Abstract
Obesity is a chronic metabolic disease caused by multiple factors and is considered to be a risk factor for type 2 diabetes, cardiovascular disease, hypertension, stroke and various cancers. Hesperidin, a flavanone glycoside, is a natural phenolic compound with a wide range of biological effects. Mounting evidence has demonstrated that hesperidin possesses inhibitory effect against obesity diseases. Our review discusses mechanisms of hesperidin in the treatment of obesity. Hesperidin regulates lipid metabolism and glucose metabolism by mediating AMPK and PPAR signaling pathways, directly regulates antioxidant index and anti-apoptosis, and indirectly mediates NF-κB signaling pathway to regulate inflammation to play a role in the treatment of obesity. In addition, hesperidin-enriched dietary supplements can significantly improve symptoms such as postprandial hyperglycemia and hyperlipidemia. Further clinical trials are also required for confirming lipid-lowering efficacy of this natural flavonoid and evaluating its safety profile.
Keywords: citrus flavonoids, lipid metabolism, glucose metabolism, anti-oxidation, anti-inflammatory
Introduction
Obesity refers to the pathological state in which the intake of energy is greater than the consumption,1 causing excessive body fat2 and making the body weight more than 20% of the standard body weight.3 Obesity is a metabolic syndrome4 that is prevalent in today’s society,5 and its prevalence is increasing worldwide.6 According to research, the global obese or overweight population has more than 30% of the global population,7 and it has reached epidemic proportions globally.8 Obesity has become a major public health problem worldwide.9
The prevalence of obesity continues to rise throughout the world, mainly due to lifestyle changes,10 urbanization11 and genetic factors,12 such as eating habits and lack of exercise.13 Food is one of the main environmental factors inducing obesity,14 excessive consumption of dietary fat leads to an increase in the number of fat cells (hyperplasia) and size (hypertrophy).15 The increase in fat cell size and the inability to store triglycerides under excessive feeding are critical for metabolic dysfunction and are characterized by activation of the inflammatory and apoptotic pathways and secretion of pro-inflammatory adipokines.16 Obesity promotes the infiltration of inflammatory cells into various tissues, leading to the development of substantial and stromal cell interactions as well as cellular and organ dysfunction.17 In addition, oxidative stress and injury are involved in the pathophysiology of obesity and its metabolic complications.18 Insulin(INS) resistance is a pathological condition in which insulin target tissues (muscle, liver, adipose tissue, and hypothalamus) are insufficiently sensitive to normal levels of insulin.19 Excessive accumulation of visceral fat is the main cause of inflammation20 and insulin resistance,9 and is closely related to the occurrence of cardiovascular,21,22 cerebrovascular diseases, hypertension,23 type 2 diabetes,24,25 hyperlipidemia, sleep apnea syndrome26 and other diseases.27 It has also increased non-alcoholic fatty liver disease,9 cancer28 and other diseases, which seriously affect the health of patients and even endanger their lives.7 Therefore, prevention and treatment of obesity29 is the key to reducing the increasing morbidity and mortality of humans.6
Flavonoids are a class of phenolic compounds widely distributed in plants. Currently, a large number of these compounds are evaluated in the form of free state and glycoside,30 and have some biological properties including antioxidant,31,32 anticancer and anti-inflammatory33 effects.34 The adipose tissue is the primary regulator of energy balance and nutrient homeostasis. White adipose tissue(WAT)35 is the main site of excess energy storage in the form of triglycerides, while brown adipose tissue(BAT)36 with multi-room fat cells contains large amounts of mitochondria. Under some stimulations such as high-fat diets, the content of mitochondria in WATs increases dramatically, a process called “browning”. Thus, it can prevent obesity and lipid accumulation through induction of brown-like adipocyte formation.9,37 Citrus flavonoids have been proven to induce browning of white adipocytes,38 reduce plasma lipid levels, improve glucose tolerance, and reduce obesity,39 and can also be used to prevent postprandial hyperglycemia.40 Studies have shown that feeding a high-fat, high-cholesterol diet for 12 weeks affects atherosclerosis, PPARs, lipoprotein receptors, and apolipoprotein-related genes in monocyte chemoattractant protein-3 mice.41 During the differentiation of adipocytes, several transcription factors, including CCAAT/enhancer binding protein(C/EBPs) and peroxisome proliferator-activated receptor gamma(PPAR-γ), activate lipogenesis.42 Extracts of citrus flavonoids inhibit intracellular triglyceride and fat accumulation and reduce the expression of PPAR-γ 243 Citrus flavonoids inhibit oleic acid-induced expression of miR-122 and miR-33, and their target mRNAs fatty acid synthase(FAS) and carnitine palmitoyltransferase 1α(TNF-α) are likely to be the main mechanisms leading to decreased lipid accumulation in HepG 2 cells.44 It has been reported that the chemical structure of flavanones is the most effective in inhibiting adipogenesis because flavonoids such as hesperidin induce a significant decrease in triacylglycerol content in preadipocytes.45 Recent studies have shown that citrus flavonoids play an important role in the treatment of dyslipidemia, insulin resistance, hepatic steatosis, obesity and atherosclerosis. Citrus flavonoids, including naringenin, hesperidin, nobiletin and hesperetin, have become promising therapeutic agents for the treatment of metabolic disorders.46
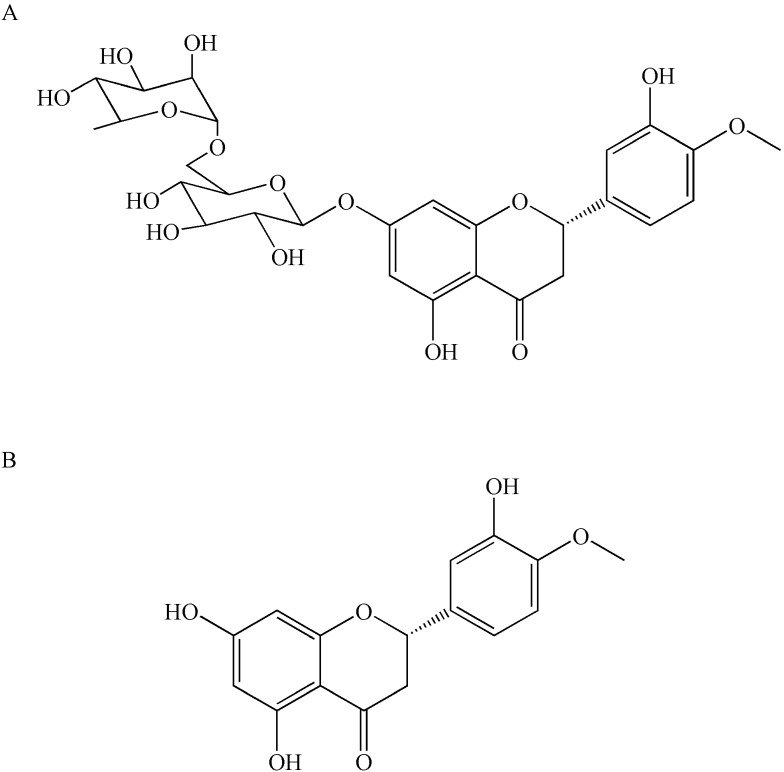
Hesperidin(C28H34O15) is a flavonoid glycoside47 which was first isolated from citrus peel by the French chemist Lebreton.48 The presence of this compound has also been proven in the genus Rutaceae, the bergamot fruit,49 the banana fruit, the lemon fruit, the lemon peel, etc.50 It may also be present in the aerial part of the genus Rubiaceae and the Cruciferous plant leeks, with roots and whole grasses. Hesperidin has an aglycon (hesperetin or methyl eriodictyol) bonded to rutinose [6-O-(α-l-Rhamnopyranosyl)-D-glucopyranose] and/or [6-O-(α-l-Rhamnosyl)-D-glucose], as a disaccharide, in its structure47 (Figure 1).
