Abstract
In an ever-changing and often ambiguous environment, organisms must use previously learned associations between antecedents and outcomes to predict future associations and make optimal choices. Chronic stress can impair one’s ability to flexibly adjust behaviors when environmental contingencies change, particularly in cases of early-life stress. In mice, exposure to elevated levels of the primary stress hormone, corticosterone (CORT), during early adolescence is sufficient to impair response-outcome decision making later in life, biasing response strategies towards inflexible habits. Nevertheless, neurobiological mechanisms are still being defined. Here, we report that exposure to excess CORT in adolescence causes a loss of dendritic spines on excitatory pyramidal neurons in the lateral, but not medial, orbital prefrontal cortex (loPFC) of mice, and spine loss correlates with the severity of habit biases in adulthood. Excess CORT also reduces the presence of ventral hippocampal (vHC) axon terminals in the loPFC. To identify functional consequences, we inactivated vHC→loPFC projections in typical healthy mice during a period when mice must update response-outcome expectations to optimally acquire food reinforcers. Inactivation impaired the animals’ subsequent ability to sustainably choose actions based on likely outcomes, causing them to defer to habit-based response strategies. Thus, vHC→loPFC projections are necessary for response-outcome expectancy updating and a target of excess glucocorticoids during early-life development. Their degradation is likely involved in long-term biases towards habit-based behaviors following glucocorticoid excess in adolescence.
Keywords: Orbital frontal, Contingency degradation, DREADDs, Action-outcome, Response-outcome, Habit
1. Introduction
An organism’s survival in an ever-changing world requires the ability to predict rewards or threats in the environment. Learned associations between antecedents and outcomes must be used and flexibly updated to orchestrate appropriate behavioral responses. An inability to update associations when environmental contingencies change can produce maladaptive behaviors resembling core symptoms of a number of psychiatric disorders, including depression, post-traumatic stress disorder (PTSD) and substance use disorder (SUD). For example, in depression, outcomes can be misattributed to current situations rather than to an individual’s chosen action; thus, depressed individuals may abstain from initiating actions to achieve goals (Griffiths et al., 2014). In SUD, continued drug abuse may have increasingly negative consequences, yet individuals struggle to modify behaviors and instead, exhibit persistent drug seeking (Everitt and Robbins, 2016). Stressor exposure, which also promotes rigid, outcome-insensitive habits (Schwabe, 2013), can trigger or exacerbate disease symptomatology. Dysfunction in the stress-sensitive circuits that guide flexible outcome-based behavior may be a common feature of seemingly disparate disorders (Griffiths et al., 2014).
The ventral hippocampus (vHC) and orbital prefrontal cortex (oPFC) are involved in guiding actions based on expected outcomes (Wikenheiser and Schoenbaum, 2016). The oPFC is thought to generate internal representations of “task spaces,” integrating abstract information about response-outcome and stimulus-outcome relationships, and even spatial information about goals (Feierstein et al., 2006), into ongoing behavior. The vHC appears to integrate context features, rules for obtaining outcomes, and predictive associations into representations that support integrative coding in the oPFC (Wikenheiser and Schoenbaum, 2016; Wikenheiser et al., 2017). Goal-relevant contextual information provided by vHC inputs (Komorowski et al., 2013) can also be used by the oPFC to help select appropriate behavioral responses for a given context (Wikenheiser and Schoenbaum, 2016; Mizumori and Tryon, 2015). Thus, the oPFC and vHC support outcome-based learning and memory necessary for flexible adaptation of behavior.
In humans, early-life adversity induces biases towards habit-based behaviors at the expense of response-outcome, “goal-directed” decision-making strategies (Patterson et al., 2013; see also Schwabe et al., 2012). Early-life stress also triggers volume atrophy and neuronal morphological alterations in the oPFC and hippocampus that are detectable in adulthood (Teicher and Samson, 2016). Developmental stress or stress hormone exposure in rodents confers similar long-term behavioral deficits (Barfield et al., 2017; Zhang et al., 2017) and cortico-limbic structural changes (Sheth et al., 2017). Stress hormones could conceivably cause decision-making biases by modifying cortico-limbic development.
Here, we examined the long-term neurobehavioral consequences of prolonged exposure to elevated levels of the primary stress hormone, corticosterone (CORT), during early adolescence in mice. We report that mice with a history of adolescent CORT exposure fail to adjust reward-seeking behavior when outcome-predictive associations change. Deficiencies are long lasting, and they coincide with the loss of dendritic spines in the lateral oPFC (loPFC), as well as inputs from the vHC. To determine causal relationships, we inactivated vHC→loPFC projections in typical healthy mice, revealing that they are indispensable for sustainably selecting actions based on their likely outcomes.
2. Materials and methods
2.1. Subjects
Group-housed male wild-type C57BL/6 mice (Jackson Labs) were used, except for dendritic spine imaging experiments, which used male mice expressing thy1-driven yellow fluorescent protein (YFP; H line from Feng et al., 2000) that were fully back-crossed onto a C57BL/6 background. Mice were maintained on a 12-h light cycle (0800 on) and provided food and water ad libitum except during instrumental conditioning when body weights were maintained at ~90% of baseline to motivate responding. Animal numbers for each experiment are indicated in the respective figure captions. Procedures were approved by the Emory University IACUC and carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals.
2.2. CORT exposure
4-pregnen-11β 21-DIOL-3 20-DIONE 21-hemisuccinate (Steraloids) was dissolved in tap water (25 μg/mL free base). CORT-exposed mice were given CORT in place of normal drinking water, while control mice consumed tap water. Water bottles were weighed daily, and mice weighed every other day to calculate average doses (~5–9 mg/kg/day) of CORT. Every 3 days, water bottles were refilled with fresh tap water or newly prepared CORT solution. Mice were exposed to CORT or water from postnatal day (P) 31–42 or P56-67, corresponding to early adolescence and adulthood in rodents (Spear, 2000). After a washout period of at least 2 weeks, instrumental conditioning began. Timelines are provided in the respective figures.
Importantly, this procedure was validated for use in adolescent mice in our prior report (Barfield et al., 2017), confirming that, as in adults, it elevates blood serum CORT to levels comparable to those following forced swim stress and causes adrenal and thymus gland atrophy that recovers when exogenous CORT is removed. Thus, the procedure allows us to isolate the neurobehavioral consequences of stress-like CORT levels (see also Gourley et al., 2012).
