Abstract
Nature is in constant flux, so animals must account for changes in their environment when making decisions. How animals learn the timescale of such changes and adapt their decision strategies accordingly is not well understood. Recent psychophysical experiments have shown humans and other animals can achieve near-optimal performance at two alternative forced choice (2AFC) tasks in dynamically changing environments. Characterization of performance requires the derivation and analysis of computational models of optimal decision-making policies on such tasks. We review recent theoretical work in this area, and discuss how models compare with subjects’ behavior in tasks where the correct choice or evidence quality changes in dynamic, but predictable, ways.
Introduction
To translate stimuli into decisions, animals interpret sequences of observations based on their prior experiences1. However, the world is fluid: The context in which a decision is made, the quality of the evidence, and even the best choice can change before a judgment is formed, or an action taken. A source of water can dry up, or a nesting site can become compromised. But even when not fully predictable, changes often have statistical structure: Some changes are rare, others are frequent, and some are more likely to occur at specific times. How have animals adapted their decision strategies to a world that is structured, but in flux?
Classic computational, behavioral, and neurophysiological studies of decision-making mostly involved tasks with fixed or statistically stable evidence1–3. To characterize the neural computations underlying decision strategies in changing environments, we must understand the dynamics of evidence accumulation4. This requires novel theoretical approaches. While normative models are a touchstone for theoretical studies5, 6, even for simple dynamic tasks the computations required to optimally translate evidence into decisions can become prohibitive7. Nonetheless, quantifying how behavior differs from normative predictions helps elucidate the assumptions animals use to make decisions8, 9.
We review normative models and compare them with experimental data from two alternative forced choice (2AFC) tasks in dynamic environments. Our focus is on tasks where subjects passively observe streams of evidence, and the evidence quality or correct choice can vary within or across trials. Humans and animals adapt their decision strategies to account for such volatile environments, often resulting in performance that is nearly optimal on average. However, neither the computations they use to do so nor their neural implementations are well understood.
Optimal evidence accumulation in changing environments
Normative models of decision-making typically assume subjects are Bayesian agents14, 15 that probabilistically compute their belief of the state of the world by combining fresh evidence with previous knowledge. Beyond normative models, notions of optimality require a defined objective. For instance, an observer may need to report the location of a sound16, or the direction of a moving cloud of dots5, and is rewarded if the report is correct. Combined with a framework to translate probabilities or beliefs into actions, normative models provide a rational way to maximize the net rewards dictated by the environment and task. Thus an optimal model combines normative computations with a policy that translates a belief into the optimal action.
How are normative models and optimal policies in dynamic environments characterized? Older observations have less relevance in rapidly changing environments than in slowly changing ones. Ideal observers account for environmental changes by adjusting the rate at which they discount prior information when making inferences and decisions17. In Box 1 we show how, in a normative model, past evidence is nonlinearly discounted at a rate dependent on environmental volatility5, 17. When this volatility8 or the underlying evidence quality13, 18 are unknown, they must also be inferred.
Box 1 – Normative evidence accumulation in dynamic environments.
Discrete time.
At times t1:n an observer receives a sequence of noisy observations, ξ1:n, of the state S1:n, governed by a two-state Markov process (Fig. 1b). Observation likelihoods, f± (ξ) = P(ξ|S±), determine the belief (log-likelihood ratio: LLR), , after observation n. If the observations are conditionally independent, the LLR can be updated recursively5, 17:
| (1) |
where h is the hazard rate (probability the state switches between times tn−1 and tn). The belief prior to the observation at time tn, yn−1, is discounted according to the environment’s volatility h. When h = 0, Eq. (1) reduces to the classic drift-diffusion model (DDM), and evidence is accumulated perfectly over time. When h = 1/2, only the latest observation, ξn, is informative. For 0 < h < 1/2, prior beliefs are discounted, so past evidence contributes less to the current belief, yn, corresponding to leaky integration. When 1/2 < h < 1, the environment alternates.
Continuous time.
When tn−tn−1 = Δt ≪ 1, and the hazard rate is defined Δt · h, LLR evolution can be approximated by the stochastic differential equation5, 17:
| (2) |
where g(t) jumps between +g and — g at a rate h, Wt is a zero mean Wiener process with variance ρ2, and the nonlinear filter — 2h sinh(y) optimally discounts prior evidence. In contrast to the classic continuum DDM, the belief, y(t), does not increase indefinitely, but saturates due to evidence-discounting.