Figure 1.
Chemical structures of hesperidin (A) and hesperetin (B).
Mechanisms Of Hesperidin In The Treatment Of Obesity
Hesperidin has anti-inflammatory, anti-oxidative and anti-cancer activities, can lower cholesterol levels and blood pressure,28 and has anti-obesity activity.43 Hesperetin and hesperidin can stimulate the release of cholecystokinin(CCK), an appetite-regulating hormone, in enteroendocrine STC-1 cells, which is ultimately used to treat obesity by suppressing appetite.51 Dietary bioflavonoid hesperidin can reduce cholesterol and triglyceride levels in broiler serum and pectoral muscle, and positively improve fatty acid and lipid metabolism in broiler breasts in a dose-dependent manner.52 The main types of HPD metabolism in rats are mainly hydrolysis, demethoxylation, dehydration, dehydrogenation, demethylation, glucuronide binding, sulfate binding and N-acetylcysteine binding.53 HPD can significantly increase the level of α-KL in serum, liver and kidney tissues of diabetic rats, and significantly reduce the levels of aspartate aminotransferase(AST), alanine aminotransferase(ALT), blood urea nitrogen(BUN) and creatinine in fibroblast growth factor-23(FGF-23) in kidney tissues and serum samples.54 High-dose hesperidin up-regulates adenosine 5ʹ-monophosphate(AMP)-activated protein kinase(AMPK) mRNA expression in mice with glycolipid metabolism disorder induced by high-fat diet, affecting insulin signaling pathway (insulin receptor(INSR), insulin receptor substrate 1(IRS-1), GLUT2/4) and lipid metabolism-related genes (sterol regulatory element-binding protein 1c(SREBP1c) and FAS and acetyl-CoA carboxylase(ACC)) gene expression also activates PPAR-α mRNA expression.55 In addition, HPD enhances the expression of genes encoding LDL receptors, which are some of the possible mechanisms by which HPD reduces blood lipids.56
The details on weight loss effect of hesperidin are shown in Table 1.
Table 1.
Studies Demonstrating The Weight Loss Effect Of Hesperidin
| Model | Dose And Treat Time | Described Effect | Weight Loss Mechanism | Ref |
|---|---|---|---|---|
| Sisolated perfused male wistar rats, ad libitum with a standard laboratory diet | 300µM; 0–70min | Glycogenolysis and glycolysis in the liver↑; glucose phosphorylation catalysed by GK↓ | G-6-Pase↓ | 57 |
| Rats | 1mL; 24h | Enzyme activities↓; production of pyruvate↓; hepatic gluconeogenes↓; α-ketoglutarate and the oxaloacetate↓ | Liver ALT↓; liver AST↓ | 58 |
| HIGH fat fed/streptozotocin- induced type 2 diabetic rats | 50mg/kg; 4w | Serum glucose and glycosylated hemoglobin↓; vitamin C and vitamin E↑ | NO↓; IL-6↓; TNF-α↓; serum INS↑; GSH↑; liver MDA↓; liver antioxidant enzymes↑ | 59 |
| Male wistar rats, high-cholesterol diet | 25g/d; 12w | Hepatic steatosis, adipose tissue and liver weights↓; serum TC ↓ | RBP, H-FABP, C-FABP in liver and adipose tissue↓ | 60 |
| Male wistar rats, high-fat/sucrose (western) diet | 100mg/kg; 8w | Blood lipid profle↑; hepatic lipid accumulation↓; non-alcoholic steatohepatitis↓ | SREBP1↓; PPAR-γ↓; SCD↓; FAS↓ | 8 |
| Type 2 diabetic rats, high fat diet | 50mg/kg; 4w | White blood cell count↓; neutrophils↓; monocytes↓; basophils↓ | IL-6↓; adipose tissue ACDC↑ | 61 |
| Streptozotocin-induced marginal type 1 diabetic rats | 10g/kg; 4w | Blood glucose↓; TC↓ | Serum ACDC↑; TG↓; G-6-Pase↓; GK↑; LDL-C↓; VLDL-C↓; HDL-C↑; serum INS↑; | 56 |
| Rats, high-cholesterol diet | 8mg/d; 6–12w | Body and liverand adipose tissue weights↓; cholesterol synthesis and absorption↓ | Lipid-related factors (RBP4, H-FABP and C-FABP)↓; ICAM-1↓; inflammatory-related factors (MCP1, CCR2 and TNF-α)↓ | 62 |
| Goto-Kakizaki weanling rats with type 2 diabetes | 0.01g/; 4w | Lipids in the serum and liver↓; blood glucose↓; HDL-C/TC↑ | The genes coding for PPARs↑; HMG-CoA reductase↓; the expression of genes encoding LDL receptor↑; serum ACDC↑; TG↓; INS↑ |
15 |
| Rats with diabetes induced by streptozotocin | 100mg/kg; 2w | Strong positive effects on diabetic toxicity in the liver and kidneys | Liver, kidney and serum α-KL ↑; FGF-23↓; MDA↓ | 20 |
| Rats subjected to isoproterenol- induced cardiotoxicity | 200mg/kg; 7d | TC↓ | LDL-C↓; TG↓; VLDL-C↓; FFA↓; plasma PL↓; HDL-C↑; PL in the heart and liver↑ | 63 |
| Rats, high-cholesterol diet | 100mg/kg; 5d | TC↓; HDL-C/TC↑; serum triglyceride levels↓ | GSH in the liver↑; serum and liver MDA↓ | 64 |
| Streptozotocin-induced hyperglycemic mice | 200mg/kg; 14d | Blood glucose↓; lipid peroxidation and total nitrate/nitrite↓ | Bad/Bcl-2↑; Bad/Bcl-XL↑; SOD↑; GSH↑ | 65 |
| C57BL/6J mice, high-fat diet | 100mg/kg/d; 4w | Serum total antioxidant capacity↑; liver TBARS levels↓; spleen mass↓; fat accumulation↓; liver damage↓ |
IL-6↓; MCP-1↓; hs-CRP↓; LDL-C↓ | 66 |
| C57 mice, high-fat diet | 100,200,400mg/kg/d; 16w | Body weight↓; body fat deposition↓; serum glucose↓; serum lipid↓; HOMA-IR index↓ | mRNA of AMPK↑; serum INS↑; impact on signaling pathway genes↑ (INSR, IRS-1, GLUT2/4) and lipid metabolism pathway genes (SREBP1↓, FAS↓, ACC↓,PPAR-α↑) | 55 |
| Mice, high-fat diet | 0.07mg/100g; 9w | Body weight and liver and adipose tissue weight↓ | PPAR-γ↑ | 67 |