2.3. Surgery
Experiments requiring intracranial surgery utilized: a fluorescent anterograde tracer, Fluoro-ruby (Millipore); a retrograde adeno-associated virus (AAV) expressing Cre (AAVrg-pmSyn1-EBFP-Cre; Addgene); a recombinant AAV encoding a Cre-driven, inhibitory Gi-coupled Designer Receptor Exclusively Activated by Designer Drugs (DREADD) (rAAV5-hSyn-DIO-hM4D(Gi)-mCherry; UNC Viral Vector Core); or a control viral vector (rAAV5-hSyn-EYFP or rAAV5-hSyn-EGFP; UNC Viral Vector Core).
Fluoro-ruby infusions occurred at P49; viral vector infusions occurred at P49 ± 1 day. Mice were anaesthetized with Ketamine/Dexdomitor (80 mg/kg/0.5 mg/kg, i.p.) and placed in a digitized stereotaxic frame (Stoelting). The head was shaved, scalp retracted, and skull leveled.
For fluoro-ruby infusions, 2 burr holes per hemisphere were drilled, and fluoro-ruby (10% solution in distilled water) was infused bilaterally into the vHC (ML ± 3.6, AP-2.4, DV-4.5 and ML ± 3.3, AP-2.8, DV-4.8) over 3 min (0.04 μl/site).
For viral vector infusions, 3 burr holes per hemisphere were drilled. A retrograde Cre-expressing virus was first infused bilaterally into the loPFC (ML ± 1.5, AP + 3.0, DV-3.1) over 5 min (0.5 μl/site). A virus expressing a Cre-driven Gi-coupled DREADD or a control viral vector was then infused bilaterally into the vHC (ML ± 3.6, AP-2.5, DV-4.5 and ML ± 3.2, AP-2.7, DV-5.0) over 5 min (0.25 μl/site). This strategy results in the Cre-driven expression of Gi-DREADDs only in vHC neurons that project to the loPFC in experimental mice, while control mice also express Cre, but not DREADDs.
Throughout, needles were left in place for 5 additional min before withdrawal and suturing of the scalp. Mice were revived with Antisedan (1 mg/kg, i.p.). Mice infused with fluoro-ruby were euthanized one week after surgery. Mice infused with viral vectors were allowed to recover for at least 2 weeks before behavioral testing.
2.4. Instrumental conditioning
Mice were food-restricted and trained to nose poke for 20 mg grain-based food reinforcers (Bio-Serv) using Med Associates conditioning chambers equipped with 2 nose poke recesses and a food magazine. Training was initiated with a fixed ratio 1 (FR1) schedule of reinforcement; mice could earn up to 30 reinforcers for responding on each of 2 apertures (60 reinforcers/session). Sessions ended when mice acquired all 60 reinforcers or at 70 min. Following 6 sessions of FR1 training (1/day), mice were shifted to a random interval (RI) 30 sec schedule of reinforcement for 4 days. 30 reinforcers were again available (60 reinforcers/session, 1 session/day), and sessions ended when all 60 pellets had been delivered, or at 70 min. Response acquisition curves represent both responses/min.
Mice were next tested in a response-outcome contingency degradation procedure, as in our prior reports (e.g., Gourley et al., 2012; Barfield et al., 2017) and also similar to the method of Barker et al. (2018). In a 25-min “non-degraded” session, one nose poke recess was occluded, and responding on the other was reinforced using a variable ratio 2 schedule of reinforcement. During a 25-min “degraded” session the next day, the opposite aperture was occluded, and responding on the available aperture produced no programmed consequences. Reinforcers were instead delivered into the magazine at a rate matched to each animal’s reinforcement rate on the previous day. Thus, the reinforcement schedule associated with one nose poke was enriched, while the causal relationship between the other response and the outcome was degraded. The order of these sessions and the location of the nose poke aperture associated with an intact or degraded response-outcome contingency were counter-balanced, except in the experiment using DREADDs, which is described in greater detail below.
Response strategies were assessed the following day in a 5-min choice test conducted in extinction. Both apertures were available. Preferential engagement of the response that was most likely to be reinforced is indicative of a goal-directed response strategy; by contrast, engagement of both responses equally is considered habit based (Balleine and O’Doherty, 2010). Response preference scores, calculated as responses on the aperture associated with a “non-degraded” contingency / “degraded” contingency, are also shown. Here, values > 1 indicate goal-directed responding, while values ~ 1 indicate no preference, habitual behavior.
For experiments utilizing DREADDs, the “non-degraded” session preceded the “degraded” session, and then all mice were administered the DREADDs ligand Clozapine N-Oxide (CNO) immediately following the “degraded” session, during a period of oPFC-dependent response-outcome memory updating (Zimmermann et al., 2018). On each of the next 2 days, mice underwent 3 successive 5-min choice tests to track response preferences over time. To minimize potential confounding effects of injection stress, all mice were given saline injections (i.p.) for several days prior to the contingency degradation procedure.
2.5. CNO preparation and administration
CNO (1 mg/kg in a volume of 1 ml/100 g, i.p., Sigma-Aldrich) was dissolved in a 2% dimethyl sulfoxide (DMSO, Sigma-Aldrich) solution in 0.9% sterile saline and prepared on the day of injection. Injections were delivered immediately following the contingency degradation training session, described above, then mice were tested the next day, drug-free. Importantly, all mice, regardless of viral vector, received CNO, equally exposing all mice to any unintended consequences of the drug, such as conversion to clozapine (Gomez et al., 2017). Notably, we have confirmed that the same dose of CNO does not itself have any effects in the same task, nor does it affect activity of the master cytoskeletal regulatory protein cofilin in the oPFC (Whyte et al., 2019). Similarly, Barker et al. (2018) confirmed that a higher 2 mg/kg dose does not impact sensitivity to instrumental contingency degradation.
2.6. Dendritic spine imaging and reconstruction
thy1-YFP-expressing mice given water or CORT-infused water from P31–42 were euthanized at P100, following behavioral testing. Mice were rapidly decapitated, brains were extracted and submerged in chilled 4% paraformaldehyde for 48 h, then transferred to 30% w/v sucrose, and sectioned at 40 μm using a freezing microtome. Dendritic segments on deep-layer pyramidal neurons in the lateral and medial oPFC (loPFC, moPFC), located between Bregma +2.8− +2.22, were imaged with a spinning disk confocal (VisiTech International) on a Leica microscope.
Between 1–5 independent segments from secondary and tertiary basilar dendritic branches, 15–20 μm in length, within 50–100 μm of the soma, were collected from each animal. Images were processed and dendritic spines were enumerated and reconstructed in 3D by a single blinded rater using Imaris software (described Gourley et al., 2013). Group sizes were 6–8 mice for the loPFC and 4–5 mice for the moPFC (less than loPFC due to sparseness of YFP signal in this region). Each mouse contributed a single density value (its average) to statistical comparison by t-test.