In 2AFC tasks, subjects accumulate evidence until they decide on one of two choices either freely or when interrogated. In these tasks, fluctuations can act on different timescales (Fig. 1a): 1) on each trial (Fig. 1b,c)5, 6, 2) unpredictably within only some trials19, 20, 3) between trials in a sequence11, 16, or 4) gradually across long blocks of trials21. We review findings in the first three cases and compare them to predictions of normative model.
Figure 1. Two alternative forced choice (2AFC) tasks in dynamic environments.
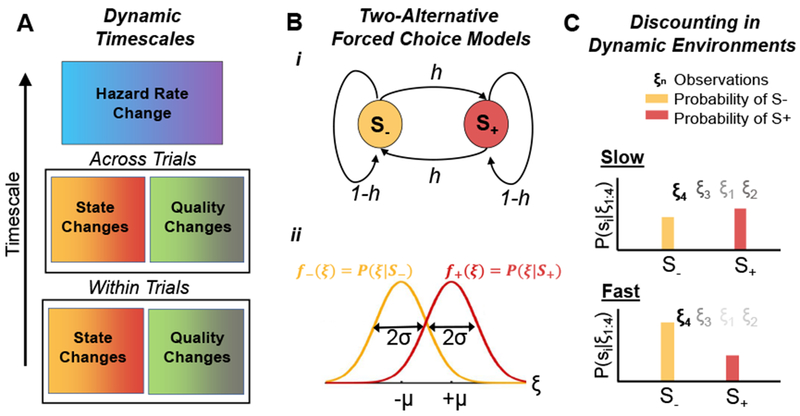
(A) Possible timescales of environmental dynamics: 1) The state (S+ or S−), or the quality of the evidence (e.g., coherence of random dot motion stimulus) may switch within a trial5, 6, 10, or 2) across trials11–13; 3) The hazard rate (switching rate, h), can change across blocks of trials6, 9. (B) In a dynamic 2AFC task, a two-state Markov chain with hazard rate h determines the state. (Bi) The current state (correct hypothesis) is either S+ (red) or S− (yellow). (Bii) Conditional densities of the observations, f±(ξ) = P(ξ|S±), shown as Gaussians with means ±μ and standard deviation σ. (C) Evidence discounting is shaped by the environmental timescale: (Top) In slow environments, posterior probabilities over the states, P(S±|ξ1:4); are more strongly influenced by the cumulative effect of past observations, ξ1:3, (darker shades of the observations, ξi, indicate higher weight) and thus points to S+. (Bottom) If changes are fast, beliefs depend more strongly on the current observation, ξ4, which outweighs older evidence and points to S−.
Within trial changes promote leaky evidence accumulation
Normative models of dynamic 2AFC tasks (Fig. 1b,c and 2a, Box 1) exhibit adaptive, nonlinear discounting of prior beliefs at a rate modified by expectations of the environment’s volatility (Fig. 1c) and saturation of certainty about each hypothesis, regardless of how much evidence is accumulated (Fig. 2a). Likewise, the performance of ideal observers at change points – times when the correct choice switches – depends sensitively on environmental volatility (Fig. 2aiii). In slowly changing environments, optimal observers assume that changes are rare, and thus adapt slowly after one has occured. Whereas, in rapidly changing environments, observers quickly update their belief after a change point. In contrast, ideal observers in static environments weigh all past observations equally, and their certainty grows without bound until a decision1, 3.
Figure 2. Dynamic State Changes.