| C2C12 cells | 0.07mg/100g; 6h | Stimulated glucose↑ | PPAR-γ↑ | |
| Pre-adipocytes of mesenchymal stem cells | 1,10,25µM; 48h-8d | Anti-adipogenic and delipidating | C/EBPβ↓; SREBP1↓; perilipin↓;PPAR-γ↓ | 68 |
| Mature adipocytes from mesenchymal stem cells | 1,10,25µM; 48h-8d | Anti-adipogenic effect and delipidating | mRNA of ATGL↑; FAS↓;TG accumulation↓ | |
| 3T3-L1 pre-adipocytes | 1,10,25µM; 0-60h-8d | Lipid accumulation↓; riacylglycerol content in pre-adipocytes↓ | SREBP1↓ | 45 |
| 3T3-L1 adipocytes | 20µM; 8d | Lipid accumulation↓ | ROS↓; PPAR-γ↓; C/EBPα↓; FABP4↓ | 28 |
| 3T3-L1 cells | 0.5mg/mL; 24h | Induction of adipolytic activity↓; key adipogenic transcription factors↓ | C/EBPα↓; PPAR-γ↓; SREBP1↓ | 42 |
| 3T3-L1 cells | 10, 50, 100µM; 8d | Anti-lipogenic capacity↑ | Binding affinity for the PPAR-γ rceptor↓; SCD↓; LPL↓ |
69 |
| RAW264.7 and 3T3-L1 cells | 1.8–8.3µM; 24h | Anti-inflammatory activity↑ | ACDC↑; IL-6↓; TNF-α↓; NO↓ | 70 |
| Enteroendocrine STC-1 cells | 0.1,0.5,1.0µM; 60min | Appetite-regulating hormones↑; cholecystokinin release↑ | Intracellular Ca(2+) concentrations↑ | 51 |
| Retinal ganglial cells −5 | 12.5,25,50µmol/L; 6h | High glucose-mediated cell loss↓; mitochondrial function↑; Cell apoptosis↓ |
ROS, MDA and protein carbonyl↓; SOD↑; CAT↑; GSH↑; caspase-9, caspase-3 and Bax/Bcl-2↓ | 71 |
| HepG2 cells | 100ug/mL; 48h | Lipid accumulation↓ | miR-122 and miR-33 expression↓; CPT1α↑; FAS↓ | 44 |
| HepG-2 cells | 50µM; 1min | Digestive enzyme activities↓; glycogen↑ |
GK activity↑; G-6-Pase↓ | 40 |
| Porcine pancreas | 100µM; 1min | Glucose consumption↑; glycogen↑; glucokinase activity↑ | α-amylase activity↓; α-glucosidase activity↓ | |
| Caenorhaditis elegans | 50µM,100µM; 0–35d | Fat accumulation↓; the ratio of oleic acid/stearic acid↓ | SCD↓; FAT-6↓; FAT-7↓; POD-2↓; MDT-15↓; ACS-2↓; KAT-1↓ | 72 |
| Broilers | 20mg/kg; 42d | Plasma antioxidant parameters↑; TC↓; total antioxidant capacity↑ | Total SOD↑; MDA↓; TG↓ | 52 |
Note: ↓indicates inhibition/reduction while ↑indicates increase/promotion.
Abbreviations: ACDC, adiponectin; hs-CRP, High-sensitivity C-reactive protein; INSR, Insulin receptor; IRS-1, Insulin receptor substrate 1; ATGL, adipose triacylglyceride lipase; PL, phospholipids; FFA, free fatty acids; TG, triglycerides; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; VLDL-C, very low density lipoprotein-cholesterol; MDA, malondialdehyde; GSH, glutathione; G-6-Pase, glucose-6-phosphatase; HMG-CoA,3-hydroxy-3-methyl-glutatyl coenzyme A; α-KL, α-Klotho; FGF-23, fibroblast growth factor-23; RBP4,retinol-binding protein 4; CCR2, C-C chemokine receptor type 2; MCP1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor alpha; TBARS, thiobarbituric acid reactive substances; ROS, reactive oxygen species; CAT, catalase; GK, glucokinase; C/EBPβ, CCAAT/enhancer-binding protein beta; PPAR-γ, peroxisome proliferator-activated receptor gamma; SREBP1, sterol regulatory element-binding protein 1; RBP, lipid metabolism–related proteins; H-FABP, heart fatty acid–binding protein; C-FABP, cutaneous fatty acid–binding protein; IL-6,interleukin-6; NF-κB, nuclear factor kappa B; SCD, stearoyl-CoA desaturase; TC, Total cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPT1α, carnitine palmitoyltransferase 1α; FAS, fatty acid synthase; LPL, lipoprotein lipase; FAT-6/7, Fatty-acid desaturase 6/7; ACS-2, acyl-CoA synthetase-2; KAT-1, ketoacyl-CoA thiolase-1; POD-2, acetyl-CoA carboxylase-2; MDT-15, mediator subunit-15.
The Effect Of Hesperidin On Lipid Metabolism
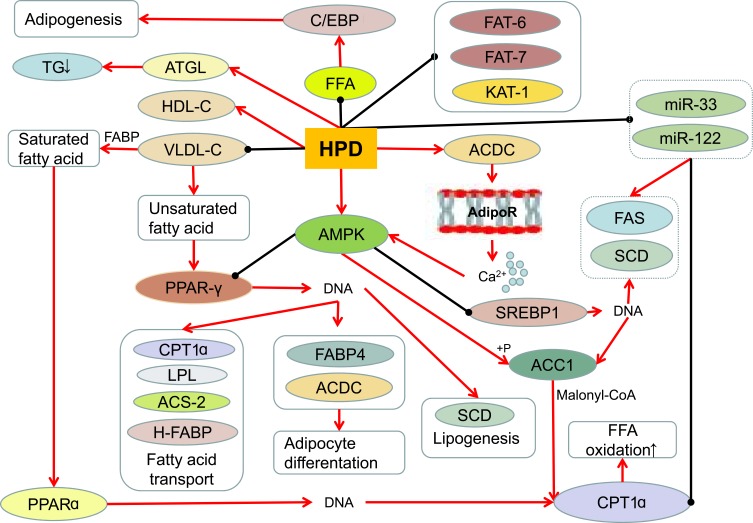
Changes in body fat content beyond a certain limit can cause obesity,73 so obesity is closely related to fat metabolism. Hesperidin can improve lipid metabolism74 (Figure 2). Adipose tissue stores lipids in the form of triglycerides, which secrete and regulate a variety of adipokines and cytokines. During obesity, in order to compensate for excessive lipid load, adipose tissue rapidly expands.46 Hesperidin (0.08%) reduces hepatic steatosis, adipose tissue and liver weight, and decreases serum total cholesterol and retinol binding protein(RBP) 4 concentrations in high-fat diets.60 Heart fatty acid–binding protein(H-FABP)and cutaneous fatty acid–binding protein(C-FABP) are thought to play key roles in fatty acid metabolism, such as fatty acid storage and transport.62 Hesperidin may improve hypercholesterolemia and fatty liver by inhibiting cholesterol synthesis and absorption, regulating RBP, C-FABP and H-FABP mRNA expression.60 HPD reduced systolic blood pressure(SBP),75 plasma total cholesterol and TG levels in obese hypertensive rats, attenuated plasma fatty acid synthase(FFA) through its anti-lipolytic activity, significantly increased high density lipoprotein-cholesterol(HDL-C), and decreased plasma low density lipoprotein-cholesterol(LDL-C) and very low density lipoprotein-cholesterol(VLDL-C).63
Figure 2.
The effect of hesperidin on lipid metabolism.
Note:
 indicate inhibition/reduction while
indicate inhibition/reduction while  indicate increase/promotion.
indicate increase/promotion.