We also classified spines as mushroom or non-mushroom in their shape, because mature mushroom-shaped spines are considered durable and likely to contain stable synapses. Mushroom-shaped spines were ≤4 μm in length and had a head:neck ratio ≥1.5 μm (Radley et al., 2013). Dendrites within the rostral vs. caudal loPFC were divided, with the rostral loPFC being defined as between Bregma + 2.8− +2.46 mm, and caudal sections posterior to 2.46. Each mouse contributed a single rostral vs. caudal value, representing the average density of mushroom-shaped spines per region per mouse.
2.7. Histology and axon terminal quantification
Mice were deeply anaesthetized and transcardially perfused with 4% paraformaldehyde one week following fluoro-ruby infusions, or after behavioral tests. Brains were extracted, stored in chilled 4% paraformaldehyde for 48 h, then stored in 30% w/v sucrose, and sectioned at 40 μm. Sections were mounted and coverslipped with Vectashield Mounting Medium; Vectashield with DAPI was used for sections containing fluoro-ruby. Infusion sites were verified by imaging fluoro-ruby, blue fluorescent protein (BFP, retro-Cre), mCherry (Gi-DREADDs), or GFP/YFP (control viral vector). Mice with mis-targeted infusions in at least 1 hemisphere were excluded from all analyses (n = 2 from fluoro-ruby experiments, n = 2 from DREADDs experiments).
Fluoro-ruby-positive axon terminal punctae in the loPFC, moPFC, and prelimbic cortex (PL) were imaged using a spinning disk confocal microscope. Z-stacks were collected with a 100 × 1.4 NA objective using a 0.2 μm step size, then collapsed into a maximum intensity projection (MIP) using ImageJ. All MIPs contained 45 serial sections. MIPs were converted to binary images using the threshold tool, then punctae were separated using the Watershed segmentation plugin and quantified (as the % of image area containing punctae) using the Analyze Particles command. Eight images were collected per animal per brain region, and each animal contributed a single value (its average) to statistical analyses. A single blinded experimenter collected and processed all images.
2.8. Statistical analyses
Two-tailed statistical analyses with α ≤ 0.05 were performed using SPSS. Response rates and response preference ratios were compared by 1- 2- or 3-factor mixed analysis of variance (ANOVA), with session, time, or aperture as a within-subjects (repeated measure) factor, as appropriate. Following interactions, post hoc t-tests were applied, and results are indicated graphically. Response preference ratios were also compared using a 1-sample t-test against 1 (no preference) for each group. When 2 groups were compared (as for dendritic spine density and axonal punctae analyses), 2-tailed unpaired t-tests were used. Correlations were analyzed by linear regression. Throughout, values + / −2 standard deviations from the mean were considered outliers and excluded. The data in Fig. 1 were sourced from a dataset originally published as part of Barfield et al., 2017. All subsequent findings are new.
Fig. 1. Excess CORT exposure during adolescence impairs the ability of mice to update outcome expectancies in adulthood.
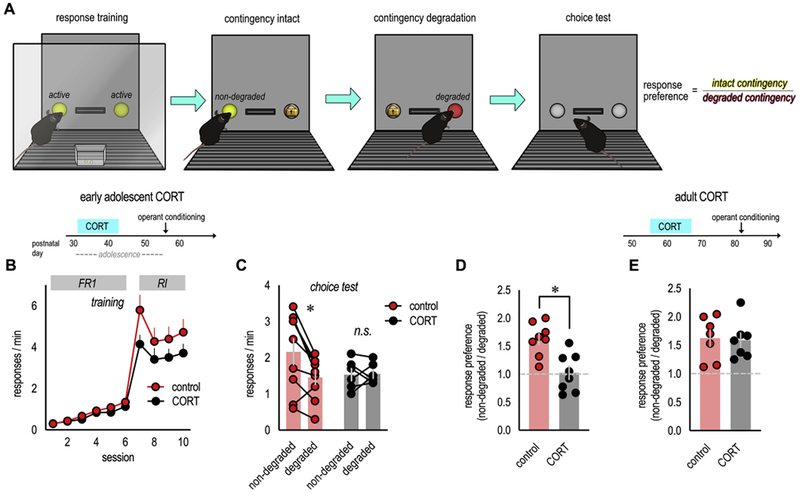
(A) Mice were trained to nose poke for food reinforcers in operant conditioning chambers. Next, the contingency between one response and its outcome was ‘degraded’ by providing food pellets independently of the mouse’s responses. The ability to update expectancies and select responses based on anticipated outcomes was then assessed in a brief choice test conducted in extinction. (B) Experimental timeline is above. First, mice exposed to excess CORT during adolescence were trained to nose poke as adults. All mice acquired the nose poke responses. “FR1” and “RI” denote the schedules of reinforcement used throughout. (C) A probe test following instrumental contingency degradation revealed that control mice preferentially engaged the response most likely to be reinforced (“non-degraded” vs. “degraded”), a goal-directed response strategy. Meanwhile, CORT-exposed mice generated habit-based response strategies, failing to differentiate between behaviors that were or were not likely to be reinforced. (D) Response rates were converted to preferences scores (“non-degraded”/“degraded”), again highlighting goal-directed responding in control mice (scores > 1) and non-preferential, habit-based responding following excess CORT (scores ~1). n = 8/group. (E) Separate mice were exposed to excess CORT in adulthood. In this case, CORT was insufficient to alter preference ratios. n = 6/group. Symbols in B represent means + SEMs, symbols in C–E represent individual mice, bars represent means ± SEMs. *p ≤ 0.05. B and C are reprinted from Barfield et al. (2017), and D and E were generated from a dataset originally reported in that manuscript. Experiments were conducted twice.
3. Results
3.1. Excess CORT during adolescence has long-term behavioral consequences
In both humans and rodents, excess glucocorticoid exposure can induce biases towards habit-based behavior, at the expense of goal-directed action (Gourley et al., 2012; Guenzel et al., 2014). Further, stressor exposure during early developmental periods appears to confer long-term habit biases across rodent and primate species (Grissom et al., 2012; Patterson et al., 2013). Here, we attempt to clarify the long-term effects of excess CORT during adolescence on key neurocircuits associated with goal-directed action, and to identify causal relationships with action/habit balance.
