and positively associated with BMI (Opel et al., 2017). The authors interpret this finding as evidence for a genetically-based trait marker of obesity risk in which reductions in grey matter in PFC precede obesity rather than serve only as an indicator of accelerated neurodegeneration precipitated by MetS.
Compatibility of the brain-body energetic trade-off with alternative explanations for the inverse association between EF and BMI.
Although we are not able to definitively rule out metabolic dysregulation as a factor in the association between BMI and EF in this analysis, it is unlikely to have a major role in our findings given the young age of the sample and correspondingly short time for adverse effects to accumulate. It is important to emphasize, however, the extent to which the proposed brain-body energetic trade-off complements, and is consistent with, additional explanations for the association between EF and BMI. Prior analyses (Francis and Susman, 2009; Graziano et al., 2013) have documented negative relations between aspects of EF, namely inhibitory control and the ability to delay gratification, and change in BMI across childhood. Such deficits in EF are theorized to lead to overeating and the increased consumption of high fat foods, resulting in metabolic dysregulation and low level inflammation that would precipitate changes in PFC structure and function leading to further impairment of EF. This is particularly the case in light of increases in self-determination and volitional decision making occurring with advancing child age that would be expected to affect food choices and levels of physical activity.
The proposed brain-body energetic trade-off is entirely consistent with the line of reasoning in which genetic and environmental pathways are likely and complementary. As such, the theorized brain-body energetic trade-off provides a basis for the identification of individuals at high risk for a vicious cycle in which genetically-mediated reductions in brain energy demand and corresponding reductions in EF increase the likelihood of high fat food consumption, leading to increased weight gain, increased risk for metabolic dysregulation, and further impairment in EF. The inverse association between EF and consumption of high fat foods is generally well established (Hall, 2016). An important direction for future research will be studies designed to examine whether genetic risk for elevated BMI is associated with reduced EF and impulsive consumption of high fat food. Further, we take it as tentative support for the theory that the association between change in BMI and change in EF was observed in this sample, given relatively low rates of obesity and overweight in early childhood seen here. Prior demonstration of associations between BMI and EF with slightly older child participants have included larger proportions of participants classified as obese or overweight using CDC reference data (Chojnacki et al., 2018; Kamijo et al., 2012). An important follow-up to this analysis with the Family Life Project sample will be to examine rates of obesity and overweight in adolescence with newly acquired longitudinal data as a function of change in BMI and change in EF in early childhood and genetic risk for obesity.
Prevention.
Given associations of early age and elevated BMI at the adiposity rebound with lifetime obesity risk, the theory of the brain-body energetic tradeoff can inform targeted prevention efforts in early childhood. Children at high risk for obesity due to polygenic risk for elevated BMI and lower EF could be identified and prioritized in analyses of prevention data and targeted for receipt of early intervention programming. Further, given the association of EF with school readiness (Blair and Raver, 2015), the proposed energetic trade-off could inform early childhood education (ECE) efforts. High quality ECE has the potential to promote school readiness as well as reduce obesity risk. The extent to which ECE programming increases the energy demand of the developing brain is currently unknown. Programs that have demonstrated effects on EF (Diamond et al., 2007; Sasser et al., 2017) would be expected to increase the brain’s need for metabolic substrate in ways that, in theory, could reduce weight gain in early childhood and thereby reduce obesity risk. An example of this combined effect is seen in a follow-up of the intensive early intervention, the Abecedarian Project, in which males in the treatment group experienced reduced weight velocity in infancy and lower BMI at the adiposity rebound relative to the control group (Campbell et al., 2014). As such, male children receiving the intervention were less likely to be overweight at 5 and 8 years in addition to exhibiting gains in IQ at these ages. Males receiving the treatment were also less likely to exhibit characteristics of MetS at age 35. Similar effects at the population level are seen in an analysis of children participating in Head Start relative to a comparison group drawn from primary health care system data (N=43,748). Children who were obese or overweight when entering Head Start had greater declines in BMI over the academic year relative to children in the comparison group (Lumeng et al., 2015).
Limitations and conclusion.
Although there are several strengths to this analysis, including a strong theoretical and empirical basis for the hypothesized association and the analysis of longitudinal data in early childhood, there are several limitations. One is the absence of information on diet and physical activity as well as other factors such as screen time and sleep that could indicate whether differences in energy intake and expenditure in early childhood might play a role in the association between BMI and EF. Although such variables are undoubtedly factors in weight gain, the inclusion of covariates (e.g., cumulative risk, sex) that might relate to variation in these variables helps to mitigate their potential role in associations reported here.