Hesperidin inhibits genes involved in the three stages of adipogenesis, C/EBPβSREBP1c, PPAR-γ and perilipin.68 Hesperidin-treated animals showed decreased expression levels of three key adipogenesis-related genes, SREBP1, FAS68 and stearoyl-CoA desaturase(SCD),32 and normalization of PKLR gene expression.8 Hesperidin showed specific inhibitory activity on 3T3-L1 preadipocytes in the intermediate stage of differentiation.32 HPD increases the expression of messenger RNA by hormone-sensitive lipases and stimulates the breakdown of mature adipocytes.76 Hesperidin significantly down-regulates the expression of stearoyl-CoA desaturase, fatty-acid desaturase(FAT-6 and FAT-7), and reduces the expression of other genes involved in lipid metabolism, including acetyl-CoA carboxylase-2(POD-2), mediator subunit-15(MDT-15), acyl-CoA synthetase-2(ACS-2) and 3-ketoacyl-CoA thiolase-1 (KAT-1), thereby reducing fat accumulation.72 Dietary bioflavonoid hesperidin can positively improve fatty acid and lipid metabolism in broiler breasts in a dose-dependent manner.52 Therefore, hesperidin can treat obesity to a certain extent by regulating adipokines, cytokines, genes, and the like in lipid metabolism.
The Effect Of Hesperidin On Glucose Metabolism
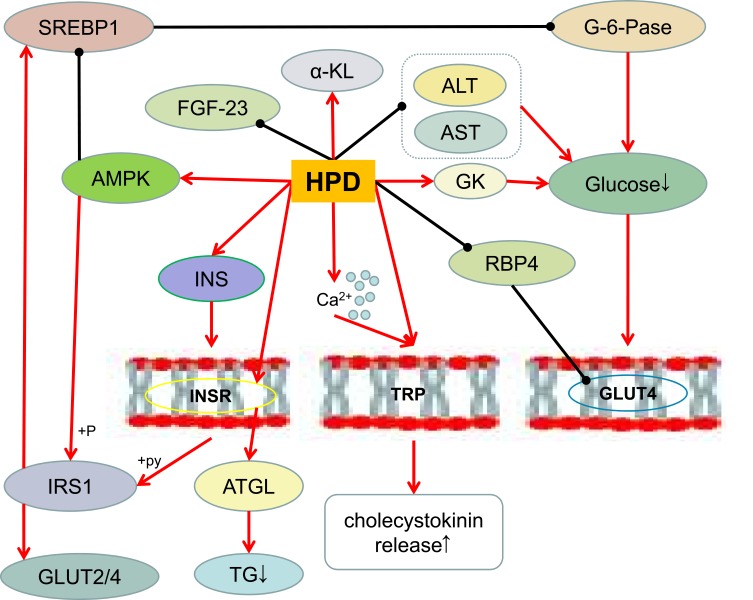
Obesity has a certain degree of impaired glucose tolerance77 and insulin dysfunction.78 Impaired glucose metabolism-related genetic variants likely interact with obesity-modifiable factors in response to glucose intolerance.79 Glucose provides most of the carbon used to construct the essential molecules of daughter cells, such as amino acids, fatty acids and nucleotides.80 Hesperidin shows moderate and selective alpha-glucosidase inhibitory activity,81 and it can inhibit the digestion of amylose and amylopectin and significantly reduce glucose-6-phosphatase activity in HepG2 cells.40 Docking simulations showed that hesperetin and hesperidin block enzyme entry into the channel, preventing the production of pyruvate, alpha-ketoglutarate and oxaloacetate, inhibiting hepatic gluconeogenesis, thereby impeding the progression of diabetes.58 In addition, hesperidin stimulates glycogenolysis and glycolysis in isolated perfused rat liver57 and reduces glucose levels induced in porcine streptozotocin-induced diabetic and diabetic rat models.82 The postprandial glycemic response of orange juice can be adjusted by partially inhibiting the intestinal glucose transporter according to the concentration of sugar and hesperidin,83 indicating that hesperidin can be used to prevent postprandial hyperglycemia.40
PPAR-c is a nuclear protein transcription factor that regulates lipid and glucose metabolism, and hesperidin maintains glucose metabolism by regulating PPAR-c activation and inhibiting fat accumulation.67 It has been demonstrated in weaned Goto-Kakizaki rats that hesperidin and cyclodextrin-clathrated hesperetin normalize blood glucose levels by altering the activity of glucose-regulating enzymes and lowering serum and liver lipid levels. These hypoglycemic and hypolipidemic effects in type 2 diabetic rats are partially altered by altering the expression of genes encoding PPAR, 3-hydroxy-3-methyl-glutatyl coenzyme A(HMG-CoA) reductase and LDL receptors.15 RBP4 has been identified as an adipokines involved in the regulation of glucose metabolism.62 The activation of GLUT4 enhances glucose uptake and increases the amount of intracellular glucose available for metabolic conversion, thereby promoting enhanced cell proliferation.80 Hesperidin can reduce the expression of RBP4 and affect GLUT460 Insulin can promote the synthesis of fatty acids in the liver, promote the entry of glucose into fat cells and convert it into triacylglycerol for storage, while inhibiting the activity of lipase and reducing the decomposition of fat.84 Insulin resistance caused by obesity inhibits insulin absorption of glucose and fat in muscle and muscle tissue.19 Hesperidin indirectly affects insulin resistance and stimulates intestinal microbial growth to increase the production of short-chain fatty acid(SCFA), thereby regulating adipose tissue, skeletal muscle and liver tissue function, and improving glucose homeostasis and insulin sensitivity.85 Hesperidin directly or indirectly regulates the metabolism of glucose and insulin (Figure 3) to improve the interaction between obesity and glucose metabolism disorders (such as hyperglycemia, diabetes, etc.), which is one of the effective ways to treat obesity.
Figure 3.
The effect of hesperidin on glucose metabolism.
Note:
 indicate inhibition/reduction while
indicate inhibition/reduction while  indicate increase/promotion.
indicate increase/promotion.