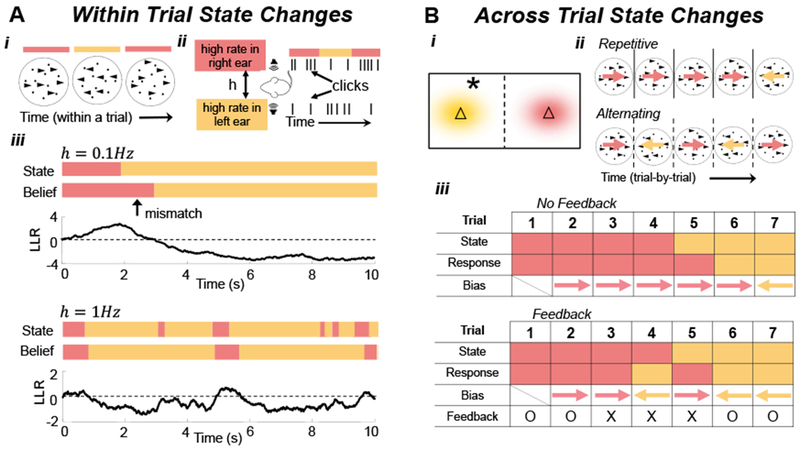
(A) State changes within trials in a (Ai) random dot motion discrimination (RDMD) task, in which drift direction switches throughout the trial5, and (Aii) dynamic auditory clicks task, in which the side of the higher rate stream alternates during the trial6. (Aiii) An ideal observer’s LLR (See Eq. (2) in Box 1) when the hazard rate is low (top panels: h=0.1Hz) and high (bottom panels: h=1Hz). Immediately after state changes, the belief typically does not match the state. (B) State changes across trials. (Bi) In the triangles task5, samples (star) are drawn from one of two Gaussian distributions (yellow and red clouds) whose centers are represented by triangles. The observers must choose the current center (triangle). (Bii) In an RDMD task, dots on each trial move in one of two directions (colored arrows) chosen according to a two-state Markov process. Depending on the switching rate, trial sequences may include excessive repetitions (Top), or alternations (Bottom). (Biii) (Top) Responses can be biased by decisions from previous trials. (Bottom) Probabilistic feedback (‘O’: correct; ‘X’: incorrect) affects initial bias (e.g., trials 3, 4, and 5), even when not completely reliable.
The responses of humans and other animals on tasks in which the correct choice changes stochastically during a trial share features with normative models: In a random dot-motion discrimination (RDMD) task, where the motion direction switches at unsignaled changepoints, humans adapt their decision-making process to the switching (hazard) rate (Fig. 2ai)5. Yet, on average, they overestimate the change rates of rapidly switching environments and underestimate the change rates of slowly switching environments, possibly due to ecologically-adaptive biases that are hard to train away. In a related experiment (Fig 2aii), rats were trained to identify which of two Poisson auditory click streams arrived at a higher rate22. When the identity of the higher-frequency stream switched unpredictably during a trial, trained rats discounted past clicks near-optimally on average, suggesting they learned to account for latent environmental dynamics6.
However, behavioral data are not uniquely explained by normative models. Linear approximations of normative models perform nearly identically17, and, under certain conditions, fit behavioral data well5, 6, 23. Do subjects implement normative decision policies or simpler strategies that approximate them? Subjects’ decision strategics can depend strongly on task design and vary across individuals5, 9, suggesting a need for sophisticated model selection techniques. Recent research suggests normative models can be robustly distinguished from coarser approximations when task difficulty and volatility are carefully tuned24.
Subjects account for correlations between trials by biasing initial beliefs
Natural environments can change over timescales that encompass multiple decisions. However, in many experimental studies, task parameters are fixed or generated independently across trials, so evidence from previous trials is irrelevant. Even so, subjects often use decisions and information from earlier trials to (serially) bias future choices25–27, reflecting ingrained assumptions about cross-trial dependencies21, 28.
To understand how subjects adapt to constancy and flux across trials, classic 2AFC experiments have been extended to include correlated cross-trial choices (Fig. 2b) where both the evidence accumulated during a trial and probabilistic reward provide information that can be used to guide subsequent decisions16, 29. When a Markov process30 (Fig. 1b) is used to generate correct choices, human observers adapt to these trial-to-trial correlations, and their response times are accurately modeled by drift diffusion11 or ballistic models16 with biased initial conditions.
Feedback or decisions across correlated trials impact different aspects of normative models31 including accumulation speed (drift)32–34, decision bounds11, or the initial belief on subsequent trials12, 35, 36. Given a sequence of dependent but statistically identical trials, optimal observers should adjust their initial belief and decision threshold16, 28, but not their accumulation speed in cases where difficulty is fixed across trials18. Thus, optimal models predict that observers should, on average, respond more quickly, but not more accurately28. Empirically, humans12, 35, 36 and other animals29 do indeed often respond faster on repeat trials, which can be modeled by per trial adjustments in initial belief. Furthermore, this bias can result from explicit feedback or subjective estimates, as demonstrated in studies where no feedback is provided (Fig. 2biii)16, 36.