A further limitation is that the sample is generally high-risk and low-income. To the extent that the range of poverty-related risk is attenuated, coefficients might under- or overestimate the magnitude of the association between change in EF and change in BMI. As well, EF can be difficult to measure in young children, placing a further limitation on the precision with which coefficients are estimated. Finally, the study is limited by the absence of direct assessment of the putative mechanism of effect, namely, the energy requirement of the developing brain. Despite these limitations, this study analyzed data from a large longitudinal sample to confirm an innovative hypothesis regarding one potential contributor to the growing obesity epidemic.
Research Highlights.
Deficits in EF associated with obesity are understood primarily as a consequence of metabolic problems and low-grade inflammation precipitated by obesity.
We find an inverse developmental relation between change in EF and change in BMI in early childhood.
Several sources of evidence support the idea that this relation is attributable to individual variation in the energy demand of the developing brain.
Results suggest an inverse relation between brain development and fat deposition in early childhood that can inform prevention efforts.
Acknowledgements:
The Family Life Project was supported by the NICHD grant P01 HD3966, with cofunding from the NIDA, and NICHD grant R01 HD051502.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Data Availability: The authors confirm that all data and analysis files underlying the findings are fully available without restriction from the first author, Clancy Blair.
References
- Attwell D, & Laughlin SB (2001). An energy budget for signaling in the grey matter of the brain. Journal of Cerebral Blood Flow and Metabolism, 21(10), 1133–1145. doi: 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2015). School readiness and self-regulation: A developmental psychobiological approach. Annual Review of Psychology, 66, 711–731. doi: 10.1146/annurev-psych-010814-015221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, … Gould E (2015). Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proceedings of the National Academy of Sciences of the United States of America, 112(51), 15731–15736. doi: 10.1073/pnas.1511593112 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, & Pan Y (2014). Early childhood investments substantially boost adult health. Science (New York, N.Y.), 343(6178), 1478–1485. doi: 10.1126/science.1248429 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki MR, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH, & Khan NA (2018). The negative influence of adiposity extends to intraindividual variability in cognitive control among preadolescent children. Obesity (Silver Spring, Md.), 26(2), 405–411. doi: 10.1002/oby.22053 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JE, McKay DR, Winkler AM, Olvera RL, Carless MA, Dyer TD, … Glahn DC (2013). Identification of pleiotropic genetic effects on obesity and brain anatomy. Human Heredity, 75(2–4), 136–143. doi: 10.1159/000353953 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, & Munro S (2007). Preschool program improves cognitive control. Science (New York, N.Y.), 318(5855), 1387–1388. doi:318/5855/1387 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Oga T, & Fujita I (2009). Spinogenesis and pruning scales across functional hierarchies. Journal of Neuroscience, 29(10), 3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2001). The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement, 61(5), 713–740. [Google Scholar]
- Francis LA, & Susman EJ (2009). Self-regulation and rapid weight gain in children from age 3 to 12 years. Archives of Pediatrics & Adolescent Medicine, 163(4), 297–302. doi: 10.1001/archpediatrics.2008.579 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, & Gao W (2018). Imaging structural and functional brain development in early childhood. Nature Reviews.Neuroscience, 19(3), 123–137. doi: 10.1038/nrn.2018.1 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, & Raichle ME (2014). Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metabolism, 19(1), 49–57. doi: 10.1016/j.cmet.2013.11.020 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Kelleher R, Calkins SD, Keane SP, & Brien MO (2013). Predicting weight outcomes in preadolescence: The role of toddlers’ self-regulation skills and the temperament dimension of pleasure. International Journal of Obesity (2005), 37(7), 937–942. doi: 10.1038/ijo.2012.165 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA (2016). Executive-control processes in high-calorie food consumption. Current Directions in Psychological Science, 25(2), 91–98. doi: 10.1177/0963721415625049 [DOI] [Google Scholar]