The Effect Of Hesperidin On Oxidation And Inflammation
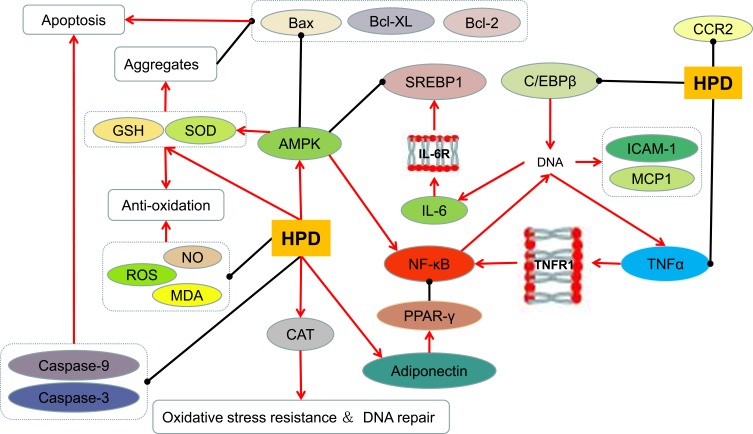
The serum oxidative index of young obese subjects increased significantly, and the antioxidant index decreased significantly, suggesting that accumulation of oxidative products in serum may be one of the causes of obesity.86,87 Hesperidin decreases the contents of glucose, glycosylated hemoglobin(HbA1c%), MDA and NO in diabetic rats, and increases levels of serum insulin, GSH, vitamin C and vitamin E. It has a protective effect on oxidative damage induced by hyperglycemia.59 The combination of hesperidin and alpha amylase has low antioxidant activity.88 Hesperidin can prevent the increase of reactive oxygen species(ROS) production in rats by exhaustive exercise, and avoid the decrease of SOD and catalase activity in thymus and spleen,89 which can effectively inhibit the formation of superoxide and oxygen.90 Hesperidin reduces ROS, MDA, caspase-9, caspase-3 and Bax/Bcl-2 levels and inhibits apoptosis, thereby protecting RGC-5 cells from high glucose-induced oxidative stress.71 Dietary application of different levels of hesperidin has a significant effect on the antioxidant capacity of mutton during cold storage,91 and can also increase plasma antioxidant parameters of broilers, including total antioxidant capacity, malondialdehyde(MDA) production, and total superoxide dismutase(SOD) activity.52 Medium doses of hesperidin(100 mg/kg) and high doses of hesperidin(200 mg/kg) improved and increased the level of endogenous antioxidant enzyme glutathione(GSH) in the liver of hyperlipidemic rats.64 Hesperidin can alter the oxidative state in hepatocytes by affecting parameters related to hepatic fatty acid oxidation, namely oxygen uptake, citrate cycling activity and ketone production.92 The bioflavonoid mixture of curcumin, hesperidin and rutin improves hepatic oxidative stress caused by streptozotocin- induced hyperglycemia, thereby improving liver function and glucose regulation.65
Obesity is a systemic low-level chronic persistent inflammatory state.93 It is currently believed that the pathophysiological basis of obesity is the early inflammatory changes in adipose tissue.94 PPAR-γ is a nuclear transcription factor involved in the inhibition of nuclear factor kappa B(NF-κB) activation and IL-6 production, which can be induced by adiponectin, while adiponectin pretreatment of porcine macrophages inhibits NF-κB activation and inhibits TNF-α secreted by LPS-stimulated macrophages.70 Injection of hesperidin can increase the content of adiponectin, thereby reducing lipid accumulation.56 Oral administration of 50 mg/kg hesperidin daily in type 2 diabetic rats for 4 weeks significantly improved red blood cells, white blood cells and their functional indicators, and significantly improved adiponectin expression downregulation and IL-6 down-regulation in adipose tissue Relationship. Hesperidin protects diabetes-related anemia by affecting adipose tissue.61 In addition, hesperidin can increase the serum total antioxidant capacity of mice with high-fat diet, inhibit IL-6, macrophage chemoattractant protein 1(MCP-1) and C-reactive protein(hs-CRP) to reduce liver thiobarba Bilobate-reactive substance(TBARS) levels and spleen mass, and prevent mouse inflammation and oxidative stress caused by a high-fat diet, thereby preventing metabolic changes associated with cardiovascular disease development in other animals.66 Hesperidin improves the degree of inflammation and oxidative damage caused by hyperglycemia and hyperlipidemia by directly affecting oxidation- related index and inflammatory factors (Figure 4), which indirectly plays a therapeutic role in the treatment of obesity- related diseases.
Figure 4.
The effect of hesperidin on oxidation and inflammation.
Note:
 indicate inhibition/reduction while
indicate inhibition/reduction while  indicate increase/promotion.
indicate increase/promotion.
Conclusions
Obesity is an abnormality in energy metabolism caused by a variety of factors,84 which in turn affects various metabolisms in the body, so the way to lose weight is also diverse. In this review, the most relevant articles were evaluated to reveal how hesperidin is effective in obesity through multi-target ways. As a cellular energy sensor, AMP activates protein kinase(AMPK), which not only restores energy balance between activities, but also plays an important role in lipid metabolism.95 PPARs are nuclear receptor proteomes, transcription factors that play important roles in lipid metabolism and glucose homeostasis.67 Hesperidin mainly regulates lipid metabolism and glucose metabolism by affecting AMPK and PPAR signaling pathways, thereby exerting a lipid-lowering effect. In addition, obesity is a systemic low-level chronic persistent inflammatory state.93Hesperidin has a therapeutic effect on obesity by mediating AMPK and PPAR pathways to regulate NF-κB inflammatory signaling pathways and reducing inflammation and apoptosis. Hesperidin can also directly regulate the oxidation index, inhibit apoptosis, thereby protecting against damage caused by oxidative stress, and improving lipid peroxidation.
Furthermore, the above-mentioned lipid-lowering effect of hesperidin can be extended to other similar flavonoids. Naturally occurring extracts and biotransformed extracts from citrus fruits can be used for the treatment of obesity, natural extracts can be used to reduce new fat cell synthesis and lipid accumulation, and biotransformation extracts can be used to induce lipolysis of adipose tissue.96 For example, citrus peel extract has potential antioxidant and lipid peroxidation and lipoxygenase inhibition.97 Citri Reticulatae Pericarpium has been investigated with a health promoting properties,98,99 which can remove moisture and protect the spleen,100 while reducing NO levels,101 exerting antioxidant effects,38,102 and lowering the liver lipid content.103 Its extract has anti-lipase activity,104 which can directly or indirectly treat obesity. Moreover, given the various biological properties of hesperidin, this phytochemical may have a wider range of biological applications in the future. Therefore, research on natural drugs or foods containing hesperidin can help expand the range of weight loss and reduce the rate of obesity in the body. Further studies on flavonoids similar to hesperidin can better reveal the preventive and therapeutic effects of hesperidin on obesity.
Although the hypoglycemic and lipid-lowering activities of hesperidin have been studied in some animals (such as rats) or cells, the lack of clinical trials on the therapeutic effect of hesperidin is a significant limitation that deserves further study. Furthermore, little is known about the clinical aspects of this compound, such as bioavailability, the appropriate dose, tolerance and efficacy of hesperidin and its metabolites for human disease.15 More investigations should be needed before hesperidin treatment is extended to humans, especially reliable clinical trials, including large-scale, rigorously controlled, and multicenter randomized controlled clinical trials are needed to assess its long-term safety.
Acknowledgments
We are indebted to our alma mater, Chengdu University of Traditional Chinese Medicine for provided convenience in the collection of documents. Thanks for all the help from everyone in our lab.
Consent For Publication
All authors have provided consent for publication.
Author Contributions
Haijun Xiong and Jin Wang are first authors and responsible for collecting materials and writing the paper. Qian Ran, Guanhua Lou, Chengyi Peng, Qingxia Gan, Ju Hu, Jilin Sun and Renchuan Yao helped with organizing the information and edited in the article pictures. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation Youth Fund (Code: 81102804), Key Research and Development Project of Sichuan Science and Technology Department (Code: 2018SZ0077) and Sichuan Colleges and Universities Research Innovation Team Construction Plan Funding (Code: 18TD0017).
Disclosure
Jilin Sun is the general manager of Sichuan Fuzheng Pharmaceutical Co. Ltd. The authors report no other conflicts of interest in this work.