The mechanism by which human subjects carry information across trials remains unclear. Different models fit to human subject data have represented inter-trial dependencies using initial bias, changes in drift rate, and updated decision thresholds11, 16, 34. Humans also tend to have strong preexisting repetition biases, even when such biases are suboptimal25–27. Can this inherent bias be overcome through training? The answer may be attainable by extending the training periods of humans or nonhuman primates5, 9, or using novel auditory decision tasks developed for rodents6, 29. Ultimately, high throughput experiments may be needed to probe how ecologically-adaptive evidence accumulation strategies change with training.
Time-varying thresholds account for heterogeneities in task difficulty
Optimal decision policies can also be shaped by unpredictable changes in decision difficulty. For instance, task difficulty can be titrated by varying the signal-to-noise ratio of the stimulus, so more observations are required to obtain the same level of certainty. Theoretical studies have shown that it is optimal to change one’s decision criterion within a trial when the difficulty of a decision varies across trials13, 18, 37. The threshold that determines how much evidence is needed to make a decision should vary during the trial (Fig. 3a) to incorporate up-to-date estimates of trial difficulty18. There is evidence that subjects use time-varying decision boundaries to balance speed and accuracy on such tasks38, 39.
Figure 3. Dynamic Evidence Quality.
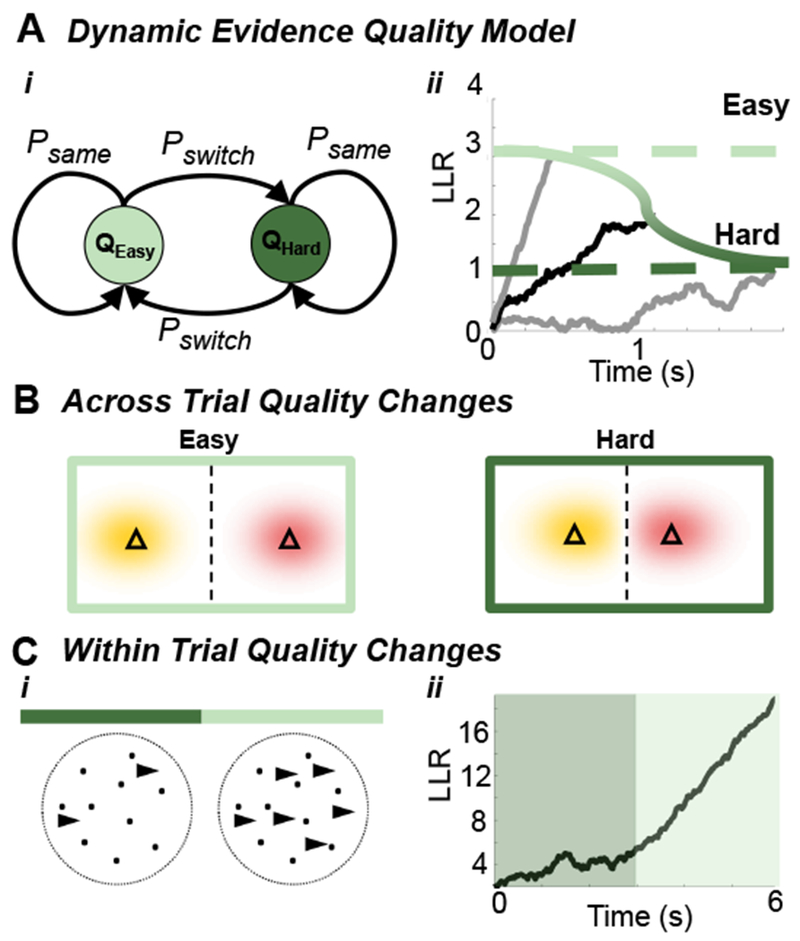
(A) Trial-to-trial two-state Markovian evidence quality switching: (Ai) Evidence quality switches between easy (Qeasy) and hard (Qhard) with probability Pswitch. (Aii) Optimal decision policies require time-varying decision thresholds. An observer who knows the evidence quality (easy or hard) uses a fixed threshold (grey traces, dashed lines) to maximize reward rate, but thresholds must vary when evidence quality is initially unknown (black trace, green gradient). (B) Different triangle task difficulties (from Fig. 2Ai): Triangles are spaced further apart in easy trials compared to hard trials. (C) Changes in quality within trials: (Ci) An RDMD task in which the drift coherence increases mid-trial, providing stronger evidence later in the trial. (Cii) The corresponding LLR increases slowly early in the trial, and more rapidly once evidence becomes stronger.