First, we exposed mice to CORT in the drinking water from P31–42, analogous to early adolescence in humans. We used a procedure that recapitulates the CORT response to forced swim stress and triggers modifications in cortio-limbic neurotrophin systems (Barfield et al., 2017). It also induces habit biases, a finding that we recapitulate here: In adulthood, we trained mice to nose poke 2 recesses for food reinforcers (Fig. 1A). Mice demonstrated no side biases, nor group differences (F < 1; F(1,15) = 2.7, p = 0.12; no time x group interaction F (9,135) = 1.6, p = 0.12), and response rates are collapsed across the two responses for simplicity (Fig. 1B). To assess whether mice behaved according to goal-directed or habit-based strategies, we used an instrumental contingency degradation procedure, in which responding on one recess becomes significantly less likely to be reinforced, while responding on the other remains reinforced (Fig. 1A). A goal-directed strategy is to preferentially engage the reinforced behavior in a subsequent probe test (also termed “choice test”). Meanwhile, habit-based responding does not change, because it is by nature insensitive to response-outcome contingencies (Balleine and O’Doherty, 2010). Control mice preferred the consistently reinforced behavior, as expected. Meanwhile, adult mice with a history of adolescent CORT exposure assumed habit-based strategies, failing to differentiate between the behaviors that were more, vs. less, likely to be reinforced (interaction F (1,13) = 6.0, p = 0.03) (Fig. 1C).
The same response rates can be converted to preference ratios (non-degraded/degraded), in which values > 1 reflect goal-directed behavior, while values ~ 1 reflect no response preference, a habitual response strategy. Again, control mice differentiated between the two responses (t(7) = 6.1, p = 0.001 vs. 1), while mice with a history of CORT exposure failed to differentiate between responses that were more or less likely to be reinforced (t(7) = 0.69, p = 0.51 vs. 1; unpaired t-test between groups: t(14) = 3.8, p = 0.002) (Fig. 1D). Thus, elevated CORT in adolescence primes mice to utilize habit-based response strategies as adults.
In contrast to adolescents, exposing adult mice to subchronic CORT is insufficient to cause habit biases (Barfield et al., 2017). To further confirm this impression, we converted response rates from our prior report (Barfield et al., 2017) to preference ratios as above. As expected, both control mice and mice exposed to CORT during adulthood could develop goal-directed response strategies (t(6) = 4.4, p = 0.005 vs. 1; t(6) = 4.6, p = 0.004 vs. 1; unpaired t-test between groups: t(12) = 0.19, p = 0.85) (Fig. 1E). Thus, adolescent mice are more vulnerable to the habit-inducing consequences of excess CORT than adults are.
3.2. Excess CORT during adolescence induces long-term dendritic spine loss on excitatory neurons in the loPFC
The oPFC (particularly the lateral subregion; see Parkes et al., 2018) is essential for goal-directed action, countering habit-based behaviors. To determine whether excess CORT during adolescence has long-term structural effects in the oPFC, which may contribute to long-lasting behavioral changes, we euthanized the thy1-YFP-expressing mice from our appetitive conditioning experiments (Fig. 1) and enumerated dendritic spines on layer V pyramidal neurons in the loPFC (Fig. 2A). As a comparison, we also enumerated spines in the moPFC (Fig. 2A). Dendritic spines in the loPFC were lost in CORT-exposed mice (t(12) = 2.8, p = 0.016) (Fig. 2B), while densities in the adjacent moPFC were apparently unaffected (t(7) = −0.6, p = 0.6) (Fig. 2C). Notably, mice were euthanized over 8 weeks following the cessation of adolescent CORT exposure, indicating that dendritic spine loss in the loPFC is detectable well into adulthood. Also notable, 3D reconstruction of dendritic spines revealed that response preference ratios positively correlated with the density of mature, mushroom-shaped spines in the rostral loPFC (r = 0.56, p = 0.048) (Fig. 2D and E). We did not identify correlations with immature spine types, or any correlations with dendritic spine densities in the caudal loPFC or moPFC (not shown).
Fig. 2. Excess CORT in adolescence induces dendritic spine loss in the loPFC: Correlations with decision-making abnormalities.
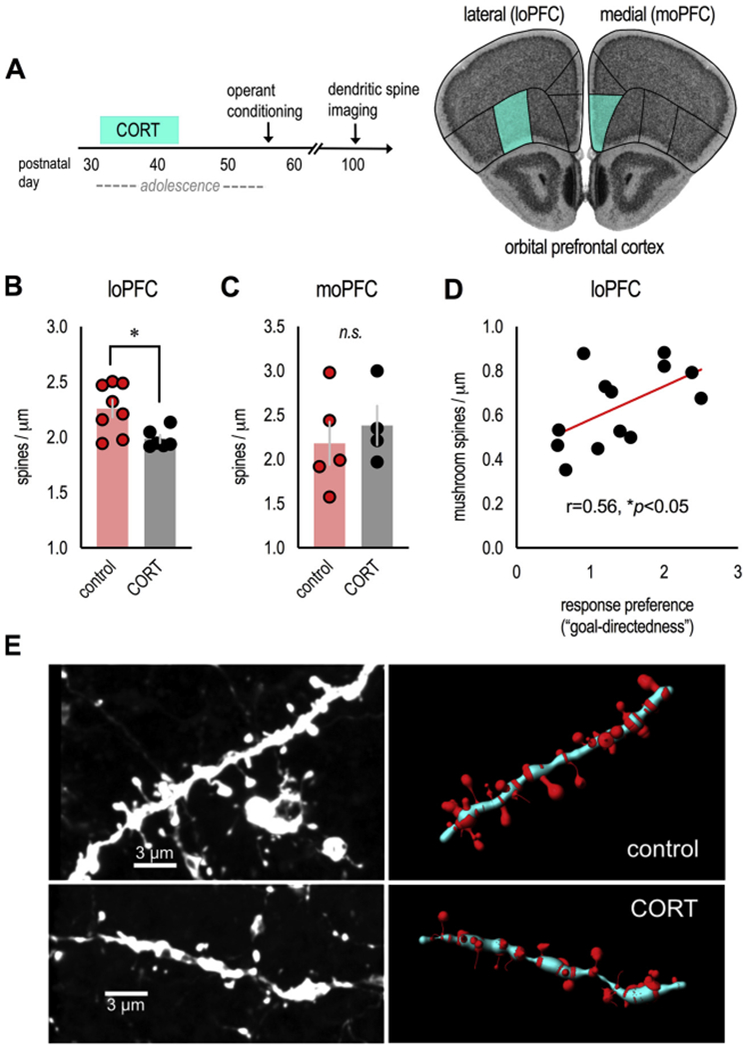
(A) Experimental time (left), thy1-YFP-expressing mice from instrumental conditioning experiments in Fig. 1 were euthanized in adulthood after behavioral testing, and basilar dendrites on excitatory neurons in the loPFC (and, for comparison, moPFC) were imaged. These regions are highlighted on images from the Mouse Brain Library (Rosen et al., 2000). (B) Adolescent CORT exposure eliminated dendritic spines in the loPFC (n = 6–8/group), (C) but not moPFC (n = 4–5/group; less than loPFC due to sparseness of YFP signal in this region). (D) In the rostral loPFC, the density of mature, mushroom-shaped spines correlated with response preference ratios from Fig. 1D. n = 6–7/group. (E) Representative dendrites from the loPFC (unprocessed images at left, reconstructions at right). Bars represent means ± SEMs, symbols represent individual mice. *p ≤ 0.05. Dendritic spines were imaged and reconstructed by a single, blinded rater.