References
- 1.Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of Ad libitum food intake. Cell Metab. 2019;30(1):67–77.e63. doi: 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uti DE, Atangwho IJ, Eyong EU, et al. African walnut (Tetracarpidium conophorum) modulates hepatic lipid accumulation in obesity via reciprocal actions on HMG-CoA reductase and paraoxonase. Endocr Metab Immune Disord Drug Targets. 2019. doi: 10.2174/1871530319666190724114729 [DOI] [PubMed] [Google Scholar]
- 3.Yan Q. Progress in research on the causes of obesity. Neijiang Technol. 2009;30(9):29. [Google Scholar]
- 4.Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi MA, Basiri ML, McHenry JA. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science. 2019;364(6447):1271–1274. doi: 10.1126/science.aax1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang YF, Yuan-Shuang WU, Song YZ, Meng QX. Research progress in the mechanism of anti-obesity effect of tea. Sci Technol Food Ind. 2013. [Google Scholar]
- 7.Xiao Y. Study of Correlation between Fat and Muscle Content of Obesity Patients and Obesity-Related Complication. Southern Medical University; 2016. [Google Scholar]
- 8.Mosqueda-Solís A, Sánchez J, Reynés B, et al. Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in western diet-fed rats. Sci Rep. 2018;8:15100. doi: 10.1038/s41598-018-32875-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ya-Chun C, Chi-Tang H, Min-Hsiung P. Immature Citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced C57BL/6 mice. J Agric Food Chem. 2018;66(37). [DOI] [PubMed] [Google Scholar]
- 10.Ohara T, Muroyama K, Yamamoto Y, Murosaki S. Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutr J. 2015;15(1):6. doi: 10.1186/s12937-016-0123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bixby H, Bentham J, Zhou B, Di Cesare M, CJ P. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569(7755):260–264. doi: 10.1038/s41586-019-1171-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandkvist M, Bjørngaard JH, Ødegård RA, Åsvold BO, Sund ER, Vie GÅ. Quantifying the impact of genes on body mass index during the obesity epidemic: longitudinal findings from the HUNT Study. BMJ. 2019;366:l4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohara T, Muroyama K, Yamamoto Y, Murosaki S. A combination of glucosyl hesperidin and caffeine exhibits an anti-obesity effect by inhibition of hepatic lipogenesis in mice. Phytother Res. 2015;29(2):310–316. doi: 10.1002/ptr.5258 [DOI] [PubMed] [Google Scholar]
- 14.Wang LH, Huang W, Li J, et al. Research progress on mechanism of acupuncture treating obese rats. Acupuncture Clin J. 2018;(2):78–81. [Google Scholar]
- 15.Akiyama S, Katsumata SI, Suzuki K. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73(12):2779–2782. doi: 10.1271/bbb.90576 [DOI] [PubMed] [Google Scholar]
- 16.Chen SS, Li Y, Xu KY, et al. Characteristics of adipocytes and their role in obesity-related inflammation. Chin J Cell Biol. 2019;41(05):979–984. [Google Scholar]
- 17.Miyachi Y, Tsuchiya K, Komiya C, et al. Roles for cell-cell adhesion and contact in obesity-induced hepatic myeloid cell accumulation and glucose intolerance. Cell Rep. 2017;18(11):2766. doi: 10.1016/j.celrep.2017.02.039 [DOI] [PubMed] [Google Scholar]
- 18.Montes‐Nieto R, Insenser M, Murri M. et al. Plasma thiobarbituric acid reactive substances (TBARS) in young adults: obesity increases fasting levels only in men whereas glucose ingestion, and not protein or lipid intake, increases postprandial concentrations regardless of sex and obesity. Mol Nutr Food Res;2017. 1700425. doi: 10.1002/mnfr.201700425 [DOI] [PubMed] [Google Scholar]
- 19.Fu XY, Guan QB. Research progress on obesity related complications. J Liaoning Univ Traditional Chin Med. 2017;(1):108. [Google Scholar]
- 20.Chagwedera DN, Ang QY, Bisanz JE, et al. Nutrient sensing in CD11c cells alters the gut microbiota to regulate food intake and body mass. Cell Metab. 2019. doi: 10.1016/j.cmet.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxton SN, Clark BJ, Withers SB. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99(4):1701–1763. doi: 10.1152/physrev.00034.2018 [DOI] [PubMed] [Google Scholar]
- 22.Robertson J, Schaufelberger M, Lindgren M, et al. Higher body mass index in adolescence predicts cardiomyopathy risk in midlife. Circulation. 2019;140(2):117–125. doi: 10.1161/CIRCULATIONAHA.118.039132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntyre HD, Catalano P, Zhang C. Gestational diabetes mellitus. Nature Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 24.Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019. doi: 10.1016/S2213-8587(19)30084-1 [DOI] [PubMed] [Google Scholar]
- 25.Neuenschwander M, Ballon A, Weber KS, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park YM, White AJ, Jackson CL, Weinberg CR, Sandler DP. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Y. Clinical Study of Obesity and Its Related Complications and Changes of Serum Related Factors in Different Glucose Metabolism States of Obese Patients. Peking Union Medical College; Chinese Academy of Medical Sciences; Tsinghua University School of Medicine; 2013. [Google Scholar]
- 28.Jeon H-J, Seo M-J, Choi H-S. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother Res. 2014;28(11):1701–1709. doi: 10.1002/ptr.5186 [DOI] [PubMed] [Google Scholar]
- 29.Torkamani A, Topol E. Polygenic risk scores expand to obesity. Cell. 2019;177(3):518–520. doi: 10.1016/j.cell.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 30.Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin — A mini-review. Life Sci. 2014;113(1–2):1–6. doi: 10.1016/j.lfs.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Chen L, Chen H, Chen S, Liu Y. Analysis of flavonoid metabolites in citrus peels (“Dahongpao”) using UPLC-ESI-MS/MS. Molecules. 2019;24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers CS, Biedermann D, Valentová K, et al. Preparation of retinoyl-flavonolignan hybrids and their antioxidant properties. Antioxidants. 2019;8(7):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toiu A, Vlase L, Vodnar DC, Gheldiu AM, Oniga I. Solidago graminifolia L. Salisb.(Asteraceae) as a valuable source of bioactive polyphenols: HPLC profile, in vitro antioxidant and antimicrobial potential. Molecules. 2019;24(14):2666. doi: 10.3390/molecules24142666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamideh P, Ali R, Fatemeh S, Ramin R, Mehrdad I. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–331. doi: 10.1002/ptr.5256 [DOI] [PubMed] [Google Scholar]
- 35.Jaitin DA, Adlung L, Thaiss CA, et al. Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Cell. 2019;178(3):686–698.e614. doi: 10.1016/j.cell.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraum TJ, Crandall JP, Ludwig DR, et al. Repeatability of quantitative brown adipose tissue imaging metrics on positron emission tomography with F-fluorodeoxyglucose in humans. Cell Metab. 2019;30(1):212–224.e214. doi: 10.1016/j.cmet.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 37.Mosqueda-Solís A, Sánchez J, Portillo MP. Combination of capsaicin and hesperidin reduces the effectiveness of each compound to decrease the adipocyte size and to induce browning features in adipose tissue of western diet fed rats. J Agric Food Chem. 2018;66(37):9679–9689. doi: 10.1021/acs.jafc.8b02611 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Li X, Fang H, et al. Flavonoids as inducers of white adipose tissue browning and thermogenesis: signalling pathways and molecular triggers. Nutr Metab (Lond). 2019;16:47. doi: 10.1186/s12986-019-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols LNA, Jackson DE, Manthey JA, Shukla SD, Holland LJ. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011;10(1):1–5. doi: 10.1186/1476-511X-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen W, Xu Y, Lu Y-H. Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J Agric Food Chem. 2012;60(38):9609–9619. doi: 10.1021/jf3032556 [DOI] [PubMed] [Google Scholar]