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, … Alzheimer’s Disease Neuroimaging Initiative. (2010). A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proceedings of the National Academy of Sciences of the United States of America, 107(18), 8404–8409. doi: 10.1073/pnas.0910878107 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, … Hillman CH (2012). The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity (Silver Spring, Md.), 20(12), 2406–2411. doi: 10.1038/oby.2012.112 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Pontifex MB, Khan NA, Raine LB, Scudder MR, Drollette ES, … Hillman CH (2014). The negative association of childhood obesity to cognitive control of action monitoring. Cerebral Cortex (New York, N.Y.: 1991), 24(3), 654–662. doi: 10.1093/cercor/bhs349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW & Blair C (in press). A hypothesis linking the energy demand of the brain to obesity risk. Proceedings of the National Academy of Sciences of the United States of America [DOI] [PMC free article] [PubMed]
- Kuzawa CW, Chugani HT, Grossman LI, Lipovich L, Muzik O, Hof PR, … Lange N (2014). Metabolic costs and evolutionary implications of human brain development. Proceedings of the National Academy of Sciences of the United States of America, 111(36), 13010–13015. doi: 10.1073/pnas.1323099111 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, … Speliotes EK (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature, 518(7538), 197–206. doi: 10.1038/nature14177 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Kaciroti N, Sturza J, Krusky AM, Miller AL, Peterson KE, Lipton R and Reischl TM, (2015). Changes in body mass index associated with head start participation. Pediatrics, 135(2), e449–e456. doi/ 10.1542/peds.2014-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, & Spencer SJ (2014). Obesity and neuroinflammation: A pathway to cognitive impairment. Brain, Behavior, and Immunity, 42, 10–21. doi: 10.1016/j.bbi.2014.04.001 [doi] [DOI] [PubMed] [Google Scholar]
- Opel N, Redlich R, Kaehler C, Grotegerd D, Dohm K, Heindel W, … Dannlowski U (2017). Prefrontal gray matter volume mediates genetic risks for obesity. Molecular Psychiatry, 22(5), 703–710. doi: 10.1038/mp.2017.51 [doi] [DOI] [PubMed] [Google Scholar]
- Peneau S, Gonzalez-Carrascosa R, Gusto G, Goxe D, Lantieri O, Fezeu L, … Rolland-Cachera MF (2016). Age at adiposity rebound: Determinants and association with nutritional status and the metabolic syndrome at adulthood. International Journal of Obesity (2005), 40(7), 1150–1156. doi: 10.1038/ijo.2016.39 [doi] [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, & Kostovic I (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13281–13286. doi: 10.1073/pnas.1105108108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille M, & Patois E (1984). Adiposity rebound in children: A simple indicator for predicting obesity. The American Journal of Clinical Nutrition, 39(1), 129–135. doi: 10.1093/ajcn/39.1.129 [DOI] [PubMed] [Google Scholar]
- Rusinek H, & Convit A (2014). Obesity: Cerebral damage in obesity-associated metabolic syndrome. Nature Reviews. Endocrinology, 10(11), 642–644. doi: 10.1038/nrendo.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser TR, Bierman KL, Heinrichs B, & Nix RL (2017). Preschool intervention can promote sustained growth in the executive-function skills of children exhibiting early deficits. Psychological Science, 28(12), 1719–1730. doi: 10.1177/0956797617711640 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, & Toga AW (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309–315. doi: 10.1038/nn1008 [doi] [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, … Schwartz MW (2012). Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation, 122(1), 153–162. doi: 10.1172/JCI59660 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M, & FLP Key Investigators. (2013). The family life project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development, 78(5), 1–150, vii. doi: 10.1111/mono.12046 [doi] [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, … Pradhan K (2009). Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring, Md.), 17(1), 60–65. doi: 10.1038/oby.2008.469 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2002). Wechsler Preschool and Primary Scale of Intelligence, third edition Psych Corp: San Antonio TX. [Google Scholar]
- Willoughby MT, Wirth RJ, Blair C, & Family Life Project Investigators. (2012). Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment, 24(2), 418–431. doi: 10.1037/a0025779 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Guo C, & Liu Y (2018). Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neuroscience and Biobehavioral Reviews, 84, 225–244. doi:S0149-7634(17)30479-7 [pii] [DOI] [PubMed] [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, & Convit A (2012). Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2060–2067. doi: 10.1161/ATVBAHA.112.252759 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, & Convit A (2012). Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics, 130(4), e856–64. doi: 10.1542/peds.2012-0324 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, & Convit A (2014). Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring, Md.), 22(8), 1865–1871. doi: 10.1002/oby.20801 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]