3.3. Excess CORT during adolescence diminishes vHC projections to the loPFC
The rostral oPFC receives input from limbic structures involved in complex decision-making processes. One such structure is the vHC (Cenquizca and Swanson, 2007) (Fig. 3A), thought to provide goal-relevant information necessary for guiding outcome-based learning and memory (Wikenheiser et al., 2017). vHC→loPFC projections mature during adolescence (Lebel and Beaulieu, 2011), making them a potential target of glucocorticoid excess during this period. To assess the long-term effects of excess CORT during adolescence on these projections, we exposed mice to exogenous CORT during early adolescence (from P31–12 as above), then infused the fluorescent tracer, fluoro-ruby, into the vHC at P49 (Fig. 3B). We then collected brains 1 week later, in early adulthood, corresponding with the onset of behavioral studies above.
Fig. 3. Excess CORT during adolescence degrades vHC→loPFC input.
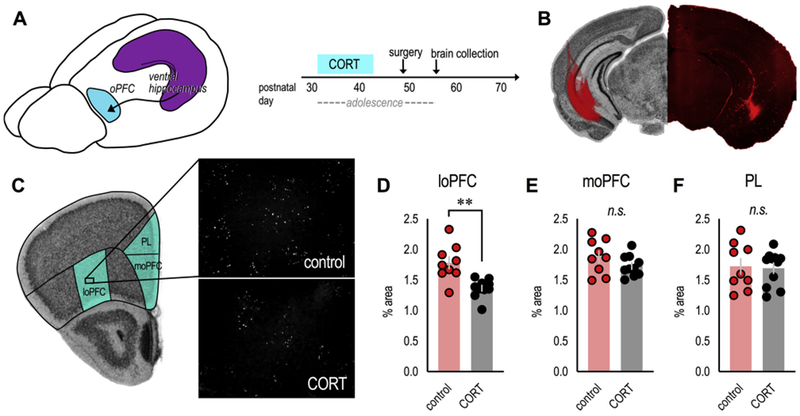
(A) Mice exposed to CORT from P31–42 received infusions of the anterograde tracer fluoro-ruby into the vHC to characterize vHC projections to the oPFC. (B) At left: The spread of fluoro-ruby in the vHC is transposed onto an image from the Mouse Brain Library (Rosen et al., 2000). At right: Representative image of fluoro-ruby in the vHC. (C) Unprocessed images of fluoro-ruby-positive axon terminal punctae in the loPFC. Comparator regions are also highlighted on an image from the Mouse Brain Library (Rosen et al., 2000). (D) Adolescent CORT exposure reduced the presence of vHC terminals in the loPFC, (E) but not the moPFC (F) or PL. n = 9–11/group. Bars represent means ± SEMs, symbols represent individual mice. **p ≤ 0.001. Punctae were imaged and enumerated by a single, blinded rater; 2 independent cohorts of mice contributed to the dataset.
Fluoro-ruby has both anterograde and retrograde properties, but connectivity between the vHC and loPFC is predominantly unidirectional, with minimal (if any) direct projections from the loPFC to the vHC (Cenquizca and Swanson, 2007). Accordingly, retrogradely labeled cell bodies in the loPFC were largely absent in our mice, while axonal punctae were abundant. Mice exposed to CORT during adolescence had fewer fluoro-ruby-positive axonal punctae in the loPFC (t(17) = 3.9, p = 0.001) (Figs. 3C and D), consistent with fewer dendritic spines in this region (Fig. 2). Meanwhile, in the moPFC, the amount of fluoro-ruby-positive punctae did not differ between groups (t(17) = 1.4, p = 0.17) (Fig. 3E), consistent with no CORT-induced changes in moPFC dendritic spines (Fig. 2). As an additional point of comparison, we also examined vHC-originating terminals in the PL. We identified no differences (t(18) = 0.2, p = 0.8) (Fig. 3F). Altogether, these patterns indicate that vHC→loPFC projections are particularly vulnerable to adolescent CORT exposure.
3.4. vHC→loPFC projections are necessary for goal-directed decision making
Exposure to excess CORT during adolescence had long-term maladaptive consequences for the ability of mice to associate actions with their outcomes (Fig. 1). Adolescent CORT also eliminated dendritic spines in the loPFC (Fig. 2) and projections from the vHC to the loPFC (Fig. 3). To determine whether the destabilization of vHC→loPFC projections could account for deficiencies in response-outcome decision making, we infused naive mice with Cre-expressing retrograde viral vectors into the loPFC and Cre-driven Gi-coupled DREADDs or a control fluorophore in the vHC (Fig. 4A and B). In this case, activation of the DREADD specifically silences vHC→loPFC projections. (We opted for this approach over an alternative strategy in which we might have attempted to rescue response preferences by stimulating vHC→loPFC projections following CORT. Our fluoro-ruby studies suggested that vHC→loPFC projections were lost with early-life CORT excess; thus, it would be difficult to interpret null effects, if any, given that they could be attributable to insufficient connectivity between the vHC and loPFC.)
Fig. 4. Inactivating vHC→loPFC projections weakens goal-directed response selection.