Dynamic programming can be used to derive optimal decision policies when trial-to-trial difficulties or reward sizes change. This method models complex decisions by breaking them into a sequence of steps, at which the value of an observer’s strategy is maximized recursively. For instance, when task difficulty changes across trials in a RDMD task, optimal decisions are modeled by a DDM with a time-varying boundary, in agreement with reaction time distributions of humans and monkeys18, 38. Both dynamic programming18 and parameterized function38, 40 based models suggest decreasing bounds maximize reward rates (Fig. 3a,b). This dynamic criterion helps participants avoid noise-triggered early decisions or extended deliberations18. An exception to this trend was identified in trial sequences without trials of extreme difficulty13, in which case the optimal strategy used a threshold that increased over time.
Time-varying decision criteria also arise when subjects perform tasks where information quality changes within trials (Fig. 3c)40, especially when initially weak evidence is followed by stronger evidence later in the trial. However, most studies use heuristic models to explain psychophysical data19, 20, suggesting a need for normative model development in these contexts. Decision threshold switches have also been observed in humans performing changepoint detection tasks, whose difficulty changes from trial-to-trial41, and in a model of value-based decisions, where the reward amounts change between trials42. Overall, optimal performance on tasks in which reward structure or decision difficulty changes across trials require time-varying decision criteria, and subject behavior approximates these normative assumptions.
One caveat is that extensive training or obvious across-trial changes are needed for subjects to learn optimal solutions. A meta-analysis of multiple studies showed that fixed threshold DDMs fit human behavior well when difficulty changes between trials were hard to perceive43. A similar conclusion holds when changes occur within trials44. However, when nonhuman primates are trained extensively on tasks where difficulty variations were likely difficult to perceive, they appear to learn a time-varying criterion strategy45. Humans also exhibit time-varying criteria in reward-free trial sequences where interrogations are interspersed with free responses46. Thus, when task design makes it difficult to perceive task heterogeneity or learn the optimal strategy, subjects seem to use fixed threshold criteria43, 44. In contrast, with sufficient training45, or when changes are easy to perceive46, subjects can learn adaptive threshold strategies.
Questions remain about how well normative models describe subject performance when difficulty changes across or within trials. How distinct do task difficulty extremes need to be for subjects to use optimal models? No systematic study has quantified performance advantages of time-varying decision thresholds. If they do not confer a significant advantage, the added complexity of dynamic thresholds may discourage their use.
When and how are normative computations learned and achieved?
Except in simple situations, or with overtrained animals, subjects can at best approximate computations of an ideal observer14. Yet, the studies we reviewed suggest that subjects often learn to do so effectively. Humans appear to use a process resembling reinforcement learning to learn the structure and parameters of decision task environments47. Such learning tracks a gradient in reward space, and subjects adapt rapidly when the task structure changes48. Subjects also switch between different near-optimal models when making inferences, which may reflect continuous task structure learning9. However, these learning strategies appear to rely on reward and could be noisier when feedback is probabilistic or absent. Alternatively, subjects may ignore feedback and learn from evidence accumulated within or across trials28, 46.
Strategy learning can be facilitated by using simplified models. For example, humans appear to use sampling strategies that approximate, but are simpler than, optimal inference9, 49. Humans also behave in ways that limit performance by, for instance, not changing their mind when faced with new evidence50. This confirmation bias may reflect interactions between decision and attention related systems that are difficult to train away51. Cognitive biases may also arise due to suboptimal applications of normative models52. For instance, recency bias can reflect an incorrect assumption of trial dependencies53. Subjects seem to continuously update latent parameters (e.g., hazard rate), perhaps assuming that these parameters are always changing21, 29.
The adaptive processes we have discussed occur on disparate timescales, and thus likely involve neural mechanisms that interact across scales. Task structure learning occurs over many sessions (days), while the volatility of the environment and other latent parameters can be learned over many trials (hours)6, 49. Trial-to-trial dependencies likely require memory processes that span minutes, while within trial changes require much faster adaptation (milliseconds to seconds).