- 41.So Jung A, J UJ, Myung-Sook C, Kyu CC, Goo Taek O, Bok PY. Functions of monocyte chemotactic protein-3 in transgenic mice fed a high-fat, high-cholesterol diet. J Microbiol Biotechnol. 2013;23(3):405. doi: 10.4014/jmb.1210.10057 [DOI] [PubMed] [Google Scholar]
- 42.Lim H, Yeo E, Song E, et al. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr Res Pract. 2015;9(6):599–605. doi: 10.4162/nrp.2015.9.6.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudhia Z, Louw J, Muller C, et al. Cyclopia maculata and Cyclopia subternata (honeybush tea) inhibits adipogenesis in 3T3-L1 pre-adipocytes. Phytomedicine. 2013;20(5):401–408. doi: 10.1016/j.phymed.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Su D, Liu H, Qi X, Dong L, Zhang R, Zhang J. Citrus peel flavonoids improve lipid metabolism by inhibiting miR-33 and miR-122 expression in HepG2 cells. Biosci Biotechnol Biochem. 2019;83(9):1–9. doi: 10.1080/09168451.2019.1608807 [DOI] [PubMed] [Google Scholar]
- 45.Mosqueda-Solís A, Lasa A, Gómez-Zorita S, Eseberri I, Picó C, Portillo MP. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017;8(10):3576–3586. doi: 10.1039/C7FO00679A [DOI] [PubMed] [Google Scholar]
- 46.Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol. 2013;24(1):34–40. doi: 10.1097/MOL.0b013e32835c07fd [DOI] [PubMed] [Google Scholar]
- 47.Hajialyani M, Hosein Farzaei M, Echeverría J, Nabavi SM, Uriarte E, Sobarzo-Sánchez E. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648. doi: 10.3390/molecules24030648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 49.Sun Q, Xu C, Liu X, et al. Simultaneous determination of aurantiamarin and 5, 7-dimethoxycoumarin in Citri sarcodactylis Fructus from Sichuan by HPLC. Huaxi Pharm J. 2017;32(6):646–648. [Google Scholar]
- 50.Huang A, Zhang Y, Huang C, et al. Research progress of synthesis and biological activity of hesperidin and hesperetin derivatives. Pharm Prog. 2018;42(7):56–65. [Google Scholar]
- 51.Kim HY, Park M, Kim K, Lee YM, Rhyu MR. Hesperetin Stimulates Cholecystokinin Secretion in Enteroendocrine STC-1 Cells. Biomol Ther (Seoul). 2013;21(2):121–125. doi: 10.4062/biomolther.2012.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamboh AA, Zhu WY. Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poult Sci. 2013;92(2):454–461. doi: 10.3382/ps.2012-02584 [DOI] [PubMed] [Google Scholar]
- 53.Jiao Q, Xu L, Jiang L, Jiang Y, Zhang J, Liu B. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MS. Xenobiotica Fate Foreign Compd Biol Sys. 2019;57(18):1–27. doi: 10.1080/00498254.2019.1567956 [DOI] [PubMed] [Google Scholar]
- 54.Dokumacioglu E, Iskender H, Musmul A. Effect of hesperidin treatment on α-Klotho/FGF-23 pathway in rats with experimentally-induced diabetes. Biomed Pharmacother. 2019;109:1206–1210. doi: 10.1016/j.biopha.2018.10.192 [DOI] [PubMed] [Google Scholar]
- 55.Pu P. [Protection mechanisms of hesperidin on mouse with insulin resistance]. Zhongguo Zhong Yao Za Zhi. 2016;41(17):3290–3295. doi: 10.4268/cjcmm20161728 [DOI] [PubMed] [Google Scholar]
- 56.Satoko A, Shin-Ichi K, Kazuharu S, Yoshiko I, Jian W, Mariko U. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2010;46(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Do Nascimento GS, Constantin RP, Gilglioni EH. et al. The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver. Toxicol Lett. 2018;291:S0378427418301279. doi: 10.1016/j.toxlet.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 58.Zareei S, Boojar MMA, Amanlou M. Inhibition of liver alanine aminotransferase and aspartate aminotransferase by hesperidin and its aglycone hesperetin: an in vitro and in silico study. Life Sci. 2017;178:49–55. doi: 10.1016/j.lfs.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 59.Mahmoud AM, Ashour MB, Abdel A. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats - journal of diabetes and its complications. J Diabetes Complications. 2012;26(6):483–490. doi: 10.1016/j.jdiacomp.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Hasegawa J, Kitamura Y, et al. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci. 2011;117:129–138. doi: 10.1254/jphs.11097FP [DOI] [PubMed] [Google Scholar]
- 61.Mahmoud AM. Hematological alterations in diabetic rats - role of adipocytokines and effect of citrus flavonoids. Excli J. 2013;12:647–657. [PMC free article] [PubMed] [Google Scholar]
- 62.Qian W, Hasegawa J, Cai X. Components of boiogito suppress the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. Yonago Acta Med. 2016;59(1):67–80. [PMC free article] [PubMed] [Google Scholar]
- 63.Selvaraj P, Pugalendi KV. Efficacy of hesperidin on plasma, heart and liver tissue lipids in rats subjected to isoproterenol-induced cardiotoxicity. Experimental Toxicol Pathol. 2012;64(5):449–452. doi: 10.1016/j.etp.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 64.Yuce B, Danis O, Sener G, Bulut M, Yarat A. Antioxidative and lipid lowering effects of 7,8-dihydroxy-3- (4-methylphenyl) coumarin in hyperlipidemic rats. Arzneimittelforschung. 2009;59(03):129–134. doi: 10.1055/s-0031-1296375 [DOI] [PubMed] [Google Scholar]
- 65.Parmar M, Syed I, Gray J. Curcumin, hesperidin, and rutin selectively interfere with apoptosis signaling and attenuate streptozotocin-induced oxidative stress-mediated hyperglycemia. <![CDATA[Current Neurovascular Research]]>. 2015;12(4):363–374. doi: 10.2174/1567202612666150812150249 [DOI] [PubMed] [Google Scholar]
- 66.Ferreira PS, Spolidorio LC, Manthey JA. Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet. Food Funct. 2016;7(6):2675–2681. doi: 10.1039/C5FO01541C [DOI] [PubMed] [Google Scholar]
- 67.Shin EJ, Hur HJ, Sung MJ. Ethanol extract of the Prunus mume fruits stimulates glucose uptake by regulating PPAR-γ in C2C12 myotubes and ameliorates glucose intolerance and fat accumulation in mice fed a high-fat diet. Food Chem. 2013;141(4):4115–4121. doi: 10.1016/j.foodchem.2013.06.059 [DOI] [PubMed] [Google Scholar]
- 68.Gómez-Zorita S, Lasa A, Abendaño N. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J Transl Med. 2017;15(1):237. doi: 10.1186/s12967-017-1343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aranaz P, Navarro-Herrera D, Zabala M, et al. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to PPARγ. Molecules. 2019;24(6):1045. doi: 10.3390/molecules24061045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakajima VM, Moala T, Caria C, et al. Biotransformed citrus extract as a source of anti-inflammatory polyphenols: effects in macrophages and adipocytes. Food Res Int. 2017;97:37–44. doi: 10.1016/j.foodres.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 71.Liu W, Shorong-Shii L, Tang-Yao H, I-Min L. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients. 2017;9(12):1312. doi: 10.3390/nu9121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng H, Wei Z, Luo H. Inhibition of fat accumulation by hesperidin in caenorhabditis elegans. J Agric Food Chem. 2016;64(25):5207–5214. doi: 10.1021/acs.jafc.6b02183 [DOI] [PubMed] [Google Scholar]