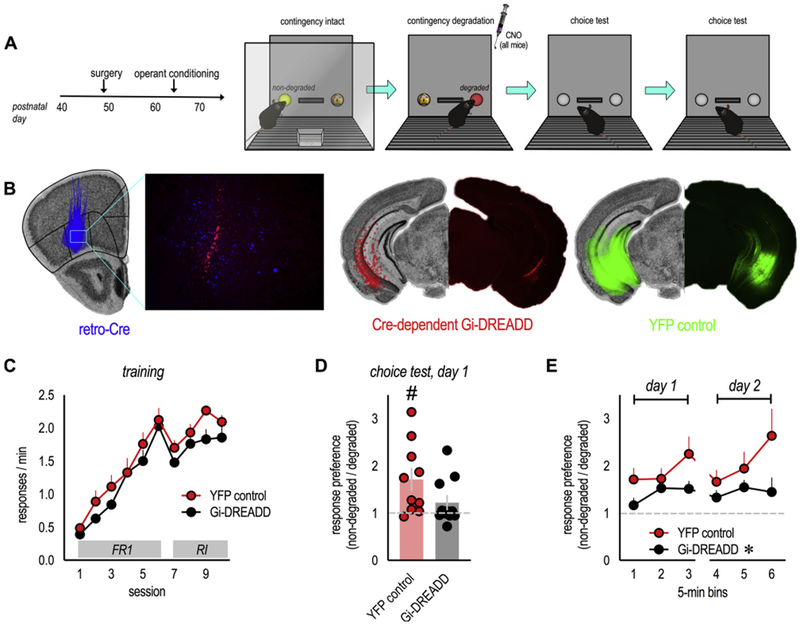
(A) Experimental timeline. Note that the DREADDs ligand CNO was administered immediately following instrumental contingency degradation. Response preferences were tested over the following 2 days when mice were drug-free. (B) Retro-Cre in the loPFC (blue), combined with a Cre-dependent Gi-DREADD in the vHC (red), was used to inactivate vHC→oPFC projections. Spread of viral vectors in the loPFC and vHC is drawn in the left hemispheres on images from the Mouse Brain Library (Rosen et al., 2000), and representative infusions are shown at right. (C) Mice were trained to nose poke for food reinforcers. “FR1” and “RI” denote the schedules of reinforcement. All mice acquired the responses during training without group differences. (D) Inactivation of vHC→loPFC projections impaired the ability of mice to generate response preferences based on the likelihood of reinforcement. (E) Impairments were persistent, detectable across several time bins and 2 test days, n = 10–11/group. Bars represent means ± SEMs. Symbols in C and E represent means + SEMs; otherwise, symbols represent individual mice. *p = 0.03 main effect of group; #p = 0.01 vs. 1 (1 reflects no change). This experiment was conducted in 2 independent cohorts of mice (for interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
All mice learned to nose poke for food reinforcement, without group differences (F(1,19) = 2.3, p = 0.15; no time x group interaction F < 1) (Fig. 4C). Immediately following response-outcome contingency degradation, during a period of response-outcome memory updating (Zimmermann et al., 2018), all mice were injected with the DREADD ligand CNO, which would inactivate vHC→loPFC projections in DREADDs-expressing mice, but have no effect in mice not expressing DREADDs.
When mice were tested the following day, drug-free, control mice overall preferred the response that was likely to be reinforced (t(9) = 3.0, p = 0.014 vs. 1) (Fig. 4D). This pattern indicates that they updated response-outcome associations to generate goal-directed response strategies. Meanwhile, Gi-DREADDs mice failed to differentiate between the responses, deferring to a habit-based response strategy (t(10) = 1.6, p = 0.15 vs. 1, no preference) (Fig. 4D). Thus, vHC→loPFC projections are necessary for selecting actions based on their outcomes. These connections are destabilized by glucocorticoid excess (Fig. 3), and inactivating them recapitulates decision-making biases caused by adolescent CORT exposure.
We also evaluated response preferences across multiple time bins over multiple days to track their stability. Over the course of 2 days, previously-inactivated mice generated consistently low preference scores (main effect of group F(1,18) = 5.5, p = 0.03; no time x group interaction F < 1) (Fig. 4E), indicating that vHC→loPFC projections are necessary for stable response-outcome memory that sustains goal-oriented response selection.
4. Discussion
We report that excess glucocorticoid exposure during adolescence impairs the ability of adult mice to modify behaviors based on response-outcome relationships. Instead, mice with a history of excess CORT defer to habitual response strategies that are insensitive to consequences. While chronic CORT exposure can induce the same behavioral deficiencies in adults (Gourley et al., 2012), we find that even subchronic exposure triggers behavioral abnormalities in adolescents and yet, is without consequence in adults (Fig. 1). This pattern suggests that adolescents are particularly vulnerable to the long-term behavioral consequences of excess CORT and provides a platform for understanding these vulnerabilities at a neurobiological level. These investigations are important because early-life stress in humans also causes habit biases later in life, behavioral attributes that can contribute to obesity, smoking, drug addiction and other negative outcomes (Patterson et al., 2013).
4.1. The loPFC bears a durable signature of early-life glucocorticoid excess
To understand the long-term consequences of excess CORT exposure during adolescence, we focus here on the oPFC. The oPFC is conceptualized as building “task spaces,” allowing organisms to link behaviors and stimuli with anticipated outcomes, even when these associations are not readily observable (Stalnaker et al., 2015), and it is necessary for response-outcome association updating, including in the specific task used here (Zimmermann et al., 2017, 2018; Whyte et al., 2019). As in many brain regions, excitatory oPFC neurons are subject to CORT-induced dendritic spine loss, but unlike in other regions, spine loss is long lasting, detectable after CORT exposure (Gourley et al., 2013). Mice in prior investigations (Gourley et al., 2013) were exposed to significantly longer periods of excess CORT, and neurons were imaged one week after CORT cessation. Here we find that < 2 weeks of exposure to stress-like CORT levels (see Barfield et al., 2017) triggers dendritic spine loss on layer V excitatory neurons in the loPFC; and importantly, spine loss is detectable multiple months later. We have identified few comparable investigations, but notably, paternal deprivation in the biparental rodent Octodon degus similarly results in long-lasting (multi-month) dendritic spine loss on layer II/III neurons in the loPFC (Helmeke et al., 2009). Thus, loPFC neurons in multiple layers appear to bear a long-term signature (dendritic spine loss) of early-life adversity.
Dendritic spines are the primary sites of postsynaptic excitatory plasticity in the brain, and the density of large, mushroom-shaped spines (those likely to contain mature synapses) in the rostral loPFC correlated with response selection strategies. Specifically, lower densities were associated with habit-like responding, the result of failing to update response-outcome associations. This pattern is consistent with evidence that successful response-outcome conditioning increases the proportion of spines with large, mature heads in the loPFC (DePoy et al., 2016; Whyte et al., 2019) and suggested to us that the loss of excitatory connections to the rostral loPFC contributed to failures in updating response-outcome associations.
The rostral loPFC receives innervation from limbic structures with cognitive functions, such as the vHC (Cenquizca and Swanson, 2007). We thus examined vHC→oPFC projections, revealing fewer axon terminals in the loPFC of adult mice with a history of excess CORT in adolescence. Considerable evidence indicates that stressors during adolescence influence hippocampal and PFC development (Sheth et al., 2017), and adolescent stressor exposure disrupts synaptic transmission in a hippocampal-PFC pathway (Koseki et al., 2009). Our findings suggest that one causal factor could be a CORT-mediated destabilization of anatomical connectivity between these structures.