This leaves us with a number of questions: How does the brain bridge timescales to learn and implement adaptive evidence integration? This likely requires coordinating fast neural activity changes with slower changes in network architecture8. Studies of decision tasks in static environments suggest that a subject’s belief and ultimate choice is reflected in evolving neural activity1–3, 54. It is unclear whether similar processes represent adaptive evidence accumulation, and, if so, how they are modulated.
Conclusions
As the range of possible descriptive models grows with task complexity8, 49, optimal observer models provide a framework for interpreting behavioral data5, 6, 34. However, understanding the computations subjects use on dynamic tasks, and when they depart from optimality, requires both careful comparison of models to data and comparisons between model classes55.
While we mainly considered optimality defined by performance, model complexity may be just as important in determining the computations used by experimental subjects56. Complex models, while more accurate, may be difficult to learn, hard to implement, and offer little advantage over simpler ones8, 9. Moreover, predictions of more complex models typically have higher variance, compared to the higher bias of more parsimonious models, resulting in a trade-off between the two9.
Invasive approaches for probing adaptive evidence accumulation are a work in progress57, 58. However, pupillometry has been shown to reflect arousal changes linked to a mismatch between expectations and observations in dynamic environments10, 27, 59. Large pupil sizes reflect high arousal after a perceived change, resulting in adaptive changes in evidence weighting. Thus, pupillometry may provide additional information for identifying computations underlying adaptive evidence accumulation.
Understanding how animals make decisions in volatile environments requires careful task design. Learning and implementing an adaptive evidence accumulation strategy needs to be both rewarding and sufficiently simple so subjects do not resign themselves to simpler computations43, 44. A range of studies have now shown that mammals can learn to use adaptive decision-making strategies in dynamic 2AFC tasks5, 6. Building on these approaches, and using them to guide invasive studies with mammals offers promising new ways of understanding the neural computations that underlie our everyday decisions.
Acknowledgements
We are grateful to Joshua Gold, Alex Piet, and Nicholas Barendregt for helpful feedback. This work was supported by an NSF/NIH CRCNS grant (R01MH115557) and an NSF grant (DMS-1517629). ZPK was also supported by an NSF grant (DMS-1615737). KJ was also supported by NSF grant DBI-1707400. WRH was supported by NSF grant SES-1556325.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gold JI & Shadlen MN The neural basis of decision making. Annu. review neuroscience 30 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Britten KH, Shadlen MN, Newsome WT & Movshon JA The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 12, 4745–4765 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogacz R, Brown E, Moehlis J, Holmes P & Cohen JD The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. review 113, 700 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Gao P et al. A theory of multineuronal dimensionality, dynamics and measurement. bioRxiv 214262 (2017). [Google Scholar]
- 5.Glaze CM, Kable JW & Gold JI Normative evidence accumulation in unpredictable environments. Elife 4, e08825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.**.Piet AT, El Hady A & Brody CD Rats adopt the optimal timescale for evidence integration in adynamic environment. Nat. Commun. 9, 4265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Rats can learn to optimally discount evidence when deciding between two dynamically switching auditory click streams, and they adapted to changes in environmental volatility.
- 7.Adams RP & MacKay DJ Bayesian online changepoint detection. arXiv preprint arXiv:0710.3742 (2007). [Google Scholar]
- 8.Radillo AE, Veliz-Cuba A, Josić K & Kilpatrick ZP Evidence accumulation and change rate inference in dynamic environments. Neural computation 29, 1561–1610 (2017). [DOI] [PubMed] [Google Scholar]
- 9.**.Glaze CM, Filipowicz AL, Kable JW, Balasubramanian V & Gold JI A bias–variance trade-off governs individual differences in on-line learning in an unpredictable environment. Nat. Hum. Behav. 2, 213 (2018). [Google Scholar]; Humans performing a dynamic triangles task use decision strategies that suggest a trade-off in which history-dependent adaptive strategies lead to higher choice variability. A sampling strategy best accounted for subject data.