- 73.Feng Q, Li Z. The causation and correlative disease of obesity and losing weight. Med Philosophy (B). 2006;27(5):65–69. [Google Scholar]
- 74.Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obesity Rev. 2016;17(7):573–586. doi: 10.1111/obr.v17.7 [DOI] [PubMed] [Google Scholar]
- 75.Guirro M, Costa A, Gual-Grau A. Multi-omics approach to elucidate the gut microbiota activity: metaproteomics and metagenomics connection. Electrophoresis. 2018;39(13):1692–1701. doi: 10.1002/elps.v39.13 [DOI] [PubMed] [Google Scholar]
- 76.Jack B, Malherbe C, Willenburg E. Polyphenol-enriched fractions of cyclopia intermedia selectively affect lipogenesis and Lipolysis in 3T3-L1 adipocytes. Planta Med. 2018;84(2):100–110. doi: 10.1055/s-0043-119463 [DOI] [PubMed] [Google Scholar]
- 77.Li E, Shan H, Chen L. et al. OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab. 2019. [DOI] [PubMed] [Google Scholar]
- 78.Zhou LL, Yang HJ, Shen W, et al. Relationship between adolescent obesity and abnormal glucose metabolism. Chin J Health Inspection. 2018;(5):626–628. [Google Scholar]
- 79.Jung SY, Sobel EM, Papp JC, Crandall CJ, Fu AN, Zhang Z-F. Obesity and associated lifestyles modify the effect of glucose metabolism-related genetic variants on impaired glucose homeostasis among postmenopausal women. Genet Epidemiol. 2016;40(6):520–530. doi: 10.1002/gepi.2016.40.issue-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, Wolfram J, Boom K, Fang X, Shen H, Ferrari M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem Funct. 2013;31(5):374–379. doi: 10.1002/cbf.v31.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loizzo MR, Falco T, Bonesi M. Ruta chalepensis L. (Rutaceae) leaf extract: chemical composition, antioxidant and hypoglicaemic activities. Nat Prod Res. 2018;32(5):521–528. doi: 10.1080/14786419.2017.1326491 [DOI] [PubMed] [Google Scholar]
- 82.FRANKE SILVIAIR, MOLZ P, MAI C. Influence of hesperidin and vitamin C on glycemic parameters, lipid profile, and DNA damage in rats treated with sucrose overload. An Acad Bras Cienc. 2018;90:2203–2210. doi: 10.1590/0001-3765201820170751 [DOI] [PubMed] [Google Scholar]
- 83.Kerimi A, Gauer JS, Crabbe S, et al. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br J Nutr. 2019;121(7):782–792. doi: 10.1017/S0007114519000084 [DOI] [PubMed] [Google Scholar]
- 84.Li S, Zhou X, Zheng T. Progress of studies of obese on mechanism and treatment by traditional Chinese medicine. Chin J Integr Traditional West Med. 2004;2(11):657–659. [Google Scholar]
- 85.Lima ACD, Cecatti C, Fidélix MP. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: controlled clinical trials. J Med Food. 2019;22(2):202–210. doi: 10.1089/jmf.2018.0080 [DOI] [PubMed] [Google Scholar]
- 86.Gong H. Regulation of Serum Partial Oxidation/Antioxidant Index in Young Obese People by 8-Week Aerobic Exercise and Its Effect on Intestinal Flora. Shandong Institute of Physical Education; 2018. [Google Scholar]
- 87.Colleluori G, Aguirre L, Phadnis U, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Luo F, Li P, She Y, Gao W. Investigation of the interaction for three Citrus flavonoids and α-amylase by surface plasmon resonance. Food Res Int. 2017;97:1–6. doi: 10.1016/j.foodres.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 89.Estruel-Amades S, Massot-Cladera M, Garcia-Cerdà P. Protective effect of hesperidin on the oxidative stress induced by an exhausting exercise in intensively trained rats. Nutrients. 2019;11(4):783. doi: 10.3390/nu11040783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caengprasath N, Ngamukote S, Mäkynen K, Adisakwattana S. The protective effects of pomelo extract (Citrus grandis L. Osbeck) against fructose-mediated protein oxidation and glycation. Excli J. 2013;12:491–502. [PMC free article] [PubMed] [Google Scholar]
- 91.Simitzis PE, Ilias-Dimopoulos V, Charismiadou MA, Biniari EE, Deligeorgis SG. Effects of dietary hesperidin supplementation on lamb performance and meat characteristics. Anim Sci J. 2013;84(2):136–143. doi: 10.1111/j.1740-0929.2012.01049.x [DOI] [PubMed] [Google Scholar]
- 92.Constantin RP, Nascimento GS, Constantin RP, et al. Citrus flavanones affect hepatic fatty acid oxidation in rats by acting as prooxidant agents. Biomed Res Int. 2013;2013:342973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang DH, Li M, Yu J. Advances in research on the relationship between chronic inflammation and obesity-type hypertension. J Clin Pathol. 2019;39(04):869–873. [Google Scholar]
- 94.Sun H, Sun LL, Pan SL, et al. The interaction of obesity on constitutional components and the status of imaging evaluation. Chin Med Imaging Technol. 2018;34(08):148–151. [Google Scholar]
- 95.Wu H, Liu Y, Chen X. Neohesperidin exerts lipid-regulating effects in vitro and in vivo via fibroblast growth factor 21 and AMP-activated protein kinase/sirtuin type 1/peroxisome proliferator-activated receptor gamma coactivator 1α signaling axis. Pharmacology. 2017;100:115–126. doi: 10.1159/000452492 [DOI] [PubMed] [Google Scholar]
- 96.Nakajima VM, Madeira JV Jr, Macedo GA, Macedo JA. Biotransformation effects on anti lipogenic activity of citrus extracts. Food Chem. 2016;11:1046–1053. [DOI] [PubMed] [Google Scholar]
- 97.Singh J, Sood S, Muthuraman A. In-vitro evaluation of bioactive compounds, anti-oxidant, lipid peroxidation and lipoxygenase inhibitory potential of Citrus karna L. peel extract. J Food Sci Technol. 2014;51(1):67–74. doi: 10.1007/s13197-011-0479-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Long T, Lv X, Xu Y. Supercritical fluid CO2 extraction of three polymethoxyflavones from Citri reticulatae pericarpium and subsequent preparative separation by continuous high-speed counter-current chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1124:284–289. doi: 10.1016/j.jchromb.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 99.Cui Y, Zhao J, Zhou J. Development of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for analysing nobiletin in citrus and herb samples. Food Chem. 2019;293:144–150. doi: 10.1016/j.foodchem.2019.04.101 [DOI] [PubMed] [Google Scholar]
- 100.Wan H-L, Chen H-Z, Shi X-Q. Study on effect of traditional Chinese medicine Jianpi Chushi decoction and ointment on chronic eczema. Asian Pac J Trop Med. 2016;9(9):920–923. doi: 10.1016/j.apjtm.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 101.Ademosun AO, Adebayo AA, Oboh G. Orange peels modulate antioxidant markers and key enzymes relevant to erection in the penile tissue of paroxetine-treated rats. Andrologia. 2019; 51:e13371. [DOI] [PubMed] [Google Scholar]
- 102.Fu M, Xu Y, Chen Y, et al. Evaluation of bioactive flavonoids and antioxidant activity in pericarpium citri reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017;230:649–656. doi: 10.1016/j.foodchem.2017.03.098 [DOI] [PubMed] [Google Scholar]
- 103.Dong L, Youngseok L, Yun K. Preventive effects of citrus unshiu peel extracts on bone and lipid metabolism in OVX rats. Molecules. 2014;19(1):783–794. doi: 10.3390/molecules19010783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng S-L, Li S-Z, Lai C-J-S. Evaluation of anti-lipase activity and bioactive flavonoids in the citri reticulatae pericarpium from different harvest time. Phytomed. 2018;43(01):103–109. doi: 10.1016/j.phymed.2018.04.008 [DOI] [PubMed] [Google Scholar]