As with dendritic spine densities, the loss of vHC axons was selective to the loPFC and not detected in medial PFC structures. Why might this be? Firstly, aspects of loPFC development are particularly prolonged relative to more medial structures (reviewed Shapiro et al., 2017), potentially opening a window of vulnerability to CORT-induced disruptions that are longer lasting or more resistant to recovery than in more medial PFC structures. Secondly, levels of several neuronally-expressed cytoskeletal regulatory elements and the neurotrophin receptor, tyrosine/tropomyosin receptor kinase B (trkB), are higher in the oPFC relative to medial PFC during adolescence (Shapiro et al., 2017). These proteins presumably serve essential functions for still-developing neurons, and insults that disrupt them could potentially render certain neuron populations particularly vulnerable to structural atrophy. For instance, prolonged CORT reduces expression of the trkB ligand Brain-derived Neurotrophic Factor in the loPFC (Gourley et al., 2009). One of many expected consequences would be the destabilization of dendritic spines and subsequent atrophy of apposing presynaptic terminals (reviewed Barfield and Gourley, 2018), which could account for the loss of vHC axon terminals here. Conversely, CORT-induced vHC damage, if any, could instead trigger spine loss in the loPFC. In support of this notion, vHC lesions degenerate vHC terminals and fibers in the loPFC (Halim and Swerdlow, 2000) and decrease dendrite lengths and dendritic spine densities on layer V pyramidal neurons in the PFC (Lipska et al., 2001). Dissecting cause-and-effect relationships could yield critical insight into circuit-level and ontogenetic mechanisms of stressor vulnerability.
4.2. vHC→loPFC connections are essential for goal-directed action
Both humans and rodents can learn to associate actions with their outcomes. When contingencies change, and expected outcomes do not match actual outcomes, internal schemas must be updated so that behaviors can be modified. Flexible, goal-directed behavior therefore necessitates updating and retaining internal representations of outcome-predictive associations, considered key functions of the loPFC. In one of many examples, inactivating the loPFC immediately following instrumental contingency degradation occludes the ability of mice to update and retain new response-outcome associations, such that subsequent responding is inefficient and habit-biased, even when the loPFC is back “on-line” (Zimmermann et al., 2017, 2018; Whyte et al., 2019). Multiple impactful reports have focused on how loPFC outputs, for example to the basolateral amygdala or dorsal striatum, coordinate goal-directed action selection, but inputs are also likely important for forming essential associations. For instance, organisms can form associations between contexts and response-outcome contingencies (Trask and Bouton, 2014). When familiar contingencies are violated and require updating, the hippocampus detects a mismatch between the expected features of the context (e.g., its association with known response-outcome contingencies) and actual features, and alerts other brain regions to update response selection strategies (Mizumori and Tryon, 2015). The vHC is likely responsible for the generation and transmission of these signals, since the ventral, and not dorsal, hippocampus encodes abstract, goal-relevant features of contexts (Komorowski et al., 2013). Thus, we envisioned that the loss of vHC→loPFC projections following excess CORT was causally related to failures in response-outcome expectancy updating.
To directly test the hypothesis that degradation of vHC→loPFC inputs triggers habit biases, we used Cre-dependent Gi-coupled DREADDs to selectively and inducibly inactivate vHC→loPFC projections in otherwise typical mice. We silenced vHC→loPFC projections immediately following instrumental contingency degradation, when mice must update, encode, and retain new expectations (i.e., the expectation that one response is no longer likely to be reinforced). Inactivated mice failed to generate adaptive response preferences in a later test, even though vHC→loPFC connections were back “on-line.” Importantly, silencing vHC→loPFC projections did not fully ablate response preferences, likely because multiple inputs (and outputs) contribute to oPFC function in this task, as has been reported in other contexts (e.g., Groman et al., 2019). The most striking consequence was that lower response preferences were sustained across multiple test sessions and days. Whatever information the vHC transmits (contextual or otherwise), it appears necessary for sustaining subsequent goal-directed response strategies.
In another report, vHC inactivation impeded optimal responding and altered the coding properties of oPFC neurons in a reversal task (Wikenheiser et al., 2017). The authors argue that vHC-oPFC connections, among others, are necessary for sustaining task states, referring to the flexible use of previously acquired information to guide future behaviors. We feel that the present findings can be explained in the same terms: The contingency degradation procedure causes mice to update response-outcome expectations as they come to recognize that a familiar behavior is no longer reinforced. In the absence of vHC-oPFC plasticity, updating is suboptimal, causing mice to defer to a more stable task representation formed during training, when responding on both nose poke apertures, equally and without preference, was the optimal strategy.
In addition to instrumental contingency degradation, reinforcer devaluation is popularly used to determine whether an animal’s behavior is goal-directed or habit-based. In this case, the value of a food reinforcer is decreased by pairing it with nausea or satiety. Inhibiting responding for the devalued outcome is considered goal-directed, while habitual behavior does not change. Interestingly, vHC inactivation does not appear to impact sensitivity to reinforcer devaluation (Yoshida et al., 2019). While these findings might seem to contradict our own, reinforcer devaluation procedures present considerably less task ambiguity relative to contingency degradation, and thus, may not recruit the vHC for “task state” updating.
It is also important to note that the vHC is anatomically positioned to coordinate both goal-directed actions (as shown here) and habits. For instance, it projects to the anterior central nucleus of the amygdala, essential to habitual behavior (Lingawi and Balleine, 2012), and vHC→nucleus accumbens shell connections support habits induced by extended response training (Barker et al., 2018). An intriguing possibility is that adolescent CORT exposure weakens some vHC connections while strengthening others, for instance, favoring connections involved in habit-based behaviors at the expense of projections involved in goal seeking.
4.3. Summary and broader implications
The loPFC is implicated in a variety of functions necessary for goal-directed behavior, including encoding outcome expectancies, attributing discrepancies between expected and actual outcomes to specific causes (i.e., credit assignment), and forming a cognitive map of task space (Stalnaker et al., 2015). The vHC, which provides a direct monosynaptic projection to the oPFC (Cenquizca and Swanson, 2007), transmits mismatch signals to the PFC, triggering learning-related plasticity that updates internal representations of outcome-predictive associations (Numan, 2015). Considered together with the observation that adolescent CORT exposure here caused loPFC dendritic spine and vHC→loPFC projection loss, and with the behavioral consequences of vHC→loPFC projection inactivation, we argue that the vHC→loPFC pathway is: 1) essential for updating response-outcome expectations necessary for sustained goal-directed behavior and 2) exceptionally vulnerable to excess glucocorticoids during adolescence, rendering CORT-exposed mice biased towards inflexible, habit-based behaviors. In humans, chronic stress or adverse experiences during childhood or adolescence increases the incidence of depression, PTSD, and SUD (Carr et al., 2013), illnesses commonly characterized by maladaptive, outcome-insensitive behaviors (resembling inflexible habits) and structural and functional abnormalities in the oPFC and vHC, and in cortico-limbic connectivity (Godsil et al., 2013). The long-term effects of adolescent glucocorticoid excess on vHC→oPFC projections in particular may contribute to risk for, or severity of, psychopathology by disrupting executive functions mediated by this connection.