- 10.Krishnamurthy K, Nassar MR, Sarode S & Gold JI Arousal-related adjustments of perceptual biases optimize perception in dynamic environments. Nat. human behaviour 1, 0107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfarb S, Wong-Lin K, Schwemmer M, Leonard NE & Holmes P Can post-error dynamics explain sequential reaction time patterns? Front. Psychol. 3, 213 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell BA & Kiani R Hierarchical decision processes that operate over distinct timescales underlie choice and changes in strategy. Proc. Natl. Acad. Sci. 113, E4531–E4540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.**.Malhotra G, Leslie DS, Ludwig CJ & Bogacz R Overcoming indecision by changing the decision boundary. J. Exp. Psychol. Gen. 146, 776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Humans’ decision strategies in tasks where difficulty varies trial-to-trial are well approximated by a drift-diffusion model with time-varying decision boundaries. Subjects’ deviations from this normative model did little to impact the reward rate.
- 14.Geisler WS Ideal observer analysis. The visual neurosciences 10, 12–12 (2003). [Google Scholar]
- 15.Knill DC & Pouget A The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 27, 712–719 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Kim TD, Kabir M & Gold JI Coupled decision processes update and maintain saccadic priors in a dynamic environment. J. Neurosci. 3078–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.*.Veliz-Cuba A, Kilpatrick ZP & Josić K Stochastic models of evidence accumulation in changing environments. SIAM Rev. 58, 264–289 (2016). [Google Scholar]; This paper presents derivations and analyses of nonlinear stochastic models of evidence accumulation in dynamic environments for decisions between two and more alternatives. It shows how optimal evidence discounting can be implemented in a multi-population model with mutual excitation.
- 18.Drugowitsch J, Moreno-Bote R, Churchland AK, Shadlen MN & Pouget A The cost of accumulating evidence in perceptual decision making. J. Neurosci. 32, 3612–3628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.*.Holmes WR, Trueblood JS & Heathcote A A new framework for modeling decisions about changing information: The piecewise linear ballistic accumulator model. Cogn. psychology 85, 1–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, humans performed a RDMD task in which the direction of dots sometimes switched midtrial. A piecewise linear accumulator model was fit to data and demonstrated that subjects react slowly to new evidence, and that the perceived strength of post-switch evidence is influenced by pre-switch evidence strength.
- 20.Holmes WR & Trueblood JS Bayesian analysis of the piecewise diffusion decision model. Behav. research methods 50, 730–743 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Yu AJ & Cohen JD Sequential effects: Superstition or rational behavior? Adv. Neural Inf. Process. Syst. 21, 1873–1880 (2008). [PMC free article] [PubMed] [Google Scholar]
- 22.Brunton BW, Botvinick MM & Brody CD Rats and humans can optimally accumulate evidence for decision-making. Sci. 340, 95–98 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Ossmy O et al. The timescale of perceptual evidence integration can be adapted to the environment. Curr. biology : CB 23, 981–986 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Tavoni G, Balasubramanian V & Gold JI The complexity dividend: when sophisticated inference matters. bioRxiv 563346 (2019). [Google Scholar]
- 25.Fernberger SW Interdependence of judgments within the series for the method of constant stimuli. J. Exp. Psychol. 3, 126 (1920). [Google Scholar]
- 26.Fründ I, Wichmann FA & Macke JH Quantifying the effect of intertrial dependence on perceptual decisions. J. vision 14, 9–9 (2014). [DOI] [PubMed] [Google Scholar]
- 27.*.Urai AE, Braun A & Donner TH Pupil-linked arousal is driven by decision uncertainty and alters serial choice bias. Nat. Commun. 8, 14637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Increases in pupil diameter can be used to predict choice alternations in serial decisions, providing a promising, non-invasive approaoch for validating theories of adaptive decision making strategies.