Acknowledgements
We thank Dr. Bryan Roth and Dr. R. Jude Samulski of the UNC Viral Vector Core for the chemogenetic materials used here.
Funding
This work was supported by the National Institutes of Health (grant numbers MH101477, MH117103, and OD011132) and the National Science Foundation Graduate Research Fellowship Program under grant number DGE-1444932.
Footnotes
Declaration of Competing Interest
None.
References
- Balleine BW, O’Doherty JP, 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Gerber KJ, Zimmermann KS, Ressler KJ, Parsons RG, Gourley SL, 2017. Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biol 15, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Gourley SL, 2018. Prefrontal trkB, glucocorticoids, and their interactions in stress and developmental contexts. Neurosci. Biobehav. Rev 95, 535–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Bryant KG, Chandler LJ, 2018. Inactivation of ventral hippocampus projections promotes sensitivity to changes in contingency. Learn. Mem 26, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF, 2013. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis 201, 1007–1020. [DOI] [PubMed] [Google Scholar]
- Cenquizca LE, Swanson LW, 2007. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev 56, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Allen AG, Gourley SL, 2016. Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl. Psychiatry 6, e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2016. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF, 2006. Representation of spatial goals in rat orbitofrontal cortex. Neuron 51, 495–507. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. , 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM, 2013. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur. Neuropsychopharmacol 23, 1165–1181. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. , 2017. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR, 2009. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 34, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, DiLeone RJ, Koleske AJ, Taylor JR, 2012. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci. USA 109, 20714–20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ, 2013. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci 33, 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths KR, Morris RW, Balleine BW, 2014. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front. Syst. Neurosci 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom EM, Hawley WR, Bromley-Dulfano SS, Marino SE, Stathopoulos NG, Dohanich GP, 2012. Learning strategy is influenced by trait anxiety and early rearing conditions in prepubertal male, but not prepubertal female rats. Neurobiol. Learn. Mem 98, 174–181. [DOI] [PubMed] [Google Scholar]
- Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, Lee D, Taylor JR, 2019. Orbitofrontal circuits control multiple reinforcement-learning processes. Neuron 103, 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzel FM, Wolf OT, Schwabe L, 2014. Glucocorticoids boost stimulus-response memory formation in humans. Psychoneuroendocrinology 45, 21–30. [DOI] [PubMed] [Google Scholar]
- Halim ND, Swerdlow NR, 2000. Distributed neurodegenerative changes 2-28 days after ventral hippocampal excitotoxic lesions in rats. Brain Res 873, 60–74. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Seidel K, Poeggel G, Bredy TW, Abraham A, Braun K, 2009. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience 163, 790–798. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H, 2013. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J. Neurosci 33, 8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H, Matsumoto M, Togashi H, Miura Y, Fukushima K, Yoshioka M, 2009. Alternation of synaptic transmission in the hippocampal-mPFC pathway during extinction trials of context-dependent fear memory in juvenile rat stress models. Synapse 63, 805–813. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C, 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci 31, 10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingawi NW, Balleine BW, 2012. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J. Neurosci 32, 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR, 2001. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur. J. Neurosci 14, 135–144. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Tryon VL, 2015. Integrative hippocampal and decision-making neurocircuitry during goal-relevant predictions and encoding. Prog. Brain Res 219, 217–242. [DOI] [PubMed] [Google Scholar]
- Numan RA, 2015. Prefrontal-hippocampal comparator for goal-directed behavior: the intentional self and episodic memory. Front. Behav. Neurosci 9, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Ravassard PM, Cerpa JC, Wolff M, Ferreira G, Coutureau E, 2018. Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cereb. Cortex 28, 2313–2325. [DOI] [PubMed] [Google Scholar]
- Patterson TK, Craske MG, Knowlton BJ, 2013. The effect of early-life stress on memory systems supporting instrumental behavior. Hippocampus 23, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA, 2013. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci 33, 14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Williams AG, Capra JA, Connolly MT, Cruz B, Lu L, et al. , 2000. The Mouse Brain Library @ www.mbl.org. Int. Mouse Genome Conference 14 pp. 166. [Google Scholar]
- Schwabe L, Bohbot VD, Wolf OT, 2012. Prenatal stress changes learning strategies in adulthood. Hippocampus 22, 2136–2143. [DOI] [PubMed] [Google Scholar]
- Schwabe L, 2013. Stress and the engagement of multiple memory systems: integration of animal and human studies. Hippocampus 23, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Parsons RG, Koleske AJ, Gourley SL, 2017. Differential expression of cytoskeletal regulatory factors in the adolescent prefrontal cortex: implications for cortical development. J. Neurosci. Res 95, 1123–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth C, McGlade E, Yurgelun-Todd D, 2017. Chronic stress in adolescents and its neurobiological and psychopathological consequences: an RDoC perspective. Chronic Stress 1 1, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G, 2015. What the orbitofrontal cortex does not do. Nat. Neurosci 18, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2016. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Bouton ME, 2014. Contextual control of operant behavior: evidence for hierarchical associations in instrumental learning. Learn. Behav 42, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte AJ, Kietzman HW, Swanson AM, Butkovich LM, Barbee BR, Bassell GJ, et al. , 2019. Reward-related expectations trigger dendritic spine plasticity in the mouse ventrolateral orbitofrontal cortex. J. Neurosci 39, 4595–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G, 2017. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron 95, 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Schoenbaum G, 2016. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat. Rev. Neurosci 17, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Drew MR, Mimura M, Tanaka KF, 2019. Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat. Neurosci 22, 770–777. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shao F, Wang Q, Xie X, Wang W, 2017. Neuroplastic correlates in the mPFC underlying the impairment of stress-coping ability and cognitive flexibility in adult rats exposed to chronic mild stress during adolescence. Neural Plast 2017, 9382797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KS, Li CC, Rainnie DG, Ressler KJ, Gourley SL, 2018. Memory retention involves the ventrolateral orbitofrontal cortex: comparison with the baso-lateral amygdala. Neuropsychopharmacology 43, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KS, Yamin JA, Rainnie DG, Ressler KJ, Gourley SL, 2017. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by BDNF-TrkB. Biol. Psychiatry 81, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