- 28.Nguyen KP, Josić K & Kilpatrick ZP Optimizing sequential decisions in the drift–diffusion model. J. Math. Psychol. 88, 32–47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermoso-Mendizabal A et al. Response outcomes gate the impact of expectations on perceptual decisions. bioRxiv 433409 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson N Effect of first-order conditional probability in two-choice learning situation. J. Exp. Psychol. 59, 73–93 (1960). [DOI] [PubMed] [Google Scholar]
- 31.White CN & Poldrack RA Decomposing bias in different types of simple decisions. J. Exp. Psychol. Learn. Mem. Cogn. 40, 385 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Ratcliff R Theoretical interpretations of the speed and accuracy of positive and negative responses. Psychol. review 92, 212 (1985). [PubMed] [Google Scholar]
- 33.Diederich A & Busemeyer JR Modeling the effects of payoff on response bias in a perceptual discrimination task: Bound-change, drift-rate-change, or two-stage-processing hypothesis. Percept. & Psychophys. 68, 194–207 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Urai AE, de Gee JW & Donner TH Choice history biases subsequent evidence accumulation. bioRxiv 251595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olianezhad F, Zabbah S, Tohidi-Moghaddam M & Ebrahimpour R Residual information of previous decision affects evidence accumulation in current decision. Front. behavioral neuroscience 13, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun A, Urai AE & Donner TH Adaptive history biases result from confidence-weighted accumulation of past choices. J. Neurosci. 2189–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deneve S Making decisions with unknown sensory reliability. Front. neuroscience 6, 75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Lee MD, Vandekerckhove J, Maris G & Wagenmakers E-J Time-varying boundaries for diffusion models of decision making and response time. Front. Psychol. 5, 1364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell BA & Kiani R Neural mechanisms of post-error adjustments of decision policy in parietal cortex. Neuron 89, 658–671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thura D, Beauregard-Racine J, Fradet C-W & Cisek P Decision making by urgency gating: theory and experimental support. J. neurophysiology 108, 2912–2930 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Johnson B, Verma R, Sun M & Hanks TD Characterization of decision commitment rule alterations during an auditory change detection task. J. neurophysiology 118, 2526–2536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajima S, Drugowitsch J & Pouget A Optimal policy for value-based decision-making. Nat. communications 7, 12400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins GE, Forstmann BU, Wagenmakers E-J, Ratcliff R & Brown SD Revisiting the evidence for collapsing boundaries and urgency signals in perceptual decision-making. J. Neurosci. 35, 2476–2484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans NJ, Hawkins GE, Boehm U, Wagenmakers E-J & Brown SD The computations that support simple decision-making: A comparison between the diffusion and urgency-gating models. Sci. reports 7, 16433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkins G, Wagenmakers E, Ratcliff R & Brown S Discriminating evidence accumulation from urgency signals in speeded decision making. J. neurophysiology 114, 40–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palestro JJ, Weichart E, Sederberg PB & Turner BM Some task demands induce collapsing bounds: Evidence from a behavioral analysis. Psychon. bulletin & review 1–24. [DOI] [PubMed] [Google Scholar]
- 47.Khodadadi A, Fakhari P & Busemeyer JR Learning to allocate limited time to decisions with different expected outcomes. Cogn. psychology 95, 17–49 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Drugowitsch J, DeAngelis GC, Angelaki DE & Pouget A Tuning the speed-accuracy trade-off to maximize reward rate in multisensory decision-making. Elife 4, e06678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson RC, Nassar MR & Gold JI Bayesian online learning of the hazard rate in change-point problems. Neural Comput. 22, 2452–2476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bronfman ZZ et al. Decisions reduce sensitivity to subsequent information. Proc. Royal Soc. B: Biol. Sci. 282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talluri BC, Urai AE, Tsetsos K, Usher M & Donner TH Confirmation bias through selective overweighting of choice-consistent evidence. Curr. Biol. 28, 3128–3135 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Beck JM, Ma WJ, Pitkow X, Latham PE & Pouget A Not noisy, just wrong: the role of suboptimal inference in behavioral variability. Neuron 74, 30–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman J & Hanna JF The structure of responses to a sequence of binary events. J. Math. Psychol. 3, 371–387 (1966). [Google Scholar]
- 54.Hanks T, Kiani R & Shadlen MN A neural mechanism of speed-accuracy tradeoff in macaque area lip. Elife 3, e02260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z, Schrater P & Pitkow X Inverse POMDP: Inferring what you think from what you do. arXiv preprint arXiv:1805.09864 (2018). [Google Scholar]
- 56.Bialek W, Nemenman I & Tishby N Predictability, complexity, and learning. Neural computation 13, 2409–2463 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Thura D & Cisek P The basal ganglia do not select reach targets but control the urgency of commitment. Neuron 95, 1160–1170 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Akrami A, Kopec CD, Diamond ME & Brody CD Posterior parietal cortex represents sensory history and mediates its effects on behaviour. Nat. 554, 368 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Nassar MR et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. neuroscience 15, 1040 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


